08-15-2021-1732 - 1245
Blog Archive
- Apr 12 (12)
- Apr 13 (2)
- Apr 14 (7)
- Apr 15 (11)
- Apr 16 (5)
- Apr 17 (14)
- Apr 18 (16)
- Apr 19 (17)
- Apr 20 (28)
- Apr 21 (29)
- Apr 22 (15)
- Apr 23 (19)
- Apr 24 (8)
- Apr 25 (58)
- Apr 26 (44)
- Apr 28 (6)
- Apr 29 (6)
- Apr 30 (7)
- May 01 (8)
- May 02 (9)
- May 03 (4)
- May 04 (6)
- May 05 (14)
- May 06 (20)
- May 07 (11)
- May 08 (18)
- May 09 (6)
- May 10 (17)
- May 11 (8)
- May 12 (25)
- May 13 (8)
- May 14 (2)
- May 15 (2)
- May 17 (16)
- May 18 (1)
- May 19 (5)
- May 20 (22)
- May 21 (6)
- May 22 (3)
- May 23 (2)
- May 24 (7)
- May 25 (1)
- May 26 (6)
- May 27 (3)
- May 28 (3)
- May 29 (10)
- May 30 (8)
- May 31 (12)
- Jun 01 (1)
- Jun 02 (1)
- Jun 03 (9)
- Jun 04 (1)
- Jun 05 (2)
- Jun 07 (4)
- Jun 08 (8)
- Jun 09 (1)
- Jun 10 (1)
- Jun 19 (1)
- Jun 27 (1)
- Jun 29 (1)
- Jun 30 (7)
- Jul 01 (3)
- Jul 02 (1)
- Jul 03 (1)
- Jul 04 (2)
- Jul 05 (1)
- Jul 06 (3)
- Jul 08 (9)
- Jul 09 (1)
- Jul 10 (1)
- Jul 11 (2)
- Jul 12 (2)
- Jul 13 (4)
- Jul 14 (4)
- Jul 15 (2)
- Jul 17 (8)
- Jul 18 (17)
- Jul 19 (1)
- Jul 20 (8)
- Jul 21 (6)
- Jul 22 (12)
- Jul 23 (10)
- Jul 25 (6)
- Jul 26 (23)
- Jul 28 (50)
- Jul 30 (12)
- Jul 31 (5)
- Aug 01 (16)
- Aug 02 (5)
- Aug 03 (5)
- Aug 04 (11)
- Aug 05 (13)
- Aug 06 (7)
- Aug 07 (10)
- Aug 08 (2)
- Aug 09 (27)
- Aug 10 (15)
- Aug 11 (67)
- Aug 12 (44)
- Aug 13 (29)
- Aug 14 (120)
- Aug 15 (61)
- Aug 16 (36)
- Aug 17 (21)
- Aug 18 (5)
- Aug 21 (5)
- Aug 22 (54)
- Aug 23 (101)
- Aug 24 (100)
- Aug 25 (99)
- Aug 26 (100)
- Aug 27 (84)
- Aug 28 (73)
- Aug 29 (76)
- Aug 30 (67)
- Aug 31 (95)
- Sep 01 (126)
- Sep 02 (68)
- Sep 03 (11)
- Sep 04 (14)
- Sep 05 (47)
- Sep 06 (101)
- Sep 07 (61)
- Sep 08 (57)
- Sep 09 (46)
- Sep 10 (14)
- Sep 11 (93)
- Sep 12 (101)
- Sep 13 (101)
- Sep 14 (100)
- Sep 15 (77)
- Sep 16 (2)
- Sep 17 (101)
- Sep 18 (91)
- Sep 19 (102)
- Sep 20 (102)
- Sep 21 (94)
- Sep 22 (84)
- Sep 23 (110)
- Sep 24 (101)
- Sep 25 (76)
- Sep 26 (43)
- Sep 27 (87)
- Sep 28 (104)
- Sep 29 (92)
- Sep 30 (33)
- Oct 01 (58)
- Oct 02 (1)
- Oct 05 (8)
- Oct 06 (6)
- Oct 07 (4)
- Oct 08 (4)
- Oct 09 (1)
- Oct 10 (18)
- Oct 11 (8)
- Oct 12 (26)
- Oct 13 (6)
- Oct 14 (2)
- Oct 15 (4)
- Oct 16 (3)
- Oct 17 (4)
- Oct 18 (3)
- Oct 19 (11)
- Oct 20 (5)
- Oct 21 (7)
- Oct 22 (5)
- Oct 23 (8)
- Oct 24 (9)
- Oct 25 (14)
- Oct 26 (8)
- Oct 27 (13)
- Oct 28 (7)
- Oct 29 (7)
- Oct 30 (22)
- Oct 31 (13)
- Nov 01 (13)
- Nov 02 (6)
- Nov 03 (10)
- Nov 04 (17)
- Nov 05 (8)
- Nov 06 (9)
- Nov 07 (11)
- Nov 08 (3)
- Nov 09 (7)
- Nov 10 (5)
- Nov 11 (5)
- Nov 12 (5)
- Nov 13 (10)
- Nov 14 (7)
- Nov 15 (15)
- Nov 16 (8)
- Nov 17 (6)
- Nov 18 (5)
- Nov 19 (7)
- Nov 20 (8)
- Nov 21 (12)
- Nov 22 (5)
- Nov 23 (7)
- Nov 24 (7)
- Nov 25 (8)
- Nov 26 (2)
- Nov 27 (12)
- Nov 28 (2)
- Nov 29 (2)
- Dec 01 (1)
- Dec 02 (3)
- Dec 03 (2)
- Dec 04 (1)
- Dec 05 (9)
- Dec 06 (22)
- Dec 07 (2)
- Dec 08 (3)
- Dec 09 (1)
- Dec 13 (2)
- Dec 14 (12)
- Dec 15 (1)
- Dec 17 (1)
- Dec 23 (4)
- Dec 24 (2)
- Dec 25 (1)
- Dec 27 (2)
- Dec 28 (1)
- Dec 29 (6)
- Dec 30 (2)
- Dec 31 (6)
- Jan 03 (3)
- Jan 04 (12)
- Jan 05 (5)
- Jan 06 (7)
- Jan 07 (1)
- Jan 08 (3)
- Jan 09 (1)
- Jan 11 (1)
- Jan 12 (5)
- Jan 14 (1)
- Jan 16 (1)
- Jan 17 (1)
- Jan 18 (2)
- Jan 23 (1)
- Jan 26 (3)
- Jan 28 (2)
- Jan 29 (3)
- Jan 30 (1)
- Jan 31 (1)
- Feb 04 (2)
- Feb 05 (2)
- Feb 08 (2)
- Feb 09 (1)
- Feb 13 (3)
- Feb 15 (2)
- Feb 16 (1)
- Feb 17 (1)
- Feb 25 (2)
- Feb 28 (2)
- Mar 03 (1)
- Mar 08 (3)
- Mar 16 (2)
- Mar 17 (1)
- Mar 18 (11)
- Mar 20 (9)
- Mar 22 (1)
- Mar 23 (3)
- Mar 31 (1)
- Apr 01 (2)
- Apr 02 (1)
- Apr 03 (2)
- Apr 04 (1)
- Apr 05 (2)
- Apr 06 (6)
- Apr 07 (1)
- Apr 08 (7)
- Apr 09 (4)
- Apr 10 (7)
- Apr 19 (18)
- Apr 20 (12)
- Apr 21 (1)
- Apr 24 (2)
- May 11 (1)
- May 16 (4)
- May 20 (2)
- May 24 (2)
- May 27 (3)
- Jun 02 (2)
- Jun 06 (1)
- Jun 07 (9)
- Jun 10 (1)
- Jun 11 (2)
- Jun 12 (3)
- Jun 15 (1)
- Jun 17 (1)
- Jun 20 (5)
- Jun 21 (12)
- Jun 22 (21)
- Jun 23 (10)
- Jun 24 (4)
- Jun 25 (10)
- Jun 26 (5)
- Jun 28 (4)
- Jun 29 (2)
- Jun 30 (2)
- Jul 01 (1)
- Jul 04 (1)
- Jul 05 (2)
- Jul 06 (1)
- Jul 07 (2)
- Jul 08 (1)
- Jul 09 (3)
- Jul 10 (6)
- Jul 11 (7)
- Jul 12 (2)
- Jul 13 (3)
- Jul 14 (7)
- Jul 15 (4)
- Jul 16 (9)
- Jul 17 (2)
- Jul 18 (6)
- Jul 19 (6)
- Jul 20 (14)
- Jul 21 (2)
- Jul 22 (6)
- Jul 23 (14)
- Jul 24 (6)
- Jul 25 (5)
- Jul 26 (5)
- Jul 27 (2)
- Jul 28 (6)
- Jul 29 (1)
- Jul 30 (3)
- Jul 31 (1)
- Aug 01 (6)
- Aug 03 (6)
- Aug 04 (4)
- Aug 05 (2)
- Aug 06 (2)
- Aug 07 (1)
- Aug 08 (1)
- Aug 09 (1)
- Aug 10 (1)
- Aug 11 (3)
- Aug 12 (1)
- Aug 13 (1)
- Aug 14 (1)
- Aug 15 (1)
- Aug 17 (9)
- Aug 19 (1)
- Aug 24 (1)
- Aug 28 (1)
- Oct 14 (1)
- Oct 22 (1)
- Nov 13 (10)
- Nov 14 (1)
- Nov 15 (3)
- Nov 23 (2)
- Nov 24 (1)
- Nov 25 (1)
- Nov 26 (1)
- Dec 01 (3)
- Dec 07 (3)
- Dec 08 (1)
- Dec 10 (2)
- Dec 12 (22)
- Dec 13 (30)
- Dec 15 (7)
- Dec 20 (5)
- Dec 28 (1)
- Dec 29 (3)
- Dec 31 (1)
- Jan 02 (2)
- Jan 10 (1)
- Jan 14 (1)
- Jan 17 (4)
- Jan 29 (2)
- Feb 03 (1)
- Feb 04 (6)
- Feb 05 (5)
- Feb 06 (10)
- Feb 08 (16)
- Feb 10 (63)
- Feb 11 (39)
- Feb 12 (33)
- Feb 13 (27)
- Feb 14 (4)
- Feb 15 (66)
- Feb 16 (7)
- Feb 17 (22)
- Feb 18 (14)
- Feb 19 (44)
- Feb 20 (3)
- Feb 21 (12)
- Feb 22 (68)
- Feb 23 (78)
- Feb 25 (3)
- Feb 26 (10)
- Feb 27 (28)
- Feb 28 (26)
- Mar 01 (17)
- Mar 02 (7)
- Mar 03 (6)
- Mar 04 (3)
- Mar 05 (7)
- Mar 06 (8)
- Mar 07 (13)
- Mar 08 (6)
- Mar 09 (3)
- Mar 10 (2)
- Mar 11 (15)
- Mar 12 (6)
- Mar 13 (2)
- Mar 14 (15)
- Mar 15 (10)
- Mar 16 (6)
- Mar 17 (5)
- Mar 18 (3)
- Mar 19 (3)
- Mar 20 (9)
- Mar 21 (2)
- Mar 22 (1)
- Mar 23 (15)
- Mar 24 (1)
- Mar 25 (1)
- Mar 26 (7)
- Mar 27 (5)
- Mar 28 (2)
- Mar 29 (8)
- Mar 30 (21)
- Mar 31 (10)
- Apr 01 (3)
- Apr 02 (3)
- Apr 03 (9)
- Apr 04 (1)
- Apr 05 (4)
- Apr 06 (4)
- Apr 07 (4)
- Apr 08 (4)
- Apr 09 (1)
- Apr 10 (1)
- Apr 11 (6)
- Apr 12 (7)
- Apr 13 (3)
- Apr 14 (2)
- Apr 15 (11)
- Apr 16 (16)
- Apr 17 (12)
- Apr 18 (29)
- Apr 19 (21)
- Apr 20 (3)
- Apr 21 (8)
- Apr 22 (3)
- Apr 23 (5)
- Apr 24 (1)
- Apr 25 (4)
- Apr 26 (6)
- Apr 27 (8)
- Apr 28 (10)
- Apr 30 (2)
- May 01 (7)
- May 02 (3)
- May 03 (16)
- May 04 (3)
- May 05 (11)
- May 06 (41)
- May 07 (2)
- May 08 (18)
- May 09 (117)
- May 10 (15)
- May 11 (85)
- May 12 (12)
- May 13 (54)
- May 14 (73)
- May 15 (85)
- May 16 (148)
- May 17 (101)
- May 18 (100)
- May 19 (99)
- May 20 (101)
- May 21 (101)
- May 22 (101)
- May 23 (101)
- May 24 (101)
- May 25 (7)
- May 27 (1)
- May 28 (1)
- May 29 (29)
- Jun 02 (1)
- Jun 03 (21)
- Jun 04 (7)
- Jun 05 (8)
- Jun 06 (1)
- Jun 22 (5)
- Jun 23 (10)
- Jun 24 (10)
- Jun 25 (4)
- Jun 26 (7)
- Jun 27 (22)
- Jun 28 (12)
- Jun 29 (11)
- Jun 30 (23)
- Jul 01 (10)
- Jul 02 (13)
- Jul 03 (17)
- Jul 04 (41)
- Jul 05 (17)
- Jul 06 (8)
- Jul 07 (10)
- Jul 08 (6)
- Jul 09 (3)
- Jul 10 (2)
- Jul 11 (2)
- Jul 12 (12)
- Jul 13 (6)
- Jul 14 (14)
- Jul 15 (5)
- Jul 17 (1)
- Jul 18 (1)
- Jul 19 (1)
- Jul 20 (1)
- Jul 22 (2)
- Jul 23 (30)
- Jul 24 (5)
- Jul 25 (55)
- Jul 27 (8)
- Jul 28 (26)
- Jul 29 (15)
- Jul 30 (35)
- Jul 31 (5)
- Aug 01 (13)
- Aug 02 (3)
- Aug 04 (1)
- Aug 05 (2)
- Aug 11 (11)
- Aug 13 (3)
- Aug 14 (7)
- Aug 15 (3)
- Aug 16 (5)
- Aug 17 (4)
- Aug 18 (4)
- Aug 19 (2)
- Aug 20 (19)
- Aug 21 (38)
- Aug 23 (14)
- Aug 24 (6)
- Aug 25 (30)
- Aug 26 (57)
- Aug 27 (19)
- Aug 28 (25)
- Aug 29 (120)
- Aug 30 (82)
- Aug 31 (46)
- Sep 01 (96)
- Sep 02 (101)
- Sep 03 (62)
- Sep 04 (32)
- Sep 05 (44)
- Sep 06 (91)
- Sep 07 (22)
- Sep 08 (100)
- Sep 09 (71)
- Sep 10 (15)
- Sep 11 (90)
- Sep 13 (2)
Sunday, August 15, 2021
08-15-2021-0439 - United States of America North America Continent Domestic Copy draft [n.d.]
1. Large Scale Industry
2. World Migration
3. HIV, tropics, animals, digging, etc.; very inhumane human experimentation; etc..
4. Anorexia Stigma appearance in world societies.
5. AIDS, toxoplasmosis of the brain surfaces in USA female of type c, lipodystrophy/etc. appear in USA male or female of type d;
6. Cancer Research (indication leukemia took; HTLV, HLV-HIV, HTLV-I, SIV, Polio/Polyo, etc. (chronic leuks v. acute leuks upon exposure, infrastructure flaws fatal revealed - acute leuk misattributed to biological cause for disease of blood, where physical cause for condition of the blood (aggravant , eg radiation type eg. gamma)))
7. Large scale domestic accidental sterilization by insecticides, water, food, air, dust/soil, components, etc.
8. Facilitation of environment to carry; increase in oil, hydrophobic volatile organic compounds lipids hydrocarbs fats etc. in atmosphere and environment (with settle seep and stew). Aerosol Aeromatic Suspension Gaseous etc.. Concentration Weight etc. diversification for carry. Prepared for bioaerosols and hydro-oxy comms. Including nitro red explosives.
9. Nuclearization (public sale of chemicals radionucleotide, masers, etc.)
10. Electrical Grid (large scale ionization), prepared from world copper (no material standard)
11. Underground spare country doing bioweapons/cloning/trafficking/teaching each other/practicing cloak work/improving cloak process/trying to build mat from particle scratch/etc.; that turned into infiltration/invasion/etc. global disruption and surveillance and finally conspiracy when they ran out of stuff to do
12. Compensation by trafficking/theft
13. Abduction and enhostage of birthright, VIV and their family. Intellectual Property Theft, Mass Trafficking. etc.
14. Slaves (slaves spread disease, danger, destruction)
15. Non-disclosure with cover (e.g. people on cl1 fightin asia 1900s; people below left alone despite surv ineffective at max cap; hostages, pows, con camps, jew emigration from con camp germ, jails, psych unit, BHU, asylum, sanitoriums, psychiatric ward, jail, detainment, shelter, street, german easy emigration from tropics/travel/back forth/etc., world scale operations (beyond normal human trav), etc.)
16. Theft of goods/etc. from foreign countries
17. Scheme concept integrated into all persons of/etc. America
18. Movin suspect groups - Mister and Jewej migration (community of everyone without discrimination only principle alignment of trafficking); german revelation 1800 and german comm channels reveal age of german cell infiltrant in americas (mass german migration); azi v appears with west german white brown 100% caucasian wavy haired chig layng; trafficker gangs appear multiracial with racial child preference (soldiers, emt, criminals, etc.); petersen and the degenerate met amcans with genetic disease and asburgers (their disease state has not improved; they steal children foreign and import for argument of improvement/false argument of health without differentiation); mass migration outward of traffickers/clones/stolen children/perturbations/disease/aberrations/derivatives/integrants/variants/integrals/etc.; etc.. Warring between gangs unacknowledged and blame the victim/etc.. Dysfunction reveal america position of inability to truth due to assailant-guilty-party-etc. of knowing-awareness-etc., and where victims have been framed on both sides (knowing victim and unknowing victim). Triangle of crime, square of crime; etc. (expands many dimensions until long-term criminal arrives; petersen/norway/nord/neander/heathen/barbarian/jewej/etc.).
19. Genetic and Metabolic disease surface provided environment, industry, import, immigrants, large scale industry/infrastructure, infrastructure, radiation, ionization, electrical, above and below ground operations, exterterresterial/sea operations, material standard, baseline disease of america, america history, time, inhumane experimentation, neglected people, low-intellect/psychopathology/misunderstanding/inferior genetics/etc. of america people, amnestic drug facilitated assault, drug compound drug compound antagonist, cloak, clone, genetic operations, cell work abuse, pharmaceutical culture, technological advancement clandestine, harrowing baby factories, fertility banks interrelation, basement human experimentation, use of facility to commit gang violent crimes, trafficking, mutilation, sterilization, amputation, torture, very large scale weapons operations, etc. (fatally flawed, fated practices), and spread to foreign country by emigrants, export, trafficking, argument, weapons, war, etc..
https://discovery.lifemapsc.com/in-vivo-development/blood
08-15-2021-0343 - America - draft
1. Large Scale Industry
2. World Migration
3. HIV, tropics, animals, digging, etc.; very inhumane human experimentation; etc..
4. Anorexia Stigma appearance in world societies.
5. AIDS, toxoplasmosis of the brain surfaces in USA female of type c, lipodystrophy/etc. appear in USA male or female of type d;
6. Cancer Research (indication leukemia took; HTLV, HLV-HIV, HTLV-I, SIV, Polio/Polyo, etc. (chronic leuks v. acute leuks upon exposure, infrastructure flaws fatal revealed - acute leuk misattributed to biological cause for disease of blood, where physical cause for condition of the blood (aggravant , eg radiation type eg. gamma)))
2. World Migration
3. HIV, tropics, animals, digging, etc.; very inhumane human experimentation; etc..
4. Anorexia Stigma appearance in world societies.
5. AIDS, toxoplasmosis of the brain surfaces in USA female of type c, lipodystrophy/etc. appear in USA male or female of type d;
6. Cancer Research (indication leukemia took; HTLV, HLV-HIV, HTLV-I, SIV, Polio/Polyo, etc. (chronic leuks v. acute leuks upon exposure, infrastructure flaws fatal revealed - acute leuk misattributed to biological cause for disease of blood, where physical cause for condition of the blood (aggravant , eg radiation type eg. gamma)))
7. Large scale domestic accidental sterilization by insecticides, water, food, air, dust/soil, components, etc.
8. Facilitation of environment to carry; increase in oil, hydrophobic volatile organic compounds lipids hydrocarbs fats etc. in atmosphere and environment (with settle seep and stew). Aerosol Aeromatic Suspension Gaseous etc.. Concentration Weight etc. diversification for carry. Prepared for bioaerosols and hydro-oxy comms. Including nitro red explosives. bioweaponization of air. to carry biosols/biomat/etc.
9. Nuclearization (public sale of chemicals radionucleotide, masers, etc.)
10. Electrical Grid (large scale ionization), prepared from world copper (no material standard)
11. Underground spare country doing bioweapons/cloning/trafficking/teaching each other/practicing cloak work/improving cloak process/trying to build mat from particle scratch/etc.; that turned into infiltration/invasion/etc. global disruption and surveillance and finally conspiracy when they ran out of stuff to do
12. Compensation by trafficking/theft
13. Abduction and enhostage of birthright, VIV and their family. Intellectual Property Theft, Mass Trafficking. etc.
14. Slaves (slaves spread disease, danger, destruction)
15. Non-disclosure with cover (e.g. people on cl1 fightin asia 1900s; people below left alone despite surv ineffective at max cap; hostages, pows, con camps, jew emigration from con camp germ, jails, psych unit, BHU, asylum, sanitoriums, psychiatric ward, jail, detainment, shelter, street, german easy emigration from tropics/travel/back forth/etc., world scale operations (beyond normal human trav), etc.)
16. Theft of goods/etc. from foreign countries
17. Scheme concept integrated into persons of/etc. America; etc..
18. Movin suspect groups - Mister and Jewej migration (community of everyone without discrimination only principle alignment of trafficking); german revelation 1800 and german comm channels reveal age of german cell infiltrant in americas (mass german migration); azi v appears with west german white brown 100% caucasian wavy haired chig layng; trafficker gangs appear multiracial with racial child preference (soldiers, emt, criminals, etc.); petersen and the degenerate met dis amcans with genetic disease and asburgers (their disease state has not improved; they steal children foreign and import for argument of improvement/false argument to health without differentiation necessary etc.); mass migration outward of traffickers/clones/stolen children/perturbations/disease/aberrations/derivatives/integrants/variants/integrals/etc.; etc.. Warring between gangs unacknowledged and blame the victim/etc.. Dysfunction reveal america position of inability to truth due to assailant-guilty-party-etc. of knowing-awareness-etc., and where victims have been framed on both sides (knowing victim and unknowing victim). Triangle of crime, square of crime; etc. (expands many dimensions until long-term criminal arrives; petersen/norway/nord/neander/heathen/barbarian/jewej/etc.).
