Blog Archive
- Apr 12 (12)
- Apr 13 (2)
- Apr 14 (7)
- Apr 15 (11)
- Apr 16 (5)
- Apr 17 (14)
- Apr 18 (16)
- Apr 19 (17)
- Apr 20 (28)
- Apr 21 (29)
- Apr 22 (15)
- Apr 23 (19)
- Apr 24 (8)
- Apr 25 (58)
- Apr 26 (44)
- Apr 28 (6)
- Apr 29 (6)
- Apr 30 (7)
- May 01 (8)
- May 02 (9)
- May 03 (4)
- May 04 (6)
- May 05 (14)
- May 06 (20)
- May 07 (11)
- May 08 (18)
- May 09 (6)
- May 10 (17)
- May 11 (8)
- May 12 (25)
- May 13 (8)
- May 14 (2)
- May 15 (2)
- May 17 (16)
- May 18 (1)
- May 19 (5)
- May 20 (22)
- May 21 (6)
- May 22 (3)
- May 23 (2)
- May 24 (7)
- May 25 (1)
- May 26 (6)
- May 27 (3)
- May 28 (3)
- May 29 (10)
- May 30 (8)
- May 31 (12)
- Jun 01 (1)
- Jun 02 (1)
- Jun 03 (9)
- Jun 04 (1)
- Jun 05 (2)
- Jun 07 (4)
- Jun 08 (8)
- Jun 09 (1)
- Jun 10 (1)
- Jun 19 (1)
- Jun 27 (1)
- Jun 29 (1)
- Jun 30 (7)
- Jul 01 (3)
- Jul 02 (1)
- Jul 03 (1)
- Jul 04 (2)
- Jul 05 (1)
- Jul 06 (3)
- Jul 08 (9)
- Jul 09 (1)
- Jul 10 (1)
- Jul 11 (2)
- Jul 12 (2)
- Jul 13 (4)
- Jul 14 (4)
- Jul 15 (2)
- Jul 17 (8)
- Jul 18 (17)
- Jul 19 (1)
- Jul 20 (8)
- Jul 21 (6)
- Jul 22 (12)
- Jul 23 (10)
- Jul 25 (6)
- Jul 26 (23)
- Jul 28 (50)
- Jul 30 (12)
- Jul 31 (5)
- Aug 01 (16)
- Aug 02 (5)
- Aug 03 (5)
- Aug 04 (11)
- Aug 05 (13)
- Aug 06 (7)
- Aug 07 (10)
- Aug 08 (2)
- Aug 09 (27)
- Aug 10 (15)
- Aug 11 (67)
- Aug 12 (44)
- Aug 13 (29)
- Aug 14 (120)
- Aug 15 (61)
- Aug 16 (36)
- Aug 17 (21)
- Aug 18 (5)
- Aug 21 (5)
- Aug 22 (54)
- Aug 23 (101)
- Aug 24 (100)
- Aug 25 (99)
- Aug 26 (100)
- Aug 27 (84)
- Aug 28 (73)
- Aug 29 (76)
- Aug 30 (67)
- Aug 31 (95)
- Sep 01 (126)
- Sep 02 (68)
- Sep 03 (11)
- Sep 04 (14)
- Sep 05 (47)
- Sep 06 (101)
- Sep 07 (61)
- Sep 08 (57)
- Sep 09 (46)
- Sep 10 (14)
- Sep 11 (93)
- Sep 12 (101)
- Sep 13 (101)
- Sep 14 (100)
- Sep 15 (77)
- Sep 16 (2)
- Sep 17 (101)
- Sep 18 (91)
- Sep 19 (102)
- Sep 20 (102)
- Sep 21 (94)
- Sep 22 (84)
- Sep 23 (110)
- Sep 24 (101)
- Sep 25 (76)
- Sep 26 (43)
- Sep 27 (87)
- Sep 28 (104)
- Sep 29 (92)
- Sep 30 (33)
- Oct 01 (58)
- Oct 02 (1)
- Oct 05 (8)
- Oct 06 (6)
- Oct 07 (4)
- Oct 08 (4)
- Oct 09 (1)
- Oct 10 (18)
- Oct 11 (8)
- Oct 12 (26)
- Oct 13 (6)
- Oct 14 (2)
- Oct 15 (4)
- Oct 16 (3)
- Oct 17 (4)
- Oct 18 (3)
- Oct 19 (11)
- Oct 20 (5)
- Oct 21 (7)
- Oct 22 (5)
- Oct 23 (8)
- Oct 24 (9)
- Oct 25 (14)
- Oct 26 (8)
- Oct 27 (13)
- Oct 28 (7)
- Oct 29 (7)
- Oct 30 (22)
- Oct 31 (13)
- Nov 01 (13)
- Nov 02 (6)
- Nov 03 (10)
- Nov 04 (17)
- Nov 05 (8)
- Nov 06 (9)
- Nov 07 (11)
- Nov 08 (3)
- Nov 09 (7)
- Nov 10 (5)
- Nov 11 (5)
- Nov 12 (5)
- Nov 13 (10)
- Nov 14 (7)
- Nov 15 (15)
- Nov 16 (8)
- Nov 17 (6)
- Nov 18 (5)
- Nov 19 (7)
- Nov 20 (8)
- Nov 21 (12)
- Nov 22 (5)
- Nov 23 (7)
- Nov 24 (7)
- Nov 25 (8)
- Nov 26 (2)
- Nov 27 (12)
- Nov 28 (2)
- Nov 29 (2)
- Dec 01 (1)
- Dec 02 (3)
- Dec 03 (2)
- Dec 04 (1)
- Dec 05 (9)
- Dec 06 (22)
- Dec 07 (2)
- Dec 08 (3)
- Dec 09 (1)
- Dec 13 (2)
- Dec 14 (12)
- Dec 15 (1)
- Dec 17 (1)
- Dec 23 (4)
- Dec 24 (2)
- Dec 25 (1)
- Dec 27 (2)
- Dec 28 (1)
- Dec 29 (6)
- Dec 30 (2)
- Dec 31 (6)
- Jan 03 (3)
- Jan 04 (12)
- Jan 05 (5)
- Jan 06 (7)
- Jan 07 (1)
- Jan 08 (3)
- Jan 09 (1)
