Chronic egg laying is {a maladaptive, behavioural disorder} commonly seen in pet birds which repeatedly lay clutches of infertile eggs in the absence of a mate. It is particularly common in cockatiels, budgerigars, lovebirds, macaws and amazon parrots.[1] Birds exhibiting chronic egg laying behavior will frequently lay eggs one after the other without stopping to brood them once the typical clutch size for their particular species has been reached.[2] Excessive egg laying places a strain on the hen's body, depleting resources such as calcium, protein and vitamins from her body and may lead to conditions such as egg binding, osteoporosis, seizures, prolapse of the oviduct or peritonitis - which may lead to her death.[3][2]
https://en.wikipedia.org/wiki/Chronic_egg_laying
Egg binding occurs in animals, such as reptiles or birds, when an egg takes longer than usual to pass out of the reproductive tract.[1]
https://en.wikipedia.org/wiki/Egg_binding
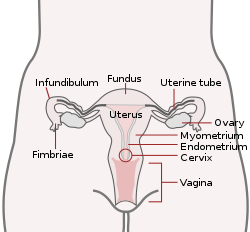
The oviduct in mammals, is the passageway from an ovary. In human females this is more usually known as the Fallopian tube or uterine tube. The eggs travel along the oviduct. These eggs will either be fertilized by spermatozoa to become a zygote, or will degenerate in the body. Normally, these are paired structures, but in birds and some cartilaginous fishes, one or the other side fails to develop (together with the corresponding ovary), and only one functional oviduct can be found.
Except in teleosts, the oviduct is not directly in contact with the ovary. Instead, the most anterior portion ends in a funnel-shaped structure called the infundibulum, which collects eggs as they are released by the ovary into the body cavity.
The only female vertebrates to lack oviducts are the jawless fishes. In these species, the single fused ovary releases eggs directly into the body cavity. The fish eventually extrudes the eggs through a small genital pore towards the rear of the body.
https://en.wikipedia.org/wiki/Oviduct
The fallopian tubes, also known as uterine tubes, oviducts[1] or salpinges (singular salpinx), are paired tubes in the human female that stretch from the uterus to the ovaries. The fallopian tubes are part of the female reproductive system. In other mammals they are only called oviducts.[2]
| Fallopian tube | |
|---|---|
 Uterus and fallopian tubes labelled as uterine tubes | |
| Details | |
| System | Reproductive system |
| Artery | tubal branches of ovarian artery, tubal branch of uterine artery via mesosalpinx |
| Lymph | lumbar lymph nodes |
| Identifiers | |
| Latin | Tuba uterina |
| Greek | Salpinx |
| MeSH | D005187 |
| TA98 | A09.1.02.001 |
| TA2 | 3486 |
| FMA | 18245 |
| Anatomical terminology | |
https://en.wikipedia.org/wiki/Fallopian_tube
Teleostei (/ˌtɛliˈɒstiaɪ/; Greek teleios "complete" + osteon "bone"), members of which are known as teleosts (/ˈtɛliɒsts, ˈtiːli-/),[4] is, by far, the largest infraclass in the class Actinopterygii, the ray-finned fishes,[a] containing 96% of all extant species of fish. Teleosts are arranged into about 40 orders and 448 families. Over 26,000 species have been described. Teleosts range from giant oarfish measuring 7.6 m (25 ft) or more, and ocean sunfish weighing over 2 t (2.0 long tons; 2.2 short tons), to the minute male anglerfish Photocorynus spiniceps, just 6.2 mm (0.24 in) long. Including not only torpedo-shaped fish built for speed, teleosts can be flattened vertically or horizontally, be elongated cylinders or take specialised shapes as in anglerfish and seahorses.
The difference between teleosts and other bony fish lies mainly in their jaw bones; teleosts have a movable premaxilla and corresponding modifications in the jaw musculature which make it possible for them to protrude their jaws outwards from the mouth. This is of great advantage, enabling them to grab prey and draw it into the mouth. In more derived teleosts, the enlarged premaxilla is the main tooth-bearing bone, and the maxilla, which is attached to the lower jaw, acts as a lever, pushing and pulling the premaxilla as the mouth is opened and closed. Other bones further back in the mouth serve to grind and swallow food. Another difference is that the upper and lower lobes of the tail (caudal) fin are about equal in size. The spine ends at the caudal peduncle, distinguishing this group from other fish in which the spine extends into the upper lobe of the tail fin.
Teleosts have adopted a range of reproductive strategies. Most use external fertilisation: the female lays a batch of eggs, the male fertilises them and the larvae develop without any further parental involvement. A fair proportion of teleosts are sequential hermaphrodites, starting life as females and transitioning to males at some stage, with a few species reversing this process. A small percentage of teleosts are viviparous and some provide parental care with typically the male fish guarding a nest and fanning the eggs to keep them well-oxygenated.