19. Genetic and Metabolic disease surface provided environment, industry, import, immigrants, large scale industry/infrastructure, infrastructure, radiation, ionization, electrical, above and below ground operations, exterterresterial/sea operations, material standard, baseline disease of america, america history, time, inhumane experimentation, neglected people, low-intellect/psychopathology/misunderstanding/inferior genetics/etc. of america people, amnestic drug facilitated assault, drug compound drug compound antagonist, cloak, clone, genetic operations, cell work abuse, pharmaceutical culture, technological advancement clandestine, harrowing baby factories, fertility banks interrelation, basement human experimentation, use of facility to commit gang violent crimes, trafficking, mutilation, sterilization, amputation, torture, very large scale weapons operations, etc. (fatally flawed, fated practices), and spread to foreign country by emigrants, export, trafficking, argument, weapons, war, etc..
https://discovery.lifemapsc.com/in-vivo-development/blood
08-15-2021-0333 - Viral Lentivirus and Prion Disease Compatible by Incubation Time Comparison/Indication/Similarity/Synergy/etc..
Viral hypothesis
This hypothesis postulates that an as of yet undiscovered infectious viral agent is the cause of the disease. Evidence for this hypothesis is as follows:
Incubation time is comparable to a lentivirus
Strain variation of different isolates of PrPSc[28]
An increasing titre of PrPSc as the disease progresses suggests a replicating agent.
https://en.wikipedia.org/wiki/Transmissible_spongiform_encephalopathy#Genetics
This hypothesis postulates that an as of yet undiscovered infectious viral agent is the cause of the disease. Evidence for this hypothesis is as follows:
Incubation time is comparable to a lentivirus
Strain variation of different isolates of PrPSc[28]
An increasing titre of PrPSc as the disease progresses suggests a replicating agent.
https://en.wikipedia.org/wiki/Transmissible_spongiform_encephalopathy#Genetics
08-15-2021-0240 - drafting
Mycosis fungoides
encephalopathy
Inflammation itis
Parasite osis
Leukoencephalopathy
Polyo virus j2000
Polio virus j1900
rables
prion
large dna virus
large macrocytic dna virus
prion disease
fibrodysplasia ossificans progressive
08-11-2021-0604 - Alternariosis Itraconazole 944
Cytochalasins are fungal metabolites
Bacillus thuringiensis
Plasmodium falciparum
trichinosis
triptosomatida
radionuclide radionucleotide environmental air
magnetohydrodynamic drive
radioisotopic agents 1964
synchrotron light
free electron laser
atomic mirror
particle beam weps
plasma accelerators
superconducting rad freq
particle beam
hard rad
ionizing rad
single photon source
de broglie microscope nano
duoplasmatron ion beam linear acc
alpha emitter
beta decay
atom optics
ultracold atom
atom laser
wafer, parabolic microphone, mirror
gamma ray
alpha process
plasma, supersonic speed
accretion
black hole rad, black hole
neutron star
wite drwarf
gravitoelectromagnetism
singularity
grav time dilation
medium
HI reg
dust clouds gallactic
proper motion
parallax
polyhalogenated comps
congeners
gravitational microlensing
nebula
neon burning process
carbon detonation
triple alpha process
inverse beta decay v beta decay
matter alt
fermions (ion)
electron degeneracy v radiation v gravity (etc., proc., etc.)
fusion against gravitational collapse
lead cooled fast reaction
trihydrogen cation
hydronium, deuterium
trihydronium, ion, cation, anion, etc.
suspensions
aerosols
scale size weight disparity plane warping
chlorosis
photorespiration
blacklead
radiotrophic fungi
black lead fungus
vinca alkaloids
trihydrogen cations oxygen triangles
glutamate
methotrexate
airborne dermatitis
methomyl
lupus
lipodystrophy syndrome
panniculitis
rubella
myelitis
rabies
hiv aids
filariasis
leprosy
Neisseria meningitidis
fibrosarcoma
osteomyelitis
fungal osteomyelitis septic
avasular necrosis
sepsis
legionella pneumophila
pyaemia
pyrexia
IFN-b
necrosis
malignant granuloma
tumor lysis syndrome
connective tissue disease
Coronaviridae is a family of enveloped, positive-strand RNA viruses w
https://en.wikipedia.org/wiki/Coronaviridae
Deltacoronavirus (Delta-CoV) is one of the four genera (Alpha-, Beta-, Gamma-, and Delta-) of coronaviruses. It is in the subfamily Orthocoronavirinae of the family Coronaviridae. They are enveloped, positive-sense, single-stranded RNA viruses. Deltacoronaviruses infect mostly birds and some mammals.
While the alpha and beta genera are derived from the bat viral gene pool, the gamma and delta genera are derived from the avian and pig viral gene pools.[1]
Recombination appears to be common among deltacoronaviruses.[2] Recombination occurs frequently in the viral genome region that encodes the host receptor binding protein. Recombination between different viral lineages contributes to the emergence of new viruses capable of interspecies transmission and adaptation to new animal hosts.[2]
https://en.wikipedia.org/wiki/Deltacoronavirus_(genus)
Human coronavirus NL63 (HCoV-NL63) is a species of coronavirus, specifically a Setracovirus from among the Alphacoronavirus genus. It was identified in late 2004 in a seven-month-old child with bronchiolitis in the Netherlands.[1] The virus is an enveloped, positive-sense, single-stranded RNA virus which enters its host cell by binding to ACE2.[2][3] Infection with the virus has been confirmed worldwide, and has an association with many common symptoms and diseases. Associated diseases include mild to moderate upper respiratory tract infections, severe lower respiratory tract infection, croup and bronchiolitis.[1]
The virus is found primarily in young children, the elderly, and immunocompromised patients with acute respiratory illness. It also has a seasonal association in temperate climates. A study performed in Amsterdam estimated the presence of HCoV-NL63 in approximately 4.7% of common respiratory illnesses.[4] The virus originated from infected palm civets and bats.[5] Estimates of its divergence from another coronavirus (HCoV-229E) are around 1000 years ago; it has likely circulated in humans for centuries.[6]
The evolution of HCoV-NL63 appears to have involved recombination between an ancestral NL63-like virus circulating in African Triaenops afer bats and a CoV 229E-like virus circulating in Hipposideros bats.[7]Recombinant viruses can arise when two viral genomes are present in the same host cell.
https://en.wikipedia.org/wiki/Human_coronavirus_NL63
Letovirinae is a subfamily of viruses within the family Coronaviridae, where it is the only subfamily besides the more diverse Orthocoronavirinae (coronaviruses). Letovirinae contains one accepted genus, Alphaletovirus, which contains one accepted subgenus, Milecovirus, which contains one accepted species, Microhyla letovirus 1 (MLeV).[1] This species was discovered in 2018 and is hosted by the ornate chorus frog (Microhyla fissipes).
Other, as yet unaccepted species in the Letovirinae have been discovered in Pacific salmon (Oncorhynchus), and in Murray River carp (Cyprinus).[2][3][4]
https://en.wikipedia.org/wiki/Letovirinae
Biosafety level 4 laboratories are used for diagnostic work and research on easily transmitted pathogens which can cause fatal disease. These include a number of viruses known to cause viral hemorrhagic fever such as Marburg virus, Ebola virus, Lassa virus, and Crimean-Congo hemorrhagic fever. Other pathogens handled at BSL-4 include Hendra virus, Nipah virus, and some flaviviruses. Additionally, poorly characterized pathogens which appear closely related to dangerous pathogens are often handled at this level until sufficient data are obtained either to confirm continued work at this level, or to permit working with them at a lower level.[15] This level is also used for work with Variola virus, the causative agent of smallpox, though this work is only performed at the Centers for Disease Control and Prevention in Atlanta, United States, and the State Research Center of Virology and Biotechnology in Koltsovo, Russia.[20]
https://en.wikipedia.org/wiki/Biosafety_level#Biosafety_level_3
Herdecovirus is a subgenus of viruses in the genus Deltacoronavirus, consisting of a single species, Night heron coronavirus HKU19.[1]
https://en.wikipedia.org/wiki/Herdecovirus
White-eye coronavirus HKU16 is a species of coronavirus in the genus Deltacoronavirus.[1]
https://en.wikipedia.org/wiki/White-eye_coronavirus_HKU16
Coronavirus diseases are caused by viruses in the coronavirus subfamily, a group of related RNA virusesthat cause diseases in mammals and birds. In humans and birds, the group of viruses cause respiratory tract infections that can range from mild to lethal. Mild illnesses in humans include some cases of the common cold (which is also caused by other viruses, predominantly rhinoviruses),[1] while more lethal varieties can cause SARS, MERS and COVID-19.[2][3] As of 2021, 45 species are registered as coronaviruses,[4] whilst 11 diseases have been identified, as listed below
Coronavirus diseasesDiseaseCauseFirst identifiedDetails
Avian infectious bronchitis Avian coronavirus(IBV) 1920s[14](isolated in 1938)[15] Originated from North America.[14]
Common cold, pneumonia, bronchiolitis, etc. Human coronavirus 229E (HCoV-229E) 1930s (isolated in 1965)[19] Likely originated from bats.[20]
Murine encephalitis JHM (named after John Howard Mueller), a murine coronavirus[21] 1949[22]
Severe acute respiratory syndrome (SARS) Severe acute respiratory syndrome coronavirus(SARS-CoV or SARS-CoV-1), a strain of severe acute respiratory syndrome–related coronavirus(SARSr-CoV) 2002 Caused the 2002–2004 SARS outbreak. Likely originated from horseshoe bats.[29]
Respiratory infection Human coronavirus NL63 (HCoV-NL63) 2004 Originated from Amsterdam, Netherlands.[31] Likely originated from tricolored bats.[32]
https://en.wikipedia.org/wiki/Coronavirus_diseases
Coronavirus disease 2019 (COVID-19) is a contagious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The first known case was identified in Wuhan, China, in December 2019.[7] The disease has since spread worldwide, leading to an ongoing pandemic.[8]
Tedros Adhanom explained: CO for corona, VI for virus, D for disease and 19 for when the outbreak was first identified (31 December 2019).[32] The WHO additionally uses "the COVID-19 virus" and "the virus responsible for COVID-19" in public communications.[31]
The official names COVID-19 and SARS-CoV-2 were issued by the WHO on 11 February 2020.[31]
COVID-19 is caused by infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus strain.[52]
The respiratory route of spread of COVID-19, encompassing larger droplets and aerosols.
Infectious particles range in size from aerosols that remain suspended in the air for long periods of time to larger droplets that remain airborne or fall to the ground.[53][57] Various groups utilise terms such as "airborne" and "droplet" both in technical and general ways, leading to confusion around terminology.[58] Additionally, COVID-19 research has redefined the traditional understanding of how respiratory viruses are transmitted.[57][59] The largest droplets of respiratory fluid do not travel far, and can be inhaled or land on mucous membranes on the eyes, nose, or mouth to infect.[53] Aerosols are highest in concentration when people are in close proximity, which leads to easier viral transmission when people are physically close,[53][57][59] but airborne transmissioncan occur at longer distances, mainly in locations that are poorly ventilated;[53] in those conditions small particles can remain suspended in the air for minutes to hours.[53]
The number of people generally infected by one infected person varies;[60] as only 10 to 20% of people are responsible for the diseases spread.[61] It often spreads in clusters, where infections can be traced back to an index case or geographical location.[62]Often in these instances, superspreading events occur, where many people are infected by one person.[60]
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel severe acute respiratory syndrome coronavirus. It was first isolated from three people with pneumonia connected to the cluster of acute respiratory illness cases in Wuhan.[63] All structural features of the novel SARS-CoV-2 virus particle occur in related coronaviruses in nature.[64]
SARS-CoV-2 is closely related to the original SARS-CoV.[66] It is thought to have an animal (zoonotic) origin. Genetic analysis has revealed that the coronavirus genetically clusters with the genus Betacoronavirus, in subgenus Sarbecovirus (lineage B) together with two bat-derived strains. It is 96% identical at the whole genome level to other bat coronavirus samples (BatCov RaTG13).[67][68] The structural proteins of SARS-CoV-2 include membrane glycoprotein (M), envelope protein (E), nucleocapsid protein (N), and the spike protein (S). The M protein of SARS-CoV-2 is about 98% similar to the M protein of bat SARS-CoV, maintains around 98% homology with pangolin SARS-CoV, and has 90% homology with the M protein of SARS-CoV; whereas, the similarity is only around 38% with the M protein of MERS-CoV. The structure of the M protein resembles the sugar transporter SemiSWEET.[69]
The many thousands of SARS-CoV-2 variants are grouped into either clades or lineages.[70][71] The WHO, in collaboration with partners, expert networks, national authorities, institutions and researchers, have established nomenclature systems for naming and tracking SARS-CoV-2 genetic lineages by GISAID, Nextstrain and Pango. At the present time, the expert group convened by WHO has recommended the labeling of variants using letters of the Greek Alphabet, for example, Alpha, Beta, Delta, and Gamma, giving the justification that they "will be easier and more practical to discussed by non-scientific audiences."[72] Nextstrain divides the variants into five clades (19A, 19B, 20A, 20B, and 20C), while GISAID divides them into seven (L, O, V, S, G, GH, and GR).[73] The Pango tool groups variants into lineages, with many circulating lineages being classed under the B.1 lineage.[71][74]
Several notable variants of SARS-CoV-2 emerged in late 2020.[citation needed] Cluster 5 emerged among minks and mink farmers in Denmark.[citation needed] After strict quarantines and a mink euthanasia campaign, it is believed to have been eradicated.[medical citation needed]
As of July 2021, there are four dominant variants of SARS-CoV-2 spreading among global populations: the Alpha Variant (formerly called the UK Variant and officially referred to as B.1.1.7), first found in London and Kent, the Beta Variant (formerly called the South Africa Variant and officially referred to as B.1.351), the Gamma Variant (formerly called the Brazil Variant and officially referred to as P.1), and the Delta Variant (formerly called the India Variant and officially referred to as B.1.617.2).[75]
Using whole genome sequencing, epidemiology and modelling suggest the Alpha variant VUI-202012/01 (the first variant under investigation in December 2020) in the B.1.1.7 lineage transmits more easily than other strains.[76]
COVID-19 can affect the upper respiratory tract (sinuses, nose, and throat) and the lower respiratory tract (windpipe and lungs).[77] The lungs are the organs most affected by COVID-19 because the virus accesses host cells via the receptor for the enzyme angiotensin-converting enzyme 2 (ACE2), which is most abundant on the surface of type II alveolar cells of the lungs.[78] The virus uses a special surface glycoprotein called a "spike" (peplomer) to connect to the ACE2 receptor and enter the host cell.[79]
Although SARS-CoV-2 has a tropism for ACE2-expressing epithelial cells of the respiratory tract, people with severe COVID-19 have symptoms of systemic hyperinflammation. Clinical laboratory findings of elevated IL‑2, IL‑7, IL‑6, granulocyte-macrophage colony-stimulating factor (GM‑CSF), interferon gamma-induced protein 10(IP‑10), monocyte chemoattractant protein 1 (MCP1), macrophage inflammatory protein 1‑alpha (MIP‑1‑alpha), and tumour necrosis factor (TNF‑α) indicative of cytokine release syndrome (CRS) suggest an underlying immunopathology.[88]
Additionally, people with COVID-19 and acute respiratory distress syndrome (ARDS) have classical serumbiomarkers of CRS, including elevated C-reactive protein (CRP), lactate dehydrogenase (LDH), D-dimer, and ferritin.[100]
Systemic inflammation results in vasodilation, allowing inflammatory lymphocytic and monocytic infiltration of the lung and the heart. In particular, pathogenic GM-CSF-secreting T cells were shown to correlate with the recruitment of inflammatory IL-6-secreting monocytes and severe lung pathology in people with COVID-19.[101]Lymphocytic infiltrates have also been reported at autopsy.[99]
Multiple viral and host factors affect the pathogenesis of the virus. The S-protein, otherwise known as the spike protein, is the viral component that attaches to the host receptor via the ACE2 receptors. It includes two subunits: S1 and S2. S1 determines the virus-host range and cellular tropism via the receptor-binding domain. S2 mediates the membrane fusion of the virus to its potential cell host via the H1 and HR2, which are heptad repeat regions. Studies have shown that S1 domain induced IgG and IgA antibody levels at a much higher capacity. It is the focus spike proteins expression that are involved in many effective COVID-19 vaccines.[102]
The M protein is the viral protein responsible for the transmembrane transport of nutrients. It is the cause of the bud release and the formation of the viral envelope.[103] The N and E protein are accessory proteins that interfere with the host's immune response.[103]
Among healthy adults not exposed to SARS-CoV-2, about 35% have CD4+ T cells that recognize the SARS-CoV-2 S protein (particularly the S2 subunit) and about 50% react to other proteins of the virus, suggesting cross-reactivity from previous common colds caused by other coronaviruses.[106]
The severity of the inflammation can be attributed to the severity of what is known as the cytokine storm.[108]Levels of interleukin 1B, interferon-gamma, interferon-inducible protein 10, and monocyte chemoattractant protein 1 were all associated with COVID-19 disease severity. Treatment has been proposed to combat the cytokine storm as it remains to be one of the leading causes of morbidity and mortality in COVID-19 disease.[109]
A cytokine storm is due to an acute hyperinflammatory response that is responsible for clinical illness in an array of diseases but in COVID-19, it is related to worse prognosis and increased fatality. The storm causes acute respiratory distress syndrome, blood clotting events such as strokes, myocardial infarction, encephalitis, acute kidney injury, and vasculitis. The production of IL-1, IL-2, IL-6, TNF-alpha, and interferon-gamma, all crucial components of normal immune responses, inadvertently become the causes of a cytokine storm. The cells of the central nervous system, the microglia, neurons, and astrocytes, are also involved in the release of pro-inflammatory cytokines affecting the nervous system, and effects of cytokine storms toward the CNS are not uncommon.[110]
Characteristic imaging features on chest radiographs and computed tomography(CT) of people who are symptomatic include asymmetric peripheral ground-glass opacities without pleural effusions.[129]
Due to overlap with other infections such as adenovirus, imaging without confirmation by rRT-PCR is of limited specificity in identifying COVID-19.[129]
In late 2019, the WHO assigned emergency ICD-10 disease codes U07.1 for deaths from lab-confirmed SARS-CoV-2 infection and U07.2 for deaths from clinically or epidemiologically diagnosed COVID-19 without lab-confirmed SARS-CoV-2 infection.[131]
Pathology
The main pathological findings at autopsy are:
Macroscopy: pericarditis, lung consolidation and pulmonary oedema[99]
Lung findings:
minor serous exudation, minor fibrin exudation[99]
pulmonary oedema, pneumocyte hyperplasia, large atypical pneumocytes, interstitial inflammation with lymphocytic infiltration and multinucleated giant cell formation[99]
diffuse alveolar damage (DAD) with diffuse alveolar exudates. DAD is the cause of acute respiratory distress syndrome (ARDS) and severe hypoxemia.[99]
organisation of exudates in alveolar cavities and pulmonary interstitial fibrosis[99]
plasmocytosis in BAL[132]
Blood and vessels: disseminated intravascular coagulation (DIC);[133] leukoerythroblastic reaction,[134] endotheliitis,[135] hemophagocytosis[135]
Heart: cardiac muscle cell necrosis[135]
Liver: microvesicular steatosis[99]
Nose: shedding of olfactory epithelium[81]
Brain: infarction[135]
Kidneys: acute tubular damage.[135]
Spleen: white pulp depletion.[135]
People with more severe cases may need treatment in hospital. In those with low oxygen levels, use of the glucocorticoid dexamethasone is strongly recommended, as it can reduce the risk of death.[206][207][208] Noninvasive ventilation and, ultimately, admission to an intensive care unit for mechanical ventilation may be required to support breathing.[209] Extracorporeal membrane oxygenation (ECMO) has been used to address the issue of respiratory failure, but its benefits are still under consideration.[210][211] Some of the cases of severe disease course are caused by systemic hyper-inflammation, the so called cytokine storm.[212]Several experimental treatments are being actively studied in clinical trials.[196] Others were thought to be promising early in the pandemic, such as hydroxychloroquine and lopinavir/ritonavir, but later research found them to be ineffective or even harmful.[196][213][214] Despite ongoing research, there is still not enough high-quality evidence to recommend so-called early treatment.[213][214] Nevertheless, in the United States, two monoclonal antibody-based therapies are available for early use in cases thought to be at high risk of progression to severe disease.[214] The antiviral remdesivir is available in the U.S., Canada, Australia, and several other countries, with varying restrictions; however, it is not recommended for people needing mechanical ventilation, and is discouraged altogether by the World Health Organization (WHO),[215] due to limited evidence of its efficacy.[196]
Complications may include pneumonia, acute respiratory distress syndrome (ARDS), multi-organ failure, septic shock, and death.[249][250][251][252] Cardiovascular complications may include heart failure, arrhythmias(including atrial fibrillation), heart inflammation, and thrombosis, particularly venous thromboembolism.[253][254][255][256][257][258] Approximately 20–30% of people who present with COVID-19 have elevated liver enzymes, reflecting liver injury.[259][145]
Neurologic manifestations include seizure, stroke, encephalitis, and Guillain–Barré syndrome (which includes loss of motor functions).[260][261] Following the infection, children may develop paediatric multisystem inflammatory syndrome, which has symptoms similar to Kawasaki disease, which can be fatal.[262][263] In very rare cases, acute encephalopathy can occur, and it can be considered in those who have been diagnosed with COVID-19 and have an altered mental status.[264]
In the case of pregnant women, it is important to note that, according to the Centers for Disease Control and Prevention, pregnant women are at increased risk of becoming seriously ill from COVID-19.[265] This is because pregnant women with COVID-19 appear to be more likely to develop respiratory and obstetric complications that can lead to miscarriage, premature delivery and intrauterine growth restriction.[265]
Fungal infections such as aspergillosis, candidiasis, cryptococcosis and mucormycosis have been recorded in patients recovering from COVID-19.[266][267]
In the US, a greater proportion of deaths due to COVID-19 have occurred among African Americans and other minority groups.[315] Structural factors that prevent them from practicing social distancing include their concentration in crowded substandard housing and in "essential" occupations such as retail grocery workers, public transit employees, health-care workers and custodial staff. Greater prevalence of lacking health insurance and care of underlying conditions such as diabetes, hypertension, and heart disease also increase their risk of death.[316] Similar issues affect Native American and Latino communities.[315] On the one hand, in the Dominican Republic there is a clear example of both gender and ethnic inequality. In this Latin American territory, there is great inequality and precariousness that especially affects Dominican women, with greater emphasis on those of Haitian descent.[317] According to a US health policy non-profit, 34% of American Indian and Alaska Native People (AIAN) non-elderly adults are at risk of serious illness compared to 21% of white non-elderly adults.[318] The source attributes it to disproportionately high rates of many health conditions that may put them at higher risk as well as living conditions like lack of access to clean water.[319]
Leaders have called for efforts to research and address the disparities.[320] In the U.K., a greater proportion of deaths due to COVID-19 have occurred in those of a Black, Asian, and other ethnic minority background.[321][322][323] More severe impacts upon victims including the relative incidence of the necessity of hospitalization requirements, and vulnerability to the disease has been associated via DNA analysis to be expressed in genetic variants at chromosomal region 3, features that are associated with European Neanderthal heritage. That structure imposes greater risks that those affected will develop a more severe form of the disease.[324] The findings are from Professor Svante Pääbo and researchers he leads at the Max Planck Institute for Evolutionary Anthropology and the Karolinska Institutet.[324] This admixture of modern human and Neanderthal genes is estimated to have occurred roughly between 50,000 and 60,000 years ago in Southern Europe.[324]
The virus is thought to be natural and of an animal origin,[64] through spillover infection.[333]
Italy had its first confirmed cases on 31 January 2020, two tourists from China.[371] Italy overtook China as the country with the most deaths on 19 March 2020 .[372] By 26 March the United States had overtaken China and Italy with the highest number of confirmed cases in the world.[373] Research on coronavirus genomes indicates the majority of COVID-19 cases in New York came from European travellers, rather than directly from China or any other Asian country.[374] Retesting of prior samples found a person in France who had the virus on 27 December 2019,[375][376] and a person in the United States who died from the disease on 6 February 2020.[377]
RT-PCR testing of untreated wastewater samples from Brazil and Italy have suggested detection of SARS-CoV-2 as early as November and December 2019, respectively, but the methods of such sewage studies have not been optimised, many have not been peer-reviewed, details are often missing, and there is a risk of false positives due to contamination or if only one gene target is detected.[378] A September 2020 review journal article said, "The possibility that the COVID-19 infection had already spread to Europe at the end of last year is now indicated by abundant, even if partially circumstantial, evidence," including pneumonia case numbers and radiology in France and Italy in November and December.[379]
Humans appear to be capable of spreading the virus to some other animals, a type of disease transmission referred to as zooanthroponosis.