- Jan 11 (1)
- Jan 12 (5)
- Jan 14 (1)
- Jan 16 (1)
- Jan 17 (1)
- Jan 18 (2)
- Jan 23 (1)
- Jan 26 (3)
- Jan 28 (2)
- Jan 29 (3)
- Jan 30 (1)
- Jan 31 (1)
- Feb 04 (2)
- Feb 05 (2)
- Feb 08 (2)
- Feb 09 (1)
- Feb 13 (3)
- Feb 15 (2)
- Feb 16 (1)
- Feb 17 (1)
- Feb 25 (2)
- Feb 28 (2)
- Mar 03 (1)
- Mar 08 (3)
- Mar 16 (2)
- Mar 17 (1)
- Mar 18 (11)
- Mar 20 (9)
- Mar 22 (1)
- Mar 23 (3)
- Mar 31 (1)
- Apr 01 (2)
- Apr 02 (1)
- Apr 03 (2)
- Apr 04 (1)
- Apr 05 (2)
- Apr 06 (6)
- Apr 07 (1)
- Apr 08 (7)
- Apr 09 (4)
- Apr 10 (7)
- Apr 19 (18)
- Apr 20 (12)
- Apr 21 (1)
- Apr 24 (2)
- May 11 (1)
- May 16 (4)
- May 20 (2)
- May 24 (2)
- May 27 (3)
- Jun 02 (2)
- Jun 06 (1)
- Jun 07 (9)
- Jun 10 (1)
- Jun 11 (2)
- Jun 12 (3)
- Jun 15 (1)
- Jun 17 (1)
- Jun 20 (5)
- Jun 21 (12)
- Jun 22 (21)
- Jun 23 (10)
- Jun 24 (4)
- Jun 25 (10)
- Jun 26 (5)
- Jun 28 (4)
- Jun 29 (2)
- Jun 30 (2)
- Jul 01 (1)
- Jul 04 (1)
- Jul 05 (2)
- Jul 06 (1)
- Jul 07 (2)
- Jul 08 (1)
- Jul 09 (3)
- Jul 10 (6)
- Jul 11 (7)
- Jul 12 (2)
- Jul 13 (3)
- Jul 14 (7)
- Jul 15 (4)
- Jul 16 (9)
- Jul 17 (2)
- Jul 18 (6)
- Jul 19 (6)
- Jul 20 (14)
- Jul 21 (2)
- Jul 22 (6)
- Jul 23 (14)
- Jul 24 (6)
- Jul 25 (5)
- Jul 26 (5)
- Jul 27 (2)
- Jul 28 (6)
- Jul 29 (1)
- Jul 30 (3)
- Jul 31 (1)
- Aug 01 (6)
- Aug 03 (6)
- Aug 04 (4)
- Aug 05 (2)
- Aug 06 (2)
- Aug 07 (1)
- Aug 08 (1)
- Aug 09 (1)
- Aug 10 (1)
- Aug 11 (3)
- Aug 12 (1)
- Aug 13 (1)
- Aug 14 (1)
- Aug 15 (1)
- Aug 17 (9)
- Aug 19 (1)
- Aug 24 (1)
- Aug 28 (1)
- Oct 14 (1)
- Oct 22 (1)
- Nov 13 (10)
- Nov 14 (1)
- Nov 15 (3)
- Nov 23 (2)
- Nov 24 (1)
- Nov 25 (1)
- Nov 26 (1)
- Dec 01 (3)
- Dec 07 (3)
- Dec 08 (1)
- Dec 10 (2)
- Dec 12 (22)
- Dec 13 (30)
- Dec 15 (7)
- Dec 20 (5)
- Dec 28 (1)
- Dec 29 (3)
- Dec 31 (1)
- Jan 02 (2)
- Jan 10 (1)
- Jan 14 (1)
- Jan 17 (4)
- Jan 29 (2)
- Feb 03 (1)
- Feb 04 (6)
- Feb 05 (5)
- Feb 06 (10)
- Feb 08 (16)
- Feb 10 (63)
- Feb 11 (39)
- Feb 12 (33)
- Feb 13 (27)
- Feb 14 (4)
- Feb 15 (66)
- Feb 16 (7)
- Feb 17 (22)
- Feb 18 (14)
- Feb 19 (44)
- Feb 20 (3)
- Feb 21 (12)
- Feb 22 (68)
- Feb 23 (78)
- Feb 25 (3)
- Feb 26 (10)
- Feb 27 (28)
- Feb 28 (26)
- Mar 01 (17)
- Mar 02 (7)
- Mar 03 (6)
- Mar 04 (3)
- Mar 05 (7)
- Mar 06 (8)
- Mar 07 (13)
- Mar 08 (6)
- Mar 09 (3)
- Mar 10 (2)
- Mar 11 (15)
- Mar 12 (6)
- Mar 13 (2)
- Mar 14 (15)
- Mar 15 (10)
- Mar 16 (6)
- Mar 17 (5)
- Mar 18 (3)
- Mar 19 (3)
- Mar 20 (9)
- Mar 21 (2)
- Mar 22 (1)
- Mar 23 (15)
- Mar 24 (1)
- Mar 25 (1)
- Mar 26 (7)
- Mar 27 (5)
- Mar 28 (2)
- Mar 29 (8)
- Mar 30 (21)
- Mar 31 (10)
- Apr 01 (3)
- Apr 02 (3)
- Apr 03 (9)
- Apr 04 (1)
- Apr 05 (4)
- Apr 06 (4)
- Apr 07 (4)
- Apr 08 (4)
- Apr 09 (1)
- Apr 10 (1)
- Apr 11 (6)
- Apr 12 (7)
- Apr 13 (3)
- Apr 14 (2)
- Apr 15 (11)
- Apr 16 (16)
- Apr 17 (12)
- Apr 18 (29)
- Apr 19 (21)
- Apr 20 (3)
- Apr 21 (8)
- Apr 22 (3)
- Apr 23 (5)
- Apr 24 (1)
- Apr 25 (4)
- Apr 26 (6)
- Apr 27 (8)
- Apr 28 (10)
- Apr 30 (2)
- May 01 (7)
- May 02 (3)
- May 03 (16)
- May 04 (3)
- May 05 (11)
- May 06 (41)
- May 07 (2)
- May 08 (18)
- May 09 (117)
- May 10 (15)
- May 11 (85)
- May 12 (12)
- May 13 (54)
- May 14 (73)
- May 15 (85)