Teleosts are economically important to humans, as is shown by their depiction in art over the centuries. The fishing industry harvests them for food, and anglers attempt to capture them for sport. Some species are farmed commercially, and this method of production is likely to be increasingly important in the future. Others are kept in aquariums or used in research, especially in the fields of genetics and developmental biology.
https://en.wikipedia.org/wiki/Teleost
A rare giant oarfish (Regalecus glesne), 7-metre (23 ft) long, captured in 1996
https://en.wikipedia.org/wiki/Teleost
Hermaphroditism
Some teleost species are hermaphroditic, which can come in two forms: simultaneous and sequential. In the former, both spermatozoa and eggs are present in the gonads. Simultaneous hermaphroditism typically occurs in species that live in the ocean depths, where potential mates are sparsely dispersed.[81][82] Self-fertilisation is rare and has only been recorded in two species, Kryptolebias marmoratus and Kryptolebias hermaphroditus.[82] With sequential hermaphroditism, individuals may function as one sex early in their adult life and switch later in life. Species with this condition include parrotfish, wrasses, sea basses, flatheads, sea breams and lightfishes.[81]
Protandry is when an individual starts out male and becomes female while the reverse condition is known as protogyny, the latter being more common. Changing sex can occur in various contexts. In the bluestreak cleaner wrasse, where males have harems of up to ten females, if the male is removed the largest and most dominant female develops male-like behaviour and eventually testes. If she is removed, the next ranking female takes her place. In the species Anthias squamipinnis, where individuals gather into large groups and females greatly outnumber males, if a certain number of males are removed from a group, the same number of females change sex and replace them. In clownfish, individuals live in groups and only the two largest in a group breed: the largest female and the largest male. If the female dies, the male switches sexes and the next largest male takes his place.[83]
In deep-sea anglerfish (sub-order Ceratioidei), the much smaller male becomes permanently attached to the female and degenerates into a sperm-producing attachment. The female and their attached male become a "semi-hermaphroditic unit".[84]
https://en.wikipedia.org/wiki/Teleost#Hermaphroditism
Spawning sites and parental care
Teleosts may spawn in the water column or, more commonly, on the substrate. Water column spawners are mostly limited to coral reefs; the fish will rush towards the surface and release their gametes. This appears to protect the eggs from some predators and allow them to disperse widely via currents. They receive no parental care. Water column spawners are more likely than substrate spawners to spawn in groups. Substrate spawning commonly occurs in nests, rock crevices or even burrows. Some eggs can stick to various surfaces like rocks, plants, wood or shells.[89]
Of the oviparous teleosts, most (79 percent) do not provide parental care.[90] Male care is far more common than female care.[90][91] Male territoriality "preadapts" a species to evolve male parental care.[92][93] One unusual example of female parental care is in discuses, which provide nutrients for their developing young in the form of mucus.[94] Some teleost species have their eggs or young attached to or carried in their bodies. For sea catfishes, cardinalfishes, jawfishes and some others, the egg may be incubated or carried in the mouth, a practice known as mouthbrooding. In some African cichlids, the eggs may be fertilised there. In species like the banded acara, young are brooded after they hatch and this may be done by both parents. The timing of the release of young varies between species; some mouthbrooders release new-hatched young while other may keep then until they are juveniles. In addition to mouthbrooding, some teleost have also developed structures to carry young. Male nurseryfish have a bony hook on their foreheads to carry fertilised eggs; they remain on the hook until they hatch. For seahorses, the male has a brooding pouch where the female deposits the fertilised eggs and they remain there until they become free-swimming juveniles. Female banjo catfishes have structures on their belly to which the eggs attach.[95]
In some parenting species, young from a previous spawning batch may stay with their parents and help care for the new young. This is known to occur in around 19 species of cichlids in Lake Tanganyika. These helpers take part in cleaning and fanning eggs and larvae, cleaning the breeding hole and protecting the territory. They have reduced growth rate but gain protection from predators. Brood parasitism also exists among teleosts; minnows may spawn in sunfish nests as well as nests of other minnow species. The cuckoo catfish is known for laying eggs on the substrate as mouthbrooding cichclids collect theirs and the young catfish will eat the cichlid larvae. Filial cannibalism occurs in some teleost families and may have evolved to combat starvation.[96]
Growth and development
Teleosts have four major life stages: the egg, the larva, the juvenile and the adult. Species may begin life in a pelagic environment or a demersal environment (near the seabed). Most marine teleosts have pelagic eggs, which are light, transparent and buoyant with thin envelopes. Pelagic eggs rely on the ocean currents to disperse and receive no parental care. When they hatch, the larvae are planktonic and unable to swim. They have a yolk sac attached to them which provides nutrients. Most freshwater species produce demersal eggs which are thick, pigmented, relatively heavy and able to stick to substrates. Parental care is much more common among freshwater fish. Unlike their pelagic counterparts, demersal larvae are able to swim and feed as soon as they hatch.[81] Larval teleosts often look very different from adults, particularly in marine species. Some larvae were even considered different species from the adults. Larvae have high mortality rates, most die from starvation or predation within their first week. As they grow, survival rates increase and there is greater physiological tolerance and sensitivity, ecological and behavioural competence.[97]
At the juvenile stage, a teleost looks more like its adult form. At this stage, its axial skeleton, internal organs, scales, pigmentation and fins are fully developed. The transition from larvae to juvenile can be short and fairly simple, lasting minutes or hours as in some damselfish, while in other species, like salmon, squirrelfish, gobies and flatfishes, the transition is more complex and takes several weeks to complete.[98] At the adult stage, a teleost is able to produce viable gametes for reproduction. Like many fish, teleosts continue to grow throughout their lives. Longevity depends on the species with some gamefish like European perch and largemouth bass living up to 25 years. Rockfish appear to be the longest living teleosts with some species living over 100 years.[99]