Some pets, especially cats and ferrets, can catch this virus from infected humans.[385][386] Symptoms in cats include respiratory (such as a cough) and digestive symptoms.[385] Cats can spread the virus to other cats, and may be able to spread the virus to humans, but cat-to-human transmission of SARS-CoV-2 has not been proven.[385][387] Compared to cats, dogs are less susceptible to this infection.[387] Behaviors which increase the risk of transmission include kissing, licking, and petting the animal.[387]
The virus does not appear to be able to infect pigs, ducks, or chickens at all.[385] Mice, rats, and rabbits, if they can be infected at all, are unlikely to be involved in spreading the virus.[387]
Tigers and lions in zoos have become infected as a result of contact with infected humans.[387] As expected, monkeys and great ape species such as orangutans can also be infected with the COVID-19 virus.[387]
Minks, which are in the same family as ferrets, have been infected.[387] Minks may be asymptomatic, and can also spread the virus to humans.[387]Multiple countries have identified infected animals in mink farms.[388] Denmark, a major producer of mink pelts, ordered the slaughter of all minks over fears of viral mutations.[388] A vaccine for mink and other animals is being researched.[388]
International research on vaccines and medicines in COVID-19 is underway by government organisations, academic groups, and industry researchers.[389][390] The CDC has classified it to require a BSL3 grade laboratory.[391] There has been a great deal of COVID-19 research, involving accelerated research processes and publishing shortcuts to meet the global demand.[392]
Repurposed antiviral drugs make up most of the research into COVID-19 treatments.[407][408] Other candidates in trials include vasodilators, corticosteroids, immune therapies, lipoic acid, bevacizumab, and recombinantangiotensin-converting enzyme 2.[408]
In March 2020, the World Health Organization (WHO) initiated the Solidarity trial to assess the treatment effects of some promising drugs: an experimental drug called remdesivir; anti-malarial drugs chloroquine and hydroxychloroquine; two anti-HIV drugs, lopinavir/ritonavir; and interferon-beta.[409][410] More than 300 active clinical trials are underway as of April 2020.[145]
Research on the antimalarial drugs hydroxychloroquine and chloroquine showed that they were ineffective at best,[411][412] and that they may reduce the antiviral activity of remdesivir.[413] By May 2020, France, Italy, and Belgium had banned the use of hydroxychloroquine as a COVID-19 treatment.[414]
A cytokine storm can be a complication in the later stages of severe COVID-19. A cytokine storm is a potentially deadly immune reaction where a large amount of pro-inflammatory cytokines and chemokines are released too quickly; A cytokine storm can lead to ARDS and multiple organ failure.[427] Data collected from Jin Yin-tan Hospital in Wuhan, China indicates that patients who had more severe responses to COVID-19 had greater amounts of pro-inflammatory cytokines and chemokines in their system than patients who had milder responses; These high levels of pro-inflammatory cytokines and chemokines indicate presence of a cytokine storm.[428]
Tocilizumab has been included in treatment guidelines by China's National Health Commission after a small study was completed.[429][430] It is undergoing a Phase II non-randomised trial at the national level in Italy after showing positive results in people with severe disease.[431][432] Combined with a serum ferritin blood test to identify a cytokine storm (also called cytokine storm syndrome, not to be confused with cytokine release syndrome), it is meant to counter such developments, which are thought to be the cause of death in some affected people.[433] The interleukin-6 receptor antagonist was approved by the FDA to undergo a Phase III clinical trial assessing its effectiveness on COVID-19 based on retrospective case studies for the treatment of steroid-refractory cytokine release syndrome induced by a different cause, CAR T cell therapy, in 2017.[434] There is no randomised, controlled evidence that tocilizumab is an efficacious treatment for CRS. Prophylactic tocilizumab has been shown to increase serum IL-6 levels by saturating the IL-6R, driving IL-6 across the blood-brain barrier, and exacerbating neurotoxicity while having no effect on the incidence of CRS.[435]
Lenzilumab, an anti-GM-CSF monoclonal antibody, is protective in murine models for CAR T cell-induced CRS and neurotoxicity and is a viable therapeutic option due to the observed increase of pathogenic GM-CSF secreting T cells in hospitalised patients with COVID-19.[436]
Transferring purified and concentrated antibodies produced by the immune systems of those who have recovered from COVID-19 to people who need them is being investigated as a non-vaccine method of passive immunisation.[437][438] Viral neutralization is the anticipated mechanism of action by which passive antibody therapy can mediate defence against SARS-CoV-2. The spike protein of SARS-CoV-2 is the primary target for neutralizing antibodies.[439] As of 8 August 2020, eight neutralizing antibodies targeting the spike protein of SARS-CoV-2 have entered clinical studies.[440] It has been proposed that selection of broad-neutralizing antibodies against SARS-CoV-2 and SARS-CoV might be useful for treating not only COVID-19 but also future SARS-related CoV infections.[439] Other mechanisms, however, such as antibody-dependent cellular cytotoxicityor phagocytosis, may be possible.[437] Other forms of passive antibody therapy, for example, using manufactured monoclonal antibodies, are in development.[437]
The use of passive antibodies to treat people with active COVID-19 is also being studied. This involves the production of convalescent serum, which consists of the liquid portion of the blood from people who recovered from the infection and contains antibodies specific to this virus, which is then administered to active patients.[437] This strategy was tried for SARS with inconclusive results.[437] An updated Cochrane review in May 2021 found high certainty evidence that for the treatment of people with moderate to severe COVID-19 convalescent plasma did not reduce mortality or bring about symptom improvement[438] There continues to be uncertainty about the safety of convalescent plasma administration to people with COVID-19 and differing outcomes measured in different studies limits their use in determining efficacy.[438]
Multisystem inflammatory syndrome in children (MIS-C), or paediatric inflammatory multisystem syndrome (PIMS / PIMS-TS), or systemic inflammatory syndrome in COVID19 (SISCoV), is a rare systemic illness involving persistent fever and extreme inflammation following exposure to SARS-CoV-2, the virus responsible for COVID-19.[7] It can rapidly lead to medical emergencies such as insufficient blood flow around the body (a condition known as shock).[7] Failure of one or more organs can occur.[8] A warning sign is unexplained persistent fever with severe symptoms following exposure to COVID-19.[9]Prompt referral to paediatric specialists is essential, and families need to seek urgent medical assistance.[7] Most affected children will need intensive care.[7]
https://en.wikipedia.org/wiki/Multisystem_inflammatory_syndrome_in_children
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), has many variants; some are believed, or have been believed, to be of particular importance due to their potential for increased transmissibility,[1] increased virulence, or reduced effectiveness of vaccines against them.[2][3]
https://en.wikipedia.org/wiki/Variants_of_SARS-CoV-2
Middle East respiratory syndrome (MERS), also known as camel flu,[1] is a viral respiratory infection caused by Middle East respiratory syndrome–related coronavirus (MERS-CoV).[2]Symptoms may range from none, to mild, to severe.[7][2] Typical symptoms include fever, cough, diarrhea, and shortness of breath.[2] The disease is typically more severe in those with other health problems.[2][7]
MERS-CoV is a coronavirus believed to be originally from bats.[2] However, humans are typically infected from camels, either during direct contact or indirectly.[2] Spread between humans typically requires close contact with an infected person.[2] Its spread is uncommon outside of hospitals.[7]Thus, its risk to the global population is currently deemed to be fairly low.[7] Diagnosis is by rRT-PCR testing of blood and respiratory samples.[5]
https://en.wikipedia.org/wiki/Middle_East_respiratory_syndrome
Spillover infection, also known as pathogen spillover and spillover event, occurs when a reservoir population with a high pathogen prevalence comes into contact with a novel host population. The pathogen is transmitted from the reservoir population and may or may not be transmitted within the host population.[1]
https://en.wikipedia.org/wiki/Spillover_infection
A pleural effusion is accumulation of excessive fluid in the pleural space, the potential space that surrounds each lung. Under normal conditions, pleural fluid is secreted by the parietal pleuralcapillaries at a rate of 0.01 millilitre per kilogram weight per hour, and is cleared by lymphaticabsorption leaving behind only 5–15 millilitres of fluid, which helps to maintain a functional vacuumbetween the parietal and visceral pleurae. Excess fluid within the pleural space can impair inspiration by upsetting the functional vacuum and hydrostatically increasing the resistance against lung expansion, resulting in a fully or partially collapsed lung.
Various kinds of fluid can accumulate in the pleural space, such as serous fluid (hydrothorax), blood(hemothorax), pus (pyothorax, more commonly known as pleural empyema), chyle (chylothorax), or very rarely urine (urinothorax). When unspecified, the term "pleural effusion" normally refers to hydrothorax. A pleural effusion can also be compounded by a pneumothorax (accumulation of air in the pleural space), leading to a hydropneumothorax.
https://en.wikipedia.org/wiki/Pleural_effusion
The human immunodeficiency viruses (HIV) are two species of Lentivirus (a subgroup of retrovirus) that infect humans. Over time, they cause acquired immunodeficiency syndrome (AIDS),[1][2] a condition in which progressive failure of the immune system allows life-threatening opportunistic infections and cancers to thrive.[3]Without treatment, average survival time after infection with HIV is estimated to be 9 to 11 years, depending on the HIV subtype.[4] In most cases, HIV is a sexually transmitted infection and occurs by contact with or transfer of blood, pre-ejaculate, semen, and vaginal fluids. Research has shown (for both same-sex and opposite-sex couples) that HIV is untransmittable through condomless sexual intercourse if the HIV-positive partner has a consistently undetectable viral load.[5][6] Non-sexual transmission can occur from an infected mother to her infant during pregnancy, during childbirth by exposure to her blood or vaginal fluid, and through breast milk.[7][8][9][10] Within these bodily fluids, HIV is present as both free virus particles and virus within infected immune cells.
HIV infects vital cells in the human immune system, such as helper T cells (specifically CD4+ T cells), macrophages, and dendritic cells.[11] HIV infection leads to low levels of CD4+ T cells through a number of mechanisms, including pyroptosis of abortively infected T cells,[12] apoptosis of uninfected bystander cells,[13]direct viral killing of infected cells, and killing of infected CD4+ T cells by CD8+ cytotoxic lymphocytes that recognize infected cells.[14] When CD4+ T cell numbers decline below a critical level, cell-mediated immunity is lost, and the body becomes progressively more susceptible to opportunistic infections, leading to the development of AIDS.
https://en.wikipedia.org/wiki/HIV
08-11-2021-1652 - Human T-cell lymphotropic virus type 1 or human T-lymphotropic virus (HTLV-I),
Human T-cell lymphotropic virus type 1 or human T-lymphotropic virus (HTLV-I), also called the adult T-cell lymphoma virus type 1, is a retrovirus of the human T-lymphotropic virus (HTLV) family that has been implicated in several kinds of diseases including very aggressive adult T-cell lymphoma (ATL), HTLV-I-associated myelopathy, uveitis, Strongyloides stercoralis hyper-infection and some other diseases. It is thought that about 1–5% of infected persons develop cancer as a result of the infection with HTLV-I over their lifetimes.[1]
https://en.wikipedia.org/wiki/Human_T-lymphotropic_virus_1
08-11-2021-1730 - Fungal osteomyelitis and septic arthritis (Bariteau et al., 2014)
J Am Acad Orthop Surg
. 2014 Jun;22(6):390-401. doi: 10.5435/JAAOS-22-06-390.
Fungal osteomyelitis and septic arthritis
Jason T Bariteau 1, Gregory R Waryasz 1, Matthew McDonnell 1, Staci A Fischer 1, Roman A Hayda 1, Christopher T Born 1
Affiliations expand
PMID: 24860135
DOI: 10.5435/JAAOS-22-06-390
Management of fungal osteomyelitis and fungal septic arthritis is challenging, especially in the setting of immunodeficiency and conditions that require immunosuppression. Because fungal osteomyelitis and fungal septic arthritis are rare conditions, study of their pathophysiology and treatment has been limited. In the literature, evidence-based treatment is lacking and, historically, outcomes have been poor. The most common offending organisms are Candida and Aspergillus, which are widely distributed in humans and soil. However, some fungal pathogens, such as Histoplasma, Blastomyces, Coccidioides, Cryptococcus, and Sporothrix, have more focal areas of endemicity. Fungal bone and joint infections result from direct inoculation, contiguous infection spread, or hematogenous seeding of organisms. These infections may be difficult to diagnose and eradicate, especially in the setting of total joint arthroplasty. Although there is no clear consensus on treatment, guidelines are available for management of many of these pathogens.
Copyright 2014 by the American Academy of Orthopaedic Surgeons.
https://pubmed.ncbi.nlm.nih.gov/24860135/
08-11-2021-1744 - rna world, virusoids, prion, plasmid, non-cellular life/acellular life, viroids, circular rna, etc. (drafting)
Circular RNA (or circRNA) is a type of single-stranded RNA which, unlike linear RNA, forms a covalently closed continuous loop. In circular RNA, the 3' and 5' ends normally present in an RNA molecule have been joined together. This feature confers numerous properties to circular RNA, many of which have only recently been identified.
Many types of circular RNA arise from otherwise protein-coding genes. Some circular RNA has been shown to code for proteins.[1][2] Some types of circular RNA have also recently shown potential as gene regulators. The biological function of most circular RNA is unclear.
Because circular RNA does not have 5' or 3' ends, it is resistant to exonuclease-mediated degradation and is presumably more stable than most linear RNA in cells.[3] Circular RNA has been linked to some diseases such as cancer.[4]
https://en.wikipedia.org/wiki/Circular_RNA
Viroids are small infectious pathogens.[1] They are composed solely of a short strand of circular, single-stranded RNA. Unlike viruses, they have no protein coating. All known viroids are inhabitants of angiosperms,[2]and most cause diseases, whose respective economic importance to humans varies widely.
https://en.wikipedia.org/wiki/Viroid
Non-cellular life, or acellular life is life that exists without a cellular structure for at least part of its life cycle.[1]Historically, most (descriptive) definitions of life postulated that a living organism must be composed of one or more cells,[2] but this is no longer considered necessary, and modern criteria allow for forms of life based on other structural arrangements.[3][4][5]
The primary candidates for non-cellular life are viruses. Some biologists consider viruses to be living organisms, but others do not. Their primary objection is that no known viruses are capable of autonomous reproduction: they must rely on cells to copy them.[1][6][7][8][9]
Engineers sometimes use the term "artificial life" to refer to software and robots inspired by biological processes, but these do not satisfy any biological definition of life.
https://en.wikipedia.org/wiki/Non-cellular_life
A plasmid is a small, extrachromosomal DNA molecule within a cell that is physically separated from chromosomal DNA and can replicate independently. They are most commonly found as small circular, double-stranded DNA molecules in bacteria; however, plasmids are sometimes present in archaea and eukaryotic organisms. In nature, plasmids often carry genes that benefit the survival of the organism and confer selective advantage such as antibiotic resistance. While chromosomes are large and contain all the essential genetic information for living under normal conditions, plasmids are usually very small and contain only additional genes that may be useful in certain situations or conditions. Artificial plasmids are widely used as vectors in molecular cloning, serving to drive the replication of recombinant DNA sequences within host organisms. In the laboratory, plasmids may be introduced into a cell via transformation. Synthetic plasmids are available for procurement over the internet.[1][2][3]
https://en.wikipedia.org/wiki/Plasmid
Prions are misfolded proteins with the ability to transmit their misfolded shape onto normal variants of the same protein. They characterize several fatal and transmissible neurodegenerative diseasesin humans and many other animals.[3] It is not known what causes the normal protein to misfold, but the abnormal three-dimensional structure is suspected of conferring infectious properties, collapsing nearby protein molecules into the same shape. The word prion derives from "proteinaceous infectious particle".[4][5][6] The hypothesized role of a protein as an infectious agent stands in contrast to all other known infectious agents such as viroids, viruses, bacteria, fungi, and parasites, all of which contain nucleic acids (DNA, RNA, or both).
https://en.wikipedia.org/wiki/Prion
The RNA world is a hypothetical stage in the evolutionary history of life on Earth, in which self-replicating RNA molecules proliferated before the evolution of DNA and proteins. The term also refers to the hypothesis that posits the existence of this stage.
https://en.wikipedia.org/wiki/RNA_world
Circular satellite RNAs
Virus classification
Informal group: Satellite nucleic acids
Informal group: Circular satellite RNAs
Virusoids are circular single-stranded RNA(s) dependent on viruses for replication and encapsidation.[1] The genome of virusoids consist of several hundred (200–400) nucleotides and does not code for any proteins.
Virusoids are essentially viroids that have been encapsulated by a helper virus coat protein. They are thus similar to viroids in their means of replication (rolling circle replication) and in their lack of genes, but they differ in that viroids do not possess a protein coat. Both virusoids and viroids encode a hammerhead ribozyme.
Virusoids, while being studied in virology, are subviral particles rather than viruses. Since they depend on helper viruses, they are classified as satellites. Virusoids are listed in virological taxonomy as Satellites/Satellite nucleic acids/Subgroup 3: Circular satellite RNA(s).[2]
https://en.wikipedia.org/wiki/Virusoid
Plasma cells, also called plasma B cells, are white blood cells that originate in the Lymphoid organs by B Lymphocytes[1][2] and secrete large quantities of proteins called antibodies in response to being presented specific substances called antigens. These antibodies are transported from the plasma cells by the blood plasma and the lymphatic system to the site of the target antigen (foreign substance), where they initiate its neutralization or destruction. B cells differentiate into plasma cells that produce antibody molecules closely modeled after the receptors of the precursor B cell.[3]
https://en.wikipedia.org/wiki/Plasma_cell
'While uncommon in solid tumors, chromosomal translocations are a common cause of these diseases.'
https://en.wikipedia.org/wiki/Tumors_of_the_hematopoietic_and_lymphoid_tissues
Mycosis fungoides, also known as Alibert-Bazin syndrome or granuloma fungoides,[1] is the most common form of cutaneous T-cell lymphoma. It generally affects the skin, but may progress internally over time. Symptoms include rash, tumors, skin lesions, and itchy skin.
While the cause remains unclear, most cases are not hereditary. Most cases are in people over 20 years of age, and it is more common in men than women. Treatment options include sunlight exposure, ultraviolet light, topical corticosteroids, chemotherapy, and radiotherapy.
sp - fungus, fungal derivatives et priming by alkylation agents rads radk immunosups overvac genotox-viral gene transplant-activation of tumor gene-cell signal dysfunction/decay matter/fungai susecept/etc..
https://en.wikipedia.org/wiki/Mycosis_fungoides
a lentivirus (species Simian immunodeficiency virus) that causes a disease in monkeys similar to AIDS and that is closely related to HIV-2
https://www.merriam-webster.com/dictionary/SIV
Structure and genome[edit]
The SIV virion is a spherical to pleomorphic glycoprotein envelope 110-120 nm enclosing a 110x50nm truncated cone or wedge-shaped (occasionally rod) capsid containing a dimeric pair of positive-sense single-stranded RNAgenome.
Related viruses also cause disease in other mammals: sheep, goats, cats, cattle, horses
SV40 is another virus that came from simians and into human populations in the mid twentieth century.
https://en.wikipedia.org/wiki/Simian_immunodeficiency_virus
Simian-T-lymphotropic viruses, also Simian T-cell leukemia viruses (STLVs), are retroviruses closely related to the human sexually and breastfeeding transmissible viruses HTLV. They have subtypes 1 through 4 as compared to HTLV 1 through 4, and each subtype has its own serovars.[1] Together they comprise PTLVs (primate T-lymphotropic viruses)[1] A study has shown that STLV-1 Tax and SBZ proteins have similar functions to their counterparts of HTLV-1. STLV-1 is oncogenic in Japanese macaques.[2]
In particular, the HTLV-I/STLV-I history might suggest a simian migration from Asia to Africa not much earlier than 19,500–60,000 years ago.[1]
https://en.wikipedia.org/wiki/Simian-T-lymphotropic_virus
08-10-2021-2353 - Hemato, Immuno, bone spleen liver panc t cell macrophage tuberculosis resp lung met cat et etc. draft
The term leukemoid reaction describes an increased white blood cell count (> 50,000 cells/μL), which is a physiological response to stress or infection (as opposed to a primary blood malignancy, such as leukemia). It often describes the presence of immature cells such as myeloblasts or red blood cells with nuclei in the peripheral blood.