- May 16 (148)
- May 17 (101)
- May 18 (100)
- May 19 (99)
- May 20 (101)
- May 21 (101)
- May 22 (101)
- May 23 (101)
- May 24 (101)
- May 25 (7)
- May 27 (1)
- May 28 (1)
- May 29 (29)
- Jun 02 (1)
- Jun 03 (21)
- Jun 04 (7)
- Jun 05 (8)
- Jun 06 (1)
- Jun 22 (5)
- Jun 23 (10)
- Jun 24 (10)
- Jun 25 (4)
- Jun 26 (7)
- Jun 27 (22)
- Jun 28 (12)
- Jun 29 (11)
- Jun 30 (23)
- Jul 01 (10)
- Jul 02 (13)
- Jul 03 (17)
- Jul 04 (41)
- Jul 05 (17)
- Jul 06 (8)
- Jul 07 (10)
- Jul 08 (6)
- Jul 09 (3)
- Jul 10 (2)
- Jul 11 (2)
- Jul 12 (12)
- Jul 13 (6)
- Jul 14 (14)
- Jul 15 (5)
- Jul 17 (1)
- Jul 18 (1)
- Jul 19 (1)
- Jul 20 (1)
- Jul 22 (2)
- Jul 23 (30)
- Jul 24 (5)
- Jul 25 (55)
- Jul 27 (8)
- Jul 28 (26)
- Jul 29 (15)
- Jul 30 (35)
- Jul 31 (5)
- Aug 01 (13)
- Aug 02 (3)
- Aug 04 (1)
- Aug 05 (2)
- Aug 11 (11)
- Aug 13 (3)
- Aug 14 (7)
- Aug 15 (3)
- Aug 16 (5)
- Aug 17 (4)
- Aug 18 (4)
- Aug 19 (2)
- Aug 20 (19)
- Aug 21 (38)
- Aug 23 (14)
- Aug 24 (6)
- Aug 25 (30)
- Aug 26 (57)
- Aug 27 (19)
- Aug 28 (25)
- Aug 29 (120)
- Aug 30 (82)
- Aug 31 (46)
- Sep 01 (96)
- Sep 02 (101)
- Sep 03 (62)
- Sep 04 (32)
- Sep 05 (44)
- Sep 06 (91)
- Sep 07 (22)
- Sep 08 (100)
- Sep 09 (71)
- Sep 10 (15)
- Sep 11 (90)
- Sep 13 (2)
Tuesday, September 7, 2021
09-07-2021-1610 - Indian Navy officer with an Indian manufactured 1A1 Carbine
09-07-2021-1555 - ion exchange resin polymer ion traps Linear quadrupole ion trap (particle activation, alignment/arrangement/organizing/etc., etc.; ionization/activation, ion alignment, charging, gradient, circle, etc.; EMR, EMF electomagnetic fielding field work matricing particle/ion/field assimilation-synchrony-desynchrony/etc., etc.)
09-07-2021-1555 - ion exchange resin polymer ion traps Linear quadrupole ion trap (particle activation, alignment/arrangement/organizing/etc., etc.; ionization/activation, ion alignment, charging, gradient, circle, etc.; EMR, EMF electomagnetic fielding field work matricing particle/ion/field assimilation-synchrony-desynchrony/etc., etc.). may use gaseous medium (e.g. standard earth air, vocs, etc.). Salt, Heavy Metal, Mercury, etc., Air (hydro trihydro cat oxytricas oxyg carbo radicals proton electron etc.; nitrogen not nec, proteins, etc.). Pressure, velocity, heat, channel, chamber, etc..
Earth's atmosphere is composed of about 78 percent nitrogen, 21 percent oxygen, 0.9 percent argon, and 0.1 percent other gases.
https://www.nationalgeographic.org/encyclopedia/atmosphere/
The trihydrogen cation or protonated molecular hydrogen is a cation (positive ion) with formula H+
3, consisting of three hydrogen nuclei (protons) sharing two electrons.
The trihydrogen cation is one of the most abundant ions in the universe. It is stable in the interstellar medium (ISM) due to the low temperature and low density of interstellar space. The role that H+
3 plays in the gas-phase chemistry of the ISM is unparalleled by any other molecular ion.
The trihydrogen cation is the simplest triatomic molecule, because its two electrons are the only valence electrons in the system. It is also the simplest example of a three-center two-electron bond system.

