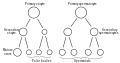
The spermatid is the haploid male gametid that results from division of secondary spermatocytes. As a result of meiosis, each spermatid contains only half of the genetic material present in the original primary spermatocyte.
Spermatids are connected by cytoplasmic material and have superfluous cytoplasmic material around their nuclei.
When formed, early round spermatids must undergo further maturational events to develop into spermatozoa, a process termed spermiogenesis (also termed spermeteliosis).
The spermatids begin to grow a living thread, develop a thickened mid-piece where the mitochondria become localised, and form an acrosome. Spermatid DNA also undergoes packaging, becoming highly condensed. The DNA is packaged firstly with specific nuclear basic proteins, which are subsequently replaced with protamines during spermatid elongation. The resultant tightly packed chromatin is transcriptionally inactive.
In 2016 scientists at Nanjing Medical University claimed they had produced cells resembling mouse spermatids artificially from stem cells. They injected these spermatids into mouse eggs and produced pups.[1]
| Spermatid | |
|---|---|
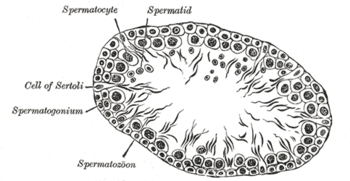
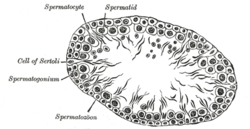
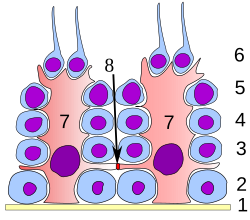 Germinal epithelium of the testicle. 1: basal lamina 2: spermatogonia 3: spermatocyte 1st order 4: spermatocyte 2nd order 5: spermatid 6: mature spermatid 7: Sertoli cell 8: tight junction (blood testis barrier) | |
 | |
| Identifiers | |
| MeSH | D013087 |
| FMA | 72294 |
| Anatomical terminology | |
https://en.wikipedia.org/wiki/Spermatid
The spermatic cord is the cord-like structure in males formed by the vas deferens (ductus deferens) and surrounding tissue that runs from the deep inguinal ring down to each testicle. Its serosal covering, the tunica vaginalis, is an extension of the peritoneum that passes through the transversalis fascia. Each testicle develops in the lower thoracic and upper lumbar region and migrates into the scrotum. During its descent it carries along with it the vas deferens, its vessels, nerves etc. There is one on each side.
| Spermatic cord | |
|---|---|
 Anatomy of the human male reproductive system | |
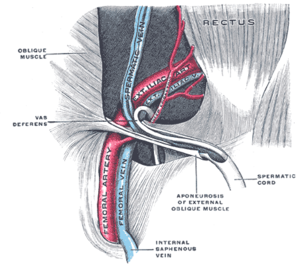 The spermatic cord in the inguinal canal (label for spermatic cord in lower right) | |
| Details | |
| Identifiers | |
| Latin | Funiculus spermaticus |
| MeSH | D013085 |
| TA98 | A09.3.04.001 |
| TA2 | 3615 |
| FMA | 19937 |
| Anatomical terminology | |
Structure[edit]
The spermatic cord is ensheathed in three layers of tissue:
- external spermatic fascia, an extension of the innominate fascia that overlies the aponeurosis of the external oblique muscle.[1]
- cremasteric muscle and fascia, formed from a continuation of the internal oblique muscle and its fascia.[1]
- internal spermatic fascia, continuous with the transversalis fascia.[1]
The normal diameter of the spermatic cord is about 16 mm (range 11 to 22 mm).[2] It is located behind the tunica vaginalis.[3]
Contents[edit]
Blood vessels[edit]
Nerves[edit]
- Nerve to cremaster (genital branch of the genitofemoral nerve)
- Testicular nerves (sympathetic nerves).
The ilioinguinal nerve is not actually located inside the spermatic cord, but runs outside it in the inguinal canal.
Other contents[edit]
The tunica vaginalis is located in front of the spermatic cord, outside it.[3]
Clinical significance[edit]
The spermatic cord is sensitive to torsion, in which the testicle rotates within its sac and blocks its own blood supply. Testicular torsion may result in irreversible damage to the testicle within hours. A collection of serous fluid in the spermatic cord is named 'funiculocele'.
The contents of the abdominal cavity may protrude into the inguinal canal, producing an indirect inguinal hernia
Varicose veins of the spermatic cord are referred to as varicocele. Though often asymptomatic, about one in four people with varicocele have negatively affected fertility.[4]
Additional images[edit]
https://en.wikipedia.org/wiki/Spermatic_cord
Spermatidogenesis is the creation of spermatids from secondary spermatocytes during spermatogenesis.
Secondary spermatocytes produced earlier rapidly enter meiosis II and divide to produce haploid spermatids.
The brevity of this stage means that secondary spermatocytes are rarely seen in histological preparations. Mouse stem cells were grown into cells resembling spermatids in 2016. These spermatids, when injected into mouse eggs, were able to produced pups.[1]
https://en.wikipedia.org/wiki/Spermatidogenesis
The glandular structure of the testis consists of numerous lobules.
Their number, in a single testis, is estimated by Berres at 250, and by Krause at 400.[1] Anatomic studies have demonstrated figures of 250–290 for the same.[2]
They differ in size according to their position, those in the middle of the gland being larger and longer.
The lobules are conical in shape, the base being directed toward the circumference of the organ, the apex toward the mediastinum testis.
Each lobule is contained in one of the intervals between the fibrous septa which extend between the mediastinum testis and the tunica albuginea, and consists of from one to three, or more, minute convoluted tubes, the tubuli seminiferi.
| Lobules of testis | |
|---|---|
 A diagram of the major components of an adult human testicle, including the following numbered items: 1. Tunica albuginea, 2. Septula testis, 3. Lobulus testis, 4. Mediastinum testis, 5. Tubuli seminiferi contorti, 6. Tubuli seminiferi recti, 7. Rete testis, 8. Ductuli efferentes testis, 9a. Head of epididymis, 9b. Body of epididymis, 9.c Tail of epididymis,10. Vas deferens, 11a. Tunica vaginalis (parietal lamina), 11b. Tunica vaginalis (visceral lamina), and 12. Cavity of tunica vaginalis. | |
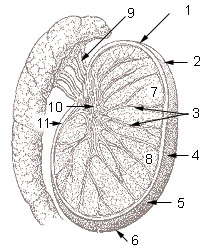 1: Head or upper pole of testis, 2: Tunica albuginea, 3: Testicular septa, 4: Anterior margin (free margin), 5: Lateral surface, 6: Tail or lower pole of testis, 7: Testicular lobules, 8: Parenchyma of testis, 9: Efferent ductules, 10: Mediastinum testis, 11: Posterior margin | |
| Details | |
| Identifiers | |
| Latin | lobuli testis |
| Anatomical terminology | |
This article incorporates text in the public domain from the 20th edition of Gray's Anatomy (1918)
- ^ Basu, SC. (2011). Male Reproductive Dysfunction. Jaypee Brothers Publishers. p. 16. ISBN 978-93-5025-703-6.
- ^ Countouris, N; Holstein, AF (Nov–Dec 1985). "[How many testicular lobules does a human testicle contain? Reexamination of an old problem]". Andrologia (in German). 17 (6): 525–31. doi:10.1111/j.1439-0272.1985.tb01707.x. PMID 4083540. S2CID 86699538.
https://en.wikipedia.org/wiki/Lobules_of_testis
Seminiferous tubules are located within the testes, and are the specific location of meiosis, and the subsequent creation of male gametes, namely spermatozoa.
| Seminiferous tubule | |
|---|---|
 | |
 | |
| Details | |
| Identifiers | |
| Latin | tubuli seminiferi |
| MeSH | D012671 |
| TA98 | A09.3.01.022 |
| TA2 | 3599 |
| FMA | 19825 |
| Anatomical terminology | |
Structure[edit]
The epithelium of the tubule consists of a type of sustentacular cells known as Sertoli cells, which are tall, columnar type cells that line the tubule.
In between the Sertoli cells are spermatogenic cells, which differentiate through meiosis to sperm cells. Sertoli cells function to nourish the developing sperm cells. They secrete androgen-binding protein, a binding protein which increases the concentration of testosterone inside the seminiferous tubules. Embryologically, they also secrete the anti-Müllerian hormone (AMH) necessary for the female Müllerian ducts to regress.
There are two types: convoluted and straight, convoluted toward the lateral side, and straight as the tubule comes medially to form ducts that will exit the testis.
The seminiferous tubules are formed from the testis cords that develop from the primitive gonadal cords, formed from the gonadal ridge.
Function[edit]
Spermatogenesis, the process for producing spermatozoa, takes place in the seminiferous tubules. During spermatogenesis, the DNA of spermatogenic cells in the seminiferous tubules is subject to damage from such sources as reactive oxygen species.[1] The genomic integrity of spermatogenic cells is protected by DNA repair processes.[2]Deficiencies in the enzymes employed in these repair processes may lead to infertility.[2]
Additional images[edit]
https://en.wikipedia.org/wiki/Seminiferous_tubule
The tunica vaginalis is the pouch of serous membrane that covers the testes. It is derived from the vaginal process of the peritoneum, which in the fetus precedes the descent of the testes from the abdomen into the scrotum.[citation needed]
After its descent, that portion of the pouch which extends from the abdominal inguinal ring to near the upper part of the gland becomes obliterated; the lower portion remains as a shut sac, which invests the surface of each testis, and is reflected on to the internal surface of the scrotum; hence it may be described as consisting of a visceral and a parietal lamina.[citation needed]
| Tunica vaginalis | |
|---|---|
 Diagram of a cross-section of a testicle. 1. Cavity of tunica vaginalis, 2. Visceral lamina, 3. Parietal lamina. | |
 The right testis, exposed by laying open the tunica vaginalis. (Tunica vaginalis is labeled at upper right.) | |
| Details | |
| Identifiers | |
| Latin | tunica vaginalis testis |
| Anatomical terminology | |
The tunica vaginalis is the pouch of serous membrane that covers the testes. It is derived from the vaginal process of the peritoneum, which in the fetus precedes the descent of the testes from the abdomen into the scrotum.[citation needed]
After its descent, that portion of the pouch which extends from the abdominal inguinal ring to near the upper part of the gland becomes obliterated; the lower portion remains as a shut sac, which invests the surface of each testis, and is reflected on to the internal surface of the scrotum; hence it may be described as consisting of a visceral and a parietal lamina.[citation needed]
Visceral lamina[edit]
The visceral lamina (lamina visceralis) covers the greater part of the testis and epididymis, connecting the latter to the testis by means of a distinct fold. From the posterior border of the gland it is reflected on to the internal surface of the scrotum.[citation needed]
Parietal lamina[edit]
The parietal lamina (lamina parietalis) is far more extensive than the visceral, extending upward for some distance in front and on the medial side of the cord, and reaching below the testis. The inner surface of the tunica vaginalis is smooth, and covered by a layer of simple squamous mesothelial cells. The interval between the visceral and parietal laminæ constitutes the cavity of the tunica vaginalis.[citation needed]
Cavum vaginale[edit]
The cavum vaginale is the cavity of the tunica vaginalis. The testicle hangs suspended in a space, the cavum vaginale, from which is separated by the visceral layer of the tunica vaginalis. The latter is continued on to the epididymis, at the lateral margins of which it is reflected forward as the parietal layer, and as this is more extensive than the visceral layer, the cavum vaginale-named cavity results. The testicle is enclosed in a thick capsule of fibrous tissue, the tunica albuginea. The tunica albuginea sends prolongations inwards, dividing the testicle into lobules. Each lobule contains the seminiferous tubules, extending from the base where they end blindly, towards the apex.[1]
Diseases[edit]
- Mesothelioma[citation needed]
- Hydrocele[2]
- Cartilaginous Bodies[3]
- Hematocele[4]
https://en.wikipedia.org/wiki/Tunica_vaginalis
The superior ligament of the epididymis is a strand of fibrous tissue which is covered by a reflection of the tunica vaginalis and connects the upper aspect of the epididymis with the testis.
https://en.wikipedia.org/wiki/Superior_ligament_of_epididymis
The fundiform ligament or fundiform ligament of the penis is a specialization or thickening of the superficial (Scarpa's) fascia extending from the linea alba of the lower abdominal wall.
It runs from the level of the pubic bone, laterally around the sides of the penis like a sling, and then unites at the base of the penis before going to the septum of the scrotum.
It is just superficial to the suspensory ligament.
Although rarely mentioned, this ligament is also found in females.
| Fundiform ligament | |
|---|---|
 | |
 Vertical section of bladder, penis, and urethra. | |
| Details | |
| Identifiers | |
| Latin | ligamentum fundiforme penis |
| TA98 | A04.5.02.023M |
| TA2 | 3692 |
| FMA | 19659 |
| Anatomical terminology | |
https://en.wikipedia.org/wiki/Fundiform_ligament
A corpus cavernosum penis (singular) (literally "cave-like body" of the penis, plural corpora cavernosa) is one of a pair of sponge-like regions of erectile tissue, which contain most of the blood in the penis during an erection.[1][2][3]
Such a corpus is homologous to the corpus cavernosum clitoridis in the female; the body of the clitoris that contains erectile tissue in a pair of corpora cavernosa with a recognisably similar structure.
| Corpus cavernosum penis | |
|---|---|
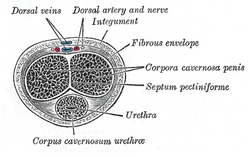 Transverse section of the penis. | |
 The constituent cavernous cylinders of the penis. | |
| Details | |
| Identifiers | |
| Latin | corpus cavernosum penis |
| TA98 | A09.4.01.014 |
| TA2 | 3678 |
| FMA | 19618 |
| Anatomical terminology | |
Anatomy[edit]
The two corpora cavernosa and corpus spongiosum (also known as the corpus cavernosum urethrae in older texts and in the adjacent diagram) are three expandable erectile tissues along the length of the penis, which fill with blood during penile erection. The two corpora cavernosa lie along the penis shaft, from the pubic bones to the head of the penis, where they join. These formations are made of a sponge-like tissue containing trabeculae, irregular blood-filled spaces lined by endothelium and separated by septum of the penis.[4][5]
The male anatomy has no vestibular bulbs, but instead a corpus spongiosum, a smaller region along the bottom of the penis, which contains the urethra and forms the glans penis.
Physiology[edit]
In some circumstances, release of nitric oxide precedes relaxation of muscles in the corpora cavernosa and corpus spongiosum, in a process similar to female arousal. The spongy tissue fills with blood, from arteries down the length of the penis. A little blood enters the corpus spongiosum; the remainder engorges the corpora cavernosa, which expand to hold 90% of the blood involved in an erection, increasing both in length and in diameter. The function of the corpus spongiosum is to prevent compression of the urethra during erection.
Blood can leave the erectile tissue only through a drainage system of veins around the outside wall of the corpus cavernosum. The expanding spongy tissue presses against a surrounding dense tissue (tunica albuginea) constricting these veins, preventing blood from leaving. The penis becomes rigid as a result. The glans penis, the expanded cap of the corpus spongiosum, remains more malleable during erection because its tunica albuginea is much thinner than elsewhere in the penis.
Additional images[edit]

Structure of the penis

The deeper branches of the internal pudendal artery.
The penis in transverse section, showing the bloodvessels.

Male pelvic organs seen from right side.

Diagram of the arteries of the penis.
Cross section of penis.