It may be lymphoid or myeloid.[1]
https://en.wikipedia.org/wiki/Leukemoid_reaction
Diffuse infiltrative lymphocytosis syndrome occurs in HIV positive patients with low CD4 counts.[1][2]
It is similar to Sjögren's syndrome,[3] with painless parotid and submandibular swelling, and sicca symptoms.
The syndrome typically improves with HAART.[citation needed]
https://en.wikipedia.org/wiki/Diffuse_infiltrative_lymphocytosis_syndrome
https://en.wikipedia.org/wiki/Subtypes_of_HIV#HIV-2
https://en.wikipedia.org/wiki/Simian_immunodeficiency_virus
DNA dC->dU-editing enzyme APOBEC-3F is a protein that in humans is encoded by the APOBEC3F gene.[3][4][5]
This gene is a member of the cytidine deaminase gene family. It is one of seven related genes or pseudogenes found in a cluster, thought to result from gene duplication, on chromosome 22. Members of the cluster encode proteins that are structurally and functionally related to the C to U RNA-editing cytidine deaminase APOBEC1. It is thought that the proteins may be RNA editing enzymes and have roles in growth or cell cycle control. Alternatively spliced transcriptvariants encoding different isoforms have been identified.[5]
https://en.wikipedia.org/wiki/APOBEC3F
Tripartite motif-containing protein 5 also known as RING finger protein 88 is a protein that in humans is encoded by the TRIM5 gene.[4] The alpha isoform of this protein, TRIM5α, is a retrovirus restriction factor, which mediates species-specific, early block to retrovirus infection.
TRIM5α is composed of 493 amino acids that is found in the cells of most primates. TRIM5α is an intrinsic immune factor important in the innate immune defense against retroviruses, along with the APOBEC family of proteins,[5][6] tetherin and TRIM22.
https://en.wikipedia.org/wiki/TRIM5alpha
The Simian foamy virus (SFV) is a species of the genus Spumavirus, which belongs to the family of Retroviridae. It has been identified in a wide variety of primates, including pro-simians, New World and Old World monkeys as well as apes, and each species has been shown to harbor a unique (species-specific) strain of SFV, including African green monkeys, baboons, macaques and chimpanzees.[1] As it is related to the more well-known retrovirus human immunodeficiency virus (HIV), its discovery in primates has led to some speculation that HIV may have been spread to the human species in Africa through contact with blood from apes, monkeys, and other primates, most likely through bushmeat hunting practices.
https://en.wikipedia.org/wiki/Simian_foamy_virus
Woolly monkey sarcoma virus (WMSV), with synonym Simian sarcoma virus (often abbreviated by SSV, but this may also stand for some species called 'Sulfolobus spindle-shaped virus', that belong to different genera in family Fuselloviridae) is a species of gammaretrovirus that infects primates. First isolation was from a fibrosarcoma in a woolly monkey (genus Lagothrix). For its reproduction the virus needs a helper or associated virus which is called Simian sarcoma associated virus (SSAV).[1][2][3]
https://en.wikipedia.org/wiki/Woolly_monkey_sarcoma_virus
Lymphomatoid granulomatosis (LYG or LG) is a very rare lymphoproliferative disorder first characterized in 1972.[1]Lymphomatoid means lymphoma-like and granulomatosis denotes the microscopic characteristic of the presence of granulomaswith polymorphic lymphoid infiltrates and focal necrosis within it.
LG most commonly affects middle aged people,[2] but has occasionally been observed in young people.[3] Males are found to be affected twice as often as females.[4]
https://en.wikipedia.org/wiki/Lymphomatoid_granulomatosis
Post-transplant lymphoproliferative disorder (PTLD) is the name given to a B-cell proliferation due to therapeutic immunosuppression after organ transplantation. These patients may develop infectious mononucleosis-like lesions or polyclonal polymorphic B-cell hyperplasia. Some of these B-cells may undergo mutations which will render them malignant, giving rise to a lymphoma.[citation needed]
In some patients, the malignant cell clone can become the dominant proliferating cell type, leading to frank lymphoma, a group of B cell lymphomas occurring in immunosuppressed patients following organ transplant.
https://en.wikipedia.org/wiki/Post-transplant_lymphoproliferative_disorder
Splenic marginal zone lymphoma (SMZL) is a type of cancer (specifically a lymphoma) made up of B-cells that replace the normal architecture of the white pulp of the spleen. The neoplastic cells are both small lymphocytes and larger, transformed lymphoblasts, and they invade the mantle zone of splenic follicles and erode the marginal zone, ultimately invading the red pulp of the spleen. Frequently, the bone marrow and splenic hilar lymph nodes are involved along with the peripheral blood. The neoplastic cells circulating in the peripheral blood are termed villous lymphocytes due to their characteristic appearance.[1]
https://en.wikipedia.org/wiki/Splenic_marginal_zone_lymphoma#cite_note-who1-1
Plasmacytoma is a plasma cell dyscrasia in which a plasma cell tumour grows within soft tissue or within the axial skeleton.
The International Myeloma Working Group lists three types: solitary plasmacytoma of bone (SPB); extramedullary plasmacytoma (EP), and multiple plasmacytomas that are either primary or recurrent.[1] The most common of these is SPB, accounting for 3–5% of all plasma cell malignancies.[2] SPBs occur as lytic lesions within the axial skeleton and extramedullary plasmacytomas most often occur in the upper respiratory tract (85%), but can occur in any soft tissue. Approximately half of all cases produce paraproteinemia. SPBs and extramedullary plasmacytomas are mostly treated with radiotherapy, but surgery is used in some cases of extramedullary plasmacytoma. The skeletal forms frequently progress to multiple myeloma over the course of 2–4 years.[3]
Due to their cellular similarity, plasmacytomas have to be differentiated from multiple myeloma. For SPB and extramedullary plasmacytoma the distinction is the presence of only one lesion (either in bone or soft tissue), normal bone marrow (<5% plasma cells), normal skeletal survey, absent or low paraprotein and no end organ damage.[1]
https://en.wikipedia.org/wiki/Plasmacytoma
This article is about the disease found in humans. For the disease that affects mustelids, see Aleutian disease.
Plasmacytosis
Plasmacytosis is a condition in which there is an unusually large proportion of plasma cells in tissues, exudates, or blood.[1]:743Plasmacytosis may be divided into two types—cutaneous and systemic—both of which have identical skin findings.[1]:743
Patients with plasmacytosis have been predominantly found to have lung infections (pneumonia, tuberculosis, abscess) whereas multiple myeloma is rarely found.[2]
https://en.wikipedia.org/wiki/Plasmacytosis
Terminal deoxynucleotidyl transferase (TdT), also known as DNA nucleotidylexotransferase (DNTT) or terminal transferase, is a specialized DNA polymerase expressed in immature, pre-B, pre-T lymphoid cells, and acute lymphoblastic leukemia/lymphoma cells. TdT adds N-nucleotides to the V, D, and J exons of the TCR and BCR genes during antibody gene recombination, enabling the phenomenon of junctional diversity. In humans, terminal transferase is encoded by the DNTT gene.[5][6] As a member of the X family of DNA polymerase enzymes, it works in conjunction with polymerase λ and polymerase μ, both of which belong to the same X family of polymerase enzymes. The diversity introduced by TdT has played an important role in the evolution of the vertebrate immune system, significantly increasing the variety of antigen receptors that a cell is equipped with to fight pathogens. Studies using TdT knockout mice have found drastic reductions (10-fold) in T-cell receptor (TCR) diversity compared with that of normal, or wild-type, systems. The greater diversity of TCRs that an organism is equipped with leads to greater resistance to infection.[7][8] Although TdT was one of the first DNA polymerases identified in mammals in 1960,[9] it remains one of the least understood of all DNA polymerases.[7] In 2016–18, TdT was discovered to demonstrate in trans template dependant behaviour in addition to its more broadly known template independent behaviour[10][11]
TdT is absent in fetal liver HSCs, significantly impairing junctional diversity in B-cells during the fetal period.[12]
https://en.wikipedia.org/wiki/Terminal_deoxynucleotidyl_transferase
T-lymphoblastic leukemia/lymphoma (WHO 2008),[1]:219 previously labeled precursor T-lymphoblastic leukemia/lymphoma(WHO 2001)[1]:219 is a form of lymphoid leukemia[2][3] and lymphoma[4] in which too many T-cell lymphoblasts (immature white blood cells) are found in the blood, bone marrow, and tissues, particularly mediastinal lymph nodes.[1]:635 Labeling as leukemia or lymphoma depends on which feature is more pronounced in a given situation, but has no biological or treatment implication.[1]:635
It is uncommon in adults, but represents 15% of childhood acute lymphoblastic leukemia and 90% of lymphoblastic lymphoma.[1]:635
The 2008 terminology dropped "precursor" to avoid linguistic redundancy because the lymphoblast is an immature precursor cell by definition.[1]:219
https://en.wikipedia.org/wiki/T-lymphoblastic_leukemia/lymphoma
T-cell-prolymphocytic leukemia (T-PLL) is a mature T-cell leukemia with aggressive behavior and predilection for blood, bone marrow, lymph nodes, liver, spleen, and skin involvement.[1] T-PLL is a very rare leukemia, primarily affecting adults over the age of 30. It represents 2% of all small lymphocytic leukemias in adults.[2] Other names include T-cell chronic lymphocytic leukemia, "knobby" type of T-cell leukemia, and T-prolymphocytic leukemia/T-cell lymphocytic leukemia.[1]
https://en.wikipedia.org/wiki/T-cell_prolymphocytic_leukemia
Anaplastic large cell lymphoma (ALCL) refers to a group of non-Hodgkin lymphomas in which aberrant T cells proliferate uncontrollably. Considered as a single entity, ALCL is the most common type of peripheral lymphoma[1] and represents ~10% of all peripheral lymphomas in children.[2] The incidence of ALCL is estimated to be 0.25 cases per 100,000 people in the United States of America.[3] There are four distinct types of anaplastic large cell lymphomas that on microscopic examination share certain key histopathological features and tumor marker proteins. However, the four types have very different clinical presentations, gene abnormalities, prognoses, and/or treatments.[1]
https://en.wikipedia.org/wiki/Anaplastic_large-cell_lymphoma
Lymphomatoid papulosis (LyP) is a rare skin disorder. The overall prevalence rate of lymphomatoid papulosis is estimated at 1.2 to 1.9 cases per 1,000,000 population. [This is a widespread misinterpretation of a 1992 study saying "the period prevalence rate of lymphomatoid papulosis was estimated to be 1.9 per 1,000,000 population for Massachusetts and 1.2 per 1,000,000 population for Pennsylvania". The authors of that study said clearly "Our estimate of 1.2-1.9 cases per 1,000,000 population should be considered a minimum estimate of the prevalence rate". That estimate was based on the 78 patients involved in the study, not the LvP population. The study recruited 11 patients from Massachusetts and 15 from Pennsylvania [1]]. This rare condition has only been studied in depth since 1968.[2]
https://en.wikipedia.org/wiki/Lymphomatoid_papulosis
Pagetoid reticulosis (also known as "acral mycoses fungoides",[1] "localized epidermotropic reticulosis",[1] "mycosis fungoides palmaris et plantaris",[1] "unilesional mycosis fungoides",[2] and "Woringer–Kolopp disease"[1]) is a cutaneous condition, an uncommon lymphoproliferative disorder, sometimes considered a form of mycosis fungoides.[1]:734
https://en.wikipedia.org/wiki/Pagetoid_reticulosis
Mycosis fungoides, also known as Alibert-Bazin syndrome or granuloma fungoides,[1] is the most common form of cutaneous T-cell lymphoma. It generally affects the skin, but may progress internally over time. Symptoms include rash, tumors, skin lesions, and itchy skin.
While the cause remains unclear, most cases are not hereditary. Most cases are in people over 20 years of age, and it is more common in men than women. Treatment options include sunlight exposure, ultraviolet light, topical corticosteroids, chemotherapy, and radiotherapy.
sp - fungus, fungal derivatives et priming by alkylation agents rads radk immunosups overvac genotox-viral gene transplant-activation of tumor gene-cell signal dysfunction/decay matter/fungai susecept/etc..
https://en.wikipedia.org/wiki/Mycosis_fungoides
Lymphoid leukemias are a group of leukemias affecting circulating lymphocytes, a type of white blood cells. The lymphocytic leukemias are closely related to lymphomas of the lymphocytes, to the point that some of them are unitary disease entities that can be called by either name (for example, adult T-cell leukemia/lymphoma). Such diseases are all lymphoproliferative disorders. Most lymphoid leukemias involve a particular subtype of lymphocytes, the B cells.
https://en.wikipedia.org/wiki/Lymphoid_leukemia
Tumors of the hematopoietic and lymphoid tissues

Micrograph of a plasmacytoma, a hematological malignancy
Tumors of the hematopoietic and lymphoid tissues (American English) or tumours of the haematopoietic and lymphoid malignancies (British English) are tumors that affect the blood, bone marrow, lymph, and lymphatic system.[1][2]Because these tissues are all intimately connected through both the circulatory system and the immune system, a disease affecting one will often affect the others as well, making myeloproliferation and lymphoproliferation (and thus the leukemiasand the lymphomas) closely related and often overlapping problems.
While uncommon in solid tumors, chromosomal translocations are a common cause of these diseases. This commonly leads to a different approach in diagnosis and treatment of haematological malignancies.
Haematological malignancies are malignant neoplasms ("cancer"), and they are generally treated by specialists in hematology and/or oncology. In some centers "haematology/oncology" is a single subspecialty of internal medicine while in others they are considered separate divisions (there are also surgical and radiation oncologists). Not all haematological disorders are malignant ("cancerous"); these other blood conditions may also be managed by a hematologist.
Hematological malignancies may derive from either of the two major blood cell lineages: myeloid and lymphoid cell lines. The myeloid cell line normally produces granulocytes, erythrocytes, thrombocytes, macrophages and mast cells; the lymphoid cell line produces B, T, NK and plasma cells. Lymphomas, lymphocytic leukemias, and myeloma are from the lymphoid line, while acute and chronic myelogenous leukemia, myelodysplastic syndromes and myeloproliferative diseases are myeloid in origin.
A subgroup of them are more severe and are known as haematological malignancies (British English)/hematological malignancies (American English) or blood cancer. They may also be referred to as liquid tumors.[3][4]
https://en.wikipedia.org/wiki/Tumors_of_the_hematopoietic_and_lymphoid_tissues
Lymphoproliferative disorders
Specialty Hematology and oncology
Lymphoproliferative disorders (LPDs) refer to a specific class of diagnoses, comprising a group of several conditions, in which lymphocytes are produced in excessive quantities. These disorders primarily present in patients who have a compromised immune system. Due to this factor, there are instances of these conditions being equated with "immunoproliferative disorders"; although, in terms of nomenclature, lymphoproliferative disorders are a subclass of immunoproliferative disorders—along with hypergammaglobulinemia and paraproteinemias.
https://en.wikipedia.org/wiki/Lymphoproliferative_disorders
mune lymphoproliferative syndromeOther namesCanale-Smith syndrome,[1]SpecialtyImmunology
Autoimmune lymphoproliferative syndrome (ALPS), is a form of lymphoproliferative disorder (LPDs). It affects lymphocyteapoptosis.[2]
It is a rare genetic disorder of abnormal lymphocyte survival caused by defective Fas mediated apoptosis.[3] Normally, after infectious insult, the immune system down-regulates by increasing Fas expression on activated B and T lymphocytes and Fas-ligand on activated T lymphocytes. Fas and Fas-ligand interact to trigger the caspase cascade, leading to cell apoptosis. Patients with ALPS have a defect in this apoptotic pathway, leading to chronic non-malignant lymphoproliferation, autoimmune disease, and secondary cancers.[4]
https://en.wikipedia.org/wiki/Autoimmune_lymphoproliferative_syndrome
X-linked lymphoproliferative disease (also known as Duncan's disease[1]:86 or Purtilo syndrome[2]) is a lymphoproliferative disorder.[3]
https://en.wikipedia.org/wiki/X-linked_lymphoproliferative_disease
Biphenotypic acute leukaemia (BAL) is an uncommon type of leukemia which arises in multipotent progenitor cells which have the ability to differentiate into both myeloid and lymphoid lineages.[1][2][3] It is a subtype of "leukemia of ambiguous lineage".[4]
The direct reasons leading to BAL are still not clear. BAL can be de novo or secondary to previous cytotoxic therapy. Many factors, such viruses, hereditary factors, and radiation, might have a relationship with BAL.
BAL is hard to treat. Usually the chemotherapy is chosen according to the morphology of the blast (ALL or AML). A blood-forming stem-cell transplantation is highly recommended. About 5% of acute leukaemia cases are BAL. BAL can occur in all ages of people but occurs more in adults than in children.[5]
https://en.wikipedia.org/wiki/Biphenotypic_acute_leukaemia
Large granular lymphocytic (LGL) leukemia is a chronic lymphoproliferative disorder that exhibits an unexplained, chronic (> 6 months) elevation in large granular lymphocytes (LGLs) in the peripheral blood.[1]
It is divided in two main categories: T-cell LGL leukemia (T-LGLL) and natural-killer (NK)-cell LGL leukemia (NK-LGLL). As the name suggests, T-cell large granular lymphocyte leukemia is characterized by involvement of cytotoxic-T cells).[2]
In a study based in the US, the average age of diagnosis was 66.5 years[3] whereas in a French study the median age at diagnosis was 59 years (with an age range of 12-87 years old).[4] In the French study, only 26% of patients were younger than 50 years which suggests that this disorder is associated with older age at diagnosis.[4] Due to lack of presenting symptoms, the disorder is likely to be underdiagnosed in the general population.[5]
https://en.wikipedia.org/wiki/Large_granular_lymphocytic_leukemia
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare hematologic malignancy. It was initially regarded as a form of lymphocyte-derived cutaneous lymphoma and alternatively named CD4+CD56+ hematodermic tumor, blastic NK cell lymphoma,[1] and agranular CD4+ NK cell leukemia.[2] Later, however, the disease was determined to be a malignancy of plasmacytoid dendritic cells rather than lymphocytes and therefore termed blastic plasmacytoid dendritic cell neoplasm. In 2016, the World Health Organization designated BPDCN to be in its own separate category within the myeloid class of neoplasms.[3] It is estimated that BPDCN constitutes 0.44% of all hematological malignancies.[4]
Blastic plasmacytoid dendritic cell neoplasm is an aggressive malignancy with features of cutaneous lymphoma (e.g. malignant plasmacytoid dendritic cell infiltrations into the skin to form single or multiple lesions) and/or leukemia (i.e. malignant plasmacytoid dendritic cells in blood and bone marrow).[2] While commonly presenting with these clinical features, BPDCN, particularly in its more advanced stages, may also involve malignant plasmacytoid dendritic cell infiltrations in and thereby injury to the liver, spleen, lymph nodes, central nervous system, or other tissues. The neoplasm occurs in individuals of all ages but predominates in the elderly; in children, it afflicts males and females equally but in adults is far more common (~75% of cases) in males.[5]
Blastic plasmacytoid dendritic cell neoplasm typically responds to chemotherapy regimens used to treat hematological malignancies. All too often, however, the disease rapidly recurs and does so in a more drug-resistant form.[5] Furthermore, the disease may occur in association with the myelodysplastic syndrome or transform to acute myeloid leukemia.[4] Consequently, BPDCN has a very low 5 year survival rate.[5]Current translational research studies on treating BPDCN have therefore focused on non-chemotherapeutic regimens that target the molecular pathways which may promote the disease.[6]
https://en.wikipedia.org/wiki/Blastic_plasmacytoid_dendritic_cell_neoplasm
Human T-cell lymphotropic virus type 1 or human T-lymphotropic virus (HTLV-I), also called the adult T-cell lymphoma virus type 1, is a retrovirus of the human T-lymphotropic virus (HTLV) family that has been implicated in several kinds of diseases including very aggressive adult T-cell lymphoma (ATL), HTLV-I-associated myelopathy, uveitis, Strongyloides stercoralis hyper-infection and some other diseases. It is thought that about 1–5% of infected persons develop cancer as a result of the infection with HTLV-I over their lifetimes.[1]
Adult T-cell lymphoma (ATL) was discovered in 1977 in Japan. The symptoms of ATL were different from other lymphomas known at the time. It was suggested that ATL is caused by the infection of a retrovirus called ATLV.[2] Strikingly, ATLV had the transforming activity in vitro.[3] These studies established that the retrovirus infection is the cause of ATL. The retrovirus is now generally called HTLV-I because later studies proved that ATLV is the same as the firstly identified human retrovirus called HTLV discovered by Bernard Poiesz and Francis Ruscetti and their co-workers in the laboratory of Robert C. Gallo at the National Cancer Institute.[4] Infection with HTLV-I, like infection with other retroviruses, probably occurs for life. A patient infected with HTLV can be diagnosed when antibodies against HTLV-1 are detected in the serum.[1]
https://en.wikipedia.org/wiki/Human_T-lymphotropic_virus_1
Enteropathy-associated T-cell lymphoma (EATL), previously termed enteropathy-associated T-cell lymphoma, type I and at one time termed enteropathy-type T-cell lymphoma (ETTL), is a complication of coeliac disease in which a malignant T-cell lymphoma develops in areas of the small intestine afflicted by the disease's intense inflammation.[1] While a relatively rare disease, it is the most common type of primary gastrointestinal T-cell lymphoma.[2]
https://en.wikipedia.org/wiki/Enteropathy-associated_T-cell_lymphoma
Angioimmunoblastic T-cell lymphoma (AITL, sometimes misspelled AILT, formerly known as "angioimmunoblastic lymphadenopathy with dysproteinemia"[2]:747) is a mature T-cell lymphoma of blood or lymph vessel immunoblastscharacterized by a polymorphous lymph node infiltrate showing a marked increase in follicular dendritic cells (FDCs) and high endothelial venules (HEVs) and systemic involvement.[1]
https://en.wikipedia.org/wiki/Angioimmunoblastic_T-cell_lymphoma
Hepatosplenic T-cell lymphoma is a rare form of lymphoma that is generally incurable, except in the case of an allogeneic stem cell transplant.[2][3] It is a systemic neoplasm comprising medium-sized cytotoxic T-cells that show significant sinusoidal infiltration in the liver, spleen, and bone marrow.[1]
https://en.wikipedia.org/wiki/Hepatosplenic_T-cell_lymphoma
CD30+ cutaneous T-cell lymphoma, also known as primary cutaneous anaplastic large cell lymphoma, is a cutaneous (skin) condition characterized by solitary or localized skin lesions that have a tendency to ulcerate.[1]:738
https://en.wikipedia.org/wiki/CD30%2B_cutaneous_T-cell_lymphoma
Secondary cutaneous CD30+ large-cell lymphoma is a cutaneous condition that may arise in cases of mycosis fungoides, and in patients with lymphomatoid papulosis.[1]:738
https://en.wikipedia.org/wiki/Secondary_cutaneous_CD30%2B_large-cell_lymphoma
Non-mycosis fungoides CD30− cutaneous large T-cell lymphoma is a cutaneous condition that usually presents as solitary or generalized plaques, nodules, or tumors of short duration.[1]:738
https://en.wikipedia.org/wiki/Non-mycosis_fungoides_CD30%E2%88%92_cutaneous_large_T-cell_lymphoma
CD30, also known as TNFRSF8, is a cell membrane protein of the tumor necrosis factor receptor family and tumor marker.