https://en.wikipedia.org/wiki/Trihydrogen_cation
An ion-exchange resin or ion-exchange polymer is a resin or polymer that acts as a medium for ion exchange. It is an insoluble matrix (or support structure) normally in the form of small (0.25–0.5 mm radius) microbeads, usually white or yellowish, fabricated from an organic polymer substrate. The beads are typically porous, providing a large surface area on and inside them the trapping of ions occurs along with the accompanying release of other ions, and thus the process is called ion exchange. There are multiple types of ion-exchange resin. Most commercial resins are made of polystyrene sulfonate.[1]
https://en.wikipedia.org/wiki/Ion-exchange_resin
Ion traps[edit]
Three-dimensional quadrupole ion trap[edit]
The quadrupole ion trap works on the same physical principles as the quadrupole mass analyzer, but the ions are trapped and sequentially ejected. Ions are trapped in a mainly quadrupole RF field, in a space defined by a ring electrode (usually connected to the main RF potential) between two endcap electrodes (typically connected to DC or auxiliary AC potentials). The sample is ionized either internally (e.g. with an electron or laser beam), or externally, in which case the ions are often introduced through an aperture in an endcap electrode.
There are many mass/charge separation and isolation methods but the most commonly used is the mass instability mode in which the RF potential is ramped so that the orbit of ions with a mass a > b are stable while ions with mass b become unstable and are ejected on the z-axis onto a detector. There are also non-destructive analysis methods.
Ions may also be ejected by the resonance excitation method, whereby a supplemental oscillatory excitation voltage is applied to the endcap electrodes, and the trapping voltage amplitude and/or excitation voltage frequency is varied to bring ions into a resonance condition in order of their mass/charge ratio.[21][22]
Cylindrical ion trap[edit]
The cylindrical ion trap mass spectrometer (CIT) is a derivative of the quadrupole ion trap where the electrodes are formed from flat rings rather than hyperbolic shaped electrodes. The architecture lends itself well to miniaturization because as the size of a trap is reduced, the shape of the electric field near the center of the trap, the region where the ions are trapped, forms a shape similar to that of a hyperbolic trap.
Linear quadrupole ion trap[edit]
A linear quadrupole ion trap is similar to a quadrupole ion trap, but it traps ions in a two dimensional quadrupole field, instead of a three-dimensional quadrupole field as in a 3D quadrupole ion trap. Thermo Fisher's LTQ ("linear trap quadrupole") is an example of the linear ion trap.[23]
A toroidal ion trap can be visualized as a linear quadrupole curved around and connected at the ends or as a cross-section of a 3D ion trap rotated on edge to form the toroid, donut-shaped trap. The trap can store large volumes of ions by distributing them throughout the ring-like trap structure. This toroidal shaped trap is a configuration that allows the increased miniaturization of an ion trap mass analyzer. Additionally, all ions are stored in the same trapping field and ejected together simplifying detection that can be complicated with array configurations due to variations in detector alignment and machining of the arrays.[24]
As with the toroidal trap, linear traps and 3D quadrupole ion traps are the most commonly miniaturized mass analyzers due to their high sensitivity, tolerance for mTorr pressure, and capabilities for single analyzer tandem mass spectrometry (e.g. product ion scans).[25]
Orbitrap[edit]
Orbitrap instruments are similar to Fourier transform ion cyclotron resonance mass spectrometers (see text below). Ions are electrostatically trapped in an orbit around a central, spindle shaped electrode. The electrode confines the ions so that they both orbit around the central electrode and oscillate back and forth along the central electrode's long axis. This oscillation generates an image current in the detector plates which is recorded by the instrument. The frequencies of these image currents depend on the mass-to-charge ratios of the ions. Mass spectra are obtained by Fourier transformation of the recorded image currents.
Orbitraps have a high mass accuracy, high sensitivity and a good dynamic range.[26]
Fourier transform ion cyclotron resonance[edit]
Fourier transform mass spectrometry (FTMS), or more precisely Fourier transform ion cyclotron resonance MS, measures mass by detecting the image current produced by ions cyclotroning in the presence of a magnetic field. Instead of measuring the deflection of ions with a detector such as an electron multiplier, the ions are injected into a Penning trap (a static electric/magnetic ion trap) where they effectively form part of a circuit. Detectors at fixed positions in space measure the electrical signal of ions which pass near them over time, producing a periodic signal. Since the frequency of an ion's cycling is determined by its mass-to-charge ratio, this can be deconvoluted by performing a Fourier transform on the signal. FTMShas the advantage of high sensitivity (since each ion is "counted" more than once) and much higher resolution and thus precision.[27][28]
Ion cyclotron resonance (ICR) is an older mass analysis technique similar to FTMS except that ions are detected with a traditional detector. Ions trapped in a Penning trap are excited by an RF electric field until they impact the wall of the trap, where the detector is located. Ions of different mass are resolved according to impact time.
https://en.wikipedia.org/wiki/Mass_spectrometry#Ion_traps
Electron ionization (EI, formerly known as electron impact ionization[1] and electron bombardment ionization[2]) is an ionization method in which energetic electrons interact with solid or gas phase atoms or molecules to produce ions.[3] EI was one of the first ionization techniques developed for mass spectrometry.[4]However, this method is still a popular ionization technique. This technique is considered a hard (high fragmentation) ionization method, since it uses highly energetic electrons to produce ions. This leads to extensive fragmentation, which can be helpful for structure determination of unknown compounds. EI is the most useful for organic compounds which have a molecular weight below 600. Also, several other thermally stable and volatile compounds in solid, liquid and gas states can be detected with the use of this technique when coupled with various separation methods.[5]
https://en.wikipedia.org/wiki/Electron_ionization
Magnetic resonance imaging (MRI) is a medical imaging technique used in radiology to form pictures of the anatomyand the physiological processes of the body. MRI scanners use strong magnetic fields, magnetic field gradients, and radio waves to generate images of the organs in the body. MRI does not involve X-rays or the use of ionizing radiation, which distinguishes it from CT and PET scans. MRI is a medical application of nuclear magnetic resonance (NMR) which can also be used for imaging in other NMR applications, such as NMR spectroscopy.