Medical ultrasonographyof a normal penis.
https://en.wikipedia.org/wiki/Corpus_cavernosum_penis
| Internal pudendal artery | |
|---|---|
 Internal iliac artery with branches, including internal pudendal artery. | |
 The superficial branches of the internal pudendal artery. | |
| Details | |
| Source | internal iliac artery |
| Vein | Internal pudendal veins |
| Supplies | external genitalia, perineum |
| Identifiers | |
| Latin | arteria pudenda interna |
| TA98 | A12.2.15.038 |
| TA2 | 4341 |
| FMA | 18835 |
| Anatomical terminology | |
Structure[edit]
The internal pudendal artery is the terminal branch of the anterior trunk of the internal iliac artery.[1] It is smaller in the female than in the male.
Path[edit]
It arises from the anterior division of internal iliac artery. It runs on the lateral pelvic wall. It exits the pelvic cavity through the greater sciatic foramen, inferior to the piriformis muscle, to enter the gluteal region.
It then curves around the sacrospinous ligament to enter the perineum through the lesser sciatic foramen.
It travels through the pudendal canal with the internal pudendal veins and the pudendal nerve.
Branches[edit]
The internal pudendal artery gives off the following branches:
The deep artery of clitoris is a branch of the internal pudendal artery and supplies the clitoral crura. Another branch of the internal pudendal artery is the dorsal artery of clitoris.
Some sources consider the urethral artery a direct branch of the internal pudendal artery,[2] while others consider it a branch of the perineal artery.[citation needed]
In males, the internal pudendal artery also gives rise to the perforating arteries of the penis.[1]
Variation[edit]
Around 70% of men have an accessory internal pudendal artery.[1] This usually does not originate from the internal iliac artery, instead originating from the external iliac artery, the obturator artery, or the vesical arteries.[1]
Function[edit]
The internal pudendal artery supplies blood to the external genitalia.
Clinical significance[edit]
In women, the internal pudendal artery may be damaged during childbirth.[3] This may cause a haematoma, which usually resolves without treatment, but may form an infected abscess.[3]
Additional images[edit]
See also[edit]
The pudendal nerve is the main nerve of the perineum.[1]: 274 It carries sensation from the external genitalia of both sexes and the skin around the anus and perineum, as well as the motor supply to various pelvic muscles, including the male or female external urethral sphincter and the external anal sphincter. If damaged, most commonly by childbirth, lesions may cause sensory loss or fecal incontinence. The nerve may be temporarily blocked as part of an anaesthetic procedure.
The pudendal canal that carries the pudendal nerve is also known by the eponymous term "Alcock's canal", after Benjamin Alcock, an Irish anatomist who documented the canal in 1836.
| Pudendal nerve | |
|---|---|
 Pudendal nerve, course and branches in a male. | |
 Cross-section of female pelvis in which nerve emerges from S2, S3, and S4 extends between the uterus and the anus and into labium minora, labium majora and the clitoris | |
| Details | |
| From | Sacral nerves S2, S3, S4 |
| To | Inferior rectal nerves perineal nerve dorsal nerve of the penis dorsal nerve of the clitoris |
| Identifiers | |
| Latin | Nervus pudendus |
| MeSH | D060525 |
| TA98 | A14.2.07.037 |
| TA2 | 6554 |
| FMA | 19037 |
| Anatomical terms of neuroanatomy | |
Structure[edit]
The pudendal nerve is paired, meaning there are two nerves, one on the left and one on the right side of the body. Each is formed as three roots immediately converge above the upper border of the sacrotuberous ligament and the coccygeus muscle.[2] The three roots become two cords when the middle and lower root join to form the lower cord, and these in turn unite to form the pudendal nerve proper just proximal to the sacrospinous ligament.[3] The three roots are derived from the ventral rami of the second, third, and fourth sacral spinal nerves, with the primary contribution coming from the fourth.[2][4]: 215 [5]: 157
The pudendal nerve passes between the piriformis muscle and coccygeus (ischiococcygeus) muscles and leaves the pelvis through the lower part of the greater sciatic foramen.[2] It crosses over the lateral part of the sacrospinous ligamentand reenters the pelvis through the lesser sciatic foramen. After reentering the pelvis, it accompanies the internal pudendal artery and internal pudendal vein upwards and forwards along the lateral wall of the ischiorectal fossa, being contained in a sheath of the obturator fascia termed the pudendal canal, along with the internal pudendal blood vessels.[6]: 8
Inside the pudendal canal, the nerve divides into branches, first giving off the inferior rectal nerve, then the perineal nerve, before continuing as the dorsal nerve of the penis (in males) or the dorsal nerve of the clitoris (in females).[6]: 34
Nucleus[edit]
The nerve is a major branch of the sacral plexus,[7]: 950 with fibers originating in Onuf's nucleus in the sacral region of the spinal cord.[3]
Variation[edit]
The pudendal nerve may vary in its origins. For example, the pudendal nerve may actually originate in the sciatic nerve.[8]Consequently, damage to the sciatic nerve can affect the pudendal nerve as well. Sometimes dorsal rami of the first sacral nerve contribute fibers to the pudendal nerve, and even more rarely S5.[3]
Function[edit]
The pudendal nerve has both motor and sensory functions. It does not carry parasympathetic fibers but does carry sympatheticfibers.[9]: 1738
The pudendal nerve supplies sensation to the penis in males, and to the clitoris in females, which travels through the branches of both the dorsal nerve of the penis and the dorsal nerve of the clitoris.[10]: 422 The posterior scrotum in males and the labia in females are also supplied, via the posterior scrotal nerves (males) or posterior labial nerves (females). The pudendal nerve is one of several nerves supplying sensation to these areas.[11] Branches also supply sensation to the anal canal.[6]: 8 By providing sensation to the penis and the clitoris, the pudendal nerve is responsible for the afferent component of penile erection and clitoral erection.[12] : 147 It is also responsible for ejaculation.[13]
Branches also innervate muscles of the perineum and the pelvic floor; namely, the bulbospongiosus and the ischiocavernosus muscles respectively[11], the levator animuscle (including the Iliococcygeus, pubococcygeus, puborectalis and either pubovaginalis in females or puboprostaticus in males)[10]: 422 [14] the external anal sphincter(via the inferior anal branch),[6]: 7 and male or female external urethral sphincter.[10]: 424–425
As it functions to innervate the external urethral sphincter it is responsible for the tone of the sphincter mediated via acetylcholine release. This means that during periods of increased acetylcholine release the skeletal muscle in the external urethral sphincter contracts, causing urinary retention. Whereas in periods of decreased acetylcholine release the skeletal muscle in the external urethral sphincter relaxes, allowing voiding of the bladder to occur.[15] (Clarification: Unlike the internal sphincter muscle, the external sphincter is made of skeletal muscle, therefore it is under voluntary control of the somatic nervous system.)
Clinical significance[edit]
Anesthesia[edit]
A pudendal nerve block, also known as a saddle nerve block, is a local anesthesia technique used in an obstetric procedure to anesthetize the perineum during labor.[16] In this procedure, an anesthetic agent such as lidocaine is injected through the inner wall of the vagina about the pudendal nerve.[17]
Damage[edit]
The pudendal nerve can be compressed or stretched, resulting in temporary or permanent neuropathy. Irreversible nerve injury may occur when nerves are stretched by 12% or more of their normal length.[6]: 655 If the pelvic floor is over-stretched, acutely (e.g. prolonged or difficult childbirth) or chronically (e.g. chronic straining during defecation caused by constipation), the pudendal nerve is vulnerable to stretch-induced neuropathy.[6]: 655 Pudendal nerve entrapment, also known as Alcock canal syndrome, is very rare and is associated with professional cycling.[18] Systemic diseases such as diabetes and multiple sclerosis can damage the pudendal nerve via demyelination or other mechanisms.[6]: 37 A pelvic tumor (most notably a large sacrococcygeal teratoma), or surgery to remove the tumor, can also cause permanent damage.[19]
Unilateral pudendal nerve neuropathy inconsistently causes fecal incontinence in some, but not others. This is because crossover innervation of the external anal sphincter occurs in some individuals.[6]: 34
Imaging[edit]
The pudendal nerve is difficult to visualize on routine CT or MR imaging, however under CT guidance, a needle may be placed adjacent to the pudendal neurovascular bundle. The ischial spine, an easily identifiable structure on CT, is used as the level of injection. A spinal needle is advanced via the gluteal muscles and advanced within several millimeters of the ischial spine. Contrast (X-ray dye) is then injected, highlighting the nerve in the canal and allowing for confirmation of correct needle placement. The nerve may then be injected with cortisone and local anesthetic to confirm and also treat chronic pain of the external genitalia (known as vulvodynia in females), pelvic and anorectal pain.[20][21]
Nerve latency testing[edit]
The time taken for a muscle supplied by the pudendal nerve to contract in response to an electrical stimulus applied to the sensory and motor fibers can be quantified. Increased conduction time (terminal motor latency) signifies damage to the nerve.[22]: 46 2 stimulating electrodes and 2 measuring electrodes are mounted on the examiner's gloved finger ("St Mark's electrode").[22]: 46
History[edit]
The term pudendal comes from Latin pudenda, meaning external genitals, derived from pudendum, meaning "parts to be ashamed of".[23] The pudendal canal is also known by the eponymous term "Alcock's canal", after Benjamin Alcock, an Irish anatomist who documented the canal in 1836. Alcock documented the existence of the canal and pudendal nerve in a contribution about iliac arteries in Robert Bentley Todd's "The Cyclopaedia of Anatomy and Physiology".[24]
Additional images[edit]

The male pelvis, showing the pudendal nerve (centre right)

Schematic showing the structures innervated by the pudendal nerve

Diagram of the course of the pudendal nerve in the male pelvis
https://en.wikipedia.org/wiki/Pudendal_nerve
The anterior divisions of the lumbar nerves, sacral nerves, and coccygeal nerve form the lumbosacral plexus, the first lumbar nerve being frequently joined by a branch from the twelfth thoracic. For descriptive purposes this plexus is usually divided into three parts:
Injuries to the lumbosacral plexus are predominantly witnessed as bone injuries. Lumbosacral trunk and sacral plexuspalsies are common injury patterns.[1]
References[edit]
![]() This article incorporates text in the public domain from page 948 of the 20th edition of Gray's Anatomy (1918)
This article incorporates text in the public domain from page 948 of the 20th edition of Gray's Anatomy (1918)
- ^ Garozzo, Debora; Zollino, Gianluca; Ferraresi, Stefano (January 11, 2014). "In lumbosacral plexus injuries can we identify indicators that predict spontaneous recovery or the need for surgical treatment? Results from a clinical study on 72 patients". Journal of Brachial Plexus and Peripheral Nerve Injury. 9 (1): 1. doi:10.1186/1749-7221-9-1. PMC 3896705. PMID 24410760.
External links[edit]
- Atlas image: abdo_wall72 at the University of Michigan Health System - "Lumbosacral Plexus"
| Lumbosacral plexus | |
|---|---|
 Plan of lumbar plexus. | |
 Plan of sacral and pudendal plexuses. | |
| Details | |
| Identifiers | |
| Latin | plexus lumbosacralis |
| MeSH | D008160 |
| TA98 | A14.2.07.001 |
| TA2 | 6516 |
| FMA | 5907 |
| Anatomical terms of neuroanatomy | |
Additional Images[edit]
| Deep external pudendal artery | |
|---|---|
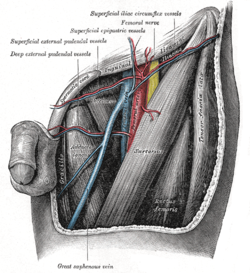 The left femoral triangle. (Deep external pudendal vessels labeled at upper left.) | |
 Scheme of the femoral artery | |
| Details | |
| Source | femoral artery |
| Vein | external pudendal vein |
| Identifiers | |
| Latin | arteria pudenda externa profunda |
| TA98 | A12.2.16.014 |
| TA2 | 4678 |
| FMA | 20739 |
| Anatomical terminology | |
| Acetabular branch | |
|---|---|
 The profunda femoris artery, femoral artery and their major branches - right thigh, frontal view (circumflex femoral arteries labeled) | |
| Details | |
| Source | Medial circumflex femoral artery |
| Identifiers | |
| Latin | Ramus acetabularis arteriae circumflexae femoris medialis |
| TA98 | A12.2.16.024 A12.2.15.010 |
| TA2 | 4689 |
| FMA | 20813 |
| Anatomical terminology | |
The circumflex fibular artery (circumflex fibular branch, circumflex branch of posterior tibial artery, or circumflex peroneal branch of posterior tibial artery) is a branch of the posterior tibial artery which supplies blood to the knee.[1]
The artery branch of the anterior tibial artery, at its initial (or superior) segment, winds around the neck of the fibula and joins patellar network.[1]
| Circumflex fibular artery | |
|---|---|
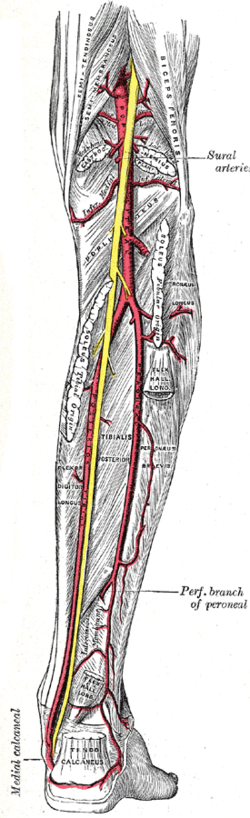 Major arteries of the leg - posterior view (circumflex fibular artery is not labeled, but region is visible) | |
| Details | |
| Source | posterior tibial artery |
| Supplies | Knee |
| Identifiers | |
| Latin | Ramus circumflexus fibularis arteriae tibialis posterioris |
| TA98 | A12.2.16.056 |
| TA2 | 4722 |
| FMA | 43918 |
| Anatomical terminology | |
The lateral plantar artery (external plantar artery), much larger than the medial, passes obliquely lateralward and forward to the base of the fifth metatarsal bone.
It then turns medialward to the interval between the bases of the first and second metatarsal bones, where it unites with the deep plantar branch of the dorsalis pedis artery, thus completing the plantar arch.
As this artery passes lateralward, it is first placed between the calcaneus and Abductor hallucis, and then between the Flexor digitorum brevis and Quadratus plantæ as it runs forward to the base of the little toe it lies more superficially between the Flexor digitorum brevis and Abductor digiti quinti, covered by the plantar aponeurosis and integument.
The remaining portion of the vessel is deeply situated; it extends from the base of the fifth metatarsal bone to the proximal part of the first interosseous space, and forms the plantar arch; it is convex forward, lies below the bases of the second, third, and fourth metatarsal bones and the corresponding Interossei, and upon the oblique part of the Adductor hallucis.
| Lateral plantar artery | |
|---|---|
 The plantar arteries. Deep view. (Lateral plantar visible at center top.) | |
| Details | |
| Source | Posterior tibial artery |
| Supplies | Sole |
| Identifiers | |
| Latin | Arteria plantaris lateralis |
| TA98 | A12.2.16.064 |
| TA2 | 4737 |
| FMA | 43926 |
| Anatomical terminology | |
| Superior genicular arteries | |
|---|---|
 The femoral artery. | |
| Details | |
| Identifiers | |
| Latin | Arteriae genus superiores |
| FMA | 22535 |
| Anatomical terminology | |
| Cremasteric artery | |
|---|---|
 The scrotum. | |
| Details | |
| Source | Inferior epigastric artery |
| Identifiers | |
| Latin | Arteria cremasterica |
| TA98 | A12.2.16.007M |
| TA2 | 4362 |
| FMA | 70192 |
| Anatomical terminology | |
Additional images[edit]

The interfoveolar ligament, seen from in front.
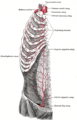
The internal mammary artery and its branches.

The arteries of the pelvis.

The iliac veins.

Dissection of side wall of pelvis showing sacral and pudendal plexuses.

Posterior view of the anterior abdominal wall in its lower half. The peritoneum is in place, and the various cords are shining through.
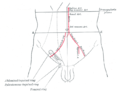
Front of abdomen, showing surface markings for arteries and inguinal canal.