Reproduction and lifecycle
Most teleost species are oviparous, having external fertilisation with both eggs and sperm being released into the water for fertilisation. Internal fertilisation occurs in 500 to 600 species of teleosts but is more typical for Chondrichthyes and many tetrapods. This involves the male inseminating the female with an intromittent organ.[72] Fewer than one in a million of externally fertilised eggs survives to develop into a mature fish, but there is a much better chance of survival among the offspring of members of about a dozen families which are viviparous. In these, the eggs are fertilised internally and retained in the female during development. Some of these species, like the live-bearing aquarium fish in the family Poeciliidae, are ovoviviparous; each egg has a yolk sac which nourishes the developing embryo, and when this is exhausted, the egg hatches and the larva is expelled into the water column. Other species, like the splitfins in the family Goodeidae, are fully viviparous, with the developing embryo nurtured from the maternal blood supply via a placenta-like structure that develops in the uterus. Oophagy is practised by a few species, such as Nomorhamphus ebrardtii; the mother lays unfertilised eggs on which the developing larvae feed in the uterus, and intrauterine cannibalism has been reported in some halfbeaks.[73]
There are two major reproductive strategies of teleosts; semelparity and iteroparity. In the former, an individual breeds once after reaching maturity and then dies. This is because the physiological changes that come with reproduction eventually lead to death.[74] Salmon of the genus Oncorhynchus are well known for this feature; they hatch in fresh water and then migrate to the sea for up to four years before travelling back to their place of birth where they spawn and die. Semelparity is also known to occur in some eels and smelts. The majority of teleost species have iteroparity, where mature individuals can breed multiple times during their lives.[75]
https://en.wikipedia.org/wiki/Teleost#Spawning_sites_and_parental_care
https://en.wikipedia.org/wiki/Oviduct
The fallopian tubes, also known as uterine tubes, oviducts[1] or salpinges (singular salpinx), are paired tubes in the human female that stretch from the uterus to the ovaries. The fallopian tubes are part of the female reproductive system. In other mammals they are only called oviducts.[2]
Each tube is a muscular hollow organ[3] that is on average between 10 and 14 cm (3.9 and 5.5 in) in length, with an external diameter of 1 cm (0.39 in).[4] It has four described parts: the intramural part, isthmus, ampulla, and infundibulum with associated fimbriae. Each tube has two openings a proximal opening nearest and opening to the uterus, and a distal opening furthest and opening to the abdomen. The fallopian tubes are held in place by the mesosalpinx, a part of the broad ligament mesentery that wraps around the tubes. Another part of the broad ligament, the mesovarium suspends the ovaries in place.[5]
An egg cell is transported from an ovary to a fallopian tube where it may be fertilized in the ampulla of the tube. The fallopian tubes are lined with simple columnar epithelium with hairlike extensions called cilia which together with peristaltic contractions from the muscular layer, move the fertilized egg (zygote) along the tube. On its journey to the uterus the zygote undergoes cell divisions that changes it to a blastocyst an early embryo, in readiness for implantation.[6]
Almost a third of cases of infertility are caused by fallopian tube pathologies. These include inflammation, and tubal obstructions. A number of tubal pathologies cause damage to the cilia of the tube which can impede movement of the sperm or egg.[7]
The name comes from the Italian Catholic priest and anatomist Gabriele Falloppio, for whom other anatomical structures are also named.[8]
Structure
Each fallopian tube leaves the uterus at an opening at the uterine horns known as the proximal tubal opening or proximal ostium.[9] The tubes have an average length of 10–14 centimeters (3.9–5.5 in)[4] that includes the intramural part of the tube. The tubes extend to near the ovaries where they open into the abdomen at the distal tubal openings. In other mammals the fallopian tube is called the oviduct which may also be used in reference to the fallopian tube in the human.[10][11] The fallopian tubes are held in place by the mesosalpinx a part of the broad ligament mesentery that wraps around the tubes. Another part of the broad ligament, the mesovarium suspends the ovaries in place.[5]
Parts
Each tube is composed of four parts: from inside the proximal tubal opening the intramural or interstitial part, that links to the narrow isthmus, the isthmus connects to the larger ampulla, which connects with the infundibulum and its associated fimbriae that opens into the peritoneal cavity from the distal tubal opening.[12]
Intramural part
The intramural part or interstitial part of the fallopian tube lies in the myometrium, the muscular wall of the uterus. This is the narrowest part of the tube that crosses the uterus wall to connect with the isthmus. The intramural part is 0.7 mm wide and 1 cm long.[12]
Isthmus
The narrow isthmus links the tube to the uterus, and connects to the ampulla. The isthmus is a rounded, and firm muscular part of the tube. The isthmus is 1–5 mm wide, and 3 cm long.[12] The isthmus contains a large number of secretory cells.[10]
Ampulla
The ampulla is the major part of the fallopian tube. The ampulla is the widest part of the tube with a maximal luminal diameter of 1 cm, and a length of 5 cm. It curves over the ovary, and is the primary site of fertilization.[12] The ampulla contains a large number of ciliated epithelial cells.[10] It is thin walled with a much folded luminal surface, and opens into the infundibulum.[12]
Infundibulum
The infundibulum opens into the abdomen at the distal tubal opening and rests above the ovary. Most cells here are ciliated epithelial cells.[10] The opening is surrounded by fimbriae, which help in the collection of the oocyte after ovulation.[4] The fimbriae (singular fimbria) is a fringe of densely ciliated tissue projections of approximately 1 mm in width around the distal tubal opening, oriented towards the ovary.[12] They are attached to the ends of the infundibulum, extending from its inner circumference, and muscular wall.[12] The cilia beat towards the fallopian tube.[12] Of all the fimbriae, one fimbria known as the ovarian fimbria is long enough to reach and make contact with the near part of the ovary during ovulation.[13][14][12] The fimbriae have a higher density of blood vessels than the other parts of the tube, and the ovarian fimbria is seen to have an even higher density.[8]
An ovary is not directly connected to its adjacent fallopian tube. When ovulation is about to occur, the sex hormones activate the fimbriae, causing them to swell with blood, extend, and hit the ovary in a gentle, sweeping motion. An oocyte is released from the ovary into the peritoneal cavity and the cilia of the fimbriae sweep it into the fallopian tube.