MRI is widely used in hospitals and clinics for medical diagnosis, staging and follow-up of disease. Compared to CT, MRI provides better contrast in images of soft-tissues, e.g. in the brain or abdomen. However, it may be perceived as less comfortable by patients, due to the usually longer and louder measurements with the subject in a long, confining tube. Additionally, implants and other non-removable metal in the body can pose a risk and may exclude some patients from undergoing an MRI examination safely.
MRI was originally called NMRI (nuclear magnetic resonance imaging), but "nuclear" was dropped to avoid negative associations.[1] Certain atomic nuclei are able to absorb radio frequency energy when placed in an external magnetic field; the resultant evolving spin polarization can induce a RF signal in a radio frequency coil and thereby be detected.[2]In clinical and research MRI, hydrogen atoms are most often used to generate a macroscopic polarization that is detected by antennae close to the subject being examined.[2] Hydrogen atoms are naturally abundant in humans and other biological organisms, particularly in water and fat. For this reason, most MRI scans essentially map the location of water and fat in the body. Pulses of radio waves excite the nuclear spin energy transition, and magnetic field gradients localize the polarization in space. By varying the parameters of the pulse sequence, different contrasts may be generated between tissues based on the relaxation properties of the hydrogen atoms therein.
Since its development in the 1970s and 1980s, MRI has proven to be a versatile imaging technique. While MRI is most prominently used in diagnostic medicine and biomedical research, it also may be used to form images of non-living objects. Diffusion MRI and Functional MRIextends the utility of MRI to capture neuronal tracts and blood flow respectively in the nervous system, in addition to detailed spatial images. The sustained increase in demand for MRI within health systems has led to concerns about cost effectiveness and overdiagnosis.[3][4]
https://en.wikipedia.org/wiki/Magnetic_resonance_imaging
In physics and materials science, the Curie temperature (TC), or Curie point, is the temperature above which certain materials lose their permanent magnetic properties, which can (in most cases) be replaced by induced magnetism. The Curie temperature is named after Pierre Curie, who showed that magnetism was lost at a critical temperature.[1]
The force of magnetism is determined by the magnetic moment, a dipole moment within an atom which originates from the angular momentum and spin of electrons. Materials have different structures of intrinsic magnetic moments that depend on temperature; the Curie temperature is the critical point at which a material's intrinsic magnetic moments change direction.
Permanent magnetism is caused by the alignment of magnetic moments and induced magnetism is created when disordered magnetic moments are forced to align in an applied magnetic field. For example, the ordered magnetic moments (ferromagnetic, Figure 1) change and become disordered (paramagnetic, Figure 2) at the Curie temperature. Higher temperatures make magnets weaker, as spontaneous magnetism only occurs below the Curie temperature. Magnetic susceptibility above the Curie temperature can be calculated from the Curie–Weiss law, which is derived from Curie's law.
In analogy to ferromagnetic and paramagnetic materials, the Curie temperature can also be used to describe the phase transition between ferroelectricity and paraelectricity. In this context, the order parameter is the electric polarization that goes from a finite value to zero when the temperature is increased above the Curie temperature.
https://en.wikipedia.org/wiki/Curie_temperature
A magnet is a material or object that produces a magnetic field. This magnetic field is invisible but is responsible for the most notable property of a magnet: a force that pulls on other ferromagnetic materials, such as iron, steel, nickel, cobalt, etc. and attracts or repels other magnets.
A permanent magnet is an object made from a material that is magnetized and creates its own persistent magnetic field. An everyday example is a refrigerator magnet used to hold notes on a refrigerator door. Materials that can be magnetized, which are also the ones that are strongly attracted to a magnet, are called ferromagnetic (or ferrimagnetic). These include the elements iron, nickel and cobalt and their alloys, some alloys of rare-earth metals, and some naturally occurring minerals such as lodestone. Although ferromagnetic (and ferrimagnetic) materials are the only ones attracted to a magnet strongly enough to be commonly considered magnetic, all other substances respond weakly to a magnetic field, by one of several other types of magnetism.
https://en.wikipedia.org/wiki/Magnet
A magnetic field is a vector field that describes the magnetic influence on moving electric charges, electric currents,[1]:ch1[2]and magnetic materials. A moving charge in a magnetic field experiences a force perpendicular to its own velocity and to the magnetic field.[1]:ch13[3] A permanent magnet's magnetic field pulls on ferromagnetic materials such as iron, and attracts or repels other magnets. In addition, a magnetic field that varies with location will exert a force on a range of non-magnetic materials by affecting the motion of their outer atomic electrons. Magnetic fields surround magnetized materials, and are created by electric currents such as those used in electromagnets, and by electric fields varying in time. Since both strength and direction of a magnetic field may vary with location, it is described mathematically by a function assigning a vector to each point of space, called a vector field.
https://en.wikipedia.org/wiki/Magnetic_field
A magnetic domain is a region within a magnetic material in which the magnetization is in a uniform direction. This means that the individual magnetic moments of the atoms are aligned with one another and they point in the same direction. When cooled below a temperature called the Curie temperature, the magnetization of a piece of ferromagneticmaterial spontaneously divides into many small regions called magnetic domains. The magnetization within each domain points in a uniform direction, but the magnetization of different domains may point in different directions. Magnetic domain structure is responsible for the magnetic behavior of ferromagnetic materials like iron, nickel, cobaltand their alloys, and ferrimagnetic materials like ferrite. This includes the formation of permanent magnets and the attraction of ferromagnetic materials to a magnetic field. The regions separating magnetic domains are called domain walls, where the magnetization rotates coherently from the direction in one domain to that in the next domain. The study of magnetic domains is called micromagnetics.