| Inguinal triangle | |
|---|---|
 Internal (from posterior to anterior) view of left> inguinal area of the male pelvis. Inguinal triangle is labeled in green. The three surrounding structures: inferior epigastric vessels: Run from upper left to center. inguinal ligament: Runs from upper right to bottom left. rectus abdominis muscle: Runs from upper left to bottom left, labeled rectus at upper left. | |
 External view. Inguinal triangle is labeled in green. Borders: inferior epigastric artery and vein: labeled at center left, and run from upper right to bottom center. inguinal ligament: not labeled on diagram, but runs a similar path to the inguinal aponeurotic falx, labeled at bottom. rectus abdominis muscle: runs from upper left to bottom left. | |
| Details | |
| Identifiers | |
| Latin | trigonum inguinale |
| TA98 | A10.1.02.433 |
| TA2 | 3795 |
| FMA | 256506 |
| Anatomical terminology | |
A fold of peritoneum, the phrenicocolic ligament is continued from the left colic flexure to the thoracic diaphragmopposite the tenth and eleventh ribs; it passes below and serves to support the spleen, and therefore has received the name of sustentaculum lienis.[1]
The phrenicocolic ligament is also called Hensing's ligament after Friedrich Wilhelm Hensing(1719–1745), a German professor for medicine in Giessen.[2][3]
Clinical significance[edit]
Knowledge of basic anatomic and the variations of suspensory ligament of the spleen it is essential in the case of open surgery or laparoscopic splenectomy.[4] Moreover, during some surgical procedures, in many cases it is necessary to exert a certain degree of traction on the spleen and on its peritoneal insertions. Unfortunately this traction may result in a rupture of the fibrous capsule of the organ, resulting in severe bleeding, very difficult to control. Particularly hazardous is the downward traction of the phrenicocolic ligament (this maneuver may be necessary for the mobilization of splenic flexure). This ligament marks the site where the colon exits the peritoneal cavity: the phrenicocolic ligament so is an important point of intersection of abdominal anatomy and, consequently, a crucial point for spread of abdominal disease.[5]
| Phrenicocolic ligament | |
|---|---|
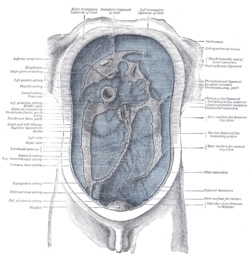 Diagram to show the lines along which the peritoneum leaves the wall of the abdomen to invest the viscera. (Phrenicocolic ligament labeled at center right.) | |
| Details | |
| Identifiers | |
| Latin | Ligamentum phrenicocolicum |
| TA98 | A10.1.02.211 |
| TA2 | 3769 |
| FMA | 16551 |
| Anatomical terminology | |
| Broad ligament of the uterus | |
|---|---|
 Uterus and right broad ligament, seen from behind. (Broad ligament visible at center.) | |
| Details | |
| Identifiers | |
| Latin | ligamentum latum uteri |
| MeSH | D001956 |
| TA98 | A10.1.02.505F |
| TA2 | 3800 |
| FMA | 16516 |
| Anatomical terminology | |
Subdivisions[edit]
| Subcomponent | Mesentery |
|---|---|
| Mesometrium[1] | uterus - the largest portion of the broad ligament |
| Mesosalpinx[1] | Fallopian tubes |
| Mesovarium[1][2] | Ovaries[2] |
Contents[edit]
The contents of the broad ligament include the following:[3]
- Reproductive
- uterine tubes (or Fallopian tube)
- ovary (some sources consider the ovary to be on the broad ligament, but not in it.)[4]
- vessels
- ovarian artery (in the suspensory ligament)[5]
- uterine artery (in reality, travels in the cardinal ligament)
- ligaments
- ovarian ligament
- round ligament of uterus
- suspensory ligament of the ovary (Some sources consider it a part of the broad ligament, while other sources just consider it a "termination" of the ligament.[6])
Relations[edit]
The peritoneum surrounds the uterus like a flat sheet that folds over its fundus, covering it anteriorly and posteriorly; on the sides of the uterus, this sheet of peritoneum comes in direct contact with itself, forming the double layer of peritoneum known as the broad ligament of the uterus.
The part where this peritoneal sheet is folded (i.e. the free edge) has the uterine tubes running between the two layers; this part is known as the mesosalpinx.
Function[edit]
The broad ligament serves as a mesentery for the uterus, ovaries, and the uterine tubes. It helps in maintaining the uterus in its position, but it is not a major contributing factor.
Clinical significance[edit]
Broad ligament hernias are rare. Due to their vague clinical presentation they are difficult to distinguish from other types of internal hernias, which can cause small bowel obstruction.[7]
Additional images[edit]
See also[edit]
In biology, antrum is a general term for a cavity or chamber, which may have specific meaning in reference to certain organs or sites in the body.
In vertebrates, it may refer specifically to:
- Antrum follicularum, the cavity in the epithelium that envelops the oocyte
- Mastoid antrum, a cavity between the middle ear and temporal bone in the skull
- Stomach antrum, either
- Pyloric antrum, the lower portion of the stomach. This is what is usually referred to as "antrum" in stomach-related topics,[citation needed]
- or Antrum cardiacum, a dilation that occurs in the esophagus near the stomach (forestomach)
- Maxillary antrum or antrum of Highmore, the maxillary sinus, a cavity in the maxilla and the largest of the paranasal sinuses
In invertebrates, it may refer specifically to:
- Antrum of female lepidoptera genitalia
The study of the genitalia of Lepidoptera is important for Lepidoptera taxonomy in addition to development, anatomy and natural history. The genitalia are complex and provide the basis for species discrimination in most families and also in family identification.[1] The genitalia are attached onto the tenth or most distal segment of the abdomen. Lepidoptera have some of the most complex genital structures in the insect groups with a wide variety of complex spines, setae, scales and tufts in males, claspers of different shapes and different modifications of the ductus bursae in females.[2][3]
The arrangement of genitalia is important in the courtship and mating as they prevent cross-specific mating and hybridisation. The uniqueness of genitalia of a species led to the use of the morphological study of genitalia as one of the most important keys in taxonomic identification of taxa below family level. With the advent of DNA analysis, the study of genitalia has now become just one of the techniques used in taxonomy.[4]
Configurations[edit]
There are three basic configurations of genitalia in the majority of the Lepidoptera based on how the arrangement in females of openings for copulation, fertilisation and egg-laying has evolved:[1]
- Exoporian : Hepialidae and related families have an external groove that carries sperm from the copulatory opening (gonopore) to the (ovipore) and are termed Exoporian.
- Monotrysian : Primitive groups have a single genital aperture near the end of the abdomen through which both copulation and egg laying occur. This character is used to designate the Monotrysia.
- Ditrysian : The remaining groups have an internal duct that carry sperm and form the Ditrysia, with two distinct openings each for copulation and egg-laying.
Male[edit]
Genitalia in male and female of any particular Lepidopteran species are adapted to fit each other like a lock (female) and key (male).[4] In males, the ninth abdominal segment is divided into a dorsal 'tegumen' and ventral 'vinculum'.[5] They form a ring-like structure for the attachment of genital parts and a pair of lateral clasping organs (claspers, valvae (singular valva), or 'harpes'). The male has a median tubular organ (called aedeagus or phallus) which is extended through an eversible sheath (or 'vesica') to inseminate the female.[3] The males have paired sperm ducts in all Lepidopterans; however, the paired testes are separate in basal taxa and fused in advanced forms.[3]
The males of many species of Papilionoidea are furnished with secondary sexual characteristics. These consist of scent-producing organs, brushes, and brands or pouches of specialised scales. These presumably meet the function of convincing the female that she is mating with a male of the correct species.[6]
Female[edit]
While the layout of internal genital ducts and openings of the female genitalia depends upon the taxonomic group that insect belongs to, the internal female reproductive system of all Lepidopterans consists of paired ovaries and accessory glands which produce the yolks and shells of the eggs. Female insects have a system of receptacles and ducts in which sperm is received, transported and stored. The oviducts of the female join together to form a common duct (called the 'oviductus communis') which leads to the vagina.[3][5]
When copulation takes place, the male butterfly or moth places a capsule of sperm (referred to as 'spermatophore') in a receptacle of the female (called the 'corpus bursae'). The sperm, when released from the capsule, swims directly into or via a small tube (the 'ductus bursae') into a special seminal receptacle (the 'spermatheca'), where the sperm is stored until it is released into the vagina for fertilisation during egg laying, which may occur hours, days, or months after mating. The eggs pass through the ovipore. The ovipore may be at the end of a modified 'ovipositor' or surrounded by a pair of broad setose anal papillae.[3][5]
Butterflies of the Parnassinae (Family Papilionidae) and some Acraeini (Family Nymphalidae) add a post-copulatory plug, called the sphragis, to the abdomen of the female after copulation preventing her from mating again.[2] The females of some moths have a scent-emitting organ located at the tip of the abdomen.[4]
Gallery[edit]
See also[edit]
https://en.wikipedia.org/wiki/Lepidoptera_genitalia
Structure[edit]
The cytoplasm of uterine epithelial cells contains typical organelles found in other cells, including a nucleus, which is located towards the bottom of the cell with one or more prominent nucleoli, mitochondria, golgi apparatus, endoplasmic reticulum, free ribosomes, lysosomes, vesicles and lipid droplets.[1] Like all epithelial cells, the uterine epithelial cells lie on a basal lamina.
Apical plasma membrane[edit]
The apical plasma membrane displays compositional variations that change at the time of implantation. The apical domain is specialized for the initial interaction with the embryo as well as controlling secretory and absorptive processes including endocytosis and pinocytosis. The apical surface of the uterine epithelial cells is covered with microvilli that are under hormonal control and vary in length and number with the oestrous cycle and during pregnancy. A hormonally dependent glycocalyx is found outside the microvilli[2] while the center of the microvilli consists of an actin filament core which is embedded into the terminal web.[3] The terminal web is a meshwork of actin filaments, which lies immediately below the microvilli and is important in maintaining the structural integrity of the cell surface as well as acting as a barrier to movement of cellular organelles.[4]
Lateral plasma membrane[edit]
The lateral plasma membrane domain is responsible for cell adhesion and is believed to control the paracellular transport of fluid and electrolytes, that is transport of fluid between the cells. A junctional complex characterises this domain and consists of three specialized areas; the zonula occludens (tight junction), zonula adherens (adherens junction) and macula adherens (desmosome). The zonula occludens and zonula adherens form a continuous belt around the cell that provides a barrier to paracellular transport and are thought to be important in cell-cell communication.[5]
Basal plasma membrane[edit]
The basal domain is essential for adhesion between the epithelium and underlying stroma as well as possible communication between these two regions. The uterine epithelial cells produce the basal lamina on which they rest.[6] The basal lamina is composed of two regions; the lamina lucida that is an electron lucent layer adjacent to the basal plasma membrane and the lamina densa that is a closely packed network of fibers.
Changes at implantation[edit]
There are dramatic changes in the morphology and biochemical characteristics of the uterine epithelial cells in preparation for blastocyst implantation.[7] These features include a loss of apical microvilli such that the apical plasma membrane becomes flattened.[8][9] There is also a decrease in the amount of glycocalyx covering the apical surface [8][10] which leads to a reduction in the negative charge of the uterine epithelial cells. Collectively, these plasma membrane changes have been termed the plasma membrane transformation.[11] Changes in the lateral junctional complex are important in the regulation of fluid movement along the paracellular pathway, between the epithelial cells.[5]
Tight junction changes during early pregnancy[edit]
During the early stages of pregnancy, prior to implantation, the tight junction complex, which is the main regulator of paracellular flow, extends 0.4 µm down the lateral plasma membrane with little cross-linking of the tight junctional strands.[12] At this time, the tight junctions are quite 'leaky' allowing movement of fluid and solutes between the epithelial cells.[5][13]
At the time of implantation the tight junctions extended further down the lateral plasma membrane (1 µm) and there was a significant increase in the cross-linking of the tight junctional strands.[12] At the time of implantation the tight junctions are electrochemically 'tighter’ and prevent the movement of fluids and electrolytes between the cells.[5][13] These changes were also found in ovariectomised rats treated with exogenous hormones. Animals treated with oestrogen displayed a picture of tight junctions similar to that seen on day 1 of pregnancy while rats treated with either progesterone alone or in combination with oestrogen had tight junctions with similar morphology to that seen at the time of implantation.[14] Various components of the tight junctions regulate the selectivity of this paracellular pathway. For example, it has been shown that it is the claudin component of tight junctions regulates the charge selectivity of the tight junctions.[15]
Fluid transport across cells[edit]
At the time of implantation in a number of species the uterine lumen closes down, which allows uterine epithelial cells to come into contact with each other and ‘fixes’ the blastocyst in place.[9][16] Uterine closure involves mild generalised oedema and reabsorption of luminal fluid.[17] Fluid absorption could occur through one or a combination of mechanisms; escape of uterine fluid through the cervix, which is unlikely, as this would have the potential to displace implanting blastocysts;[18]endocytosis by pinopods, which develop at the time of attachment,[16][19] or by transcellular means. This is influenced by the tight junction molecules and ion/water channels in the apical plasma membrane of uterine epithelial cells.
Studies have found an increase in claudin-4 within the tight junctions of uterine epithelial cells at the time of implantation[5] and an increase in ENaC in the apical membrane of uterine epithelial cells.[20][21][22] The increase in claudin-4 prevents the movement of Na+ ions between the cells, and the appearance of ENaC in the apical membrane allows movement of Na+ ions through the cell, from the lumen into the underlying stroma.[23] There is also an increase in AQP5 in the apical plasma membrane of uterine epithelial cells at time of implantation.[13][24] The osmotic gradient created by the reabsorption of Na+ ions leads to reabsorption of water through AQP5 channels in the apical plasma membrane, which causes the uterine epithelial cells to come into contact with each other and the blastocyst.[13][24]
| Uterine appendages | |
|---|---|
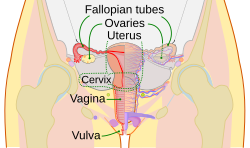 Schematic frontal view of female anatomy | |
 Uterus and right broad ligament, seen from behind. (In this orientation, the contents "in front" of the broad ligament are posterior to it.) | |
| Details | |
| Identifiers | |
| Latin | adnexa uteri |
| MeSH | D000290 |
| Anatomical terminology | |
Terminology[edit]
They can be defined in slightly different ways:
- Some sources define the adnexa as the fallopian tubes and ovaries.[1]
- Others include the supporting tissues".[2]
- Another source defines the appendages as the "regions of the true pelvis posterior to the broad ligaments".[3]
- One dictionary includes the fallopian tubes, ovaries, and ligaments (without specifying precisely which ligaments are included).[4]
Clinical significance[edit]
The term "adnexitis" is sometimes used to describe an inflammation of the uterine appendages (adnexa).[5] In this context, it replaces the terms oophoritis and salpingitis.
The term adnexal mass is sometimes used when the location of a uterine mass is not yet more precisely known.
63% of ectopic pregnancies present with an adnexal mass. Depending on the size of the mass, it could be a medical emergency.
Term "Adnexectomy" in Gynaecology is often used for Salpingo-Oophorectomy (removal of both: fallopian tubes and ovaries).
Additional images[edit]
In anatomy, adnexa refers to the appendages of an organ. The term adnexa stems from a Latin word meaning appendages.
More specifically, it can refer to:Adnexa of eye (accessory visual structures)
Adnexa of uterus (uterine appendages)Adnexa of skin (skin appendages)
https://en.wikipedia.org/wiki/Adnexa
The tubuli seminiferi recti (also known as the tubuli recti, tubulus rectus, or straight seminiferous tubules) are structures in the testicle connecting the convoluted region of the seminiferous tubules to the rete testis, although the tubuli recti have a different appearance distinguishing them from these two structures.