Microanatomy
When viewed under the microscope, the fallopian tube has three layers.[6] From outer to inner, these are the serosa, muscularis mucosae, and the mucosa.[15][16]
The outermost covering layer of serous membrane is known as the serosa.[6] The serosa is derived from the visceral peritoneum.[14]
The muscularis mucosae consists of an outer ring of smooth muscle arranged longitudinally, and a thick inner circular ring of smooth muscle.[6] This layer is responsible for the rhythmic peristaltic contractions of the fallopian tubes, that with the cilia move the egg cell towards the uterus.[14]
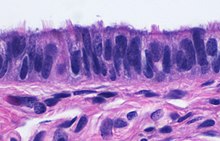
The innermost mucosa is made up of a layer of luminal epithelium, and an underlying thin layer of loose connective tissue the lamina propria.[16] There are three different cell types in the epithelium. Around 25% of the cells are ciliated columnar cells; around 60% are secretory cells, and the rest are peg cells thought to be a secretory cell variant.[4] The ciliated cells are most numerous in the infundibulum, and the ampulla. Estrogen increases the formation of cilia on these cells. Peg cells are shorter, have surface microvilli, and are located between the other epithelial cells.[6] The presence of immune cells in the mucosa has also been reported with the main type being CD8+ T-cells. Other cells found are B lymphocytes, macrophages, NK cells, and dendritic cells.[16]
The histological features of tube vary along its length. The mucosa of the ampulla contains an extensive array of complex folds, whereas the relatively narrow isthmus has a thick muscular coat and simple mucosal folds.[14]
Development
Embryos develop a genital ridge that forms at their tail end and eventually forms the basis for the urinary system and reproductive tracts. Either side and to the front of this tract, around the sixth week develops a duct called the paramesonephric duct, also called the Müllerian duct.[17] A second duct, the mesonephric duct, develops adjacent to this. Both ducts become longer over the next two weeks, and the paramesonephric ducts around the eighth week cross to meet in the midline and fuse.[17] One duct then regresses, with this depending on whether the embryo is genetically female or male. In females, the paramesonephric duct remains, and eventually forms the female reproductive tract.[17] The portions of the paramesonephric duct which are more cranial—that is, further from the tail-end, end up forming the fallopian tubes.[17] In males, because of the presence of the Y sex chromosome, anti-Müllerian hormone is produced. This leads to the degeneration of the paramesonephric duct.[17]
As the uterus develops, the part of the fallopian tubes closer to the uterus, the ampulla, becomes larger. Extensions from the fallopian tubes, the fimbriae, develop over time. Cell markers have been identified in the fimbriae which suggests that their embryonic origin is different from that of the other tube segments.[8]
Apart from the presence of sex chromosomes, specific genes associated with the development of the fallopian tubes include the Wnt and Hox groups of genes, Lim1, Pax2, and Emx2.[17]
Embryos have two pairs of ducts that will let gametes out of the body when they are adults; one pair (the Müllerian ducts) develops in females into the fallopian tubes, uterus, and vagina, while the other pair (the Wolffian ducts) develops in males into the epididymis and vas deferens.
The homologous organ in the male is the appendix testis.[18]
Function
Fertilization
The fallopian tube allows the passage of an egg from the ovary to the uterus. When an oocyte is developing in an ovary, it is surrounded by a spherical collection of cells known as an ovarian follicle. Just before ovulation, the primary oocyte completes meiosis I to form the first polar body and a secondary oocyte which is arrested in metaphase of meiosis II.
At the time of ovulation in the menstrual cycle, the secondary oocyte is released from the ovary. The follicle and the ovary's wall rupture, allowing the secondary oocyte to escape. The secondary oocyte is caught by the fimbriated end of the fallopian tube and travels to the ampulla. Here, the egg is able to become fertilized with sperm. The ampulla is typically where the sperm are met and fertilization occurs; meiosis II is promptly completed. After fertilization, the ovum is now called a zygote and travels toward the uterus with the aid of the hairlike cilia and the activity of the muscle of the fallopian tube. The early embryo requires critical development in the fallopian tube.[10] After about five days, the new embryo enters the uterine cavity and, on about the sixth day, begins to implant on the wall of the uterus.
The release of an oocyte does not alternate between the two ovaries and seems to be random. After removal of an ovary, the remaining one produces an egg every month.[19]
Clinical significance
Almost a third of cases of infertility are caused by fallopian tube pathologies. These include inflammation, and tubal obstructions. A number of tubal pathologies cause damage to the cilia of the tube which can impede movement of the sperm or egg. A number of sexually transmitted infections can lead to infertility.[7]
Inflammation
Salpingitis is inflammation of the fallopian tubes and may be found alone, or with other pelvic inflammatory diseases (PIDs). A thickening of the fallopian tube at its narrow isthmus portion, due to inflammation, is known as salpingitis isthmica nodosa. Like another PID endometriosis, it may lead to fallopian tube obstruction. Fallopian tube obstruction may be a cause of infertility or ectopic pregnancy.[20]
Blockage or narrowing
If a blocked fallopian tube has affected fertility, its repair where possible may increase the chances of becoming pregnant.[21] Tubal obstruction can be proximal, distal or mid-segmental.Tubal obstruction is a major cause of infertility but full testing of tubal functions is not possible. However, the testing of patency – whether or not the tubes are open can be carried out using hysterosalpingography, laparoscopy and dye, or hystero contrast sonography (HyCoSy). During surgery, the condition of the tubes may be inspected and a dye such as methylene blue can be injected into the uterus and shown to pass through the tubes when the cervix is occluded. As tubal disease is often related to Chlamydia infection, testing for Chlamydia antibodies has become a cost-effective screening device for tubal pathology.[22]
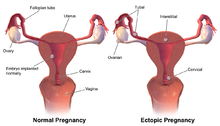
Ectopic pregnancy
Occasionally the embryo implants outside of the uterus, creating an ectopic pregnancy. Most ectopic pregnancies occur in the fallopian tube, and are commonly known as tubal pregnancies.[23]
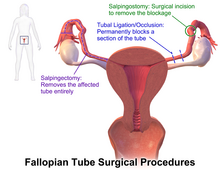
Surgery
The surgical removal of a fallopian tube is called a salpingectomy. To remove both tubes is a bilateral salpingectomy. An operation that combines the removal of a fallopian tube with the removal of at least one ovary is a salpingo-oophorectomy. An operation to remove a fallopian tube obstruction is called a tuboplasty. A surgical procedure to permanently prevent conception is tubal ligation.