Magnetic domains form in materials which have magnetic ordering; that is, their dipoles spontaneously align due to the exchange interaction. These are the ferromagnetic, ferrimagnetic and antiferromagnetic materials. Paramagnetic and diamagnetic materials, in which the dipoles align in response to an external field but do not spontaneously align, do not have magnetic domains.
https://en.wikipedia.org/wiki/Magnetic_domain
A neodymium magnet (also known as NdFeB, NIB or Neo magnet) is the most widely used[1] type of rare-earth magnet. It is a permanent magnet made from an alloy of neodymium, iron, and boron to form the Nd2Fe14B tetragonal crystalline structure.[2] Developed independently in 1984 by General Motors and Sumitomo Special Metals,[3][4][5] neodymium magnets are the strongest type of permanent magnet available commercially.[2][6] Because of different manufacturing processes, they are divided into two subcategories, namely sintered NdFeB magnets and bonded NdFeB magnets.[7][8] They have replaced other types of magnets in many applications in modern products that require strong permanent magnets, such as electric motors in cordless tools, hard disk drives and magnetic fasteners.
Sintered Nd2Fe14B tends to be vulnerable to corrosion, especially along grain boundaries of a sintered magnet. This type of corrosion can cause serious deterioration, including crumbling of a magnet into a powder of small magnetic particles, or spalling of a surface layer.
This vulnerability is addressed in many commercial products by adding a protective coating to prevent exposure to the atmosphere. Nickel plating or two-layered copper-nickel plating are the standard methods, although plating with other metals, or polymer and lacquer protective coatings, are also in use.[21]
https://en.wikipedia.org/wiki/Neodymium_magnet
Spall are fragments of a material that are broken off a larger solid body. It can be produced by a variety of mechanisms, including as a result of projectile impact, corrosion, weathering, cavitation, or excessive rolling pressure (as in a ball bearing). Spalling and spallationboth describe the process of surface failure in which spall is shed.

The terms spall, spalling, and spallation have been adopted by particle physicists; in neutron scattering instruments, neutrons are generated by bombarding a uranium target with a stream of atoms. The neutrons that are ejected from the target are known as "spall".
In anti-tank warfare, spalling through mechanical stress is an intended effect of high-explosive squash head (HESH) anti-tank shells and many other munitions which may not be powerful enough to pierce the armor of a target. The relatively soft warhead, containing or made of plastic explosive, flattens against the armor plating on tanks and other armored fighting vehicles (AFVs) and explodes, creating a shock wave that travels through the armor as a compression wave and is reflected at the free surface as a tensile wave breaking (tensile stress/strain fracture) the metal on the inside. The resulting spall is dangerous to crew and equipment, and may result in a partial or complete disablement of a vehicle and/or its crew. Many AFVs are equipped with spall liners inside their armor for protection.
A kinetic energy penetrator, if it can defeat the armor, generally causes spalling within the target as well, which helps to destroy or disable the vehicle and its crew.[1]
Mechanical Spalling
Mechanical spalling occurs at high stress contact points, for example, in a ball bearing. Spalling occurs in preference to brinelling where the maximal shear stress occurs not at the surface, but just below, shearing the spall off.
One of the simplest forms of mechanical spalling is plate impact, in which two waves of compression are reflected on the free-surfaces of the plates and then interact to generate a region of high tensile stress inside one of the plates.
Spalling can also occur as an effect of cavitation, where fluids are subjected to localized low pressures that cause vapor bubbles to form, typically in pumps, water turbines, vessel propellers, and even piping under some conditions. When such bubbles collapse, a localized high pressure can cause spalling on adjacent surfaces.
Unloading
Unloading is the release of pressure due to the removal of an overburden. When the pressure is reduced rapidly, the rapid expansion of the rock causes high surface stress and spalling.
Freeze–thaw weathering[edit]
Freeze–thaw weathering is caused by moisture freezing inside cracks in rock. Upon freezing its volume expands, causing large forces which cracks spall off the outer surface. As this cycle repeats the outer surface repeatedly undergoes spalling, resulting in weathering.
Some stone and masonry surfaces used as building surfaces will absorb moisture at their surface. If exposed to severe freezing conditions, the surface may flake off due to the expansion of the water. This effect can also be seen in terra-cotta surfaces (even if glazed) if there is an entrance for water at the edges.
Salt spalling[edit]
Salt spalling is a specific type of weathering which occurs in porous building materials, such as brick, natural stone, tiles and concrete. Dissolved salt is carried through the material in water and crystallizes inside the material near the surface as the water evaporates. As the salt crystals expand this builds up shear stresses which break away spall from the surface.
Some engineers[weasel words] believe that porous building materials can be protected against salt spalling by treatment with penetrating sealants which are hydrophobic (water repellent) and will penetrate deeply enough to keep water with dissolved salts well away from the surface.[citation needed] Great care and expert advice must be consulted, however, to ensure that any coating is compatible with the substrate in terms of breathability (ability to allow the release of vapors from inside while preventing water intrusion), or other serious problems can be created.
Chimneys show spalling damage before other portions of buildings because they are more exposed to the elements.
Spalling in refractory concrete[edit]
There are two drivers for spalling of concrete: thermal strain caused by rapid heating and internal pressures due to the removal of water. Being able to predict the outcome of different heating rates on thermal stresses and internal pressure during water removal is particularly important to industry and other concrete structures.