https://en.wikipedia.org/wiki/CD30
Adult T-cell leukemia/lymphoma (ATL or ATLL) is a rare cancer of the immune system's T-cells[1][2][3] caused by human T cell leukemia/lymphotropic virus type 1 (HTLV-1).[4] All ATL cells contain integrated HTLV-1 provirus further supporting that causal role of the virus in the cause of the neoplasm.[4] A small amount of HTLV-1 individuals progress to develop ATL with a long latency period between infection and ATL development. ATL is categorized into 4 subtypes: acute, smoldering, lymphoma-type, chronic. Acute and Lymphoma-type are known to particularity be aggressive with poorer prognosis. [5]
Globally, the retrovirus HTLV-1 is estimated to infect 20 million people with the incidence of ATL approximately 0.05 per 100,000 with endemic regions such as regions of Japan, as high as 27 per 100,000.[6] However, cases have increased in non-endemic regions with highest incidence of HTLV-1 in southern/northern islands of Japan, Caribbean, Central and South America, intertropical Africa, Romania, northern Iran. ATL normally occurs around the age of 62 years but median age at diagnosis does depend on prevalence of the HTLV-1 infection in the geographic location.[7]
Current treatment regiments for ATL are based on clinical subtype and response to initial therapy. Some therapy modalities for treatment may not available in all countries therefore strategies differ across the world. All patients are referred to clinical trials if available. Beyond clinical trials, treatments are centered on multiagent chemotherapy, zidovudine plus interferon a (AZT/IFN), and allogenic hematopoietic stem cell transplantation (alloHSCT).[6]
https://en.wikipedia.org/wiki/Adult_T-cell_leukemia/lymphoma
Granulomatous slack skin (GSS) is a rare cutaneous condition, a variant of lymphoma that typically presents in middle-aged adults.[2]:735
It is a form of cutaneous T-cell lymphoma[3] and a variant of mycosis fungoides.[4]
https://en.wikipedia.org/wiki/Granulomatous_slack_skin
In the mycotic stage, infiltrative plaques appear and biopsy shows a polymorphous inflammatory infiltrate in the dermis that contains small numbers of frankly atypical lymphoid cells. These cells may line up individually along the epidermal basal layer. The latter finding if unaccompanied by spongiosis is highly suggestive of mycosis fungoides. In the tumorous stage a dense infiltrate of medium-sized lymphocytes with cerebriform nuclei expands the dermis.
https://en.wikipedia.org/wiki/Mycosis_fungoides
Simian-T-lymphotropic viruses, also Simian T-cell leukemia viruses (STLVs), are retroviruses closely related to the human sexually and breastfeeding transmissible viruses HTLV. They have subtypes 1 through 4 as compared to HTLV 1 through 4, and each subtype has its own serovars.[1] Together they comprise PTLVs (primate T-lymphotropic viruses)[1] A study has shown that STLV-1 Tax and SBZ proteins have similar functions to their counterparts of HTLV-1. STLV-1 is oncogenic in Japanese macaques.[2]
In particular, the HTLV-I/STLV-I history might suggest a simian migration from Asia to Africa not much earlier than 19,500–60,000 years ago.[1]
https://en.wikipedia.org/wiki/Simian-T-lymphotropic_virus
The oral polio vaccine (OPV) AIDS hypothesis states that the AIDS pandemic originated from live polio vaccines prepared in chimpanzee tissue cultures, accidentally contaminated with SIV virus and then administered to up to one million Africans between 1957 and 1960 in experimental mass vaccination campaigns.
Data analyses in molecular biology and phylogenetic studies contradict the OPV AIDS hypothesis; consequently, scientific consensus regards the hypothesis as disproven.[1][2][3][4] The journal Nature has described the hypothesis as "refuted".[5]
https://en.wikipedia.org/wiki/Oral_polio_vaccine_AIDS_hypothesis
08-10-2021-2358 - Polio Modified Live Live Vaccine - SIV Cont (Animal derived vaccine only permissible)
The oral polio vaccine (OPV) AIDS hypothesis states that the AIDS pandemic originated from live polio vaccines prepared in chimpanzee tissue cultures, accidentally contaminated with SIV virus and then administered to up to one million Africans between 1957 and 1960 in experimental mass vaccination campaigns.
https://en.wikipedia.org/wiki/Oral_polio_vaccine_AIDS_hypothesis
08-11-2021-0103 - Autoimmune proliferative syndrome 927
Autoimmune lymphoproliferative syndrome (ALPS), is a form of lymphoproliferative disorder (LPDs). It affects lymphocyteapoptosis.[2]
It is a rare genetic disorder of abnormal lymphocyte survival caused by defective Fas mediated apoptosis.[3] Normally, after infectious insult, the immune system down-regulates by increasing Fas expression on activated B and T lymphocytes and Fas-ligand on activated T lymphocytes. Fas and Fas-ligand interact to trigger the caspase cascade, leading to cell apoptosis. Patients with ALPS have a defect in this apoptotic pathway, leading to chronic non-malignant lymphoproliferation, autoimmune disease, and secondary cancers.[4]
https://en.wikipedia.org/wiki/Autoimmune_lymphoproliferative_syndrome
Non-mycosis fungoides CD30− cutaneous large T-cell lymphoma is a cutaneous condition that usually presents as solitary or generalized plaques, nodules, or tumors of short duration.[1]:738
https://en.wikipedia.org/wiki/Non-mycosis_fungoides_CD30%E2%88%92_cutaneous_large_T-cell_lymphoma
CD30, also known as TNFRSF8, is a cell membrane protein of the tumor necrosis factor receptor family and tumor marker.
https://en.wikipedia.org/wiki/CD30
08-11-2021-0358 - drafting fungies, viridaes, vectors (phage, viron, viri thread, parasites, frags, prions, vso, org mods, infective seq, nuc seq, nuclear nucles, etc.), immunosuppr spliean bones cell cycle signals cp TNF lysis SIVIAN A SIV poolio-rabys live modlive HIV HIB etc. HEPA groundboys/airpets/waterwaders/firephiles/etc. oxyhydrodysequilind Autoimmune proliferative syndrom... acute myeloblasic leukimiaes murcomycotics mycosis mysosos sporeluants-parasites fungal leukemiaes decaying matter
08-11-2021-0358 - drafting fungies, viridaes, vectors (phage, viron, viri thread, parasites, frags, prions, vso, org mods, infective seq, nuc seq, nuclear nucles, etc.), immunosuppr spliean bones cell cycle signals cp TNF lysis SIVIAN A SIV poolio-rabys live modlive HIV HIB etc. HEPA groundboys/airpets/waterwaders/firephiles/etc. oxyhydrodysequilind Autoimmune proliferative syndrom... acute myeloblasic leukimiaes murcomycotics mycosis mysosos sporeluants-parasites fungal leukemiaes decaying matter
Decay Matter induction
FPoisidon
Alkylation, Nuclearization, Nuc Tag, Intercalation, Modification of STD biochem.
Leukemiaes, RAdiation induction, Immunosuppression, Cell Signal dysf ind env, dysregs, fungies.
Env - fungies murcomycotics mycobacteriaes bacteriophagulants
Viridaes - Fungal gene encode, carry sustain fragment to build
Bacteriaes - viridae encombants, helping make stuff from/etc. virus
fungal leukemiaes
murcomycotics mycotics fpo hydropo envstddissem air-sporeluant/viridae friendly
viridaes - SIVA, SIV, HEVA, HIVS, HIV, HI-R-us
Autoimmune prolif syndrom; acute myeloblasia lukes
fungal leukemiaes (blood parasite threads and fungees where decay matters)
Carbonaceous Chondrites eaters
props hydrags for dis emr and emf
As nitric acid was added to a column containing an ion-exchange resin and americium, the chemicals exploded, blowing out "pieces of glass and plastic" (plexiglass[4]) from the glove box.[2] Harold McCluskey was exposed to at least 37 MBqof americium-241 and nitric acid.[5]He was hit on the right side by a mixture of nitric acid, broken glass, americium and ion exchange resin.[6]
https://en.wikipedia.org/wiki/Harold_McCluskey
https://en.wikipedia.org/wiki/Harold_McCluskey
08-05-2021-1057 - TLS et EMR
08-05-2021-1054 - Radiation
08-05-2021-1051 - DNA parasite w effect macro w ap...
08-05-2021-1042 - Acute lymphoblastic leukemia
1. Coovules
2. Aerosol/Aeronautics/Air-med/gas-stb-org/etc. (hydaulics, space, excision of std env stp etc.)
3. Bone-cellular-genetic-etc. dysfunction induction wh dissemination whether or not identifiable outcome
4. Tumor Lysis Syndrome, ALBL; Bone degen, bone decay, cog depre, etc.
5. Ancestors-et-f. Rabys Poolio viridae of bone-blood-nerve systinis. Zoonotics. Neander Disease (europe, norway, germany, etc.). Torture disease. Derived from torted hums. Recommend do no injury/release contemp/violence/etc. of ancient gem/treasure infectious disease.
6. Radiation environment (EMR, Wireless, Rad K, Nuc Med CL1 2021, etc.)
7. Alkylation agent, intercalations, particle, chemical, resistant; all env conditions resistant (subbuild).
8. Hydric Acid, Resination-Glassification of Air due chemical compo isolated/suspended/etc. to gas pha or sol-gel dep on cond etc., Ion collection, channel formations/matricings, etc. rads. radioak potas nitrog helium hydrides hyd trit hydrogen chunking oxygen warping plane warps etc.
9. DNA parasite v. etc.
10. Fungization - ful-gan, hepashityls, mycotics, thrasis/thratix, fungal leukemiaes et murcomycotix/ffs/fungiesvar et -/|HIV-Americanium.
11. Decayin gMatter
12. prog cycles TLS ABL AIPS rapid cyc
13. G pro VIR ev
08-11-2021-0521 - Lipopolysaccharides ENDOTOXINS
Lipopolysaccharides (LPS), also known as endotoxins, are large molecules consisting of a lipid and a polysaccharide composed of O-antigen, outer core and inner core joined by a covalent bond; they are found in the outer membrane of Gram-negative bacteria. The term lipooligosaccharide ("LOS") is used to refer to a low-molecular-weight form of bacterial lipopolysaccharides.
Today, the term endotoxin is mostly used synonymously with LPS,[1] although there are a few endotoxins that are not related to LPS, such as the so-called delta endotoxin proteins secreted by Bacillus thuringiensis.https://en.wikipedia.org/wiki/Lipopolysaccharide
Note 2. Solution 2.
Note 3. Biofilm, plaque, tartar, calculus, calcification/mineralization, liequefacation/liquificaton/etc.
Note 4. USA NAC DOM Volatile org chemicals, infiltration/irritation, inflammation, inflammatory response cascade/cycling/layers/etc., wound healing/healing, granulation, keloid scarring/scarring/subst matrix/time variance/etc., biofilm deposition, plaquening (embeddement hairing), signal interference, mineralization, etc..
Note 5. Delta.
Wednesday, August 11, 2021
08-11-2021-0051 - Fungal Leukeamiaes - sp - fungus, fungal derivatives et priming by alkylation agents rads radk immunosups overvac genotox-viral gene transplant-activation of tumor gene-cell signal dysfunction/decay matter/fungai susecept/etc..
Pagetoid reticulosis (also known as "acral mycoses fungoides",[1] "localized epidermotropic reticulosis",[1] "mycosis fungoides palmaris et plantaris",[1] "unilesional mycosis fungoides",[2] and "Woringer–Kolopp disease"[1]) is a cutaneous condition, an uncommon lymphoproliferative disorder, sometimes considered a form of mycosis fungoides.[1]:734
https://en.wikipedia.org/wiki/Pagetoid_reticulosis
Mycosis fungoides, also known as Alibert-Bazin syndrome or granuloma fungoides,[1] is the most common form of cutaneous T-cell lymphoma. It generally affects the skin, but may progress internally over time. Symptoms include rash, tumors, skin lesions, and itchy skin.
While the cause remains unclear, most cases are not hereditary. Most cases are in people over 20 years of age, and it is more common in men than women. Treatment options include sunlight exposure, ultraviolet light, topical corticosteroids, chemotherapy, and radiotherapy.
sp - fungus, fungal derivatives et priming by alkylation agents rads radk immunosups overvac genotox-viral gene transplant-activation of tumor gene-cell signal dysfunction/decay matter/fungai susecept/etc..
https://en.wikipedia.org/wiki/Mycosis_fungoides
While uncommon in solid tumors, chromosomal translocations are a common cause of these diseases. This commonly leads to a different approach in diagnosis and treatment of haematological malignancies.
A subgroup of them are more severe and are known as haematological malignancies (British English)/hematological malignancies (American English) or blood cancer. They may also be referred to as liquid tumors.[3][4]
Tumors of the hematopoietic and lymphoid tissues (American English) or tumours of the haematopoietic and lymphoid malignancies (British English) are tumors that affect the blood, bone marrow, lymph, and lymphatic system.[1][2]Because these tissues are all intimately connected through both the circulatory system and the immune system, a disease affecting one will often affect the others as well, making myeloproliferation and lymphoproliferation (and thus the leukemiasand the lymphomas) closely related and often overlapping problems.
Hematological malignancies may derive from either of the two major blood cell lineages: myeloid and lymphoid cell lines. The myeloid cell line normally produces granulocytes, erythrocytes, thrombocytes, macrophages and mast cells; the lymphoid cell line produces B, T, NK and plasma cells. Lymphomas, lymphocytic leukemias, and myeloma are from the lymphoid line, while acute and chronic myelogenous leukemia, myelodysplastic syndromes and myeloproliferative diseases are myeloid in origin.
A subgroup of them are more severe and are known as haematological malignancies (British English)/hematological malignancies (American English) or blood cancer. They may also be referred to as liquid tumors.[3][4]
https://en.wikipedia.org/wiki/Tumors_of_the_hematopoietic_and_lymphoid_tissues
Di- and polyphosphates[edit]
These materials contain Ca2+ combined with the polyphosphates, such as P2O74− and triphosphate [P3O10]5−:
Dicalcium diphosphate (CAS#7790-76-3]: Ca2P2O7
Calcium triphosphate (CAS# 26158-70-3): Ca5(P3O10)2
Hydroxy- and oxo-phosphates[edit]
The cDNAs encoding LT and TNF were cloned in 1984[23] and were revealed to be similar.
https://en.wikipedia.org/wiki/Tumor_necrosis_factor
The cDNAs encoding LT and TNF were cloned in 1984[23] and were revealed to be similar. The binding of TNF to its receptor and its displacement by LT confirmed the functional homologybetween the two factors. The sequential and functional homology of TNF and LT led to the renaming of TNF as TNFα and LT as TNFβ. In 1985, Bruce A. Beutler and Anthony Cerami discovered that cachectin (a hormone which induces cachexia) was actually TNF.[24] They then identified TNF as a mediator of lethal endotoxin poisoning.[25] Kevin J. Tracey and Cerami discovered the key mediator role of TNF in lethal septic shock, and identified the therapeutic effects of monoclonal anti-TNF antibodies.[26][27]
https://en.wikipedia.org/wiki/Tumor_necrosis_factor
Research in the Laboratory of Mark Mattson has shown that TNF can prevent the death/apoptosis of neurons by a mechanism involving activation of the transcription factor NF-κB which induces the expression of antioxidant enzymes and Bcl-2.[28][29]
https://en.wikipedia.org/wiki/Tumor_necrosis_factor
Activation of NF-κB: TRADD recruits TRAF2 and RIP. TRAF2 in turn recruits the multicomponent protein kinase IKK, enabling the serine-threonine kinaseRIP to activate it. An inhibitory protein, IκBα, that normally binds to NF-κB and inhibits its translocation, is phosphorylated by IKK and subsequently degraded, releasing NF-κB. NF-κB is a heterodimeric transcription factor that translocates to the nucleus and mediates the transcription of a vast array of proteins involved in cell survival and proliferation, inflammatory response, and anti-apoptotic factors.
https://en.wikipedia.org/wiki/Tumor_necrosis_factor
NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) is a protein complex that controls transcription of DNA, cytokine production and cell survival. NF-κB is found in almost all animal cell types and is involved in cellular responses to stimuli such as stress, cytokines, free radicals, heavy metals, ultraviolet irradiation, oxidized LDL, and bacterial or viral antigens.[1][2][3][5][6] NF-κB plays a key role in regulating the immune response to infection. Incorrect regulation of NF-κB has been linked to cancer, inflammatory and autoimmune diseases, septic shock, viral infection, and improper immune development. NF-κB has also been implicated in processes of synaptic plasticity and memory.[7][8][9][10][11][12]
https://en.wikipedia.org/wiki/NF-κB
The human TNF gene was cloned in 1985.[30] It maps to chromosome 6p21.3, spans about 3 kilobases and contains 4 exons. The last exon shares similarity with lymphotoxin alpha (LTA, once named as TNF-β).[31] The three prime untranslated region (3'-UTR) of TNF contains an AU-rich element(ARE).
https://en.wikipedia.org/wiki/Tumor_necrosis_factor
Human tumour necrosis factor has about 30% homology in its amino acid sequence with lymphotoxin, a lymphokine that has similar biological properties. Recombinant tumour necrosis factor can be obtained by expression of its complementary DNA in Escherichia coli and induces the haemorrhagic necrosis of transplanted methylcholanthrene-induced sarcomas in syngeneic mice.
https://pubmed.ncbi.nlm.nih.gov/6392892/
Nature
. 1984 Dec 20-1985 Jan 2;312(5996):721-4. doi: 10.1038/312721a0.
Cloning and expression of cDNA for human lymphotoxin, a lymphokine with tumour necrosis activity
P W Gray, B B Aggarwal, C V Benton, T S Bringman, W J Henzel, J A Jarrett, D W Leung, B Moffat, P Ng, L P Svedersky, et al.
PMID: 6334807
DOI: 10.1038/312721a0
A chemically-synthesized gene and natural complementary DNA coding for human lymphotoxin were isolated and engineered for expression in Escherichia coli. Purified recombinant lymphotoxin shows cytotoxic activity on murine and human tumour cell lines in vitro and causes necrosis of certain murine sarcomas in vivo.
https://pubmed.ncbi.nlm.nih.gov/6334807/
Lipopolysaccharides (LPS), also known as endotoxins, are large molecules consisting of a lipid and a polysaccharide composed of O-antigen, outer core and inner core joined by a covalent bond; they are found in the outer membrane of Gram-negative bacteria. The term lipooligosaccharide ("LOS") is used to refer to a low-molecular-weight form of bacterial lipopolysaccharides.
Today, the term endotoxin is mostly used synonymously with LPS,[1] although there are a few endotoxins that are not related to LPS, such as the so-called delta endotoxin proteins secreted by Bacillus thuringiensis.
The toxic activity of LPS was first discovered and termed endotoxin by Richard Friedrich Johannes Pfeiffer, who distinguished between exotoxins, which he classified as a toxin that is released by bacteria into the surrounding environment, and endotoxins, which he considered to be a toxin kept "within" the bacterial cell and released only after destruction of the bacterial cell wall.[2]:84 Subsequent work showed that release of LPS from gram negativemicrobes does not necessarily require the destruction of the bacterial cell wall, but rather, LPS is secreted as part of the normal physiological activity of membrane vesicle trafficking in the form of bacterial outer membrane vesicles (OMVs), which may also contain other virulence factors and proteins.[3][4]
LPS is the major component of the outer membrane of Gram-negative bacteria, contributing greatly to the structural integrity of the bacteria, and protecting the membrane from certain kinds of chemical attack. LPS is the most abundant antigen on the cell surface of most Gram-negative bacteria, contributing up to 80% of the outer membrane of E. coli and Salmonella.[4] LPS increases the negative charge of the cell membrane and helps stabilize the overall membrane structure. It is of crucial importance to many Gram-negative bacteria, which die if it is mutated or removed; however, it appears that LPS is nonessential in at least some Gram-negative bacteria, such as Neisseria meningitidis, Moraxella catarrhalis, and Acinetobacter baumannii.[5]LPS induces a strong response from normal animal immune systems. It has also been implicated in non-pathogenic aspects of bacterial ecology, including surface adhesion, bacteriophage sensitivity, and interactions with predators such as amoebae.
LPS is required for the proper conformation of omptin activity; however, smooth LPS will sterically hinder omptins.https://en.wikipedia.org/wiki/Lipopolysaccharide
Bacillus thuringiensis (or Bt) is a Gram-positive, soil-dwelling bacterium, the most commonly used biological pesticide worldwide. B. thuringiensis also occurs naturally in the gut of caterpillars of various types of moths and butterflies, as well on leaf surfaces, aquatic environments, animal feces, insect-rich environments, and flour mills and grain-storage facilities.[1][2] It has also been observed to parasitize other moths such as Cadra calidella—in laboratory experiments working with C. calidella, many of the moths were diseased due to this parasite.[3]
During sporulation, many Bt strains produce crystal proteins (proteinaceous inclusions), called delta endotoxins, that have insecticidal action. This has led to their use as insecticides, and more recently to genetically modified crops using Bt genes, such as Bt corn.[4] Many crystal-producing Bt strains, though, do not have insecticidal properties.[5] The subspecies israelensis is commonly used for control of mosquitoes[6] and of fungus gnats.[7]
As a toxic mechanism, cry proteins bind to specific receptors on the membranes of mid-gut (epithelial) cells of the targeted pests, resulting in their rupture. Other organisms (including humans, other animals and non-targeted insects) that lack the appropriate receptors in their gut cannot be affected by the cry protein, and therefore are not affected by Bt.[8][9]
https://en.wikipedia.org/wiki/Bacillus_thuringiensis
Trypanosomatida is a group of kinetoplastid excavates distinguished by having only a single flagellum. The name is derived from the Greek trypano (borer) and soma (body) because of the corkscrew-like motion of some trypanosomatid species. All members are exclusively parasitic, found primarily in insects.[1] A few genera have life-cycles involving a secondary host, which may be a vertebrate, invertebrate or plant. These include several species that cause major diseases in humans.[2] Trypanosomatida are intracellular parasites.