Cancer
Fallopian tube cancer, which typically arises from the epithelial lining of the fallopian tube, has historically been considered to be a very rare malignancy. Evidence suggests it probably represents a significant portion of what has previously been classified as ovarian cancer, as much as 80 per cent. These are classed as serous carcinomas, and are usually located in the fimbriated distal tube.[24]
Other
In rare cases a fallopian tube may prolapse into the vaginal canal following a hysterectomy. The swollen fimbriae can have the appearance of an adenocarcinoma.[25]
History
The fallopian tube was first described as a structure linked to fertilization by the Greek physician Soranus of Ephesus (1st century AD).[8] The fallopian tubes were named by Vesalius after his assistant the 16th-century Italian anatomist Gabriele Falloppio, the first person to provide a detailed description of the tubes.[26][8] He thought they resembled trumpets, tube in Italian, which was misunderstood and became the English "tube".[27]
Though the name Fallopian tube is eponymous, it is often spelt with a lower case f from the assumption that the adjective fallopian has been absorbed into modern English as the de facto name for the structure. Merriam-Webster dictionary for example lists fallopian tube, often spelt Fallopian tube.[28]
Additional images
Cross-section of fallopian tube, stained and viewed under microscope
References
![]() This article incorporates text in the public domain from page 1257 of the 20th edition of Gray's Anatomy (1918)
This article incorporates text in the public domain from page 1257 of the 20th edition of Gray's Anatomy (1918)
He thought they reminded him of what were in those days rather long musical instruments with an end like a trumpet's bell, these were tubas. And so he called them tubas. And if you have a tuba, if you have a word ending in A in Italian, how do you pluralise it? What is two tuba? ... Tube. With an E on the end, spelled T-U-B-E. So, when it went around the world as his tube, his tubas, people saw the word tube. But, in fact, he had called them tubas.
- "Definition of FALLOPIAN TUBE". www.merriam-webster.com. Retrieved 26 September 2022.
External links
- Histology image: 18501loa – Histology Learning System at Boston University
- Menstrual Cycle - Merck
https://en.wikipedia.org/wiki/Fallopian_tube
The veiled chameleon (Chamaeleo calyptratus) is a species of chameleon (family Chamaeleonidae) native to the Arabian Peninsula in Yemen and Saudi Arabia. Other common names include cone-head chameleon, Yemen chameleon, and Yemeni chameleon[1] They are born pastel green and without their distinctive casques on their head. They grow this as well as become more colorful as they mature. They are known for their variable color changes due to a variety of factors, including to show aggression, social status, reproduction, and stress. Females live around 5 years and males live for around 8 and they breed a few times a year.
| Veiled chameleon | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Reptilia |
| Order: | Squamata |
| Suborder: | Iguania |
| Family: | Chamaeleonidae |
| Genus: | Chamaeleo |
| Species: | C. calyptratus
|
| Binomial name | |
| Chamaeleo calyptratus | |
 | |
https://en.wikipedia.org/wiki/Veiled_chameleon
A gonopore, sometimes called a gonadopore, is a genital pore in many invertebrates. Hexapods, including insects have a single common gonopore, except mayflies, which have a pair of gonopores.[1] More specifically, in the unmodified female it is the opening of the common oviduct, and in the male, it is the opening of the ejaculatory duct.
The position of the gonopore varies considerably between groups, but is generally constant within groups, allowing its position to be used as a "segmental marker". In Malacostraca, it is on the sixth thoracic segment; in Symphyla it is on the fourth trunk segment; in arachnids, it is on the second segment of the opisthosoma.[2] In insects and centipedes, the gonopores are close to the animal's tail,[2] while in millipedes they are on the third body segment behind the head, near the second pair of legs.[3]
https://en.wikipedia.org/wiki/Gonopore
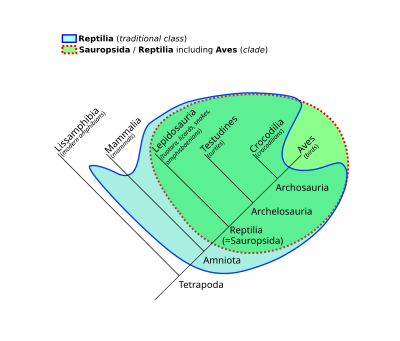
Sauropsida ("lizard faces") is a clade of amniotes, broadly equivalent to the class Reptilia. Sauropsida is the sister taxon to Synapsida, the other clade of amniotes which includes mammals as its only modern representatives. Although early synapsids have historically been referred to as "mammal-like reptiles", all synapsids are more closely related to mammals than to any modern reptile. Sauropsids, on the other hand, include all amniotes more closely related to modern reptiles than to mammals. This includes Aves (birds), which are now recognized as a subgroup of archosaurian reptiles despite originally being named as a separate class in Linnaean taxonomy.