Explosive spalling events of refractory concrete can result in serious problems. If an explosive spalling occurs, projectiles of reasonable mass (1–10 kg) can be thrust violently over many metres, which will have safety implications and render the refractory structure unfit for service. Repairs will then be required resulting in significant costs to industry.[2]
https://en.wikipedia.org/wiki/Spall
There are several variations on the simple Whipple shield. Multi-shock shields,[2][3] like the one used on the Stardust spacecraft, use multiple bumpers spaced apart to increase the shield's ability to protect the spacecraft. Whipple shields that have a filling in between the rigid layers of the shield are called stuffed Whipple shields.[4][5] The filling in these shields is usually a high-strength material like Kevlar or Nextel aluminium oxide fiber.[6] The type of shield, the material, thickness and distance between layers are varied to produce a shield with minimal mass that will also minimize the probability of penetration. There are over 100 shield configurations on the International Space Station alone,[7] with higher-risk areas having better shielding.
https://en.wikipedia.org/wiki/Whipple_shield
A substance is pyrophoric (from Greek: πυροφόρος, pyrophoros, 'fire-bearing') if it ignites spontaneously in air at or below 54 °C (129 °F) (for gases) or within 5 minutes after coming into contact with air (for liquids and solids).[1] Examples are iron sulfide and many reactive metals including plutonium and uranium, when powdered or thinly sliced. Pyrophoric materials are often water-reactive as well and will ignite when they contact water or humid air. They can be handled safely in atmospheres of argon or (with a few exceptions) nitrogen. Class D fire extinguishers are designated for use in fires involving pyrophoric materials.
https://en.wikipedia.org/wiki/Pyrophoricity
Space debris (also known as space junk, space pollution,[1] space waste, space trash, or space garbage) is defunct artificial objects in space—principally in Earth orbit—which no longer serve a useful function. These include derelict spacecraft—nonfunctional spacecraft and abandoned launch vehicle stages—mission-related debris, and particularly numerous in Earth orbit, fragmentation debris from the breakup of derelict rocket bodies and spacecraft. In addition to derelict man-made objects left in orbit, other examples of space debris include fragments from their disintegration, erosion and collisions or even paint flecks, solidified liquids expelled from spacecraft, and unburned particles from solid rocket motors. Space debris represents a risk to spacecraft.[2]
Space debris is typically a negative externality—it creates an external cost on others from the initial action to launch or use a spacecraft in near-Earth orbit—a cost that is typically not taken into account nor fully accounted for in the cost[3][4] by the launcher or payload owner.[5][1][6] The measurement, mitigation, and potential removal of debris are conducted by some participants in the space industry.[7]
As of October 2019, the US Space Surveillance Network reported nearly 20,000 artificial objects in orbit above the Earth,[8] including 2,218 operational satellites.[9] However, these are just the objects large enough to be tracked. As of January 2019, more than 128 million pieces of debris smaller than 1 cm (0.4 in), about 900,000 pieces of debris 1–10 cm, and around 34,000 of pieces larger than 10 cm (3.9 in) were estimated to be in orbit around the Earth.[7] When the smallest objects of artificial space debris (paint flecks, solid rocket exhaust particles, etc.) are grouped with micrometeoroids, they are together sometimes referred to by space agencies as MMOD (Micrometeoroid and Orbital Debris). Collisions with debris have become a hazard to spacecraft; the smallest objects cause damage akin to sandblasting, especially to solar panels and optics like telescopes or star trackers that cannot easily be protected by a ballistic shield.[10]
Below 2,000 km (1,200 mi) Earth-altitude, pieces of debris are denser than meteoroids; most are dust from solid rocket motors, surface erosion debris like paint flakes, and frozen coolant from RORSAT (nuclear-powered satellites).[citation needed] For comparison, the International Space Station orbits in the 300–400 kilometres (190–250 mi) range, while the two most recent large debris events—the 2007 Chinese antisat weapon test and the 2009 satellite collision—occurred at 800 to 900 kilometres (500 to 560 mi) altitude.[11] The ISS has Whipple shielding to resist damage from small MMOD; however, known debris with a collision chance over 1/10,000 are avoided by maneuveringthe station.
https://en.wikipedia.org/wiki/Space_debris#Micrometeoroid_shielding
The Misznay–Schardin effect, or platter effect, is a characteristic of the detonation of a broad sheet of explosive.
Explosive blasts expand directly away from, and perpendicular to, the surface of an explosive. Unlike the blast from a rounded explosive charge, which expands in all directions, the blast produced by an explosive sheet expands primarily perpendicular to its plane, in both directions. However, if one side is backed by a heavy or fixed mass, most of the blast (i.e. most of the rapidly expanding gas and its kinetic energy) will be reflected in the direction away from the mass.[1][2]
https://en.wikipedia.org/wiki/Misznay–Schardin_effect
An explosively formed penetrator (EFP), also known as an explosively formed projectile, a self-forging warhead, or a self-forging fragment, is a special type of shaped charge designed to penetrate armoreffectively. As the name suggests, the effect of the explosive charge is to deform a metal plate into a slug or rod shape and accelerate it toward a target. They were first developed as oil well perforators by American oil companies in the 1930s, and were deployed as weapons in World War II.[1][2]
https://en.wikipedia.org/wiki/Explosively_formed_penetrator
Munroe effect[edit]
The Munroe or Neumann effect is the focusing of blast energy by a hollow or void cut on a surface of an explosive. The earliest mention of hollow charges occurred in 1792. Franz Xaver von Baader (1765–1841) was a German mining engineer at that time; in a mining journal, he advocated a conical space at the forward end of a blasting charge to increase the explosive's effect and thereby save powder.[4] The idea was adopted, for a time, in Norway and in the mines of the Harz mountains of Germany, although the only available explosive at the time was gunpowder, which is not a high explosive and hence incapable of producing the shock wave that the shaped-charge effect requires.[5]
The first true hollow charge effect was achieved in 1883, by Max von Foerster (1845–1905),[6] chief of the nitrocellulose factory of Wolff & Co. in Walsrode, Germany.[7][8]
By 1886, Gustav Bloem of Düsseldorf, Germany had filed U.S. Patent 342,423 for hemispherical cavity metal detonators to concentrate the effect of the explosion in an axial direction.[9] The Munroe effect is named after Charles E. Munroe, who discovered it in 1888. As a civilian chemist working at the U.S. Naval Torpedo Station at Newport, Rhode Island, he noticed that when a block of explosive guncotton with the manufacturer's name stamped into it was detonated next to a metal plate, the lettering was cut into the plate. Conversely, if letters were raised in relief above the surface of the explosive, then the letters on the plate would also be raised above its surface.[10] In 1894, Munroe constructed the first crude shaped charge:[11][12]
Among the experiments made ... was one upon a safe twenty-nine inches cube, with walls four inches and three quarters thick, made up of plates of iron and steel ... [W]hen a hollow charge of dynamite nine pounds and a half in weight and untamped was detonated on it, a hole three inches in diameter was blown clear through the wall ... The hollow cartridge was made by tying the sticks of dynamite around a tin can, the open mouth of the latter being placed downward.[13]
Although Munroe's discovery of the shaped charge was widely publicized in 1900 in Popular Science Monthly, the importance of the tin can "liner" of the hollow charge remained unrecognized for another 44 years.[14] Part of that 1900 article was reprinted in the February 1945 issue of Popular Science,[15] describing how shaped-charge warheads worked. It was this article that at last revealed to the general public how the fabled Bazooka actually worked against armored vehicles during WWII.