They enter the fibrous tissue of the mediastinum, and pass upward and backward, forming, in their ascent, a close network of anastomosing tubes which are merely channels in the fibrous stroma, lined by flattened epithelium, and having no proper walls; this constitutes the rete testis. Only Sertoli cells line the terminal ends of the seminiferous tubules (tubuli recti).
https://en.wikipedia.org/wiki/Tubuli_seminiferi_recti
An anastomosis (/əˌnæstəˈmoʊsɪs/, plural anastomoses) is a connection or opening between two things (especially cavities or passages) that are normally diverging or branching, such as between blood vessels, leaf veins, or streams. Such a connection may be normal (such as the foramen ovale in a fetus's heart) or abnormal (such as the patent foramen ovale in an adult's heart); it may be acquired (such as an arteriovenous fistula) or innate (such as the arteriovenous shunt of a metarteriole); and it may be natural (such as the aforementioned examples) or artificial (such as a surgical anastomosis). The reestablishment of an anastomosis that had become blocked is called a reanastomosis. Anastomoses that are abnormal, whether congenital or acquired, are often called fistulas.
The term is used in medicine,[1] biology, mycology, geology, and geography.
Etymology[edit]
Anastomosis: medical or Modern Latin, from Greek ἀναστόμωσις, anastomosis, "outlet, opening", Gr ana- "up, on, upon", stoma "mouth", "to furnish with a mouth".[2]Thus the -stom- syllable is cognate with that of stoma in botany or stoma in medicine.
Medical anatomy[edit]
An anastomosis is the connection of two normally divergent structures.[3] It refers to connections between blood vessels or between other tubular structures such as loops of intestine.
Circulatory[edit]
In circulatory anastomoses, many arteries naturally anastomose with each other; for example, the inferior epigastric artery and superior epigastric artery, or the anterior and/or posterior communicating arteries in the Circle of Willis in the brain. The circulatory anastomosis is further divided into arterial and venous anastomosis. Arterial anastomosis includes actual arterial anastomosis (e.g., palmar arch, plantar arch) and potential arterial anastomosis (e.g. coronary arteries and cortical branch of cerebral arteries). Anastomoses also form alternative routes around capillary beds in areas that don't need a large blood supply, thus helping regulate systemic blood flow.
Surgical[edit]
Surgical anastomosis occurs when segments of intestine, blood vessel, or any other structure are connected together surgically (anastomosed). Examples include arterial anastomosis in bypass surgery, intestinal anastomosis after a piece of intestine has been resected, Roux-en-Y anastomosis and ureteroureterostomy. Surgical anastomosis techniques include Linear Stapled Anastomosis,[4] Hand Sewn Anastomosis,[4] End-to-End Anastomosis (EEA).[5] Anastomosis can be performed by hand or with an anastomosis assist device.[6] Studies have been performed comparing various anastomosis approaches taking into account surgical "time and cost, postoperative anastomotic bleeding, leakage, and stricture".[7]
Pathological[edit]
Pathological anastomosis results from trauma or disease and may involve veins, arteries, or intestines. These are usually referred to as fistulas. In the cases of veins or arteries, traumatic fistulas usually occur between artery and vein. Traumatic intestinal fistulas usually occur between two loops of intestine (entero-enteric fistula) or intestine and skin (enterocutaneous fistula). Portacaval anastomosis, by contrast, is an anastomosis between a vein of the portal circulation and a vein of the systemic circulation, which allows blood to bypass the liver in patients with portal hypertension, often resulting in hemorrhoids, esophageal varices, or caput medusae.
Biology[edit]
Evolution[edit]
In evolution, anastomosis is a recombination of evolutionary lineage. Conventional accounts of evolutionary lineage present themselves as the branching out of species into novel forms. Under anastomosis, species might recombine after initial branching out, such as in the case of recent research that shows that ancestral populations along human and chimpanzee lineages may have interbred after an initial branching event.[8] The concept of anastomosis also applies to the theory of symbiogenesis, in which new species emerge from the formation of novel symbiotic relationships.
Mycology[edit]
In mycology, anastomosis is the fusion between branches of the same or different hyphae.[9] Hence the bifurcating fungal hyphae can form true reticulating networks. By sharing materials in the form of dissolved ions, hormones, and nucleotides, the fungus maintains bidirectional communication with itself. The fungal network might begin from several origins; several spores (i.e. by means of conidial anastomosis tubes), several points of penetration, each a spreading circumference of absorption and assimilation. Once encountering the tip of another expanding, exploring self, the tips press against each other in pheromonal recognition or by an unknown recognition system, fusing to form a genetic singular clonal colony that can cover hectares called a genet or just microscopical areas.[10]
For fungi, anastomosis is also a component of reproduction. In some fungi, two different haploid mating types – if compatible – merge. Somatically, they form a morphologically similar mycelial wave front that continues to grow and explore. The significant difference is that each septated unit is binucleate, containing two unfused nuclei, i.e. one from each parent that eventually undergoes karyogamy and meiosis to complete the sexual cycle.
Also the term "anastomosing" is used for mushroom gills which interlink and separate to form a network.[11]
Botany[edit]
The growth of a strangler fig around a host tree, with tendrils fusing together to form a mesh, is called anastomosing.[12]
Geology[edit]
In geology, anastomosis refers to quartz (or other) veins displaying this property, which is often related to shearing in metamorphic regions.[citation needed]
Geography[edit]
Anastomosing streams consist of multiple channels that divide and reconnect and are separated by semi-permanent banks formed of cohesive material, such that they are unlikely to migrate from one channel position to another. They can be confused with braided rivers based on their planforms alone, but braided rivers are much shallower and more dynamic than anastomosing rivers. Some definitions require that an anastomosing river be made up of interconnected channels that enclose floodbasins,[13] again in contrast with braided rivers. Rivers with anastomosed reaches include the Magdalena River in Colombia,[14] the upper Columbia River in British Columbia, Canada,[15] the Drumheller Channels of the Channeled Scablands of the state of Washington, USA, and the upper Narew River in Poland.[16] The term anabranch has been used for segments of anastamosing rivers.
In cave systems anastomosis is the splitting of cave passages that later reconnect.[17
https://en.wikipedia.org/wiki/Anastomosis
The prostatic ducts (or prostatic ductules) open into the floor of the prostatic portion of the urethra, and are lined by two layers of epithelium, the inner layer consisting of columnar and the outer of small cubical cells.
Small colloid masses, known as amyloid bodies are often found in the gland tubes.
They open onto the prostatic sinus.
https://en.wikipedia.org/wiki/Prostatic_ducts
The monotremes (egg laying mammals) represent the order of extant mammals most distantly related to humans. The platypus (Ornithorhynchus anatinus) is indigenous to eastern Australia; the short-beaked echidna (Tachyglossus aculeatus) is indigenous to Australia and Papua New Guinea; whereas the long-beaked echidna (Zaglossus bruijni) is restricted to Papua New Guinea and Irian Jaya.[1][2] Since monotremes exhibit characteristics common with both reptiles (e.g. presence of a cloaca) and therian mammals (e.g. mammary glands), they are of great interest for the study of mammalian evolution.[1][2]
Monotremes exhibit a combination of reptilian and mammalian characteristics[edit]
Male monotremes are testicond (have intraabdominal testes) with the testes undergoing seasonal emergence during winter. The fully developed seminiferous tubules exhibit distinctly small stages of spermatogenesis in that more than one stage is often observed in a cross section of the tubule,[3] a characteristic of spermatogenesis that has also been observed in a reptile,[4] common in birds[5][6] and man.[7]
The monotreme paired excurrent ducts (ductuli efferentes, epididymides[8] and vas deferens) empty into a single urethra.[2] Glandular tissue surrounds the urethra into which a pair of bulbourethral glands (Cowper's glands) empty at the base of the penis. The intraabdominal testes and excurrent ducts, along with the presence of a cloaca exhibit homology to the reptilian male reproductive tract.[1][2]
The combination of reptilian and mammalian structures within the monotreme reproductive tract has informed the evolution of the male reproductive tract in mammals. For example, the intraabdominal low sperm storage capacity of the echidna epididymis[8] informed the role of the epididymis as a prime mover in the evolution of descended testes in mammals as it relates to lower extragonadal temperatures enhancing epididymal sperm storage in scrotal mammals.[9]
The glandular designation of periurethral tissue within the monotreme male reproductive tract has been a matter of scientific discussion for approximately two centuries.[10][11][12][13] Examination of the monotreme periurethral tissue has been limited by the availability of these protected and relatively rare mammals, hence, the long time line for scientific research of reproductive tissue between studies.
Structure of the monotreme prostate[edit]
The glandular tissue surrounding the monotreme urethra most likely represents a rudimentary prostate.[13] There are no periurethral glands in reptiles. Hence, the evolution of the prostate gland is unique to mammals. Primordial periurethral glands have been described in the platypus as secretory glandular tissue surrounding the length of the urethra.[12] The periurethral tissue exhibits regional swelling, being widest immediately beneath the bladder and progressively reducing in thickness along the length of the urethra.[12] Surrounding the periurethral glands is a urethral muscularis.[12] Observations in the platypus of the periurethral glands were non-committal as to homology with the prostate.[11][12] Subsequently, the periurethral tissue in the echidna was definitively identified as a rudimentary prostate.[13] This is supported by:
- anatomically these glands are placed at their widest at the base of the bladder,[10][11] which is similar in location for the prostate in other mammals,
- histologic lining of the echidna urethra with transitional epithelium (Figures 1 & 2), and not post-prostate urethral lining of pseudostratified or stratified columnar epithelium observed in eutherian mammals,
- location of periurethral glands with 9-11 buds per cross section surrounding and exiting into the urethra,[13]
- histology of simple columnar secretory cells lining compound alveolar glands,[12][13]
- the glandular tissue is surrounded by a muscularis to facilitate expulsion of secretory products into the urethra,[12][13]
- seasonal changes in echidna glandular development,[13] whereby in aspermatogenic animals the glandular tissue surrounding the urethra is less developed (Figure 1) than during the breeding season when the secretory glands surrounding the urethra of spermatogenic animals appear well developed (Figure 2) coinciding with elevated levels of testosterone and active spermatogenesis.[1][2]
In aggregate, these characteristics of glandular tissue surrounding the urethra identify a rudimentary disseminate prostate in monotremes.[13]
https://en.wikipedia.org/wiki/Prostate_evolution_in_monotreme_mammals
The spongy urethra (cavernous portion of urethra, penile urethra) is the longest part of the male urethra, and is contained in the corpus spongiosum of the penis.
It is about 15 cm long, and extends from the termination of the membranous portion to the external urethral orifice.
Commencing below the inferior fascia of the urogenital diaphragm it passes forward and upward to the front of the pubic symphysis; and then, in the flaccid condition of the penis, it bends downward and forward.
It is narrow, and of uniform size in the body of the penis, measuring about 6 mm in diameter; it is dilated behind, within the bulb, and again anteriorly within the glans penis, where it forms the fossa navicularis urethrae.
The spongy urethra runs along the length of the penis on its ventral (underneath) surface. It is about 15–16 cm in length, and travels through the corpus spongiosum. The ducts from the urethral gland (gland of Littre) enter here. The openings of the bulbourethral glands are also found here.[1] Some textbooks will subdivide the spongy urethra into two parts, the bulbous and pendulous urethra. The urethral lumen (interior) runs effectively parallel to the penis, except at the narrowest point, the external urethral meatus, where it is vertical. This produces a spiral stream of urine and has the effect of cleaning the external urethral meatus.[citation needed] The lack of an equivalent mechanism in the female urethra partly explains why urinary tract infections occur so much more frequently in females.[citation needed]
| Spongy urethra | |
|---|---|
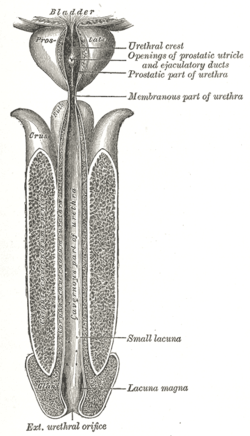 The human male urethra laid open on its anterior (upper) surface. | |
| Details | |
| Precursor | Urogenital folds |
| Identifiers | |
| Latin | Pars spongiosa urethrae masculinae, pars cavernosa urethrae masculinae |
| TA98 | A09.4.02.021 |
| TA2 | 3682, 3455 |
| FMA | 19675 |
| Anatomical terminology | |
Epithelium[edit]
Pseudostratified columnar – proximally, Stratified squamous – distally
https://en.wikipedia.org/wiki/Spongy_urethra
| Tunica vasculosa testis | |
|---|---|
| Details | |
| Identifiers | |
| Latin | Tunica vasculosa testis |
| Anatomical terminology | |
The tunica vasculosa is the vascular layer of the testis, consisting of a plexus of blood vessels, held together by delicate areolar tissue.
It clothes the inner surface of the tunica albuginea and the different septa in the interior of the gland, and therefore forms an internal investment to all the spaces of which the gland is composed.
https://en.wikipedia.org/wiki/Tunica_vasculosa_testis
Histology[edit]
The epididymis is covered by a two layered pseudostratified epithelium. The epithelium is separated by a basement membrane from the connective tissue wall which has smooth muscle cells. The major cell types in the epithelium are:
- Principal cells: columnar cells that, with the basal cells, form the majority of the epithelium. In the caput (head) region these cells have long stereocilia that are tuft-like extensions that project into the lumen.[4] The sterocilia are much shorter in the cauda (tail) segment.[4] They also secrete carnitine, sialic acid, glycoproteins, and glycerylphosphorylcholine into the lumen.
- Basal cells: shorter, pyramid-shaped cells which contact the basal lamina but taper off before their apical surfaces reach the lumen. These are thought to be undifferentiated precursors of principal cells.
- Apical cells: predominantly found in the head region
- Clear cells: predominant in the tail region
- Intraepithelial lymphocytes: distributed throughout the tissue.
- Intraepithelial macrophages[5][6]
Stereocilia[edit]
The stereocilia of the epididymis are long cytoplasmic projections that have an actin filament backbone.[4] These filaments have been visualized at high resolution using fluorescent phalloidin that binds to actin filaments.[4] The stereocilia in the epididymis are non-motile. These membrane extensions increase the surface area of the cell, allowing for greater absorption and secretion. It has been shown that epithelial sodium channel ENaC that allows the flow of Na+ ions into the cell is localized on stereocilia.[4]
Because sperm are initially non-motile as they leave the seminiferous tubules, large volumes of fluid are secreted to propel them to the epididymis. The core function of the stereocilia is to resorb 90% of this fluid as the spermatozoa start to become motile. This absorption creates a fluid current that moves the immobile sperm from the seminiferous tubules to the epididymis. Spermatozoa do not reach full motility until they reach the vagina, where the alkaline pH is neutralized by acidic vaginal fluids.
Development[edit]
In the embryo, the epididymis develops from tissue that once formed the mesonephros, a primitive kidney found in many aquatic vertebrates. Persistence of the cranial end of the mesonephric duct will leave behind a remnant called the appendix of the epididymis. In addition, some mesonephric tubules can persist as the paradidymis, a small body caudal to the efferent ductules.
A Gartner's duct is a homologous remnant in the female.
https://en.wikipedia.org/wiki/Epididymis#Histology
The mesonephros (Greek: middle kidney) is one of three excretory organs that develop in vertebrates. It serves as the main excretory organ of aquatic vertebrates and as a temporary kidney in reptiles, birds, and mammals. The mesonephros is included in the Wolffian body after Caspar Friedrich Wolff who described it in 1759. (The Wolffian body is composed of: mesonephros + paramesonephrotic blastema)
https://en.wikipedia.org/wiki/Mesonephros
| Precursors | |
|---|---|
| Urinary system | |
| See also | |
https://en.wikipedia.org/wiki/Mesonephros
Mesonephric tubules are genital ridges that are next to the mesonephros.
In males, some of the mesonephric kidney tubules, instead of being used to filter blood like the rest, "grow" over to the developing testes, penetrate them, and become connected to the seminiferous tubules of the testes. They also form the epididymis and the paradidymis.[1]