The base of Sauropsida forks into two main groups of "reptiles": Eureptilia ("true reptiles") and Parareptilia ("next to reptiles"). Eureptilia encompasses all living reptiles (including birds), as well as various extinct groups. Parareptilia is typically considered to be an entirely extinct group, though a few hypotheses for the origin of turtles have suggested that they belong to the parareptiles. The clades Recumbirostra and Varanopidae, traditionally thought to be lepospondyls and synapsids respectively, may also be basal sauropsids. The term "Sauropsida" originated in 1864 with Thomas Henry Huxley,[1] who grouped birds with reptiles based on fossil evidence.
History of classification
Huxley and the fossil gaps
The term Sauropsida ("lizard faces") has a long history, and hails back to Thomas Henry Huxley, and his opinion that birds had risen from the dinosaurs. He based this chiefly on the fossils of Hesperornis and Archaeopteryx, that were starting to become known at the time.[2] In the Hunterian lectures delivered at the Royal College of Surgeons in 1863, Huxley grouped the vertebrate classes informally into mammals, sauroids, and ichthyoids (the latter containing the anamniotes), based on the gaps in physiological traits and lack of transitional fossils that seemed to exist between the three groups. Early in the following year he proposed the names Sauropsida and Ichthyopsida for the two latter.[1] Huxley did however include groups on the mammalian line (synapsids) like Dicynodon among the sauropsids. Thus, under the original definition, Sauropsida contained not only the groups usually associated with it today, but also several groups that today are known to be in the mammalian side of the tree.[3]
Sauropsids redefined
By the early 20th century, the fossils of Permian synapsids from South Africa had become well known, allowing palaeontologists to trace synapsid evolution in much greater detail. The term Sauropsida was taken up by E.S. Goodrich in 1916 much like Huxley's, to include lizards, birds and their relatives. He distinguished them from mammals and their extinct relatives, which he included in the sister group Theropsida (now usually replaced with the name Synapsida). Goodrich's classification thus differs somewhat from Huxley's, in which the non-mammalian synapsids (or at least the dicynodontians) fell under the sauropsids. Goodrich supported this division by the nature of the hearts and blood vessels in each group, and other features such as the structure of the forebrain. According to Goodrich, both lineages evolved from an earlier stem group, the Protosauria ("first lizards"), which included some Paleozoic amphibians as well as early reptiles predating the sauropsid/synapsid split (and thus not true sauropsids).[3]
Detailing the reptile family tree
In 1956, D.M.S. Watson observed that sauropsids and synapsids diverged very early in the reptilian evolutionary history, and so he divided Goodrich's Protosauria between the two groups. He also reinterpreted the Sauropsida and Theropsida to exclude birds and mammals respectively, making them paraphyletic, unlike Goodrich's definition. Thus his Sauropsida included Procolophonia, Eosuchia, Millerosauria, Chelonia (turtles), Squamata[4] (lizards and snakes), Rhynchocephalia, Crocodilia, "thecodonts" (paraphyletic basal Archosauria), non-avian dinosaurs, pterosaurs, ichthyosaurs, and sauropyterygians.[5]
This classification supplemented, but was never as popular as, the classification of the reptiles (according to Romer's classic Vertebrate Paleontology[6]) into four subclasses according to the positioning of temporal fenestrae, openings in the sides of the skull behind the eyes. Since the advent of phylogenetic nomenclature, the term Reptilia has fallen out of favor with many taxonomists, who have used Sauropsida in its place to include a monophyletic group containing the traditional reptiles and the birds.
Cladistics and the Sauropsida
The class Reptilia has been known to be an evolutionary grade rather than a clade for as long as evolution has been recognised. Reclassifying reptiles has been among the key aims of phylogenetic nomenclature.[7] The term Sauropsida had from the mid 20th century been used to denote all species not on the synapsid side after the synapsid/sauropsid split, a branch-based clade. This group encompasses all now-living reptiles as well as birds, and as such is comparable to Goodrich's classification, the difference being that better resolution of the early amniote tree has split up most of Goodrich's "Protosauria", though definitions of Sauropsida essentially identical to Huxley's (i.e. including the mammal-like reptiles) are also forwarded.[8][9] Some later cladistic work has used Sauropsida more restrictively, to signify the crown group, i.e. all descendants of the last common ancestor of extant reptiles and birds. A number of phylogenetic stem, node and crown definitions have been published, anchored in a variety of fossil and extant organisms, thus there is currently no consensus of the actual definition (and thus content) of Sauropsida as a phylogenetic unit.[10]
Some taxonomists, such as Benton (2004), have co-opted the term to fit into traditional rank-based classifications, making Sauropsida and Synapsida class-level taxa to replace the traditional Class Reptilia, while Modesto and Anderson (2004), using the PhyloCode standard, have suggested replacing the name Sauropsida with their redefinition of Reptilia, arguing that the latter is by far better known and should have priority.[10]
Evolutionary history
Sauropsids evolved from basal amniotes stock approximately 320 million years ago in the Paleozoic Era. In the Mesozoic Era (from about 250 million years ago to about 66 million years ago), sauropsids were the largest animals on land, in the water, and in the air. The Mesozoic is sometimes called the Age of Reptiles. Sixty-six million years ago, the large-bodied sauropsids died out in the global extinction event at the end of the Mesozoic era. With the exception of a few species of birds, the entire dinosaur lineage became extinct; in the following era, the Cenozoic, the remaining birds diversified so extensively that, today, nearly one out of every three species of land vertebrate is a bird species.