In 1910, Egon Neumann of Germany discovered that a block of TNT, which would normally dent a steel plate, punched a hole through it if the explosive had a conical indentation.[16][17] The military usefulness of Munroe's and Neumann's work was unappreciated for a long time. Between the world wars, academics in several countries – Myron Yakovlevich Sukharevskii (Мирон Яковлевич Сухаревский) in the Soviet Union,[18] William H. Payment and Donald Whitley Woodhead in Britain,[19] and Robert Williams Wood in the U.S.[20] – recognized that projectiles could form during explosions. However, it was not until 1932 that Franz Rudolf Thomanek, a student of physics at Vienna's Technische Hochschule, conceived an anti-tank round that was based on the hollow charge effect. When the Austrian government showed no interest in pursuing the idea, Thomanek moved to Berlin's Technische Hochschule, where he continued his studies under the ballistics expert Carl Julius Cranz.[21] There in 1935, he and Hellmuth von Huttern developed a prototype anti-tank round. Although the weapon's performance proved disappointing, Thomanek continued his developmental work, collaborating with Hubert Schardin at the Waffeninstitut der Luftwaffe (Air Force Weapons Institute) in Braunschweig.[22]
By 1937, Schardin believed that hollow-charge effects were due to the interactions of shock waves. It was during the testing of this idea that, on February 4, 1938, Thomanek conceived the shaped-charge explosive (or Hohlladungs-Auskleidungseffekt (hollow-charge liner effect)).[23] (It was Gustav Adolf Thomer who in 1938 first visualized, by flash radiography, the metallic jet produced by a shaped-charge explosion.[24]) Meanwhile, Henry Hans Mohaupt, a chemical engineer in Switzerland, had independently developed a shaped-charge munition in 1935, which was demonstrated to the Swiss, French, British, and U.S. militaries.[25]
During World War II, shaped-charge munitions were developed by Germany (Panzerschreck, Panzerfaust, Panzerwurfmine, Mistel), Britain (PIAT, Beehive cratering charge), the Soviet Union (RPG-43, RPG-6), the U.S. (bazooka),[26][27] and Italy (Effetto Pronto Speciale shells for various artillery pieces).[28] The development of shaped charges revolutionized anti-tank warfare. Tanks faced a serious vulnerability from a weapon that could be carried by an infantryman or aircraft.
One of the earliest uses of shaped charges was by German glider-borne troops against the Belgian Fort Eben-Emael in 1940.[29] These demolition charges – developed by Dr. Wuelfken of the German Ordnance Office – were unlined explosive charges[30] and didn't produce a metal jet like the modern HEAT warheads. Due to the lack of metal liner they shook the turrets but they did not destroy them, and other airborne troops were forced to climb on the turrets and smash the gun barrels.[31]
https://en.wikipedia.org/wiki/Shaped_charge#Munroe_effect
High-explosive anti-tank (HEAT) is a type of shaped charge explosive that uses the Munroe effect to penetrate heavy armor. The warhead functions by having an explosive charge collapse a metal liner inside the warhead into a high-velocity superplastic jet; this superplastic jet is capable of penetrating armor steel to a depth of seven or more times the diameter of the charge (charge diameters, CD). The jet's effect is purely kinetic in nature; the round has no explosive or incendiary effect on the target.
Because they rely on the chemical energy in the warhead rather than on the kinetic energy of the entire round for their penetration performance, HEAT warheads do not have to be delivered with high velocity, as an armor-piercing round does. Thus they generate less recoil.
The HEAT warhead has become less effective against tanks and other armored vehicles due to the use of composite armor, explosive-reactive armor, and active-protection systems which destroy the HEAT warhead before it functions. While unitary HEAT warheads may pose little threat to any modern tank,[citation needed] they are still deadly against lighter vehicles and aircraft, and multiple warheads can be combined in a tandem configuration to defeat some advanced armors.
The performance of HEAT weapons has nothing to do with thermal effects.
https://en.wikipedia.org/wiki/High-explosive_anti-tank

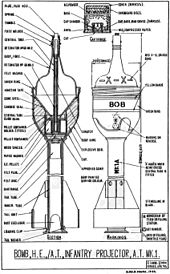
above. bob
https://en.wikipedia.org/wiki/PIAT
The De Lisle carbine or De Lisle Commando carbine was a British firearm used during World War II that was designed with an integrated suppressor. That, combined with its use of subsonic ammunition, made it extremely quiet in action, possibly one of the quietest firearms ever made.[3][page needed]
Few were manufactured as their use was limited to specialist military units.
| De Lisle Commando Carbine | |
|---|---|
  De Lisle Carbine. Top, with wooden stock. Bottom, with folding stock from a Patchett submachine gun | |
| Type | Carbine |
| Place of origin | United Kingdom |
https://en.wikipedia.org/wiki/De_Lisle_carbine