The sperm differentiate inside the seminiferous tubules, then swim down these tubes, then through these special mesonephric tubules, and go down inside Wolffian duct, to the coelom and finally to the organ the animal uses to transport sperm into females.
In females, it gives rise to the epoophoron and the paroöphoron.[2]
https://en.wikipedia.org/wiki/Mesonephric_tubules
The metanephrogenic blastema or metanephric blastema (or metanephric mesenchyme, or metanephric mesoderm) is one of the two embryological structures that give rise to the kidney, the other being the ureteric bud.
The metanephric blastema mostly develops into nephrons, but can also form parts of the collecting duct system.
The system of tissue induction between the ureteric bud and the metanephric blastema is a reciprocal control system. GDNF, gonadal derived neurotrophic factor, is produced by the metanephric blastema and is essential in binding to the Ret receptor on the ureteric bud, which bifurcates and coalesces as a result to form the renal pelvis, major and minor calyces and collecting ducts. Mutations in the EYA1 gene, which is a GDNF transcription factor, lead to the renal abnormalities of BOR syndrome (branchio-oto-renal syndrome).
See also[edit]
The cloacal membrane is the membrane that covers the embryonic cloaca during the development of the urinary and reproductive organs.
It is formed by ectoderm and endoderm coming into contact with each other.[1] As the human embryo grows and caudal folding continues, the urorectal septum divides the cloaca into a ventral urogenital sinus and dorsal anorectal canal. Before the urorectal septum has an opportunity to fuse with the cloacal membrane, the membrane ruptures, exposing the urogenital sinus and dorsal anorectal canal to the exterior. Later on, an ectodermal plug, the anal membrane, forms to create the lower third of the rectum. It ruptures in the seventh week of gestation.
https://en.wikipedia.org/wiki/Cloacal_membrane
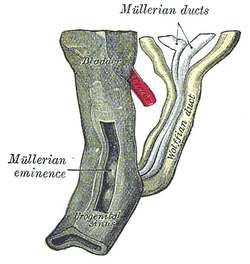
The cloaca is a structure in the development of the urinary and reproductive organs.
The hind-gut is at first prolonged backward into the body-stalk as the tube of the allantois; but, with the growth and flexure of the tail-end of the embryo, the body-stalk, with its contained allantoic tube, is carried forward to the ventral aspect of the body, and consequently a bend is formed at the junction of the hind-gut and allantois.
This bend becomes dilated into a pouch, which constitutes the endodermal cloaca; into its dorsal part the hind-gut opens, and from its ventral part the allantois passes forward.
At a later stage the Wolffian duct and Müllerian duct open into its ventral portion.
The cloaca is, for a time, shut off from the anterior by the cloacal membrane, formed by the apposition of the ectodermand endoderm, and reaching, at first, as far forward as the future umbilicus.
Behind the umbilicus, however, the mesoderm subsequently extends to form the lower part of the abdominal wall and pubic symphysis.
By the growth of the surrounding tissues the cloacal membrane comes to lie at the bottom of a depression, which is lined by ectoderm and named the ectodermal cloaca.
https://en.wikipedia.org/wiki/Cloaca_(embryology)
The lateral plate mesoderm is the mesoderm that is found at the periphery of the embryo. It is to the side of the paraxial mesoderm, and further to the axial mesoderm. The lateral plate mesoderm is separated from the paraxial mesoderm by a narrow region of intermediate mesoderm. The mesoderm is the middle layer of the three germ layers, between the outer ectoderm and inner endoderm.
During the third week of embryonic development the lateral plate mesoderm splits into two layers forming the intraembryonic coelom.
The outer layer of lateral plate mesoderm adheres to the ectoderm to become the somatic or parietal layer known as the somatopleure. The inner layer adheres to the endoderm to become the splanchnic or visceral layer known as the splanchnopleure.
| Lateral plate mesoderm | |
|---|---|
 Transverse section of a chick embryo of forty-five hours’ incubation. * Axial mesoderm: yellow, at notochord. * Paraxial mesoderm: red, at somite. * Intermediate mesoderm: purple, near Wolffian duct. * Lateral plate mesoderm: purple, divided into "Somatic mesoderm" and "Splanchic mesoderm". | |
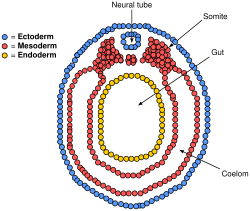 Diagram of vertebrate embryo showing divided lateral plate mesoderm forming the intraembryonic coelom. Somatic mesoderm at outer layer, splanchnic at inner layer. | |
| Details | |
| Carnegie stage | 9 |
| Precursor | mesoderm |
| Gives rise to | somatopleure, splanchnopleure |
| Identifiers | |
| Latin | mesoderma laminae lateralis |
| TE | plate mesoderm_by_E5.0.3.0.0.0.2 E5.0.3.0.0.0.2 |
| Anatomical terminology | |
Development[edit]
The lateral plate mesoderm will split into two layers, the somatopleuric mesenchyme, and the splanchnopleuric mesenchyme.
- The somatopleuric layer forms the future body wall.
- The splanchnopleuric layer forms the circulatory system.
Spaces within the lateral plate are enclosed and forms the intraembryonic coelom.
It is formed by the secretion of BMP-4 by the ectoderm.[1]
Serosal mesoderms[edit]
Lateral plate mesoderm gives rise to the serosal mesoderms.[2]
- forms a ventral layer associated with endoderm, the splanchnopleuric mesoderm. This forms the viscera and heart
- forms a dorsal layer associated with ectoderm, the somatopleuric mesoderm. This forms the body wall lining and dermis.
- Abdominal portion becomes contained in dorsal mesentery, part of the serosal mesoderm.
- When the two layers form, a cardiogenic plate is visible. Later, this will form the myocardial primordium, which will contribute to the tubular heart.
Cavities[edit]
In the 4th week the coelom divides into pericardial, pleural and peritoneal cavities.[2]
- First partition: is the septum transversum.
- This will be translocated later into the diaphragm and ventral mesentery.
- Divides the coelom into primitive pericardial and peritoneal cavities
- Pleuropericardial folds appear on the lateral wall of primitive pericardial cavity, which will eventually cause a partition to form the pericardial and pleural cavities.
- Communication between these partitions formed by the pericardioperitoneal canals. However, pleuroperitoneal membranes will grow to fuse with the septum transversum to close off these canals.
- At day 22, lung buds form, remaining ensheathed in a splanchnopleuric mesoderm
Limb development[edit]
Cells from the lateral plate mesoderm and the myotome migrate to the limb field and proliferate to create the limb bud. The lateral plate cells produce the cartilaginous and skeletal portions of the limb while the myotome cells produce the muscle components. The lateral plate mesodermal cells secrete a fibroblast growth factor (FGF7 and FGF10, presumably) to induce the overlying ectoderm to form an important organizing structure called the apical ectodermal ridge (AER).The AER reciprocatively secretes FGF8 and FGF4 which maintains the FGF10 signal and induces proliferation in the mesoderm.[citation needed] The position of FGF10 expression is regulated by Wnt8c in the hindlimb and Wnt2b in the forelimb. The forelimb and the hindlimb are specified by their position along the anterior/posterior axis and possibly by two T-box containing transcription factors: Tbx5 and Tbx4, respectively.
Additional images[edit]
See also[edit]
https://en.wikipedia.org/wiki/Lateral_plate_mesoderm
The nephrogenic cord is a portion of the urogenital ridge which is the source of much of the urinary system.[1]
The nephrogenic cords are bilateral condensations of the intermediate mesoderm.[2] The cords extend from the cervical segments to the sacral segments of the embryo. The nephrogenic cords are located on the posterior wall of the embryo, which is where the kidneys are located. The nephrogenic cords go through three phases of development which overlap to some extent, both in space and in time. The 1st phase is the pronephros, the 2nd phase is the mesonephros and the 3rd and final stage is the metanephros.
https://en.wikipedia.org/wiki/Nephrogenic_cord| Paramesonephric duct | |
|---|---|
 Urogenital sinus of female human embryo of eight and a half to nine weeks old. | |
 Tail end of human embryo, from eight and a half to nine weeks old. |
Human embryonic development, or human embryogenesis, is the development and formation of the human embryo. It is characterised by the processes of cell division and cellular differentiation of the embryo that occurs during the early stages of development. In biological terms, the development of the human body entails growth from a one-celled zygote to an adult human being. Fertilisation occurs when the sperm cell successfully enters and fuses with an egg cell (ovum). The genetic material of the sperm and egg then combine to form a single cell called a zygote and the germinal stage of development commences.[1] Embryonic development in the human, covers the first eight weeks of development; at the beginning of the ninth week the embryo is termed a fetus. Human embryology is the study of this development during the first eight weeks after fertilisation. The normal period of gestation (pregnancy) is about nine months or 40 weeks.
The germinal stage refers to the time from fertilization through the development of the early embryo until implantation is completed in the uterus. The germinal stage takes around 10 days.[2]During this stage, the zygote begins to divide, in a process called cleavage. A blastocyst is then formed and implanted in the uterus. Embryogenesis continues with the next stage of gastrulation, when the three germ layers of the embryo form in a process called histogenesis, and the processes of neurulation and organogenesis follow.
In comparison to the embryo, the fetus has more recognizable external features and a more complete set of developing organs. The entire process of embryogenesis involves coordinated spatial and temporal changes in gene expression, cell growth and cellular differentiation. A nearly identical process occurs in other species, especially among chordates.
https://en.wikipedia.org/wiki/Human_embryonic_development
A chordate (/ˈkɔːrdeɪt/) is an animal of the phylum Chordata (/kɔːrˈdeɪtə/). All chordates possess five synapomorphies, or primary characteristics, at some point during their larval or adulthood stages that distinguish them from all other taxa. These five synapomorphies include a notochord, dorsal hollow nerve cord, endostyle or thyroid, pharyngeal slits, and a post-anal tail. The name “chordate” comes from the first of these synapomorphies, the notochord, which plays a significant role in chordate structure and movement. Chordates are also bilaterally symmetric, have a coelom, possess a circulatory system, and exhibit metameric segmentation.
In addition to the morphological characteristics used to define chordates, analysis of genome sequences has identified two conserved signature indels (CSIs) in the proteins cyclophilin-like protein and mitochondrial inner membrane protease ATP23, which are exclusively shared by all vertebrates, tunicates and cephalochordates.[5]These CSIs provide molecular means to reliably distinguish chordates from all other metazoan.
The Chordata and Ambulacraria together form the superphylum Deuterostomia. Chordates are divided into three subphyla: Vertebrata (fish, amphibians, reptiles, birds, and mammals); Tunicata or Urochordata (sea squirts, salps); and Cephalochordata (which includes lancelets). There are also extinct taxa such as the Vetulicolia. Hemichordata (which includes the acorn worms) has been presented as a fourth chordate subphylum, but now is treated as a separate phylum: hemichordates and Echinodermata form the Ambulacraria, the sister phylum of the Chordates. Of the more than 65,000 living species of chordates, about half are ray-finned fishes that are members of the class Actinopterygii.
Chordate fossils have been found from as early as the Cambrian explosion, 539 million years ago.[6] Cladistically(phylogenetically), vertebrates – chordates with the notochord replaced by a vertebral column during development – are considered to be a subgroup of the clade Craniata, which consists of chordates with a skull. The Craniata and Tunicata compose the clade Olfactores. (See diagram under Phylogeny.)
Anatomy[edit]
Chordates form a phylum of animals that are defined by having at some stage in their lives all of the following anatomical features:[7]
- A notochord, a stiff rod of cartilage that extends along the inside of the body. Among the vertebrate sub-group of chordates the notochord develops into the spine, and in wholly aquatic species this helps the animal to swim by flexing its tail.
- A dorsal neural tube. In fish and other vertebrates, this develops into the spinal cord, the main communications trunk of the nervous system.
- Pharyngeal slits. The pharynx is the part of the throat immediately behind the mouth. In fish, the slits are modified to form gills, but in some other chordates they are part of a filter-feeding system that extracts particles of food from the water in which the animals live.
- Post-anal tail. A muscular tail that extends backwards behind the anus. In some chordates such as humans, this is only present in the embryonic stage.
- An endostyle. This is a groove in the ventral wall of the pharynx. In filter-feeding species it produces mucus to gather food particles, which helps in transporting food to the esophagus.[8] It also stores iodine, and may be a precursor of the vertebrate thyroid gland.[7]
There are soft constraints that separate chordates from other biological lineages, but are not part of the formal definition:
- All chordates are deuterostomes. This means that, during the embryo development stage, the anus forms before the mouth.
- All chordates are based on a bilateral body plan.[9]
- All chordates are coelomates, and have a fluid-filled body cavity called a coelom with a complete lining called peritoneum derived from mesoderm (see Brusca and Brusca).[10]
Classification[edit]
The following schema is from the 2015 edition of Vertebrate Palaeontology.[11][12] The invertebrate chordate classes are from Fishes of the World.[13] While it is structured so as to reflect evolutionary relationships (similar to a cladogram), it also retains the traditional ranks used in Linnaean taxonomy.
- Phylum Chordata
- Subphylum Cephalochordata (Acraniata) – (lancelets; 30 species)
- Class Leptocardii (lancelets)
- Clade Olfactores
- Subphylum Tunicata (Urochordata) – (tunicates; 3,000 species)
- Class Ascidiacea (sea squirts)
- Class Thaliacea (salps)
- Class Appendicularia (larvaceans)
- Class Sorberacea
- Subphylum Vertebrata (Craniata) (vertebrates – animals with backbones; 66,100+ species)
- Superclass 'Agnatha' paraphyletic (jawless vertebrates; 100+ species)
- Class Cyclostomata
- Infraclass Myxinoidea or Myxini (hagfish; 65 species)
- Infraclass Petromyzontida or Hyperoartia (lampreys)
- Class †Conodonta
- Class †Myllokunmingiida
- Class †Pteraspidomorphi
- Class †Thelodonti
- Class †Anaspida
- Class †Cephalaspidomorphi
- Class Cyclostomata
- Infraphylum Gnathostomata (jawed vertebrates)
- Class †Placodermi (Paleozoic armoured forms; paraphyletic in relation to all other gnathostomes)
- Class Chondrichthyes (cartilaginous fish; 900+ species)
- Class †Acanthodii (Paleozoic "spiny sharks"; paraphyletic in relation to Chondrichthyes)
- Class Osteichthyes (bony fish; 30,000+ species)
- Subclass Actinopterygii (ray-finned fish; about 30,000 species)
- Subclass Sarcopterygii (lobe-finned fish: 8 species)
- Superclass Tetrapoda (four-limbed vertebrates; 35,100+ species) (The classification below follows Benton 2004, and uses a synthesis of rank-based Linnaean taxonomy and also reflects evolutionary relationships. Benton included the Superclass Tetrapoda in the Subclass Sarcopterygii in order to reflect the direct descent of tetrapods from lobe-finned fish, despite the former being assigned a higher taxonomic rank.)[14]
- Superclass 'Agnatha' paraphyletic (jawless vertebrates; 100+ species)
- Subphylum Tunicata (Urochordata) – (tunicates; 3,000 species)
- Subphylum Cephalochordata (Acraniata) – (lancelets; 30 species)
Subphyla[edit]
Cephalochordata: Lancelets[edit]
Cephalochordates, one of the three subdivisions of chordates, are small, "vaguely fish-shaped" animals that lack brains, clearly defined heads and specialized sense organs.[19] These burrowing filter-feeders compose the earliest-branching chordate sub-phylum.[20][21]
Tunicata (Urochordata)[edit]
Most tunicates appear as adults in two major forms, known as "sea squirts" and salps, both of which are soft-bodied filter-feeders that lack the standard features of chordates. Sea squirts are sessile and consist mainly of water pumps and filter-feeding apparatus;[22] salps float in mid-water, feeding on plankton, and have a two-generation cycle in which one generation is solitary and the next forms chain-like colonies.[23] However, all tunicate larvae have the standard chordate features, including long, tadpole-like tails; they also have rudimentary brains, light sensors and tilt sensors.[22] The third main group of tunicates, Appendicularia (also known as Larvacea), retain tadpole-like shapes and active swimming all their lives, and were for a long time regarded as larvae of sea squirts or salps.[24] The etymology of the term Urochordata (Balfour 1881) is from the ancient Greek οὐρά (oura, "tail") + Latin chorda ("cord"), because the notochord is only found in the tail.[25] The term Tunicata(Lamarck 1816) is recognised as having precedence and is now more commonly used.[22]
Craniata (Vertebrata)[edit]
Craniates all have distinct skulls. They include the hagfish, which have no vertebrae. Michael J. Benton commented that "craniates are characterized by their heads, just as chordates, or possibly all deuterostomes, are by their tails".[26]