Phylogeny
The cladogram presented here illustrates the "family tree" of sauropsids, and follows a simplified version of the relationships found by M.S. Lee, in 2013.[11] All genetic studies have supported the hypothesis that turtles (formerly categorized together with ancient anapsids) are diapsid reptiles, despite lacking any skull openings behind their eye sockets; some studies have even placed turtles among the archosaurs,[11][12][13][14][15][16] though a few have recovered turtles as lepidosauromorphs instead.[17] The cladogram below used a combination of genetic (molecular) and fossil (morphological) data to obtain its results.[11]
| Amniota |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
Laurin & Piñeiro (2017) and Modesto (2019) proposed an alternate phylogeny of basal sauropsids. In this tree, parareptiles include turtles and are closely related to non-araeoscelidian diapsids. The family Varanopidae, otherwise included in Synapsida, is considered by Modesto a sauropsid group.[18][19]
|
|
| |||||||||||||||||||||||||||||||||||||||
|
|
In recent studies, the "microsaur" clade Recumbirostra, historically considered lepospondyl reptiliomorphs, have been recovered as early sauropsids.[20][21]
Structure difference with synapsids[edit]
The last common ancestor of synapsids and Sauropsida lived at around 320mya during Carboniferous, known as Reptiliomorpha.
Thermal and secretion
The early synapsids inherited abundant glands on their skins from their amphibian ancestors. Those glands evolved into sweat glands in synapsids, which granted them the ability to maintain constant body temperature but made them unable to save water from evaporation. Moreover, the way synapsids discharge nitrogenous waste is though urea, which is toxic and must be dissolved in water to be secreted. Unfortunately, the upcoming Permian and Triassic periods were arid periods. As a result, only a small percent of early synapsids survived in the land from South Africa to Antarctica in today's geography. Unlike synapsids, sauropsids do not have those glands on the skin; their way of nitrogenous waste emission is though uric acid which does not require water and can be excreted with feces. As a result, sauropsids were able to expand to all environments and reach their pinnacle. Even today, most vertebrates that live in arid environments are sauropsids, snakes and desert lizards for example.
Brain structure[edit]
Different from how synapsids have their cortex in 6 different layers of neurons which is called neocortex, the cerebrum of Sauropsida has a completely different structure. For the corresponding structure of the cerebrum in the classic view, the neocortex of synapsids is homology with only the Archicortex of the avian brain. However, in the modern view appeared since the 1960s, behavioral studies suggested that avian neostriatum and hyperstriatum can receive signals of vision, hearing, and body sensations, which means, they act just like neocortex. Comparing an avian brain to that to a mammal, nuclear-to-layered hypothesis proposed by Karten(1969), suggested that the cells which form layers in synapsids' neocortex, gather individually by type and form several nuclei. For synapsids, when one new function is adapted in evolution it will be assigned to a separate area of cortex, so for each function, synapsids will have to develop a separate area of cortex, and damage to that specific cortex may cause disability[22]. However, for Sauropsida functions are disassembled and assigned to all nuclei. In this case, brain function is highly flexible for Sauropsida, even with a small brain, many Sauropsida can still have a relatively high intelligence compared to mammals, for example, Corvidae. So, it is possible that dinosaurs, like T-rex which we know have tiny brains compared to their enormous body size, are much more intelligent than what people imagined.[23]
References
- 1. Jarvis, Güntürkün, O., Bruce, L., Csillag, A., Karten, H., Kuenzel, W., Medina, L., Paxinos, G., Perkel, D. J., Shimizu, T., Striedter, G., Wild, J. M., Ball, G. F., Dugas-Ford, J., Durand, S. E., Hough, G. E., Husband, S., Kubikova, L., Lee, D. W., … Butler, A. B. (2005). Avian brains and a new understanding of vertebrate brain evolution. Nature Reviews. Neuroscience, 6(2), 151–159. https://doi.org/10.1038/nrn1606
https://en.wikipedia.org/wiki/Sauropsida
Lungfish are freshwater vertebrates belonging to the class Dipnoi.[1] Lungfish are best known for retaining ancestral characteristics within the Osteichthyes, including the ability to breathe air, and ancestral structures within Sarcopterygii, including the presence of lobed fins with a well-developed internal skeleton. Lungfish represent the closest living relatives of the tetrapods. The mouths of lungfish typically bear tooth plates, which are used to crush hard shelled organisms.
Today there are only six known species of lungfish, living in Africa, South America, and Australia, though they were formerly globally distributed. The fossil record of the group extends into the Early Devonian, over 410 million years ago. The earliest known members of the group were marine, while almost all post-Carboniferous representatives inhabit freshwater environments.[2]
https://en.wikipedia.org/wiki/Lungfish
Chondrostei is a group of non-neopterygian ray-finned fish. While the term originally referred to the paraphyletic grouping of all non-neopterygian ray-finned fish, it was redefined by Patterson in 1982 to be a clade comprising the Acipenseriformes (which includes sturgeon and paddlefish) and their extinct relatives.[1]
Taxa commonly suggested to represent relatives of the Acipenseriformes include the Triassic marine fish Birgeria and the Saurichthyiformes, but their relationship with the Acipenseriformes has been strongly challenged on cladistical grounds.[2] Coccolepididae, a group of small weakly ossified Jurassic and Cretaceous fish found in both marine and freshwater environments, have been suggested to be close relatives of the Acipenseriformes. However, this has never been subject to cladistical analysis.[1]
https://en.wikipedia.org/wiki/Chondrostei
Eels are ray-finned fish belonging to the order Anguilliformes (/æŋˈɡwɪlɪfɔːrmiːz/), which consists of eight suborders, 19 families, 111 genera, and about 800 species. Eels undergo considerable development from the early larval stage to the eventual adult stage and are usually predators.