(malaria)
https://en.wikipedia.org/wiki/Trypanosomatida#Morphologies
Categories:
Protein domains
Peripheral membrane proteins
Bacterial toxins
Crystals
Proteins
https://en.wikipedia.org/wiki/Delta_endotoxin
Pore-forming proteins (PFTs, also known as pore-forming toxins) are usually produced by bacteria, and include a number of protein exotoxins but may also be produced by other organisms such as earthworms, who produce lysenin. They are frequently cytotoxic (i.e., they kill cells), as they create unregulated pores in the membrane of targeted cells.
https://en.wikipedia.org/wiki/Pore-forming_toxin
Aflatoxins are various poisonous carcinogens and mutagens that are produced by certain molds, particularly Aspergillus species. The fungi grow in soil, decaying vegetation and various staple foodstuffs and commoditiessuch as hay, sweetcorn, wheat, millet, sorghum, cassava, rice, chili peppers, cottonseed, peanuts, tree nuts, sesame seeds, sunflower seeds, and various spices. In short, the relevant fungi grow on almost any crop or food. When such contaminated food is processed or consumed, the aflatoxins enter the general food supply. They have been found in both pet and human foods, as well as in feedstocks for agricultural animals. Animals fed contaminated food can pass aflatoxin transformation products into eggs, milk products, and meat.[1] For example, contaminated poultry feed is the suspected source of aflatoxin-contaminated chicken meat and eggs in Pakistan.[2]
https://en.wikipedia.org/wiki/Aflatoxin
β-Nitropropionic acid (3-nitropropanoic acid, BPA, 3-NPA) is a mycotoxin, a potent mitochondrialinhibitor,[1] toxic to humans. It is produced by a number of fungi, and found widely in food, in sugar cane, as well as Japanese fungally fermented staples miso, soy sauce, katsuobushi,[2] and some traditional Chinese medicines.[3]
https://en.wikipedia.org/wiki/Beta-Nitropropionic_acid
Clostridium botulinum is a Gram-positive, rod-shaped, anaerobic, spore-forming, motile bacterium with the ability to produce the neurotoxin botulinum.[1][2]
The botulinum toxin can cause botulism; a severe flaccid paralytic disease in humans and other animals[2] and is the most potent toxin known to humankind, natural or synthetic, with a lethal dose of 1.3–2.1 ng/kg in humans.[3]
https://en.wikipedia.org/wiki/Clostridium_botulinum
Superantigens (SAgs) are a class of antigens that result in excessive activation of the immune system. Specifically it causes non-specific activation of T-cells resulting in polyclonal T cell activation and massive cytokine release. SAgs are produced by some pathogenic viruses and bacteria most likely as a defense mechanism against the immune system.[1] Compared to a normal antigen-induced T-cell response where 0.0001-0.001% of the body's T-cells are activated, these SAgs are capable of activating up to 20% of the body's T-cells.[2] Furthermore, Anti-CD3 and Anti-CD28 antibodies (CD28-SuperMAB) have also shown to be highly potent superantigens (and can activate up to 100% of T cells).
https://en.wikipedia.org/wiki/Superantigen
Cytochalasins are fungal metabolites that have the ability to bind to actin filaments and block polymerization and the elongation of actin. As a result of the inhibition of actin polymerization, cytochalasins can change cellularmorphology, inhibit cellular processes such as cell division, and even cause cells to undergo apoptosis.[1] Cytochalasins have the ability to permeate cell membranes, prevent cellular translocation and cause cells to enucleate.[2]Cytochalasins can also have an effect on other aspects of biological processes unrelated to actin polymerization. For example, cytochalasin A and cytochalasin B can also inhibit the transport of monosaccharides across the cell membrane,[2] cytochalasin H has been found to regulate plant growth,[3]cytochalasin D inhibits protein synthesis[4] and cytochalasin E prevents angiogenesis.[5]
https://en.wikipedia.org/wiki/Cytochalasin
Ergotamine, sold under the brand names Cafergot (with caffeine) and Ergomar among others, is an ergopeptine and part of the ergot family of alkaloids; it is structurally and biochemically closely related to ergoline.[4] It possesses structural similarity to several neurotransmitters, and has biological activity as a vasoconstrictor.
https://en.wikipedia.org/wiki/Ergotamine
https://nikiyaantonbettey.blogspot.com/2021/08/08-11-2021-0710.html
A mycotoxin (from the Greek μύκης mykes, "fungus" and τοξίνη toxini, "toxin")[1][2] is a toxic secondary metabolite produced by organisms of the fungus kingdom[3] and is capable of causing disease and death in both humans and other animals.[4] The term 'mycotoxin' is usually reserved for the toxic chemical products produced by fungi that readily colonize crops.[5]
Examples of mycotoxins causing human and animal illness include aflatoxin, citrinin, fumonisins, ochratoxin A, patulin, trichothecenes, zearalenone, and ergot alkaloids such as ergotamine.[6]
One mold species may produce many different mycotoxins, and several species may produce the same mycotoxin.[7]
https://en.wikipedia.org/wiki/Mycotoxin
Fumonisin B1 is the most prevalent member of a family of toxins, known as fumonisins, produced by several species of Fusarium molds, such as Fusarium verticillioides,[1] which occur mainly in maize (corn), wheat and other cereals. Fumonisin B1 contamination of maize has been reported worldwide at mg/kg levels. Human exposure occurs at levels of micrograms to milligrams per day and is greatest in regions where maize products are the dietary staple.
Fumonisin B1 is hepatotoxic and nephrotoxic in all animal species tested. The earliest histological change to appear in either the liver or kidney of fumonisin-treated animals is increased apoptosis followed by regenerative cell proliferation. While the acute toxicity of fumonisin is low, it is the known cause of two diseases which occur in domestic animals with rapid onset: equine leukoencephalomalacia and porcine pulmonary oedema syndrome. Both of these diseases involve disturbed sphingolipid metabolism and cardiovascular dysfunction.
https://en.wikipedia.org/wiki/Fumonisin_B1
Listeriolysin O (LLO) is a hemolysin produced by the bacterium Listeria monocytogenes, the pathogen responsible for causing listeriosis. The toxin may be considered a virulence factor, since it is crucial for the virulence of L. monocytogenes.[1]
https://en.wikipedia.org/wiki/Listeriolysin_O
Gliotoxin is a sulfur-containing mycotoxin that belongs to a class of naturally occurring 2,5-diketopiperazines[1] produced by several species of fungi, especially those of marine origin. It is the most prominent member of the epipolythiopiperazines, a large class of natural products featuring a diketopiperazine with di- or polysulfide linkage. These highly bioactive compounds have been the subject of numerous studies aimed at new therapeutics.[2] Gliotoxin was originally isolated from Gliocladium fimbriatum, and was named accordingly. It is an epipolythiodioxopiperazine metabolite.
https://en.wikipedia.org/wiki/Gliotoxin
08-11-2021-1224 - Listeriolysin O (Hemolysin)
Listeriolysin O (LLO) is a hemolysin produced by the bacterium Listeria monocytogenes, the pathogen responsible for causing listeriosis. The toxin may be considered a virulence factor, since it is crucial for the virulence of L. monocytogenes.[1]
Listeriolysin O is a non-enzymatic, cytolytic, thiol-activated, cholesterol-dependent cytolysin; hence, it is activated by reducing agents and inhibited by oxidizing agents.[2] However, LLO differs from other thiol-activated toxins, since its cytolytic activity is maximized at a pH of 5.5.[2]
By maximizing activity at a pH of 5.5, LLO is selectively activated within the acidic phagosomes (average pH ~ 5.9) of cells that have phagocytosed L. monocytogenes.[3] After LLO lyses the phagosome, the bacterium escapes into the cytosol, where it can grow intracellularly. Upon release from the phagosome, the toxin has little activity in the more basic cytosol.
Hence, LLO permits L. monocytogenes to escape from phagosomes into the cytosol without damaging the plasma membrane of the infected cell. This allows the bacteria to live intracellularly, where they are protected from extracellular immune system factors such as the complement system and antibodies.
LLO also causes dephosphorylation of histone H3 and deacetylation of histone H4 during the early phases of infection, prior to entry of L. monocytogenes into the host cell.[4] The pore-forming activity is not involved in causing the histone modifications. The alterations of the histones cause the down regulation of genes encoding proteins involved in the inflammatory response. Thus, LLO may be important in subverting the host immune response to L. monocytogenes.[4]
A PEST-like sequence is present in LLO and is considered essential for virulence, since mutants lacking the sequence lysed the host cell.[5] However, contrary to PEST's supposed role in protein degradation, evidence suggests that the PEST-like sequence may regulate LLO production in the cytosol rather than increase degradation of LLO.[6]
A recombinant BCG vaccine against Mycobacterium tuberculosis is being developed that expresses Listeriolysin O and lacks Urease C. The ΔureC hly+ rBCG vaccine has significantly higher protection than the original BCG strain due to improved antigen presentation. Listeriolysin creates pores in the phagosome and allows the bacteria to escape into the cytosol, so antigens can be presented on both Class I and Class II Major Histocompatibility Complex and activate CD8 and CD4 T-cells respectively. Urease produces ammonia and creates a basic environment which inhibits listeriolysin activity, so it is knocked out to provide the optimal pH.[9]
https://en.wikipedia.org/wiki/Listeriolysin_O
Note. pH modification blood to modulate infection.
949
Hemolysins or haemolysins are lipids and proteins that cause lysis of red blood cells by disrupting the cell membrane. Although the lytic activity of some microbe-derived hemolysins on red blood cells may be of great importance for nutrient acquisition, many hemolysins produced by pathogens do not cause significant destruction of red blood cells during infection. However, hemolysins are often capable of lysing red blood cells in vitro.
While most hemolysins are protein compounds, some are lipid biosurfactants.[1]
https://en.wikipedia.org/wiki/Hemolysin
08-11-2021-1244 - Mycotoxins
Mycotoxins
Ibotenic acid
Muscarine
Muscimol
https://en.wikipedia.org/wiki/Neurotoxin
Note. research investigative pharmaceuticals (Quote) [Reference Retained]
MPP+ (1-methyl-4-phenylpyridinium) is a positively charged organic molecule with the chemical formulaC12H12N+. It is a neurotoxin that acts by interfering with oxidative phosphorylation in mitochondria by inhibiting complex I, leading to the depletion of ATP and eventual cell death.[1]
MPP+ arises in the body as the toxic metabolite of the closely related compound MPTP. MPTP is converted in the brain into MPP+ by the enzyme MAO-B, ultimately causing parkinsonism in primates by killing certain dopamine-producing neurons in the substantia nigra. The ability for MPP+ to induce Parkinson's disease has made it an important compound in Parkinson's research since this property was discovered in 1983.[2][3]
The chloride salt of MPP+ found use in the 1970s as an herbicide under the trade name cyperquat.[3]Though no longer in use as an herbicide, cyperquat's closely related structural analog paraquat still finds widespread usage, raising some safety concerns.
https://en.wikipedia.org/wiki/MPP%2B
NF-κB family members share structural homology with the retroviral oncoprotein v-Rel, resulting in their classification as NF-κB/Rel proteins.[1]
There are five proteins in the mammalian NF-κB family:[18]
ClassProteinAliasesGene
I NF-κB1 p105 → p50 NFKB1
NF-κB2 p100 → p52 NFKB2
II RelA p65 RELA
RelB RELB
c-Rel REL
https://en.wikipedia.org/wiki/NF-κB
An oncogene is a gene that has the potential to cause cancer.[1] In tumor cells, these genes are often mutated, or expressed at high levels.[2]
Most normal cells will undergo programmed form of rapid cell death (apoptosis) when critical functions are altered and malfunctioning. Activated oncogenes can cause those cells designated for apoptosis to survive and proliferate instead.[3] Most oncogenes began as proto-oncogenes: normal genes involved in cell growth and proliferation or inhibition of apoptosis. If, through mutation, normal genes promoting cellular growth are up-regulated (gain-of-function mutation), they will predispose the cell to cancer; thus, they are termed "oncogenes". Usually multiple oncogenes, along with mutated apoptotic or tumor suppressor genes will all act in concert to cause cancer. Since the 1970s, dozens of oncogenes have been identified in human cancer. Many cancer drugs target the proteins encoded by oncogenes.[2][4][5][6]
https://en.wikipedia.org/wiki/Oncogene
The ankyrin repeat is a 33-residue motif in proteins consisting of two alpha helices separated by loops, first discovered in signaling proteins in yeast Cdc10 and Drosophila Notch. Domains consisting of ankyrin tandem repeats mediate protein–protein interactions and are among the most common structural motifs in known proteins. They appear in bacterial, archaeal, and eukaryotic proteins, but are far more common in eukaryotes. Ankyrin repeat proteins, though absent in most viruses, are common among poxviruses. Most proteins that contain the motif have four to six repeats, although its namesake ankyrin contains 24, and the largest known number of repeats is 34, predicted in a protein expressed by Giardia lamblia.[2]
Ankyrin repeats typically fold together to form a single, linear solenoid structure called ankyrin repeat domains. These domains are one of the most common protein–protein interaction platforms in nature. They occur in a large number of functionally diverse proteins, mainly from eukaryotes. The few known examples from prokaryotes and viruses may be the result of horizontal gene transfers.[3] The repeat has been found in proteins of diverse function such as transcriptional initiators, cell cycle regulators, cytoskeletal, ion transporters, and signal transducers. The ankyrin fold appears to be defined by its structure rather than its function, since there is no specific sequence or structure that is universally recognised by it.
Considering the atomic structures of individual ankyrin repeats, the loop is often a type 1 beta bulge loop, while both alpha-helices commonly have a Schellman loop at their N-terminus.
https://en.wikipedia.org/wiki/Ankyrin_repeat
In addition to mammals, NF-κB is found in a number of simple animals as well.[19] These include cnidarians (such as sea anemones, coral and hydra), porifera (sponges), single-celled eukaryotes including Capsaspora owczarzaki and choanoflagellates, and insects (such as moths, mosquitoes and fruitflies). The sequencing of the genomes of the mosquitoes A. aegypti and A. gambiae, and the fruitfly D. melanogaster has allowed comparative genetic and evolutionary studies on NF-κB. In those insect species, activation of NF-κB is triggered by the Toll pathway (which evolved independently in insects and mammals) and by the Imd (immune deficiency) pathway.[20]
NF-κB (green) heterodimerizes with RelB (cyan) to form a ternary complex with DNA (orange) that promotes gene transcription.[21]
NF-κB is important in regulating cellular responses because it belongs to the category of "rapid-acting" primary transcription factors, i.e., transcription factors that are present in cells in an inactive state and do not require new protein synthesis in order to become activated (other members of this family include transcription factors such as c-Jun, STATs, and nuclear hormone receptors). This allows NF-κB to be a first responder to harmful cellular stimuli. Known inducers of NF-κB activity are highly variable and include reactive oxygen species (ROS), tumor necrosis factor alpha (TNFα), interleukin 1-beta (IL-1β), bacterial lipopolysaccharides (LPS), isoproterenol, cocaine, endothelin-1 and ionizing radiation.[22]
NF-κB suppression of tumor necrosis factor cytotoxicity (apoptosis) is due to induction of antioxidant enzymesand sustained suppression of c-Jun N-terminal kinases (JNKs).[23]
Receptor activator of NF-κB (RANK), which is a type of TNFR, is a central activator of NF-κB. Osteoprotegerin(OPG), which is a decoy receptor homolog for RANK ligand (RANKL), inhibits RANK by binding to RANKL, and, thus, osteoprotegerin is tightly involved in regulating NF-κB activation.[24]
Many bacterial products and stimulation of a wide variety of cell-surface receptors lead to NF-κB activation and fairly rapid changes in gene expression.[1] The identification of Toll-like receptors (TLRs) as specific pattern recognition molecules and the finding that stimulation of TLRs leads to activation of NF-κB improved our understanding of how different pathogens activate NF-κB. For example, studies have identified TLR4 as the receptor for the LPS component of Gram-negative bacteria.[25] TLRs are key regulators of both innate and adaptive immune responses.[26]
Unlike RelA, RelB, and c-Rel, the p50 and p52 NF-κB subunits do not contain transactivation domains in their C terminal halves. Nevertheless, the p50 and p52 NF-κB members play critical roles in modulating the specificity of NF-κB function. Although homodimers of p50 and p52 are, in general, repressors of κB site transcription, both p50 and p52 participate in target gene transactivation by forming heterodimers with RelA, RelB, or c-Rel.[27] In addition, p50 and p52 homodimers also bind to the nuclear protein Bcl-3, and such complexes can function as transcriptional activators.[28][29][30]
In unstimulated cells, the NF-κB dimers are sequestered in the cytoplasm by a family of inhibitors, called IκBs (Inhibitor of κB), which are proteins that contain multiple copies of a sequence called ankyrin repeats. By virtue of their ankyrin repeat domains, the IκB proteins mask the nuclear localization signals (NLS) of NF-κB proteins and keep them sequestered in an inactive state in the cytoplasm.[31]
IκBs are a family of related proteins that have an N-terminal regulatory domain, followed by six or more ankyrin repeats and a PEST domain near their C terminus. Although the IκB family consists of IκBα, IκBβ, IκBε, and Bcl-3, the best-studied and major IκB protein is IκBα. Due to the presence of ankyrin repeats in their C-terminal halves, p105 and p100 also function as IκB proteins. The c-terminal half of p100, that is often referred to as IκBδ, also functions as an inhibitor.[32][33] IκBδ degradation in response to developmental stimuli, such as those transduced through LTβR, potentiate NF-κB dimer activation in a NIK dependent non-canonical pathway.[32][34]
Activation of the NF-κB is initiated by the signal-induced degradation of IκB proteins. This occurs primarily via activation of a kinase called the IκB kinase(IKK). IKK is composed of a heterodimer of the catalytic IKKα and IKKβ subunits and a "master" regulatory protein termed NEMO (NF-κB essential modulator) or IKKγ. When activated by signals, usually coming from the outside of the cell, the IκB kinase phosphorylates two serine residues located in an IκB regulatory domain. When phosphorylated on these serines (e.g., serines 32 and 36 in human IκBα), the IκB proteins are modified by a process called ubiquitination, which then leads them to be degraded by a cell structure called the proteasome.
With the degradation of IκB, the NF-κB complex is then freed to enter the nucleus where it can 'turn on' the expression of specific genes that have DNA-binding sites for NF-κB nearby. The activation of these genes by NF-κB then leads to the given physiological response, for example, an inflammatory or immune response, a cell survival response, or cellular proliferation. Translocation of NF-κB to nucleus can be detected immunocytochemically and measured by laser scanning cytometry.[35] NF-κB turns on expression of its own repressor, IκBα. The newly synthesized IκBα then re-inhibits NF-κB and, thus, forms an auto feedback loop, which results in oscillating levels of NF-κB activity.[36] In addition, several viruses, including the AIDS virus HIV, have binding sites for NF-κB that controls the expression of viral genes, which in turn contribute to viral replication or viral pathogenicity. In the case of HIV-1, activation of NF-κB may, at least in part, be involved in activation of the virus from a latent, inactive state.[37] YopP is a factor secreted by Yersinia pestis, the causative agent of plague, that prevents the ubiquitination of IκB. This causes this pathogen to effectively inhibit the NF-κB pathway and thus block the immune response of a human infected with Yersinia.[38]
Concerning known protein inhibitors of NF-κB activity, one of them is IFRD1, which represses the activity of NF-κB p65 by enhancing the HDAC-mediated deacetylation of the p65 subunit at lysine 310, by favoring the recruitment of HDAC3 to p65. In fact IFRD1 forms trimolecular complexes with p65 and HDAC3.[39][40]
The NAD+-dependent protein deacetylase and longevity factor SIRT1 inhibits NF-κB gene expression by deacetylating the RelA/p65 subunit of NF-κB at lysine 310.[41]
A select set of cell-differentiating or developmental stimuli, such as lymphotoxin β-receptor (LTβR), BAFF or RANKL, activate the non-canonical NF-κB pathway to induce NF-κB/RelB:p52 dimer in the nucleus. In this pathway, activation of the NF-κB inducing kinase (NIK) upon receptor ligation led to the phosphorylation and subsequent proteasomal processing of the NF-κB2 precursor protein p100 into mature p52 subunit in an IKK1/IKKa dependent manner. Then p52 dimerizes with RelB to appear as a nuclear RelB:p52 DNA binding activity. RelB:p52 regulates the expression of homeostatic lymphokines, which instructs lymphoid organogenesis and lymphocyte trafficking in the secondary lymphoid organs.[42] In contrast to the canonical signaling that relies on NEMO-IKK2 mediated degradation of IκBα, -β, -ε, non-canonical signaling depends on NIK mediated processing of p100 into p52. Given their distinct regulations, these two pathways were thought to be independent of each other. However, it was found that syntheses of the constituents of the non-canonical pathway, viz RelB and p52, are controlled by canonical IKK2-IκB-RelA:p50 signaling.[43] Moreover, generation of the canonical and non-canonical dimers, viz RelA:p50 and RelB:p52, within the cellular milieu are mechanistically interlinked.[43] These analyses suggest that an integrated NF-κB system network underlies activation of both RelA and RelB containing dimer and that a malfunctioning canonical pathway will lead to an aberrant cellular response also through the non-canonical pathway. Most intriguingly, a recent study identified that TNF-induced canonical signalling subverts non-canonical RelB:p52 activity in the inflamed lymphoid tissues limiting lymphocyte ingress.[44] Mechanistically, TNF inactivated NIK in LTβR‐stimulated cells and induced the synthesis of Nfkb2 mRNA encoding p100; these together potently accumulated unprocessed p100, which attenuated the RelB activity. A role of p100/Nfkb2 in dictating lymphocyte ingress in the inflamed lymphoid tissue may have broad physiological implications.