Most craniates are vertebrates, in which the notochord is replaced by the vertebral column.[27] These consist of a series of bony or cartilaginous cylindrical vertebrae, generally with neural arches that protect the spinal cord, and with projections that link the vertebrae. However hagfish have incomplete braincases and no vertebrae, and are therefore not regarded as vertebrates,[28] but as members of the craniates, the group from which vertebrates are thought to have evolved.[29] However the cladistic exclusion of hagfish from the vertebrates is controversial, as they may be degenerate vertebrates who have lost their vertebral columns.[30]
The position of lampreys is ambiguous. They have complete braincases and rudimentary vertebrae, and therefore may be regarded as vertebrates and true fish.[31] However, molecular phylogenetics, which uses biochemical features to classify organisms, has produced both results that group them with vertebrates and others that group them with hagfish.[32] If lampreys are more closely related to the hagfish than the other vertebrates, this would suggest that they form a clade, which has been named the Cyclostomata.[33]
Phylogeny[edit]
Overview[edit]
There is still much ongoing differential (DNA sequence based) comparison research that is trying to separate out the simplest forms of chordates. As some lineages of the 90% of species that lack a backbone or notochord might have lost these structures over time, this complicates the classification of chordates. Some chordate lineages may only be found by DNA analysis, when there is no physical trace of any chordate-like structures.[35]
Attempts to work out the evolutionary relationships of the chordates have produced several hypotheses. The current consensus is that chordates are monophyletic, meaning that the Chordata include all and only the descendants of a single common ancestor, which is itself a chordate, and that craniates' nearest relatives are tunicates. Recent identification of two conserved signature indels (CSIs) in the proteins cyclophilin-like protein and mitochondrial inner membrane protease ATP23, which are exclusively shared by all vertebrates, tunicates and cephalochordates also provide strong evidence of the monophyly of Chordata.[5]
All of the earliest chordate fossils have been found in the Early Cambrian Chengjiang fauna, and include two species that are regarded as fish, which implies that they are vertebrates. Because the fossil record of early chordates is poor, only molecular phylogenetics offers a reasonable prospect of dating their emergence. However, the use of molecular phylogenetics for dating evolutionary transitions is controversial.
It has also proved difficult to produce a detailed classification within the living chordates. Attempts to produce evolutionary "family trees" shows that many of the traditional classes are paraphyletic.
| Deuterostomes |
| |||||||||||||||||||||||||||
While this has been well known since the 19th century, an insistence on only monophyletic taxa has resulted in vertebrate classification being in a state of flux.[36]
The majority of animals more complex than jellyfish and other Cnidarians are split into two groups, the protostomes and deuterostomes, the latter of which contains chordates.[37] It seems very likely the 555 million-year-old Kimberella was a member of the protostomes.[38][39]If so, this means the protostome and deuterostome lineages must have split some time before Kimberella appeared—at least 558 million years ago, and hence well before the start of the Cambrian 538.8 million years ago.[37] The Ediacaran fossil Ernietta, from about 549 to 543 million years ago, may represent a deuterostome animal.[40]
Fossils of one major deuterostome group, the echinoderms (whose modern members include starfish, sea urchins and crinoids), are quite common from the start of the Cambrian, 542 million years ago.[41] The Mid Cambrian fossil Rhabdotubus johanssoni has been interpreted as a pterobranch hemichordate.[42] Opinions differ about whether the Chengjiang fauna fossil Yunnanozoon, from the earlier Cambrian, was a hemichordate or chordate.[43][44] Another fossil, Haikouella lanceolata, also from the Chengjiang fauna, is interpreted as a chordate and possibly a craniate, as it shows signs of a heart, arteries, gill filaments, a tail, a neural chord with a brain at the front end, and possibly eyes—although it also had short tentacles round its mouth.[44] Haikouichthys and Myllokunmingia, also from the Chengjiang fauna, are regarded as fish.[34][45] Pikaia, discovered much earlier (1911) but from the Mid Cambrian Burgess Shale (505 Ma), is also regarded as a primitive chordate.[46] On the other hand, fossils of early chordates are very rare, since invertebrate chordates have no bones or teeth, and only one has been reported for the rest of the Cambrian.[47]
The evolutionary relationships between the chordate groups and between chordates as a whole and their closest deuterostome relatives have been debated since 1890. Studies based on anatomical, embryological, and paleontological data have produced different "family trees". Some closely linked chordates and hemichordates, but that idea is now rejected.[8] Combining such analyses with data from a small set of ribosome RNA genes eliminated some older ideas, but opened up the possibility that tunicates (urochordates) are "basal deuterostomes", surviving members of the group from which echinoderms, hemichordates and chordates evolved.[48] Some researchers believe that, within the chordates, craniates are most closely related to cephalochordates, but there are also reasons for regarding tunicates (urochordates) as craniates' closest relatives.[8][49]
Since early chordates have left a poor fossil record, attempts have been made to calculate the key dates in their evolution by molecular phylogenetics techniques—by analyzing biochemical differences, mainly in RNA. One such study suggested that deuterostomes arose before 900 million years ago and the earliest chordates around 896 million years ago.[49] However, molecular estimates of dates often disagree with each other and with the fossil record,[49] and their assumption that the molecular clock runs at a known constant rate has been challenged.[50][51]
Traditionally, Cephalochordata and Craniata were grouped into the proposed clade "Euchordata", which would have been the sister group to Tunicata/Urochordata. More recently, Cephalochordata has been thought of as a sister group to the "Olfactores", which includes the craniates and tunicates. The matter is not yet settled.
A specific relationship between Vertebrates and Tunicates is also strongly supported by two CSIs found in the proteins predicted exosome complex RRP44 and serine palmitoyltransferase, that are exclusively shared by species from these two subphyla but not Cephalochordates, indicating Vertebrates are more closely related to Tunicates than Cephalochordates.[5]
Cladogram[edit]
Phylogenetic tree of the chordate phylum. Lines of the cladogram show probable evolutionary relationships between both extinct taxa, which are denoted with a dagger(†), and extant taxa. Relatives of vertebrates are invertebrates. The positions (relationships) of the lancelets, tunicates, and craniates/vertebrates are based on the following studies:[52][53][54][55]
| Chordata |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Closest nonchordate relatives[edit]
Hemichordates[edit]
Hemichordates ("half chordates") have some features similar to those of chordates: branchial openings that open into the pharynx and look rather like gill slits; stomochords, similar in composition to notochords, but running in a circle round the "collar", which is ahead of the mouth; and a dorsal nerve cord—but also a smaller ventral nerve cord.
There are two living groups of hemichordates. The solitary enteropneusts, commonly known as "acorn worms", have long proboscises and worm-like bodies with up to 200 branchial slits, are up to 2.5 metres (8.2 ft) long, and burrow though seafloor sediments. Pterobranchs are colonial animals, often less than 1 millimetre (0.039 in) long individually, whose dwellings are interconnected. Each filter feeds by means of a pair of branched tentacles, and has a short, shield-shaped proboscis. The extinct graptolites, colonial animals whose fossils look like tiny hacksaw blades, lived in tubes similar to those of pterobranchs.[56]
Echinoderms[edit]
Echinoderms differ from chordates and their other relatives in three conspicuous ways: they possess bilateral symmetry only as larvae – in adulthood they have radial symmetry, meaning that their body pattern is shaped like a wheel; they have tube feet; and their bodies are supported by skeletons made of calcite, a material not used by chordates. Their hard, calcified shells keep their bodies well protected from the environment, and these skeletons enclose their bodies, but are also covered by thin skins. The feet are powered by another unique feature of echinoderms, a water vascular system of canals that also functions as a "lung" and surrounded by muscles that act as pumps. Crinoids look rather like flowers, and use their feather-like arms to filter food particles out of the water; most live anchored to rocks, but a few can move very slowly. Other echinoderms are mobile and take a variety of body shapes, for example starfish, sea urchins and sea cucumbers.[57]
History of name[edit]
Although the name Chordata is attributed to William Bateson (1885), it was already in prevalent use by 1880. Ernst Haeckeldescribed a taxon comprising tunicates, cephalochordates, and vertebrates in 1866. Though he used the German vernacular form, it is allowed under the ICZN codebecause of its subsequent latinization.[4]
See also[edit]
- Chordate genomics
- List of chordate orders – All the classes and orders of phylum Chordata
https://en.wikipedia.org/wiki/Chordate
https://en.wikipedia.org/wiki/Category:Embryology_of_urogenital_system
The rete ovarii is a structure formed from the primary sex cords in females.[1]
It is the counterpart of the rete testis in males.[2] It is a narrow hilus, at which nerves and vessels enter the ovary. In the medulla of the mammalian ovary near the hilus are small masses of blind tubules or solid cords—the rete ovarii—which are homologous (i.e., of the same embryonic origin) with the rete testis in the male. The microscopic right ovary of birds usually consists only of medullary tissue.
https://en.wikipedia.org/wiki/Rete_ovarii
In biology, a tubule is a general term referring to small tube or similar type of structure. Specifically, tubule can refer to:
- a small tube or fistular structure
- a minute tube lined with glandular epithelium[1]
- any hollow cylindrical body structure
- a minute canal found in various structures or organs of the body[2]
- a slender elongated anatomical channel[3]
- a minute tube, especially as an anatomical structure.
Examples of tubules[edit]
- Collecting tubules: terminal channels of the nephrons[4]
- Cuvierian tubules: clusters of sticky tubules located at the base of the respiratory tree, which may be discharged by some sea cucumbers (holothurians) when mechanically stimulated (i.e. being threatened by a predator)[5]
- Dentinal tubules or dental canaliculi: minute channels in the dentine of a tooth that extend from the pulp cavity to the cementum or the enamel[4]
- Distal convoluted tubule: the convoluted portion of the vertebrate nephron that lies between the loop of Henle and the nonsecretory part of the nephron and that is concerned especially with the concentration of urine[3]
- Galactophorous tubule or lactiferous ducts: small channels for the passage of milk from the secreting cells in the mammary gland to the nipple[4]
- Loop of Henle: the long U-shaped part of the renal tubule, extending through the medulla from the end of the proximal convoluted tubule. It begins with a descending limb (comprising the proximal straight tubule and the thin tubule ), followed by the ascending limb (the distal straight tubule ), and ending at the distal convoluted tubule[2]
- Malpighian tubule: any of the excretory organs in insects that lie in the abdominal body cavity and empty into the junction between the midgut and hindgut[6]
- Mesonephric tubule: tubules comprising the mesonephros or temporary kidney of amniotes
- Metanephric tubule: tubules comprising the permanent kidney of amniotes[4]
- Microtubule: a microscopically small tubule[2]
- Proximal convoluted tubule: the portion of the nephron immediately after the glomerulus
- Renal tubule: the minute canals made up of basement membrane and lined with epithelium, composing the substance of the kidney and secreting, reabsorbing, collecting and conducting the urine. Include proximal convoluted, the nephron loop (containing the proximal straight tubule, descending and ascending thin, and the distal straight tubules) and the distal convoluted tubules[4]
- Seminiferous tubules: any of the numerous long convoluted tubules in the testis which are the sites where spermatozoa mature
- T tubule: transverse intracellular tubules invaginating from the cell membrane and surrounding the myofibrils of the T system of skeletal and cardiac muscle, serving as a pathway for the spread of electrical excitation within a muscle cell
- Trachea: tubules forming the respiratory system of most insects and many arachnids
- Uriniferous tubules: any of the small tubules that are the excretory units of the vertebrate kidney
- Uveoscleral pathway: a tubule that drains excess aqueous humor
- Vasa efferentia: convoluted tubules that lead from the rete testis to the vas deferens and form the head of the epididymis[2]
- Vesicular-tubular clusters between the golgi apparatus and endoplasmic reticulum which assist in transport.[7]
https://en.wikipedia.org/wiki/Tubule
A fistula (plural: fistulas or fistulae /-li, -laɪ/; from Latin fistula, "tube, pipe") in anatomy is an abnormal connectionbetween two hollow spaces (technically, two epithelialized surfaces), such as blood vessels, intestines, or other hollow organs.[2][3][4] Types of fistula can be described by their location. Anal fistulas connect between the anal canal and the perianal skin. Anovaginal or rectovaginal fistulas occur when a hole develops between the anus or rectum and the vagina. Colovaginal fistulas occur between the colon and the vagina. Urinary tract fistulas are abnormal openings within the urinary tract or an abnormal connection between the urinary tract and another organ such as between the bladder and the uterus in a vesicouterine fistula, between the bladder and the vagina in a vesicovaginal fistula, and between the urethra and the vagina in urethrovaginal fistula. When occurring between two parts of the intestine, it is known as an enteroenteral fistula, between the small intestine and the skin as an enterocutaneous fistula, and between the colon and the skin as a colocutaneous fistula.[3]
Fistulas can result from an infection or inflammation, injury or surgery.[5] Fistulas are sometimes surgically created as part of a treatment, for example arteriovenous fistulas for hemodialysis.[6]
Treatment for fistula varies depending on the cause and extent of the fistula, but often involves surgical intervention combined with antibiotic therapy. In some cases the fistula is temporarily covered using a fibrin glue or plug. Catheters may be required to drain a fistula.[3]
Globally, every year between 50,000 and 100,000 women are affected by fistula relating to childbirth.[7] In botany, the term is most common in its adjectival forms, where it is used in binomial names to refer to species that are distinguished by hollow or tubular structures. Monarda fistulosa, for example, has tubular flowers.[8] The term was first used in the 14th century.[2]
| Fistula | |
|---|---|
 | |
| Abdominal CT scan with right colocutaneous fistula and associated subcutaneous pneumatosis | |
| Pronunciation | |
| Specialty | General surgery |
https://en.wikipedia.org/wiki/Fistula
In embryogenesis, the sex cords (primitive sex cords, primitive seminiferous cords, or gonadal cords) are structures that develop from the gonadal ridges that further differentiate based on an embryo's sex. After sexual differentiation, at day 49, the sex cords in females become the cortical cords, also called secondary cords. After further development, they become the ovarian follicles. The sex cords in males become the testis cords by the action of the testis-determining factor protein, which helps to develop and nourish the Sertoli cells.
The testis cords are precursors to the rete testis. They play several different roles in the development of the male genitals.[1] The primitive sex cords originate from the proliferation of the epithelium of the two gonadal ridges. These epithelial cells (from the gonadal ridges) penetrate and invade the underlying mesenchyme to form the primitive sex cords.[2] This occurs shortly before and during the arrival of the primordial germ cells (PGCs) to the paired gonadal ridges.[3]
See also[edit]
In embryology, the gonadal ridge (or urogenital ridge) is the precursor to the gonads. The gonadal ridge initially consists mainly of mesenchyme and cells of underlying mesonephric origin. Once oogonia enter this area they attempt to associate with these somatic cells. Development proceeds and the oogonia become fully surrounded by a layer of cells (pre-granulosa cells).
The gonadal ridge appears at approximately five weeks, and gives rise to the sex cords.
| Gonadal ridge | |
|---|---|
 Section of the fold in the mesonephros of a chick embryo of the fourth day. ("Genital ridge" labeled at left.) | |
| Details | |
| Precursor | urogenital folds |
| Gives rise to | sex cords |
| System | Reproductive system |
| Identifiers | |
| Latin | crista gonadalis |
| TE | ridge_by_E5.7.1.0.0.0.5 E5.7.1.0.0.0.5 |
| Anatomical terminology | |