The term "eel" is also used for some other eel-shaped fish, such as electric eels (genus Electrophorus), spiny eels (family Mastacembelidae), swamp eels (family Synbranchidae), and deep-sea spiny eels (family Notacanthidae). However, these other clades evolved their eel-like shapes independently from the true eels. Eels live both in salt and fresh water, and some species are catadromous.
https://en.wikipedia.org/wiki/Eel
The Notacanthiformes /nɒtəˈkænθɪfɔːrmiːz/ are an order of deep-sea ray-finned fishes, consisting of the families Halosauridae and Notacanthidae (spiny eels).[1]
The order is of relatively recent vintage; Fishes of the World (2006) lists it as the suborder Notacanthoidei of Albuliformes.[2] The notacanthiforms are much more eel-like than the albuliforms; for instance, the caudal fin has disappeared.
Fish of the order are found in oceans worldwide, at depths from 120 to 4,900 metres (390 to 16,080 ft). They are elongated fish, although not as much so as the true eels. They typically feed on slow-moving or sessile animals, such as molluscs, echinoderms, and sea anemones. Like the true eels, they have a leptocephalus larva that floats in the surface waters before transforming into an adult. Unusually, the larva can often be larger than the adult.[3]
https://en.wikipedia.org/wiki/Notacanthiformes
The Gonorynchiformes /ɡɒnəˈrɪŋkɪfɔːrmiːz/ are an order of ray-finned fish that includes the important food source, the milkfish (Chanos chanos, family Chanidae), and a number of lesser-known types, both marine and freshwater.
The alternate spelling "Gonorhynchiformes", with an "h", is frequently seen but not official.
Gonorynchiformes have small mouths and no teeth. They are the sole group in the clade Anotophysi, a subgroup of the superorder Ostariophysi. They are characterized by a primitive Weberian apparatus formed by the first three vertebrae and one or more cephalic ribs within the head. This apparatus is believed to be a hearing organ, and is found in a more advanced and complex form in the related cypriniform fish, such as carp.[1] Also like the cypriniforms, the gonorynchiforms produce a substance from their skin when injured that dissolves into the water and acts an alarm signal to other fish.[2]
https://en.wikipedia.org/wiki/Gonorynchiformes
The Gymnotiformes /dʒɪmˈnɒtɪfɔːrmiːz/ are an order of teleost bony fishes commonly known as Neotropical knifefish or South American knifefish. They have long bodies and swim using undulations of their elongated anal fin. Found almost exclusively in fresh water (the only exceptions are species that occasionally may visit brackish water to feed), these mostly nocturnal fish are capable of producing electric fields to detect prey, for navigation, communication, and, in the case of the electric eel (Electrophorus electricus), attack and defense.[2] A few species are familiar to the aquarium trade, such as the black ghost knifefish (Apteronotus albifrons), the glass knifefish (Eigenmannia virescens), and the banded knifefish (Gymnotus carapo).
https://en.wikipedia.org/wiki/Gymnotiformes
Stomiiformes /ˈstɒmi.ɪfɔːrmiːz/ is an order of deep-sea ray-finned fishes of very diverse morphology. It includes, for example, dragonfishes, lightfishes (Gonostomatidae and Phosichthyidae), loosejaws, marine hatchetfishes and viperfishes. The order contains 4 families (5 according to some authors) with more than 50 genera and at least 410 species. As usual for deep-sea fishes, there are few common names for species of the order, but the Stomiiformes as a whole are often called dragonfishes and allies or simply stomiiforms.[1]
The scientific name means "Stomias-shaped", from Stomias (the type genus) + the standard fish order suffix "-formes". It ultimately derives from Ancient Greek stóma (στόμᾶ, "mouth") + Latin forma ("external form"), the former in reference to the huge mouth opening of these fishes.[2]
https://en.wikipedia.org/wiki/Stomiiformes
The jellynose fishes or tadpole fishes are the small order Ateleopodiformes. This group of ray-finned fish is monotypic, containing a single family Ateleopodidae. It has about a dozen species in four genera, but these enigmatic fishes are in need of taxonomic revision. [1]
The scientific name means "Ateleopus-shaped", from Ateleopus (the type genus) + the standard fish order suffix "-formes". It ultimately derives from Ancient Greek atelēs (ἀτελής, "imperfect") + pous (πούς, "foot") + Latin forma ("external form"), the Greek part in reference to the reduced pectoral and ventral fins of the jellynoses.[2]
https://en.wikipedia.org/wiki/Jellynose_fish
The beardfishes consist of a single extant genus, Polymixia, of deep-sea marine ray-finned fish named for their pair of long hyoid barbels. They are classified in their own order Polymixiiformes /pɒliˈmɪksi.ɪfɔːrmiːz/.[1] But as Nelson says, "few groups have been shifted back and forth as frequently as this one, and they were recently added to Paracanthoptergii".[2] For instance, they have previously been classified as belonging to the Beryciformes. They are of little economic importance.[3]
They are found in tropical and subtropical waters of the Atlantic, Indian and western Pacific Ocean. They are bottom-dwelling fish, found down to about 800 m (2,600 ft) depth. Most are relatively small fish, although one species, Polymixia berndti, is over 40 cm (16 in) in length.[3]
https://en.wikipedia.org/wiki/Beardfish












No comments:
Post a Comment