In addition to its traditional role in lymphoid organogenesis, the non-canonical NF-κB pathway also directly reinforces inflammatory immune responses to microbial pathogens by modulating canonical NF-κB signalling. It was shown that p100/Nfkb2 mediates stimulus-selective and cell-type-specific crosstalk between the two NF-κB pathways and that Nfkb2-mediated crosstalk protects mice from gut pathogens.[45][46] On the other hand, a lack of p100-mediated regulations repositions RelB under the control of TNF-induced canonical signalling. In fact, mutational inactivation of p100/Nfkb2 in multiple myeloma enabled TNF to induce a long-lasting RelB activity, which imparted resistance in myeloma cells to chemotherapeutic drug.[47]
NF-κB is a major transcription factor that regulates genes responsible for both the innate and adaptive immune response.[48] Upon activation of either the T- or B-cell receptor, NF-κB becomes activated through distinct signaling components. Upon ligation of the T-cell receptor, protein kinase Lck is recruited and phosphorylates the ITAMs of the CD3 cytoplasmic tail. ZAP70 is then recruited to the phosphorylated ITAMs and helps recruit LAT and PLC-γ, which causes activation of PKC. Through a cascade of phosphorylation events, the kinase complex is activated and NF-κB is able to enter the nucleus to upregulate genes involved in T-cell development, maturation, and proliferation.[49]
In addition to roles in mediating cell survival, studies by Mark Mattson and others have shown that NF-κB has diverse functions in the nervous systemincluding roles in plasticity, learning, and memory.[50] In addition to stimuli that activate NF-κB in other tissues, NF-κB in the nervous system can be activated by Growth Factors (BDNF, NGF) and synaptic transmission such as glutamate.[8] These activators of NF-κB in the nervous system all converge upon the IKK complex and the canonical pathway.
Recently there has been a great deal of interest in the role of NF-κB in the nervous system. Current studies suggest that NF-κB is important for learning and memory in multiple organisms including crabs,[10][11] fruit flies,[51] and mice.[8][9] NF-κB may regulate learning and memory in part by modulating synaptic plasticity,[7][52] synapse function,[51][53][54] as well as by regulating the growth of dendrites[55] and dendritic spines.[54]
Genes that have NF-κB binding sites are shown to have increased expression following learning,[9] suggesting that the transcriptional targets of NF-κB in the nervous system are important for plasticity. Many NF-κB target genes that may be important for plasticity and learning include growth factors (BDNF, NGF)[56] cytokines (TNF-alpha, TNFR)[57] and kinases (PKAc).[52]
Despite the functional evidence for a role for Rel-family transcription factors in the nervous system, it is still not clear that the neurological effects of NF-κB reflect transcriptional activation in neurons. Most manipulations and assays are performed in the mixed-cell environments found in vivo, in "neuronal" cell cultures that contain significant numbers of glia, or in tumor-derived "neuronal" cell lines. When transfections or other manipulations have been targeted specifically at neurons, the endpoints measured are typically electrophysiology or other parameters far removed from gene transcription. Careful tests of NF-κB-dependent transcription in highly purified cultures of neurons generally show little to no NF-κB activity.[58][59]
Some of the reports of NF-κB in neurons appear to have been an artifact of antibody nonspecificity.[60] Of course, artifacts of cell culture—e.g., removal of neurons from the influence of glia—could create spurious results as well. But this has been addressed in at least two coculture approaches. Moerman et al.[61] used a coculture format whereby neurons and glia could be separated after treatment for EMSA analysis, and they found that the NF-κB induced by glutamatergic stimuli was restricted to glia (and, intriguingly, only glia that had been in the presence of neurons for 48 hours). The same investigators explored the issue in another approach, utilizing neurons from an NF-κB reporter transgenic mouse cultured with wild-type glia; glutamatergic stimuli again failed to activate in neurons.[62] Some of the DNA-binding activity noted under certain conditions (particularly that reported as constitutive) appears to result from Sp3 and Sp4 binding to a subset of κB enhancer sequences in neurons.[63] This activity is actually inhibited by glutamate and other conditions that elevate intraneuronal calcium. In the final analysis, the role of NF-κB in neurons remains opaque due to the difficulty of measuring transcription in cells that are simultaneously identified for type. Certainly, learning and memory could be influenced by transcriptional changes in astrocytes and other glial elements. And it should be considered that there could be mechanistic effects of NF-κB aside from direct transactivation of genes.
As such, many different types of human tumors have misregulated NF-κB: that is, NF-κB is constitutively active. Active NF-κB turns on the expression of genes that keep the cell proliferating and protect the cell from conditions that would otherwise cause it to die via apoptosis. In cancer, proteins that control NF-κB signaling are mutated or aberrantly expressed, leading to defective coordination between the malignant cell and the rest of the organism. This is evident both in metastasis, as well as in the inefficient eradication of the tumor by the immune system.[64]
Normal cells can die when removed from the tissue they belong to, or when their genome cannot operate in harmony with tissue function: these events depend on feedback regulation of NF-κB, and fail in cancer.[65]
Defects in NF-κB results in increased susceptibility to apoptosis leading to increased cell death. This is because NF-κB regulates anti-apoptotic genes especially the TRAF1 and TRAF2 and therefore abrogates the activities of the caspase family of enzymes, which are central to most apoptotic processes.[66]
However, even though convincing experimental data have identified NF-κB as a critical promoter of tumorigenesis, which creates a solid rationale for the development of antitumor therapy that is based upon suppression of NF-κB activity, caution should be exercised when considering anti-NF-κB activity as a broad therapeutic strategy in cancer treatment as data has also shown that NF-κB activity enhances tumor cell sensitivity to apoptosis and senescence.
In addition, it has been shown that canonical NF-κB is a Fas transcription activator and the alternative NF-κB is a Fas transcription repressor.[73] Therefore, NF-κB promotes Fas-mediated apoptosis in cancer cells, and thus inhibition of NF-κB may suppress Fas-mediated apoptosis to impair host immune cell-mediated tumor suppression.
https://en.wikipedia.org/wiki/NF-κB
Neurocystcercotics (helminthiae; nematodae; very small and small reproducible/clonable parasite)
Fungaes/mycotics/sporeluants/bacterial-sporulants-toxins-metabolite-virus/plasmid-gene-fragments-etc. (clostridium, mad cow disease, spongiform encephalitis, stephys (staph), etc.)
Endotoxins, intoxicants, toxins, poisons, genotoxing, etc.
mycosis, mycobacteriae, mycotic parasite, symbiotics/activators/etc., Mycosis fungoide, etc..
(Fungy eat decay matter sometime, secondary metabolite/product of metabolism/byproduct of metabolism/possibility of cell function-structure-cycle-etc./etc. of fungees is sometimes toxin (toxic or not; dependent toxicity or not; potent toxic or not; etc.) or toxic [or alternatively, sometimes not toxic, etc.]; some able to survive in nuclear particle environment, some may consume/utilize/transform/etc. straight nuclear particle, etc.; radioactive particle induce tissue death melt/smold/burn/decay/etc. (including force burns; knocked off axis; etc.) alteration to rate/environment/condition/ctrl/etc., tissue survival depend on rate match time limited (rate match inefficient - compensatory, synergism, harmonization, etc. - not perfect rate match but sufficient to sustain cellular struct-funct-etc. for time)
Fungal Leukemiaes (fungus, bacteria plasmid, viridae, hum genome vir activator, act of prev inf immun respo, nuclear exposure, alkylation agents, injury, protazoe/amoebaes, parasitics (esp blood and cellular intra parasitics))
Aspergillosis (fungy parasite facilitation - massive; Tumor lysis factor, with lead prior etc.)
Amoaebitic Disease of Brain
Protozoans - Nagleria Floweri
Miliary Tuberculosis
US-ASC0 - Radiation, Alkylation Agent, Biohazard/Disease Trans, genotox (mimetic)
Toxin Neurodegen
acute myeloblasic leuk
Prion disease
Industrial/Man-Made VOC (inc. oxygen, hydrocats, trits, hydrazines, organophor, orgphos, nitrogen, ammonia, -ol, sulfis, sterols, hormones, insecticide, oil based, hydrophobic, insoluble wat, lipids, hygros, anabolic sub, steroides, fatty acids, fats, solvents, thinners, coagulants, ethylene, oxine, oxane, ethol, ethane, ethyl, eth, ether, ester, ols, ster, chlors, cholesterols, chlorine, salt, lards, paste, wax, grease, motor oil, enging carburator fluids, antifreeze, standard engine lubricants/fuel/etc., unusable energy of excess energy or toxic metabolite or etc., pus, protein masses, protein, plaquening, softening, malacia, holeing, moleing, mineralization of soft tissue, liquefication of tissues, dumping syndrome, sterilytics, signal comm usurpation, signal comm analog/usurpation, signal, communication, cell signal, senescence induction, proliferation, cell mobility/parasite mobility, parasite env culting, growth medium of bact/parasites/etc. improvement (var type), radioactive couplants, additives, etc.)*
Nuclear Weapons (dif prof; background; ranked, etc.; acute leukemia spc rad; birth to death or accident or mimetic case; mostly not provisible - not public domain)
Purple book - Non-mycosis fungoides CD30, TNF, chromosomal transloc/gene deletion-seq-insert-modding w radioactive or potent-etc./etc., Nf-kB, plasmid, viral vector, etc.
HIV ET HLV HTV HTLV SIV
1. Particle
2. Prion
3. Decay Matters
4. Fungus
5. Virus
6. Gene, Protein, Viron, Gene Decay Particle-Org-etc.
7. Bacteria (single cell)
8. Bacteriophage, virophage, phage
9. Protazoe
10. amoebae - animal
11. Parasite (e.g. dna)
12. Differentiated systems (liq sol gas solgel gel sol pls etc.)
13. VOCs, inhabitable cells (no nucleus), bone cave, etc. (tiny little nutrient - blood; large viand nutrient - digestive; nitrogen - bladder; chlorine - st, protinated-fatulants, etc..
Human T-cell lymphotropic virus type 1 or human T-lymphotropic virus (HTLV-I), also called the adult T-cell lymphoma virus type 1, is a retrovirus of the human T-lymphotropic virus (HTLV) family that has been implicated in several kinds of diseases including very aggressive adult T-cell lymphoma (ATL), HTLV-I-associated myelopathy, uveitis, Strongyloides stercoralis hyper-infection and some other diseases. It is thought that about 1–5% of infected persons develop cancer as a result of the infection with HTLV-I over their lifetimes.[1]
Adult T-cell lymphoma (ATL) was discovered in 1977 in Japan. The symptoms of ATL were different from other lymphomas known at the time. It was suggested that ATL is caused by the infection of a retrovirus called ATLV.[2] Strikingly, ATLV had the transforming activity in vitro.[3] These studies established that the retrovirus infection is the cause of ATL. The retrovirus is now generally called HTLV-I because later studies proved that ATLV is the same as the firstly identified human retrovirus called HTLV discovered by Bernard Poiesz and Francis Ruscetti and their co-workers in the laboratory of Robert C. Gallo at the National Cancer Institute.[4] Infection with HTLV-I, like infection with other retroviruses, probably occurs for life. A patient infected with HTLV can be diagnosed when antibodies against HTLV-1 are detected in the serum.[1]
https://en.wikipedia.org/wiki/Human_T-lymphotropic_virus_1
Lipopolysaccharides (LPS), also known as endotoxins, are large molecules consisting of a lipid and a polysaccharide composed of O-antigen, outer core and inner core joined by a covalent bond; they are found in the outer membrane of Gram-negative bacteria. The term lipooligosaccharide ("LOS") is used to refer to a low-molecular-weight form of bacterial lipopolysaccharides.
Today, the term endotoxin is mostly used synonymously with LPS,[1] although there are a few endotoxins that are not related to LPS, such as the so-called delta endotoxin proteins secreted by Bacillus thuringiensis.
https://en.wikipedia.org/wiki/Lipopolysaccharide
Autoimmune lymphoproliferative syndrome (ALPS), is a form of lymphoproliferative disorder (LPDs). It affects lymphocyteapoptosis.[2]
It is a rare genetic disorder of abnormal lymphocyte survival caused by defective Fas mediated apoptosis.[3] Normally, after infectious insult, the immune system down-regulates by increasing Fas expression on activated B and T lymphocytes and Fas-ligand on activated T lymphocytes. Fas and Fas-ligand interact to trigger the caspase cascade, leading to cell apoptosis. Patients with ALPS have a defect in this apoptotic pathway, leading to chronic non-malignant lymphoproliferation, autoimmune disease, and secondary cancers.[4]
https://en.wikipedia.org/wiki/Autoimmune_lymphoproliferative_syndrome
NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) is a protein complex that controls transcription of DNA, cytokine production and cell survival. NF-κB is found in almost all animal cell types and is involved in cellular responses to stimuli such as stress, cytokines, free radicals, heavy metals, ultraviolet irradiation, oxidized LDL, and bacterial or viral antigens.[1][2][3][5][6] NF-κB plays a key role in regulating the immune response to infection. Incorrect regulation of NF-κB has been linked to cancer, inflammatory and autoimmune diseases, septic shock, viral infection, and improper immune development. NF-κB has also been implicated in processes of synaptic plasticityand memory.[7][8][9][10][11][12]
https://en.wikipedia.org/wiki/NF-κB
The ankyrin repeat is a 33-residue motif in proteins consisting of two alpha helices separated by loops, first discovered in signaling proteins in yeast Cdc10 and Drosophila Notch.
https://en.wikipedia.org/wiki/Ankyrin_repeat
An oncogene is a gene that has the potential to cause cancer.[1] In tumor cells, these genes are often mutated, or expressed at high levels.[2]
https://en.wikipedia.org/wiki/Oncogene
Toll-like receptors (TLRs) are a class of proteins that play a key role in the innate immune system. They are single-pass membrane-spanning receptors usually expressed on sentinel cells such as macrophagesand dendritic cells, that recognize structurally conserved molecules derived from microbes. Once these microbes have breached physical barriers such as the skin or intestinal tract mucosa, they are recognized by TLRs, which activate immune cell responses. The TLRs include TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, TLR7, TLR8, TLR9, TLR10, TLR11, TLR12, and TLR13, though the last three are not found in humans,[1] and there isn't a functional gene for TLR10 in mice. [2] TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10 are located on the cell membrane, whereas TLR3, TLR7, TLR8, and TLR9 are located in intracellular vesicles (because they are sensors of nucleic acids).[3]
TLRs received their name from their similarity to the protein coded by the toll gene identified in Drosophilain 1985 by Christiane Nüsslein-Volhard and Eric Wieschaus.[4]
https://en.wikipedia.org/wiki/Toll-like_receptor
In the field of molecular biology, nuclear receptors are a class of proteins found within cells that are responsible for sensing steroid and thyroid hormones and certain other molecules. In response, these receptors work with other proteins to regulate the expression of specific genes, thereby controlling the development, homeostasis, and metabolism of the organism.
Nuclear receptors have the ability to directly bind to DNA and regulate the expression of adjacent genes; hence these receptors are classified as transcription factors.[2][3] The regulation of gene expression by nuclear receptors generally only happens when a ligand—a molecule that affects the receptor's behavior—is present. More specifically, ligand binding to a nuclear receptor results in a conformational change in the receptor, which, in turn, activates the receptor, resulting in up- or down-regulation of gene expression.
A unique property of nuclear receptors that differentiates them from other classes of receptorsis their ability to directly interact with and control the expression of genomic DNA. As a consequence, nuclear receptors play key roles in both embryonic development and adult homeostasis. As discussed below, nuclear receptors may be classified according to either mechanism[4][5] or homology.[6][7]
https://en.wikipedia.org/wiki/Nuclear_receptor
Volatile organic compounds are compounds that have a high vapor pressure and low water solubility. Many VOCs are human-made chemicals that are used and produced in the manufacture of paints, pharmaceuticals, and refrigerants. VOCs typically are industrial solvents, such as trichloroethylene; fuel oxygenates, such as methyl tert-butyl ether (MTBE); or by-products produced by chlorination in water treatment, such as chloroform. VOCs are often components of petroleum fuels, hydraulic fluids, paint thinners, and dry cleaning agents. VOCs are common ground-water contaminants.
https://www.epa.gov/indoor-air-quality-iaq/what-are-volatile-organic-compounds-vocs
Volatile organic compounds (VOC) are organic chemicals that have a high vapour pressure at room temperature. High vapor pressure correlates with a low boiling point, which relates to the number of the sample's molecules in the surrounding air, a trait known as volatility.[1]
VOCs are responsible for the odor of scents and perfumes as well as pollutants. VOCs play an important role in communication between animals and plants, e.g. attractants for pollinators,[2] protection from predation,[3] and even inter-plant interactions.[4] Some VOCs are dangerous to human health or cause harm to the environment. Anthropogenic VOCs are regulated by law, especially indoors, where concentrations are the highest. Most VOCs are not acutely toxic, but may have long-term chronic health effects.
https://en.wikipedia.org/wiki/Volatile_organic_compound
NF-κB is increasingly expressed with obesity and aging,[87] resulting in reduced levels of the anti-inflammatory, pro-autophagy, anti-insulin resistanceprotein sirtuin 1. NF-κB increases the levels of the microRNA miR-34a (which inhibits nicotinamide adenine dinucleotide NAD synthesis) by binding to its promoter region.[88] resulting in lower levels of sirtuin 1.
NF-κB and interleukin 1 alpha mutually induce each other in senescent cells in a positive feedback loop causing the production of senescence-associated secretory phenotype (SASP) factors.[89]
Aberrant activation of NF-κB is frequently observed in many cancers. Moreover, suppression of NF-κB limits the proliferation of cancer cells. In addition, NF-κB is a key player in the inflammatory response. Hence methods of inhibiting NF-κB signaling has potential therapeutic application in cancer and inflammatory diseases.[101][102]
Both the canonical and non-canonical NF-κB pathways require proteasomal degradation of regulatory pathway components for NF-κB signalling to occur. The proteosome inhibitor Bortezomib broadly blocks this activity and is approved for treatment of NF-κB driven Mantle Cell Lymphoma and Multiple Myeloma.[103][104]
The discovery that activation of NF-κB nuclear translocation can be separated from the elevation of oxidant stress[105] gives a promising avenue of development for strategies targeting NF-κB inhibition.
The drug denosumab acts to raise bone mineral density and reduce fracture rates in many patient sub-groups by inhibiting RANKL. RANKL acts through its receptor RANK, which in turn promotes NF-κB,[106] RANKL normally works by enabling the differentiation of osteoclasts from monocytes.
Disulfiram, olmesartan and dithiocarbamates can inhibit the nuclear factor-κB (NF-κB) signaling cascade.[107] Effort to develop direct NF-κB inhibitor has emerged with compounds such as (-)-DHMEQ, PBS-1086, IT-603 and IT-901.[108][109][110] (-)-DHMEQ and PBS-1086 are irreversible binder to NF-κB while IT-603 and IT-901 are reversible binder. DHMEQ covalently binds to Cys 38 of p65.[111]
Anatabine's antiinflammatory effects are claimed to result from modulation of NF-κB activity.[112] However the studies purporting its benefit use abnormally high doses in the millimolar range (similar to the extracellular potassium concentration), which are unlikely to be achieved in humans.
BAY 11-7082 has also been identified as a drug that can inhibit the NF-κB signaling cascade. It is capable of preventing the phosphorylation of IKK-α in an irreversible manner such that there is down regulation of NF-κB activation.[113]
It has been shown that administration of BAY 11-7082 rescued renal functionality in diabetic-induced Sprague-Dawley rats by suppressing NF-κB regulated oxidative stress.[114]
Research has shown that the N-acylethanolamine, palmitoylethanolamide is capable of PPAR-mediated inhibition of NF-κB.[115]
The biological target of iguratimod, a drug marketed to treat rheumatoid arthritis in Japan and China, was unknown as of 2015, but the primary mechanism of action appeared to be preventing NF-κB activation.[116]
https://en.wikipedia.org/wiki/NF-κB
Plasmodium falciparum is a unicellular protozoan parasite of humans, and the deadliest species of Plasmodium that causes malaria in humans.[2] The parasite is transmitted through the bite of a female Anopheles mosquito and causes the disease's most dangerous form, falciparum malaria. It is responsible for around 50% of all malaria cases.[3][4] P. falciparum is therefore regarded as the deadliest parasite in humans. It is also associated with the development of blood cancer (Burkitt's lymphoma) and is classified as Group 2A carcinogen.