Structure and function[edit]
In the primordial ovarian follicle, and later in follicle development (folliculogenesis), granulosa cells advance to form a multilayered cumulus oophorus surrounding the oocyte in the preovulatory or antral (or Graafian) follicle.
The major functions of granulosa cells include the production of sex steroids, as well as myriad growth factors thought to interact with the oocyte during its development. The sex steroid production begins with follicle-stimulating hormone (FSH) from the anterior pituitary, stimulating granulosa cells to convert androgens(coming from the thecal cells) to estradiol by aromatase during the follicular phase of the menstrual cycle.[1] However, after ovulation the granulosa cells turn into granulosa lutein cells that produce progesterone. The progesterone may maintain a potential pregnancy and causes production of a thick cervical mucus that inhibits sperm entry into the uterus.
Embryology of ovarian granulosa cells[edit]
In the development of the urinary and reproductive organs, the oogonia become invaginated in the gonadal ridge.
The embryological origin of granulosa cells remains controversial. In the 1970s, evidence emerged that the first cells to make contact with the oogonia were of mesonephric origin. It was suggested that mesonephric cells already closely associated with the oogonia proliferated throughout development to form the granulosa cell layer.[2][3][4] Recently, this hypothesis has been challenged with some thorough histology. Sawyer et al. hypothesized that in sheep most of the granulosa cells develop from cells of the mesothelium (i.e., epithelial cells from the presumptive surface epithelium of the ovary).[5] In 2013, it was proposed that both granulosa cells and the ovarian surface epithelial cells are instead derived from a precursor cell called gonadal-ridge epithelial-like cell. [6]
Granulosa Cell Types[edit]
Cumulus Cells (CC) vs Mural Granulosa Cells (MGC)
Cumulus cells surround the oocyte. They provide nutrients to the oocyte and influence the development of the oocyte in a paracrine fashion. Mural granulosa cells line the follicular wall and surround the fluid-filled antrum. The oocyte secretes factors that determine the functional differences between CCs and MGGs. CCs primarily support growth and development of the oocyte whereas MGCs primarily serve an endocrine function and support the growth of the follicle. Cumulus cells aid in oocyte development and show higher expression of SLC38A3, a transporter for amino acids, and Aldoa, Eno1, Ldh1, Pfkp, Pkm2, and Tpi1, enzymes responsible for glycolysis.[7] MGCs are more steroidogenically active and have higher levels of mRNA expression of steroidogenic enzymes such as cytochrome P450.[8] MGCs produce an increasing amount of estrogen which leads to the LH surge.[9] Following the LH surge, cumulus cells undergo cumulus expansion, in which they proliferate at a ten-fold higher rate than MGCs in response to FSH.[10] During expansion CCs also produce a mucified matrix required for ovulation.[11]
Cell culture[edit]
Cell culture of granulosa cells can be performed in vitro. Plating density (number of cells per volume of culture medium) plays a critical role for the differentiation. A lower plating density makes granulosa cells exhibit estrogen production, while a higher plating density makes them appear as progesterone producing theca lutein cells.[12]
Additional images[edit]
See also[edit]
Maintaining cells in culture[edit]
For the majority of isolated primary cells, they undergo the process of senescence and stop dividing after a certain number of population doublings while generally retaining their viability (described as the Hayflick limit).
Aside from temperature and gas mixture, the most commonly varied factor in culture systems is the cell growth medium. Recipes for growth media can vary in pH, glucose concentration, growth factors, and the presence of other nutrients. The growth factors used to supplement media are often derived from the serum of animal blood, such as fetal bovine serum (FBS), bovine calf serum, equine serum, and porcine serum. One complication of these blood-derived ingredients is the potential for contamination of the culture with viruses or prions, particularly in medical biotechnology applications. Current practice is to minimize or eliminate the use of these ingredients wherever possible and use human platelet lysate (hPL).[9] This eliminates the worry of cross-species contamination when using FBS with human cells. hPL has emerged as a safe and reliable alternative as a direct replacement for FBS or other animal serum. In addition, chemically defined media can be used to eliminate any serum trace (human or animal), but this cannot always be accomplished with different cell types. Alternative strategies involve sourcing the animal blood from countries with minimum BSE/TSE risk, such as The United States, Australia and New Zealand,[10] and using purified nutrient concentrates derived from serum in place of whole animal serum for cell culture.[11]
Plating density (number of cells per volume of culture medium) plays a critical role for some cell types. For example, a lower plating density makes granulosa cells exhibit estrogen production, while a higher plating density makes them appear as progesterone-producing theca lutein cells.[12]
Cells can be grown either in suspension or adherent cultures.[13] Some cells naturally live in suspension, without being attached to a surface, such as cells that exist in the bloodstream. There are also cell lines that have been modified to be able to survive in suspension cultures so they can be grown to a higher density than adherent conditions would allow. Adherent cells require a surface, such as tissue culture plastic or microcarrier, which may be coated with extracellular matrix (such as collagen and laminin) components to increase adhesion properties and provide other signals needed for growth and differentiation. Most cells derived from solid tissues are adherent. Another type of adherent culture is organotypic culture, which involves growing cells in a three-dimensional (3-D) environment as opposed to two-dimensional culture dishes. This 3D culture system is biochemically and physiologically more similar to in vivo tissue, but is technically challenging to maintain because of many factors (e.g. diffusion).[14]
Cell culture basal media[edit]
There are different kinds of cell culture media which being used routinely in life science including the following:
- MEM
- DMEM
- RPMI 1640
- Ham's f-12
- IMDM
- Leibovitz L-15
- DMEM/F-12
Components of cell culture media[edit]
| Component | Function |
|---|---|
| Carbon source (glucose/glutamine) | Source of energy |
| Amino acid | Building blocks of protein |
| Vitamins | Promote cell survival and growth |
| Balanced salt solution | An isotonic mixture of ions to maintain optimum osmotic pressure within the cells and provide essential metal ions to act as cofactors for enzymatic reactions, cell adhesion etc. |
| Phenol red dye | pH indicator. The color of phenol red changes from orange/red at pH 7–7.4 to yellow at acidic (lower) pH and purple at basic (higher) pH. |
| Bicarbonate /HEPES buffer | It is used to maintain a balanced pH in the media |
Typical Growth conditions[edit]
| Parameter | |
|---|---|
| Temperature | 37 °C |
| CO2 | 5% |
| Relative Humidity | 95% |
Cell line cross-contamination[edit]
Cell line cross-contamination can be a problem for scientists working with cultured cells.[15] Studies suggest anywhere from 15–20% of the time, cells used in experiments have been misidentified or contaminated with another cell line.[16][17][18] Problems with cell line cross-contamination have even been detected in lines from the NCI-60 panel, which are used routinely for drug-screening studies.[19][20] Major cell line repositories, including the American Type Culture Collection (ATCC), the European Collection of Cell Cultures (ECACC) and the German Collection of Microorganisms and Cell Cultures (DSMZ), have received cell line submissions from researchers that were misidentified by them.[19][21] Such contamination poses a problem for the quality of research produced using cell culture lines, and the major repositories are now authenticating all cell line submissions.[22] ATCC uses short tandem repeat (STR) DNA fingerprinting to authenticate its cell lines.[23]
To address this problem of cell line cross-contamination, researchers are encouraged to authenticate their cell lines at an early passage to establish the identity of the cell line. Authentication should be repeated before freezing cell line stocks, every two months during active culturing and before any publication of research data generated using the cell lines. Many methods are used to identify cell lines, including isoenzyme analysis, human lymphocyte antigen (HLA) typing, chromosomal analysis, karyotyping, morphology and STR analysis.[23]
One significant cell-line cross contaminant is the immortal HeLa cell line.
Other technical issues[edit]
As cells generally continue to divide in culture, they generally grow to fill the available area or volume. This can generate several issues:
- Nutrient depletion in the growth media
- Changes in pH of the growth media
- Accumulation of apoptotic/necrotic (dead) cells
- Cell-to-cell contact can stimulate cell cycle arrest, causing cells to stop dividing, known as contact inhibition.
- Cell-to-cell contact can stimulate cellular differentiation.
- Genetic and epigenetic alterations, with a natural selection of the altered cells potentially leading to overgrowth of abnormal, culture-adapted cells with decreased differentiation and increased proliferative capacity.[24]
The choice of culture medium might affect the physiological relevance of findings from cell culture experiments due to the differences in the nutrient composition and concentrations.[25] A systematic bias in generated datasets was recently shown for CRISPR and RNAi gene silencing screens,[26] and for metabolic profiling of cancer cell lines.[25] Using a growth medium that better represents the physiological levels of nutrients can improve the physiological relevance of in vitro studies and recently such media types, as Plasmax[27] and Human Plasma Like Medium (HPLM),[28] were developed.
Manipulation of cultured cells[edit]
Among the common manipulations carried out on culture cells are media changes, passaging cells, and transfecting cells. These are generally performed using tissue culture methods that rely on aseptic technique. Aseptic technique aims to avoid contamination with bacteria, yeast, or other cell lines. Manipulations are typically carried out in a biosafety cabinet or laminar flow cabinet to exclude contaminating micro-organisms. Antibiotics (e.g. penicillin and streptomycin) and antifungals (e.g.amphotericin B and Antibiotic-Antimycotic solution) can also be added to the growth media.
As cells undergo metabolic processes, acid is produced and the pH decreases. Often, a pH indicator is added to the medium to measure nutrient depletion.
Media changes[edit]
In the case of adherent cultures, the media can be removed directly by aspiration, and then is replaced. Media changes in non-adherent cultures involve centrifuging the culture and resuspending the cells in fresh media.
Passaging cells[edit]
Passaging (also known as subculture or splitting cells) involves transferring a small number of cells into a new vessel. Cells can be cultured for a longer time if they are split regularly, as it avoids the senescence associated with prolonged high cell density. Suspension cultures are easily passaged with a small amount of culture containing a few cells diluted in a larger volume of fresh media. For adherent cultures, cells first need to be detached; this is commonly done with a mixture of trypsin-EDTA; however, other enzyme mixes are now available for this purpose. A small number of detached cells can then be used to seed a new culture. Some cell cultures, such as RAW cells are mechanically scraped from the surface of their vessel with rubber scrapers.
Transfection and transduction[edit]
Another common method for manipulating cells involves the introduction of foreign DNA by transfection. This is often performed to cause cells to express a gene of interest. More recently, the transfection of RNAi constructs have been realized as a convenient mechanism for suppressing the expression of a particular gene/protein. DNA can also be inserted into cells using viruses, in methods referred to as transduction, infection or transformation. Viruses, as parasitic agents, are well suited to introducing DNA into cells, as this is a part of their normal course of reproduction.
Established human cell lines[edit]
Cell lines that originate with humans have been somewhat controversial in bioethics, as they may outlive their parent organism and later be used in the discovery of lucrative medical treatments. In the pioneering decision in this area, the Supreme Court of California held in Moore v. Regents of the University of California that human patients have no property rights in cell lines derived from organs removed with their consent.[29]
It is possible to fuse normal cells with an immortalised cell line. This method is used to produce monoclonal antibodies. In brief, lymphocytes isolated from the spleen (or possibly blood) of an immunised animal are combined with an immortal myeloma cell line (B cell lineage) to produce a hybridoma which has the antibody specificity of the primary lymphocyte and the immortality of the myeloma. Selective growth medium (HA or HAT) is used to select against unfused myeloma cells; primary lymphoctyes die quickly in culture and only the fused cells survive. These are screened for production of the required antibody, generally in pools to start with and then after single cloning.
Cell strains[edit]
A cell strain is derived either from a primary culture or a cell line by the selection or cloning of cells having specific properties or characteristics which must be defined. Cell strains are cells that have been adapted to culture but, unlike cell lines, have a finite division potential. Non-immortalized cells stop dividing after 40 to 60 population doublings[30] and, after this, they lose their ability to proliferate (a genetically determined event known as senescence).[31]
Applications of cell culture[edit]
Mass culture of animal cell lines is fundamental to the manufacture of viral vaccines and other products of biotechnology. Culture of human stem cells is used to expand the number of cells and differentiate the cells into various somatic cell types for transplantation.[32] Stem cell culture is also used to harvest the molecules and exosomes that the stem cells release for the purposes of therapeutic development.[33]
Biological products produced by recombinant DNA (rDNA) technology in animal cell cultures include enzymes, synthetic hormones, immunobiologicals (monoclonal antibodies, interleukins, lymphokines), and anticancer agents. Although many simpler proteins can be produced using rDNA in bacterial cultures, more complex proteins that are glycosylated (carbohydrate-modified) currently must be made in animal cells. An important example of such a complex protein is the hormone erythropoietin. The cost of growing mammalian cell cultures is high, so research is underway to produce such complex proteins in insect cells or in higher plants, use of single embryonic cell and somatic embryos as a source for direct gene transfer via particle bombardment, transit gene expression and confocal microscopy observation is one of its applications. It also offers to confirm single cell origin of somatic embryos and the asymmetry of the first cell division, which starts the process.
Cell culture is also a key technique for cellular agriculture, which aims to provide both new products and new ways of producing existing agricultural products like milk, (cultured) meat, fragrances, and rhino horn from cells and microorganisms. It is therefore considered one means of achieving animal-free agriculture. It is also a central tool for teaching cell biology.[34]
Cell culture in two dimensions[edit]
Research in tissue engineering, stem cells and molecular biology primarily involves cultures of cells on flat plastic dishes. This technique is known as two-dimensional (2D) cell culture, and was first developed by Wilhelm Roux who, in 1885, removed a portion of the medullary plate of an embryonic chicken and maintained it in warm saline for several days on a flat glass plate. From the advance of polymer technology arose today's standard plastic dish for 2D cell culture, commonly known as the Petri dish. Julius Richard Petri, a German bacteriologist, is generally credited with this invention while working as an assistant to Robert Koch. Various researchers today also utilize culturing laboratory flasks, conicals, and even disposable bags like those used in single-use bioreactors.
Aside from Petri dishes, scientists have long been growing cells within biologically derived matrices such as collagen or fibrin, and more recently, on synthetic hydrogels such as polyacrylamide or PEG. They do this in order to elicit phenotypes that are not expressed on conventionally rigid substrates. There is growing interest in controlling matrix stiffness,[35] a concept that has led to discoveries in fields such as:
- Stem cell self-renewal[36][37]
- Lineage specification[38]
- Cancer cell phenotype[39][40][41]
- Fibrosis[42][43]
- Hepatocyte function[44][45][46]
- Mechanosensing[47][48][49]
Cell culture in three dimensions[edit]
Cell culture in three dimensions has been touted as "Biology's New Dimension".[50] At present, the practice of cell culture remains based on varying combinations of single or multiple cell structures in 2D.[51] Currently, there is an increase in use of 3D cell cultures in research areas including drug discovery, cancer biology, regenerative medicine, nanomaterials assessment and basic life science research.[52][53][54] 3D cell cultures can be grown using a scaffold or matrix, or in a scaffold-free manner. Scaffold based cultures utilize an acellular 3D matrix or a liquid matrix. Scaffold-free methods are normally generated in suspensions.[55] There are a variety of platforms used to facilitate the growth of three-dimensional cellular structures including scaffold systems such as hydrogel matrices[56] and solid scaffolds, and scaffold-free systems such as low-adhesion plates, nanoparticle facilitated magnetic levitation,[57] and hanging drop plates.[58][59] Culturing cells in 3D leads to wide variation in gene expression signatures and partly mimics tissues in the physiological states.[60]
3D cell culture in scaffolds
Eric Simon, in a 1988 NIH SBIR grant report, showed that electrospinning could be used to produced nano- and submicron-scale polystyrene and polycarbonate fibrous scaffolds specifically intended for use as in vitro cell substrates. This early use of electrospun fibrous lattices for cell culture and tissue engineering showed that various cell types including Human Foreskin Fibroblasts (HFF), transformed Human Carcinoma (HEp-2), and Mink Lung Epithelium (MLE) would adhere to and proliferate upon polycarbonate fibers. It was noted that, as opposed to the flattened morphology typically seen in 2D culture, cells grown on the electrospun fibers exhibited a more histotypic rounded 3-dimensional morphology generally observed in vivo.[61]
3D cell culture in hydrogels[edit]
As the natural extracellular matrix (ECM) is important in the survival, proliferation, differentiation and migration of cells, different hydrogel culture matrices mimicking natural ECM structure are seen as potential approaches to in vivo –like cell culturing.[62] Hydrogels are composed of interconnected pores with high water retention, which enables efficient transport of substances such as nutrients and gases. Several different types of hydrogels from natural and synthetic materials are available for 3D cell culture, including animal ECM extract hydrogels, protein hydrogels, peptide hydrogels, polymer hydrogels, and wood-based nanocellulose hydrogel.
3D Cell Culturing by Magnetic Levitation[edit]
The 3D Cell Culturing by Magnetic Levitation method (MLM) is the application of growing 3D tissue by inducing cells treated with magnetic nanoparticle assemblies in spatially varying magnetic fields using neodymium magnetic drivers and promoting cell to cell interactions by levitating the cells up to the air/liquid interface of a standard petri dish. The magnetic nanoparticle assemblies consist of magnetic iron oxide nanoparticles, gold nanoparticles, and the polymer polylysine. 3D cell culturing is scalable, with the capability for culturing 500 cells to millions of cells or from single dish to high-throughput low volume systems.
Tissue culture and engineering[edit]
Cell culture is a fundamental component of tissue culture and tissue engineering, as it establishes the basics of growing and maintaining cells in vitro. The major application of human cell culture is in stem cell industry, where mesenchymal stem cells can be cultured and cryopreserved for future use. Tissue engineering potentially offers dramatic improvements in low cost medical care for hundreds of thousands of patients annually.
Vaccines[edit]
Vaccines for polio, measles, mumps, rubella, and chickenpox are currently made in cell cultures. Due to the H5N1 pandemic threat, research into using cell culture for influenza vaccines is being funded by the United States government. Novel ideas in the field include recombinant DNA-based vaccines, such as one made using human adenovirus (a common cold virus) as a vector,[63][64] and novel adjuvants.[65]
Cell culture in microfluidic device[edit]
Culture of non-mammalian cells[edit]
Besides the culture of well-established immortalised cell lines, cells from primary explants of a plethora of organisms can be cultured for a limited period of time before senescence occurs (see Hayflick's limit). Cultured primary cells have been extensively used in research, as is the case of fish keratocytes in cell migration studies.[66][34][67]
Plant cell culture methods[edit]
Plant cell cultures are typically grown as cell suspension cultures in a liquid medium or as callus cultures on a solid medium. The culturing of undifferentiated plant cells and calli requires the proper balance of the plant growth hormones auxin and cytokinin.
Insect cell culture[edit]
Cells derived from Drosophila melanogaster (most prominently, Schneider 2 cells) can be used for experiments which may be hard to do on live flies or larvae, such as biochemical studies or studies using siRNA. Cell lines derived from the army worm Spodoptera frugiperda, including Sf9 and Sf21, and from the cabbage looper Trichoplusia ni, High Five cells, are commonly used for expression of recombinant proteins using baculovirus.[68]
Bacterial and yeast culture methods[edit]
For bacteria and yeasts, small quantities of cells are usually grown on a solid support that contains nutrients embedded in it, usually a gel such as agar, while large-scale cultures are grown with the cells suspended in a nutrient broth.
Viral culture methods[edit]
The culture of viruses requires the culture of cells of mammalian, plant, fungal or bacterial origin as hosts for the growth and replication of the virus. Whole wild typeviruses, recombinant viruses or viral products may be generated in cell types other than their natural hosts under the right conditions. Depending on the species of the virus, infection and viral replication may result in host cell lysis and formation of a viral plaque.
https://en.wikipedia.org/wiki/Cell_culture#Maintaining_cells_in_culture
A viral plaque is a visible structure formed after introducing a viral sample to a cell culture grown on some nutrient medium. The virus will replicate and spread, generating regions of cell destruction known as plaques. For example, Vero cell or other tissue cultures may be used to investigate an influenza virus or coronavirus, while various bacterial cultures would be used for bacteriophages.
Counting the number of plaques can be used as a method of virus quantification. These plaques can sometimes be detected visually using colony counters, in much the same way as bacterial colonies are counted; however, they are not always visible to the naked eye, and sometimes can only be seen through a microscope, or using techniques such as staining (e.g. neutral red for eukaryotes[1] or giemsa for bacteria[2]) or immunofluorescence. Special computer systems have been designed with the ability to scan samples in batches.
The appearance of the plaque depends on the host strain, virus and the conditions. Highly virulent or lytic strains create plaques that look clear (due to total cell destruction), while strains that only kill a fraction of their hosts (due to partial resistance/lysogeny), or only reduce the rate of cell growth, give turbid plaques. Some partially lysogenic phages give bull's-eye plaques with spots or rings of growth in the middle of clear regions of complete lysis.
https://en.wikipedia.org/wiki/Viral_plaque
An assay is an investigative (analytic) procedure in laboratory medicine, mining, pharmacology, environmental biology and molecular biology for qualitatively assessing or quantitatively measuring the presence, amount, or functional activity of a target entity. The measured entity is often called the analyte, the measurand, or the targetof the assay. The analyte can be a drug, biochemical substance, chemical element or compound, or cell in an organism or organic sample.[1][2] An assay usually aims to measure an analyte's intensive property and express it in the relevant measurement unit (e.g. molarity, density, functional activity in enzyme international units, degree of effect in comparison to a standard, etc.).
If the assay involves exogenous reactants (the reagents), then their quantities are kept fixed (or in excess) so that the quantity and quality of the target are the only limiting factors. The difference in the assay outcome is used to deduce the unknown quality or quantity of the target in question. Some assays (e.g., biochemical assays) may be similar to chemical analysis and titration. However, assays typically involve biological material or phenomena that are intrinsically more complex in composition or behavior, or both. Thus, reading of an assay may be noisy and involve greater difficulties in interpretation than an accurate chemical titration. On the other hand, older generation qualitative assays, especially bioassays, may be much more gross and less quantitative (e.g., counting death or dysfunction of an organism or cells in a population, or some descriptive change in some body part of a group of animals).
Assays have become a routine part of modern medical, environmental, pharmaceutical, and forensic technology. Other businesses may also employ them at the industrial, curbside, or field levels. Assays in high commercial demand have been well investigated in research and development sectors of professional industries. They have also undergone generations of development and sophistication. In some cases, they are protected by intellectual property regulations such as patentsgranted for inventions. Such industrial-scale assays are often performed in well-equipped laboratories and with automated organization of the procedure, from ordering an assay to pre-analytic sample processing (sample collection, necessary manipulations e.g. spinning for separation, aliquoting[definition needed] if necessary, storage, retrieval, pipetting, aspiration, etc.). Analytes are generally tested in high-throughput autoanalyzers, and the results are verified and automatically returned to ordering service providers and end-users. These are made possible through the use of an advanced laboratory informatics system that interfaces with multiple computer terminals with end-users, central servers, the physical autoanalyzer instruments, and other automata.[clarification needed]
https://en.wikipedia.org/wiki/Assay
Biological immortality (sometimes referred to as bio-indefinite mortality) is a state in which the rate of mortality from senescence is stable or decreasing, thus decoupling it from chronological age. Various unicellular and multicellular species, including some vertebrates, achieve this state either throughout their existence or after living long enough. A biologically immortal living being can still die from means other than senescence, such as through injury, poison, disease, lack of available resources, or changes to environment.
This definition of immortality has been challenged in the Handbook of the Biology of Aging,[1] because the increase in rate of mortality as a function of chronological age may be negligible at extremely old ages, an idea referred to as the late-life mortality plateau. The rate of mortality may cease to increase in old age, but in most cases that rate is typically very high.[2]
The term is also used by biologists to describe cells that are not subject to the Hayflick limit on how many times they can divide.
Cell lines[edit]
Biologists chose the word "immortal" to designate cells that are not subject to the Hayflick limit, the point at which cells can no longer divide due to DNA damage or shortened telomeres. Prior to Leonard Hayflick's theory, Alexis Carrel hypothesized that all normal somatic cells were immortal.[3]
The term "immortalization" was first applied to cancer cells that expressed the telomere-lengthening enzyme telomerase, and thereby avoided apoptosis—i.e. cell death caused by intracellular mechanisms. Among the most commonly used cell lines are HeLa and Jurkat, both of which are immortalized cancer cell lines. HeLa cellsoriginated from a sample of cervical cancer taken from Henrietta Lacks in 1951.[4] These cells have been and still are widely used in biological research such as creation of the polio vaccine,[5] sex hormone steroid research,[6] and cell metabolism.[7] Embryonic stem cells and germ cells have also been described as immortal.[8][9]
Immortal cell lines of cancer cells can be created by induction of oncogenes or loss of tumor suppressor genes. One way to induce immortality is through viral-mediated induction of the large T-antigen,[10] commonly introduced through simian virus 40 (SV-40).[11]
Organisms[edit]
According to the Animal Aging and Longevity Database, the list of animals with negligible aging (along with estimated longevity in the wild) includes:[12]
- Blanding's turtle (Emydoidea blandingii) – 77 years
- Olm (Proteus anguinus) – 102 years
- Eastern box turtle (Terrapene carolina) – 138 years
- Red sea urchin (Strongylocentrotus franciscanus) – 200 years
- Rougheye rockfish (Sebastes aleutianus) – 205 years
- Ocean quahog clam (Arctica islandica) – 507 years
- Greenland shark (Somniosus microcephalus) - 250 to 500 years
In 2018, scientists working for Calico, a company owned by Alphabet, published a paper in the journal eLife which presents possible evidence that Heterocephalus glaber (Naked mole rat) do not face increased mortality risk due to aging.[13][14][15]
Bacteria and some yeast[edit]
Many unicellular organisms age: as time passes, they divide more slowly and ultimately die. Asymmetrically dividing bacteria and yeast also age. However, symmetrically dividing bacteria and yeast can be biologically immortal under ideal growing conditions.[16] In these conditions, when a cell splits symmetrically to produce two daughter cells, the process of cell division can restore the cell to a youthful state. However, if the parent asymmetrically buds off a daughter only the daughter is reset to the youthful state—the parent isn't restored and will go on to age and die. In a similar manner stem cells and gametes can be regarded as "immortal".
Hydra[edit]
Hydras are a genus of the Cnidaria phylum. All cnidarians can regenerate, allowing them to recover from injury and to reproduce asexually. Hydras are simple, freshwater animals possessing radial symmetry and contain post-mitotic cells (cells that will never divide again) only in the extremities.[17] All hydra cells continually divide.[18] It has been suggested that hydras do not undergo senescence, and, as such, are biologically immortal. In a four-year study, 3 cohorts of hydra did not show an increase in mortality with age. It is possible that these animals live much longer, considering that they reach maturity in 5 to 10 days.[19] However, this does not explain how hydras are consequently able to maintain telomere lengths.
Jellyfish[edit]
Turritopsis dohrnii, or Turritopsis nutricula, is a small (5 millimeters (0.20 in)) species of jellyfish that uses transdifferentiation to replenish cells after sexual reproduction. This cycle can repeat indefinitely, potentially rendering it biologically immortal. This organism originated in the Caribbean sea, but has now spread around the world.[citation needed] Similar cases include hydrozoan Laodicea undulata[20] and scyphozoan Aureliasp.1.[21]
Lobsters[edit]
Research suggests that lobsters may not slow down, weaken, or lose fertility with age, and that older lobsters may be more fertile than younger lobsters. This does not however make them immortal in the traditional sense, as they are significantly more likely to die at a shell moult the older they get (as detailed below).
Their longevity may be due to telomerase, an enzyme that repairs long repetitive sections of DNA sequences at the ends of chromosomes, referred to as telomeres. Telomerase is expressed by most vertebrates during embryonic stages but is generally absent from adult stages of life.[22] However, unlike vertebrates, lobsters express telomerase as adults through most tissue, which has been suggested to be related to their longevity.[23][24][25] Contrary to popular belief, lobsters are not immortal. Lobsters grow by moulting which requires considerable energy, and the larger the shell the more energy is required.[26] Eventually, the lobster will die from exhaustion during a moult. Older lobsters are also known to stop moulting, which means that the shell will eventually become damaged, infected, or fall apart and they die.[27] The European lobster has an average life span of 31 years for males and 54 years for females.
Planarian flatworms[edit]
Planarian flatworms have both sexually and asexually reproducing types. Studies on genus Schmidtea mediterraneasuggest these planarians appear to regenerate (i.e. heal) indefinitely, and asexual individuals have an "apparently limitless [telomere] regenerative capacity fueled by a population of highly proliferative adult stem cells". "Both asexual and sexual animals display age-related decline in telomere length; however, asexual animals are able to maintain telomere lengths somatically (i.e. during reproduction by fission or when regeneration is induced by amputation), whereas sexual animals restore telomeres by extension during sexual reproduction or during embryogenesis like other sexual species. Homeostatic telomerase activity observed in both asexual and sexual animals is not sufficient to maintain telomere length, whereas the increased activity in regenerating asexuals is sufficient to renew telomere length... "[28]
For sexually reproducing planaria: "the lifespan of individual planarian can be as long as 3 years, likely due to the ability of neoblasts to constantly replace aging cells". Whereas for asexually reproducing planaria: "individual animals in clonal lines of some planarian species replicating by fission have been maintained for over 15 years".[29][30]
See also[edit]
- Aging brain
- American Academy of Anti-Aging Medicine
- Calico (company)
- Cryptobiosis
- DNA damage theory of aging
- Maximum life span
- Methuselah Foundation
- Reliability theory of aging and longevity
- Rejuvenation (aging)
- Strategies for engineered negligible senescence (SENS)
- Telomerase in cancer cell
- Timeline of senescence research
https://en.wikipedia.org/wiki/Biological_immortality
Strategies for engineered negligible senescence (SENS) is a range of proposed regenerative medical therapies, either planned or currently in development, for the periodical repair of all age-related damage to human tissue. These therapies have the ultimate aim of maintaining a state of negligible senescence in patients and postponing age-associated disease.[1] SENS was first defined by British biogerontologist Aubrey de Grey.
While some biogerontologists support the SENS program, many contend that the ultimate goals of de Grey's programme are too speculative given the current state of technology and refer to it as "fantasy rather than science".[2][3]
Strategies[edit]
As described by SENS, the following table details major ailments and the program's proposed preventative strategies:[7]
| Issue | Proposed countermeasures |
|---|---|
| Extracellular aggregates | Immunotherapeutic clearance |
| Accumulation of senescent cells | Senescence marker-targeted toxins, immunotherapy |
| Extracellular matrix stiffening | AGE-breaking molecules, tissue engineering |
| Intracellular aggregates | Novel lysosomal hydrolases |
| Mitochondrial mutations | Allotopic expression of 13 proteins |
| Cancerous cells | Removal of telomere-lengthening machinery |
| Cell loss, tissue atrophy | Stem cells and tissue engineering |
Scientific reception[edit]
While some fields mentioned as branches of SENS are supported by the medical research community, e.g., stem cell research, anti-Alzheimers research and oncogenomics, the SENS programme as a whole has been a highly controversial proposal. Many of its critics argue that the SENS agenda is fanciful and that the complicated biomedical phenomena involved in aging contain too many unknowns for SENS to be fully implementable in the foreseeable future.[8]
Cancer may deserve special attention as an aging-associated disease, but the SENS claim that nuclear DNA damage only matters for aging because of cancer has been challenged in the literature,[9] as well as by material in the DNA damage theory of aging. More recently, biogerontologist Marios Kyriazis has criticised the clinical applicability of SENS[10][11] by claiming that such therapies, even if developed in the laboratory, would be practically unusable by the general public.[12] De Grey responded to one such criticism.[further explanation needed][13]
2005 EMBO Reports statement[edit]
In November 2005, 28 biogerontologists published a statement of criticism in EMBO Reports, "Science fact and the SENS agenda: what can we reasonably expect from ageing research?,"[8] arguing "each one of the specific proposals that comprise the SENS agenda is, at our present stage of ignorance, exceptionally optimistic,"[8]and that some of the specific proposals "will take decades of hard work [to be medically integrated], if [they] ever prove to be useful."[8] The researchers argue that while there is "a rationale for thinking that we might eventually learn how to postpone human illnesses to an important degree,"[8] increased basic research, rather than the goal-directed approach of SENS, is currently the scientifically appropriate goal.
https://en.wikipedia.org/wiki/Strategies_for_engineered_negligible_senescence
A senolytic (from the words senescence and -lytic, "destroying") is among a class of small molecules under basic research to determine if they can selectively induce death of senescent cells and improve health in humans.[1] A goal of this research is to discover or develop agents to delay, prevent, alleviate, or reverse age-related diseases.[2][3] A related concept is "senostatic", which means to suppress senescence.
https://en.wikipedia.org/wiki/Senolytic
Dasatinib, sold under the brand name Sprycel among others, is a targeted therapy medication used to treat certain cases of chronic myelogenous leukemia (CML) and acute lymphoblastic leukemia (ALL).[3]Specifically it is used to treat cases that are Philadelphia chromosome-positive (Ph+).[3] It is taken by mouth.[3]
Common adverse effects include low white blood cells, low blood platelets, anemia, swelling, rash, and diarrhea.[3] Severe adverse effects may include bleeding, pulmonary edema, heart failure, and prolonged QT syndrome.[3] Use during pregnancy may result in harm to the baby.[3] It is a tyrosine-kinase inhibitorand works by blocking a number of tyrosine kinases such as Bcr-Abl and the Src kinase family.[3]
Dasatinib was approved for medical use in the United States and in the European Union in 2006.[3][2] It is on the World Health Organization's List of Essential Medicines.[4]
Medical uses[edit]
Dasatinib is used to treat people with chronic myeloid leukemia and people with acute lymphoblastic leukemia who are positive for the Philadelphia chromosome.[5]
In the EU dasatinib is indicated for children with
- newly diagnosed Philadelphia chromosome-positive chronic myelogenous leukaemia in chronic phase (Ph+ CML CP) or Ph+ CML CP resistant or intolerant to prior therapy including imatinib.[2]
- newly diagnosed Ph+ acute lymphoblastic leukaemia (ALL) in combination with chemotherapy.[2]
- newly diagnosed Ph+ CML in chronic phase (Ph+ CML-CP) or Ph+ CML-CP resistant or intolerant to prior therapy including imatinib.[2]
and adults with
- newly diagnosed Philadelphia-chromosome-positive (Ph+) chronic myelogenous leukaemia (CML) in the chronic phase;[2]
- chronic, accelerated or blast phase CML with resistance or intolerance to prior therapy including imatinib mesilate;[2]
- Ph+ acute lymphoblastic leukaemia (ALL) and lymphoid blast CML with resistance or intolerance to prior therapy.[2]
Adverse effects[edit]
The most common side effects are infection, suppression of the bone marrow (decreasing numbers of leukocytes, erythrocytes, and thrombocytes),[6] headache, hemorrhage (bleeding), pleural effusion (fluid around the lungs), dyspnea (difficulty breathing), diarrhea, vomiting, nausea (feeling sick), abdominal pain(belly ache), skin rash, musculoskeletal pain, tiredness, swelling in the legs and arms and in the face, fever.[2] Neutropenia and myelosuppression were common toxic effects. Fifteen people (of 84, i.e. 18%) in the above-mentioned study developed pleural effusions, which was a suspected side effect of dasatinib. Some of these people required thoracentesis or pleurodesis to treat the effusions. Other adverse events included mild to moderate diarrhea, peripheral edema, and headache. A small number of people developed abnormal liver function tests which returned to normal without dose adjustments. Mild hypocalcemia was also noted, but did not appear to cause any significant problems. Several cases of pulmonary arterial hypertension (PAH) were found in people treated with dasatinib,[7]possibly due to pulmonary endothelial cell damage.[8]
On October 11, 2011, the U.S. Food and Drug Administration (FDA) announced that dasatinib may increase the risk of a rare but serious condition in which there is abnormally high blood pressure in the arteries of the lungs (pulmonary hypertension, PAH).[9] Symptoms of PAH may include shortness of breath, fatigue, and swelling of the body (such as the ankles and legs).[9] In reported cases, people developed PAH after starting dasatinib, including after more than one year of treatment.[9]Information about the risk was added to the Warnings and Precautions section of the Sprycel drug label.[9]
Research[edit]
Dasatinib has been shown to eliminate senescent cells in cultured adipocyte progenitor cells.[22] Dasatinib has been shown to induce apoptosis in senescent cells by inhibiting Src kinase, whereas quercetin inhibits the anti-apoptotic protein Bcl-xL.[22] Administration of dasatinib along with quercetin to mice improved cardiovascularfunction and eliminated senescent cells.[23] Aged mice given dasatinib with quercetin showed improved health and survival.[23]
Giving dasatinib and quercetin to mice eliminated senescent cells and caused a long-term resolution of frailty.[24] A study of fourteen human patients with idiopathic pulmonary fibrosis (a disease characterized by increased numbers of senescent cells) given dasatinib and quercetin showed improved physical function and evidence of reduced senescent cells.[22]
https://en.wikipedia.org/wiki/Dasatinib
Piperazine (/paɪˈpɛrəziːn/) is an organic compound that consists of a six-membered ring containing two nitrogen atoms at opposite positions in the ring. Piperazine exists as small alkaline deliquescent crystals with a saline taste.
The piperazines are a broad class of chemical compounds, many with important pharmacological properties, which contain a core piperazine functional group.
As an anthelmintic[edit]
Piperazine was marketed by Bayer as an anthelmintic in the early 20th century, and was featured in print ads alongside other popular Bayer products at the time, including heroin.[5] In fact, a large number of piperazine compounds have an anthelmintic action. Their mode of action is generally by paralysing parasites, which allows the host body to easily expel the invasive organism. The neuromuscular effects are thought to be caused by blocking acetylcholine at the myoneural junction. This action is mediated by its agonist effects upon the inhibitory GABA (γ-aminobutyric acid) receptor. Its selectivity for helminths is because vertebrates use GABA only in the CNS, and the GABA receptor of helminths is of a different isoform from that of vertebrates.[6]
Piperazine hydrate, piperazine adipate and piperazine citrate (used to treat ascariasis and enterobiasis[7]) are the most common anthelmintic piperazine compounds. These drugs are often referred to simply as "piperazine" which may cause confusion between the specific anthelmintic drugs, the entire class of piperazine-containing compounds, and the compound itself.
Diethylcarbamazine, a derivative of piperazine, is used to treat some types of filariasis.
https://en.wikipedia.org/wiki/Piperazine
Paramesonephric ducts (or Müllerian ducts) are paired ducts of the embryo that run down the lateral sides of the urogenital ridge and terminate at the sinus tubercle in the primitive urogenital sinus. In the female, they will develop to form the fallopian tubes, uterus, cervix, and the upper one-third of the vagina.
https://en.wikipedia.org/wiki/Paramesonephric_duct
Carbon capture and storage[edit]
Amine blends that are activated by concentrated piperazine are used extensively in commercial CO2 removal for carbon capture and storage (CCS) because piperazine advantageously allows for protection from significant thermal and oxidative degradation at typical coal flue gas conditions. The thermal degradation rates for methyl diethanolamine (MDEA) and piperazine (PZ) are negligible, and PZ, unlike other metals, protect MDEA from oxidative degradation.[8] This increased stability of the MDEA/PZ solvent blend over MDEA and other amine solvents provides for greater capacity for and requires less work to capture a given amount of CO2.
Piperazine's solubility is low, so it is often used in relatively small amounts to supplement another amine solvent. One or more of piperazine's performance advantages are often compromised in practice due to its low concentration; nonetheless, the CO2 absorption rate, heat of absorption, and solvent capacity are increased through the addition of piperazine to amine gas treating solvents, the most common of which is MDEA due to its unmatched high rate and capacity efficiency. For example, a 5 m PZ/5 m MDEA blend yields an 11% larger difference in CO2 concentration than 8 m PZ between the lean (inlet absorbent) and rich (outlet absorbent) amine solvent streams, or in other words, more CO2 is removed from the sour (flue) gas stream per unit mass of solvent, and an almost 100% larger concentration difference than 7 m MEA.[9]
Given that typical amine-based absorption processes run at temperatures from 45 °C to 55 °C, the capabilities of piperazine are well within the bounds of and thus favored for carbon capture. Piperazine can be thermally regenerated through multi-stage flash distillation and other methods after being used in operating temperatures up to 150 °C and recycled back into the absorption process, providing for higher overall energy performance in amine gas treating processes.[10]
The advantages to using concentrated piperazine (CPZ) as an additive had been confirmed through, for example, three pilot plants in Australia that are operated by CSIRO. This program was launched to explore remedies to the high costs of post-combustion carbon capture, and the results were positive. Using CPZ, which is more reactive and thermally stable than standard MEA solutions, capital and compression (energy) costs were lowered through size reductions in absorber columns and solvent regeneration at higher temperatures.[11]
Chemistry[edit]
The amine groups on piperazine react readily with carbon dioxide to produce PZ carbamate at a low loading (mol CO2/equiv PZ) range and PZ bicarbamate at an operating range of 0.31-0.41 mol CO2/equiv PZ, enhancing the rate of overall CO2 absorbed under operating conditions (refer to Figure 1 below). Due to these reactions, there is limited free piperazine present in the solvent, resulting in its low volatility and rates of precipitation as PZ-6H2O.[10]
Most of these agents can be classified as either phenylpiperazines, benzylpiperazines, diphenylmethylpiperazines (benzhydrylpiperazines), pyridinylpiperazines, pyrimidinylpiperazines, or tricyclics (with the piperazine ring attached to the heterocyclic moiety via a side chain).
https://en.wikipedia.org/wiki/Piperazine