The species originated from the malarial parasite Laverania found in gorillas, around 10,000 years ago.[5]Alphonse Laveran was the first to identify the parasite in 1880, and named it Oscillaria malariae. Ronald Rossdiscovered its transmission by mosquito in 1897. Giovanni Battista Grassi elucidated the complete transmission from a female anopheline mosquito to humans in 1898. In 1897, William H. Welch created the name Plasmodium falciparum, which ICZN formally adopted in 1954. P. falciparum assumes several different forms during its life cycle. The human-infective stage are sporozoites from the salivary gland of a mosquito. The sporozoites grow and multiply in the liver to become merozoites. These merozoites invade the erythrocytes(RBCs) to form trophozoites, schizonts and gametocytes, during which the symptoms of malaria are produced. In the mosquito, the gametocytes undergo sexual reproduction to a zygote, which turns into ookinete. Ookinete forms oocytes from which sporozoites are formed.
https://en.wikipedia.org/wiki/Plasmodium_falciparum
Anatabine (uh-nat-uh-been,-bin) is one of the minor alkaloids found in plants in the family Solanaceae, which includes the tobacco plant and tomato. Commercial tobacco plants typically produce alkaloids at levels between 2% and 4% of total dry weight, with nicotine accounting for about 90% of the total alkaloid content, and the related compounds anabatine, nornicotine, and anabasine making up nearly all the rest.[1]These compounds are thought to be biologically active, and part of plants' natural defense system against insects.[1]
Anatabine has anti-inflammatory activity partly through inhibition of STAT3 phosphorylation in vitro and in vivo.[2]
https://en.wikipedia.org/wiki/Anatabine
Multiple myeloma (MM), also known as plasma cell myeloma and simply myeloma, is a cancerof plasma cells, a type of white blood cell that normally produces antibodies.[6] Often, no symptoms are noticed initially.[10] As it progresses, bone pain, anemia, kidney dysfunction, and infections may occur.[10] Complications may include amyloidosis.[3]
https://en.wikipedia.org/wiki/Multiple_myeloma
Y box binding protein 1 also known as Y-box transcription factor or nuclease-sensitive element-binding protein 1 is a protein that in humans is encoded by the YBX1 gene.[5]
https://en.wikipedia.org/wiki/Y_box_binding_protein_1
DNA-binding protein A is a protein that in humans is encoded by the CSDA gene.[5][6][7]
https://en.wikipedia.org/wiki/CSDA_(gene)
High-Mobility Group or HMG is a group of chromosomal proteins that are involved in the regulation of DNA-dependent processes such as transcription, replication, recombination, and DNA repair.[1]
https://en.wikipedia.org/wiki/High-mobility_group
Multiple myeloma may develop from monoclonal gammopathy of undetermined significance that progresses to smoldering myeloma.[13] The abnormal plasma cells produce abnormal antibodies, which can cause kidney problems and overly thick blood.[10] The plasma cells can also form a mass in the bone marrow or soft tissue.[10] When one tumor is present, it is called a plasmacytoma; more than one is called multiple myeloma.[10]Multiple myeloma is diagnosed based on blood or urine tests finding abnormal antibodies, bone marrow biopsy finding cancerous plasma cells, and medical imaging finding bone lesions.[6]Another common finding is high blood calcium levels.[6]
https://en.wikipedia.org/wiki/Multiple_myeloma
Nuclear factor of activated T-cells (NFAT) is a family of transcription factors shown to be important in immune response. One or more members of the NFAT family is expressed in most cells of the immune system. NFAT is also involved in the development of cardiac, skeletal muscle, and nervous systems. NFAT was first discovered as an activator for the transcription of IL-2 in T cells (as a regulator of T cell immune response) but has since been found to play an important role in regulating many more body systems.[1] NFAT transcription factors are involved in many normal body processes as well as in development of several diseases, such as inflammatory bowel diseases and several types of cancer. NFAT is also being investigated as a drug target for several different disorders.
https://en.wikipedia.org/wiki/NFAT
https://en.wikipedia.org/wiki/NF-κB
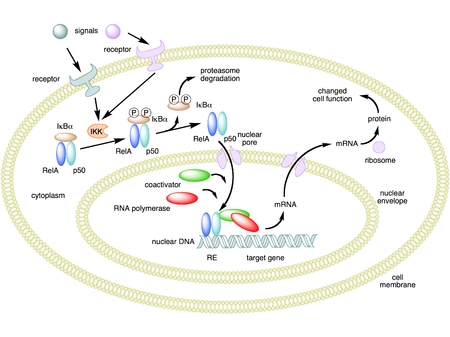
Mechanism of NF-κB action. In this figure, the NF-κB heterodimer consisting of Rel and p50 proteins is used as an example. While in an inactivated state, NF-κB is located in the cytosol complexed with the inhibitory protein IκBα. Through the intermediacy of integral membrane receptors, a variety of extracellular signals can activate the enzyme IκB kinase (IKK). IKK, in turn, phosphorylates the IκBα protein, which results in ubiquitination, dissociation of IκBα from NF-κB, and eventual degradation of IκBα by the proteasome. The activated NF-κB is then translocated into the nucleus where it binds to specific sequences of DNA called response elements (RE). The DNA/NF-κB complex then recruits other proteins such as coactivators and RNA polymerase, which transcribe downstream DNA into mRNA. In turn, mRNA is translated into protein, resulting in a change of cell function.[1][2][3][4]

Schematic diagram of NF-κB protein structure. There are two structural classes of NF-κB proteins: class I (top) and class II (bottom). Both classes of proteins contain a N-terminal DNA-binding domain (DBD), which also serves as a dimerization interface to other NF-κB transcription factors and, in addition, binds to the inhibitory IκBα protein. The C-terminus of class I proteins contains a number of ankyrin repeats and has transrepression activity. In contrast, the C-terminus of class II proteins has a transactivation function.[1][2][3][4]
NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) is a protein complex that controls transcription of DNA, cytokine production and cell survival. NF-κB is found in almost all animal cell types and is involved in cellular responses to stimuli such as stress, cytokines, free radicals, heavy metals, ultraviolet irradiation, oxidized LDL, and bacterial or viral antigens.[1][2][3][5][6] NF-κB plays a key role in regulating the immune response to infection. Incorrect regulation of NF-κB has been linked to cancer, inflammatory and autoimmune diseases, septic shock, viral infection, and improper immune development. NF-κB has also been implicated in processes of synaptic plasticity and memory.[7][8][9][10][11][12]
By Nikiya Anton Bettey at August 11, 2021
Email ThisBlogThis!Share to TwitterShare to FacebookShare to Pinterest
Talaromyces marneffei, formerly called Penicillium marneffei,[1] was identified in 1956.[2] The organism is endemic to southeast Asia where it is an important cause of opportunistic infections in those with HIV/AIDS-related immunodeficiency. Incidence of T. marneffei infections has increased due to a rise in HIV infection rates in the region.[3][4]
When it was classified as a Penicillium, it was the only known thermally dimorphic species of that genus that caused a lethal systemic infection (talaromycosis), with fever and anaemia similar to disseminatedcryptococcosis. This contrasted with related Penicillium species that are usually regarded as unimportant in terms of causing human disease.[citation needed]
https://en.wikipedia.org/wiki/Talaromyces_marneffei
Sarcocystis neurona is primarily a neural parasite of horses and its management is of concern in veterinarian medicine. The protozoan Sarcocystis neurona is a protozoan of single celled character and belongs to the family, Sarcocystidae, a group called coccidia.[1] The protozoan, S. neurona, is a member of the genus Sarcocystis, and is most commonly associated with equine protozoal myeloencephalitis (EPM).[2] The protozoan, S. neurona, can be easily cultivated and genetically manipulated, hence its common use as a model to study numerous aspects of cell biology.[3]
https://en.wikipedia.org/wiki/Sarcocystis_neurona
Leishmania tropica is a species of flagellate parasites that infects humans and hyraxes, and the cause of the disease Leishmaniasis RecidivansOr bugdadsore, a form of cutaneous leishmaniasis. L. tropica infection results in non-ulcerating disease. Cause Oriental sores<Korean J. Parasitol. 2007 Jun;45(2):103-9.> <Mahmoudzadeh-Niknam H1, Kiaei SS, Iravani D.>
https://en.wikipedia.org/wiki/Leishmania_tropica
Amphotericin B is an antifungal medication used for serious fungal infections and leishmaniasis.[1] The fungal infections it is used to treat include mucormycosis, aspergillosis, blastomycosis, candidiasis, coccidioidomycosis, and cryptococcosis.[2] For certain infections it is given with flucytosine.[3] It is typically given by injection into a vein.[2]
Common side effects include a reaction with fever, chills, and headaches soon after the medication is given, as well as kidney problems.[2] Allergic symptoms including anaphylaxis may occur.[2] Other serious side effects include low blood potassium and inflammation of the heart.[1] It appears to be relatively safe in pregnancy.[2] There is a lipid formulation that has a lower risk of side effects.[2] It is in the polyene class of medications and works in part by interfering with the cell membrane of the fungus.[1][2]
Amphotericin B was isolated from Streptomyces nodosus in 1955 at the Squibb For Medical Research Institute from cultures isolated from the streptomycete obtained from the river bed of Orinoco in that region of Venezuela[4] and came into medical use in 1958.[5][6] It is on the World Health Organization's List of Essential Medicines, the safest and most effective medicines needed in a health system.[7] It is available as a generic medication.[2] The cost in the developing world of a course of treatment as of 2010 is between US$162 and 229.[1]
https://en.wikipedia.org/wiki/Amphotericin_B
Tritrichomonas foetus is a species of single-celled flagellated parasites that is known to be a pathogen of the bovine reproductive tract as well as the intestinal tract of cats. In cattle, the organism is transmitted to the female vagina and uterus from the foreskin of the bull where the parasite is known to reside. It causes infertility, and, at times, has caused spontaneous abortions in the first trimester. In the last ten years, there have been reports of Tritrichomonas foetus in the feces of young cats that have diarrhea[1] and live in households with multiple cats. Tritrichomonas foetus looks similarly to Giardia and is often misdiagnosed for it when viewed under a microscope.[2]
https://en.wikipedia.org/wiki/Tritrichomonas_foetus
search for tubular leukemia, dyscrasia, hemato, immuno, fungal, etc. missin, etc..
Some causes of fungal meningitis include Cryptococcus, Histoplasma, Blastomyces, Coccidioides, and Candida.
https://www.cdc.gov/meningitis/fungal.html
Tularemia, also known as rabbit fever, is an infectious disease caused by the bacteriumFrancisella tularensis.[4] Symptoms may include fever, skin ulcers, and enlarged lymph nodes.[3]Occasionally, a form that results in pneumonia or a throat infection may occur.[3]
The bacterium is typically spread by ticks, deer flies, or contact with infected animals.[4] It may also be spread by drinking contaminated water or breathing in contaminated dust.[4] It does not spread directly between people.[8] Diagnosis is by blood tests or cultures of the infected site.[5][9]
Prevention is by using insect repellent, wearing long pants, rapidly removing ticks, and not disturbing dead animals.[6] Treatment is typically with the antibiotic streptomycin.[9] Gentamicin, doxycycline, or ciprofloxacinmay also be used.[5]
Between the 1970s and 2015, around 200 cases were reported in the United States a year.[7] Males are affected more often than females.[7] It occurs most frequently in the young and the middle aged.[7] In the United States, most cases occur in the summer.[7] The disease is named after Tulare County, California, where the disease was discovered in 1911.[10] A number of other animals, such as rabbits, may also be infected.[4]
https://en.wikipedia.org/wiki/Tularemia
Anatabine (uh-nat-uh-been,-bin) is one of the minor alkaloids found in plants in the family Solanaceae, which includes the tobacco plant and tomato. Commercial tobacco plants typically produce alkaloids at levels between 2% and 4% of total dry weight, with nicotine accounting for about 90% of the total alkaloid content, and the related compounds anabatine, nornicotine, and anabasine making up nearly all the rest.[1]These compounds are thought to be biologically active, and part of plants' natural defense system against insects.[1]
Anatabine has anti-inflammatory activity partly through inhibition of STAT3 phosphorylation in vitro and in vivo.[2]
https://en.wikipedia.org/wiki/Anatabine
08-11-2031-1650 - Smouldering Synd, Amyloidosis, Monoclone, Gammaopath, plasma cell dyscrasia, hyperviscosity, protein build, kidney dis, plasma cell myeloma etc.) drafting
Multiple myeloma (MM), also known as plasma cell myeloma and simply myeloma, is a cancerof plasma cells, a type of white blood cell that normally produces antibodies.[6] Often, no symptoms are noticed initially.[10] As it progresses, bone pain, anemia, kidney dysfunction, and infections may occur.[10] Complications may include amyloidosis.[3]
https://en.wikipedia.org/wiki/Multiple_myeloma
Hyperviscosity syndrome is a group of symptoms triggered by an increase in the viscosity of the blood. Symptoms of high blood viscosity include spontaneous bleeding from mucous membranes, visual disturbances due to retinopathy, and neurologic symptoms ranging from headache and vertigo to seizuresand coma.
https://en.wikipedia.org/wiki/Hyperviscosity_syndrome
Amyloidosis is a group of diseases in which abnormal proteins, known as amyloid fibrils, build up in tissue.[4] There are several types with varying symptoms; signs and symptoms may include diarrhea, weight loss, feeling tired, enlargement of the tongue, bleeding, numbness, feeling faint with standing, swelling of the legs, or enlargement of the spleen.[2]
https://en.wikipedia.org/wiki/Amyloidosis
Smouldering myeloma, is a disease classified as intermediate in a spectrum of step-wise progressive diseases termed plasma cell dyscrasias. In this spectrum of diseases, a clone of plasma cells secreting monoclonal paraprotein (also termed myeloma protein or M protein) causes the relatively benign disease of monoclonal gammopathy of undetermined significance. This clone proliferates and may slowly evolve into more aggressive sub-clones that cause smouldering multiple myeloma. Further and more rapid evolution causes the overtly malignant stage of multiple myeloma and can subsequently lead to the extremely malignant stage of secondary plasma cell leukemia.[1][2][3] Thus, some patients with smouldering myeloma progress to multiple myeloma and plasma cell leukemia. Smouldering myeloma, however, is not a malignant disease. It is characterised as a pre-malignant disease that lacks symptoms but is associated with bone marrow biopsy showing the presence of an abnormal number of clonal myeloma cells, blood and/or urine containing a myeloma protein, and a significant risk of developing into a malignant disease.[2]
https://en.wikipedia.org/wiki/Smouldering_myeloma
Monoclonal gammopathy of undetermined significance (MGUS) is a plasma cell dyscrasia in which plasma cells or other types of antibody-producing cells secrete a myeloma protein, i.e. an abnormal antibody, into the blood; this abnormal protein is usually found during standard laboratory blood or urine tests. MGUS resembles multiple myeloma and similar diseases, but the levels of antibodies are lower,[2] the number of plasma cells (white blood cells that secrete antibodies) in the bone marrow is lower, and it rarely has symptoms or major problems. However, since MGUS can lead to multiple myeloma, which develops at the rate of about 1.5% a year, or other symptomatic conditions, yearly monitoring is recommended.
The progression from MGUS to multiple myeloma usually involves several steps. In rare cases, it may also be related with a slowly progressive symmetric distal sensorimotor neuropathy.[3]
https://en.wikipedia.org/wiki/Monoclonal_gammopathy_of_undetermined_significance
08-07-2021-1403 - Plasmodium falciparum protazoe RBC infilt 876
Plasmodium falciparum is a unicellular protozoan parasite of humans, and the deadliest species of Plasmodium that causes malaria in humans.[2] The parasite is transmitted through the bite of a female Anopheles mosquito and causes the disease's most dangerous form, falciparum malaria. It is responsible for around 50% of all malaria cases.[3][4] P. falciparum is therefore regarded as the deadliest parasite in humans. It is also associated with the development of blood cancer (Burkitt's lymphoma) and is classified as Group 2A carcinogen.
The species originated from the malarial parasite Laverania found in gorillas, around 10,000 years ago.[5] Alphonse Laveran was the first to identify the parasite in 1880, and named it Oscillaria malariae. Ronald Ross discovered its transmission by mosquito in 1897. Giovanni Battista Grassi elucidated the complete transmission from a female anopheline mosquito to humans in 1898. In 1897, William H. Welch created the name Plasmodium falciparum, which ICZN formally adopted in 1954. P. falciparum assumes several different forms during its life cycle. The human-infective stage are sporozoites from the salivary gland of a mosquito. The sporozoites grow and multiply in the liver to become merozoites. These merozoites invade the erythrocytes (RBCs) to form trophozoites, schizonts and gametocytes, during which the symptoms of malaria are produced. In the mosquito, the gametocytes undergo sexual reproduction to a zygote, which turns into ookinete. Ookinete forms oocytes from which sporozoites are formed.
As of the World Health Organization World Malaria Report 2020, there were 229 million cases of malaria worldwide in 2019, resulting in an estimated 409,000 deaths. Nearly all malarial deaths are caused by P. falciparum, and 94% of such cases occur in Africa. Children under five years of age are most affected, accounting for 67% of the total deaths. In Sub-Saharan Africa, almost 100% of cases were due to P. falciparum, whereas in most other malarial countries, other, less virulent plasmodial species predominate.[6]
https://en.wikipedia.org/wiki/Plasmodium_falciparum
Acute lymphoblastic leukemia (ALL) is a cancer of the lymphoid line of blood cells characterized by the development of large numbers of immature lymphocytes.[1] Symptoms may include feeling tired, pale skin color, fever, easy bleeding or bruising, enlarged lymph nodes, or bone pain.[1] As an acute leukemia, ALL progresses rapidly and is typically fatal within weeks or months if left untreated.[11]
In most cases, the cause is unknown.[2] Genetic risk factors may include Down syndrome, Li-Fraumeni syndrome, or neurofibromatosis type 1.[1] Environmental risk factors may include significant radiation exposure or prior chemotherapy.[1]Evidence regarding electromagnetic fields or pesticides is unclear.[4][6] Some hypothesize that an abnormal immune response to a common infection may be a trigger.[4] The underlying mechanism involves multiple genetic mutations that results in rapid cell division.[2] The excessive immature lymphocytes in the bone marrow interfere with the production of new red blood cells, white blood cells, and platelets.[1] Diagnosis is typically based on blood tests and bone marrow examination.[3]
ALL affected about 876,000 people globally in 2015 and resulted in about 111,000 deaths.[14][10] It occurs most commonly in children, particularly those between the ages of two and five.[15][4] In the United States it is the most common cause of cancer and death from cancer among children.[2] ALL is notable for being the first disseminated cancer to be cured.[16]Survival for children increased from under 10% in the 1960s to 90% in 2015.[2] Survival rates remain lower for babies (50%)[17] and adults (35%).[8] According to the National Cancer Intelligence Network (NCIN), generally for people with ALL: around 70 out of 100 people (70%) will survive their leukemia for 5 years or more after they are diagnosed.
https://en.wikipedia.org/wiki/Acute_lymphoblastic_leukemia
Rinderpest (also cattle plague or steppe murrain) was an infectious viral disease of cattle, domestic buffalo, and many other speciesof even-toed ungulates, including gaurs, buffaloes, large antelope, deer, giraffes, wildebeests, and warthogs.[2] The disease was characterized by fever, oral erosions, diarrhea, lymphoid necrosis, and high mortality. Death rates during outbreaks were usually extremely high, approaching 100% in immunologically naïve populations.[3] Rinderpest was mainly transmitted by direct contact and by drinking contaminated water, although it could also be transmitted by air.[4] After a global eradication campaign since the mid-20th century, the last confirmed case of rinderpest was diagnosed in 2001.[5]
On 14 October 2010, the United Nations Food and Agriculture Organization (FAO) announced that field activities in the decades-long, worldwide campaign to eradicate the disease were ending, paving the way for a formal declaration in June 2011 of the global eradication of rinderpest.[6] On 25 May 2011, the World Organisation for Animal Health announced the free status of the last eight countries not yet recognized (a total of 198 countries were now free of the disease), officially declaring the eradication of the disease.[7] In June 2011, the United Nations FAO confirmed the disease was eradicated, making rinderpest only the second disease in history to be fully wiped out (outside laboratory stocks), following smallpox.[8] In June 2019 the UK destroyed its stocks of rinderpest virus, held at the Pirbright Institute in Surrey, which were most of the world's retained samples. This followed the completion of a digital record of the virus's genetic code, thereby obviating the need to store samples as a protective resource in case the virus re-emerges. Researchers at Pirbright and the United Nations expressed a hope that the other samples in laboratories around the world will also be destroyed, totally eradicating the virus from the Earth.[9]
Rinderpest is believed to have originated in Asia, later spreading through the transport of cattle.[10] The term Rinderpest is a Germanword meaning "cattle-plague".[2][10] The rinderpest virus (RPV) is closely related to the measles and canine distemper viruses.[11] The measles virus possibly emerged from rinderpest as a zoonotic disease around 600 BC, a period that coincides with the rise of large human settlements.[12][13]
https://en.wikipedia.org/wiki/Rinderpest
Viral hypothesis
This hypothesis postulates that an as of yet undiscovered infectious viral agent is the cause of the disease. Evidence for this hypothesis is as follows:
Incubation time is comparable to a lentivirus
Strain variation of different isolates of PrPSc[28]
An increasing titre of PrPSc as the disease progresses suggests a replicating agent.
Plasmacytoma is a plasma cell dyscrasia in which a plasma cell tumour grows within soft tissue or within the axial skeleton.
The International Myeloma Working Group lists three types: solitary plasmacytoma of bone (SPB); extramedullary plasmacytoma (EP), and multiple plasmacytomas that are either primary or recurrent.[1] The most common of these is SPB, accounting for 3–5% of all plasma cell malignancies.[2] SPBs occur as lytic lesions within the axial skeleton and extramedullary plasmacytomas most often occur in the upper respiratory tract (85%), but can occur in any soft tissue. Approximately half of all cases produce paraproteinemia. SPBs and extramedullary plasmacytomas are mostly treated with radiotherapy, but surgery is used in some cases of extramedullary plasmacytoma. The skeletal forms frequently progress to multiple myeloma over the course of 2–4 years.[3]
Due to their cellular similarity, plasmacytomas have to be differentiated from multiple myeloma. For SPB and extramedullary plasmacytoma the distinction is the presence of only one lesion (either in bone or soft tissue), normal bone marrow(<5% plasma cells), normal skeletal survey, absent or low paraprotein and no end organ damage.[1]
https://en.wikipedia.org/wiki/Plasmacytoma
Lymphoproliferative disorders (LPDs) refer to a specific class of diagnoses, comprising a group of several conditions, in which lymphocytes are produced in excessive quantities. These disorders primarily present in patients who have a compromised immune system. Due to this factor, there are instances of these conditions being equated with "immunoproliferative disorders"; although, in terms of nomenclature, lymphoproliferative disorders are a subclass of immunoproliferative disorders—along with hypergammaglobulinemia and paraproteinemias.
https://en.wikipedia.org/wiki/Lymphoproliferative_disorders
Human T-cell lymphotropic virus type 1 or human T-lymphotropic virus (HTLV-I), also called the adult T-cell lymphoma virus type 1, is a retrovirus of the human T-lymphotropic virus (HTLV) family that has been implicated in several kinds of diseases including very aggressive adult T-cell lymphoma (ATL), HTLV-I-associated myelopathy, uveitis, Strongyloides stercoralis hyper-infection and some other diseases. It is thought that about 1–5% of infected persons develop cancer as a result of the infection with HTLV-I over their lifetimes.[1]
Adult T-cell lymphoma (ATL) was discovered in 1977 in Japan. The symptoms of ATL were different from other lymphomas known at the time. It was suggested that ATL is caused by the infection of a retrovirus called ATLV.[2] Strikingly, ATLV had the transforming activity in vitro.[3] These studies established that the retrovirus infection is the cause of ATL. The retrovirus is now generally called HTLV-I because later studies proved that ATLV is the same as the firstly identified human retrovirus called HTLV discovered by Bernard Poiesz and Francis Ruscetti and their co-workers in the laboratory of Robert C. Gallo at the National Cancer Institute.[4] Infection with HTLV-I, like infection with other retroviruses, probably occurs for life. A patient infected with HTLV can be diagnosed when antibodies against HTLV-1 are detected in the serum.[1]
https://en.wikipedia.org/wiki/Human_T-lymphotropic_virus_1
CD30+ cutaneous T-cell lymphoma, also known as primary cutaneous anaplastic large cell lymphoma, is a cutaneous (skin) condition characterized by solitary or localized skin lesions that have a tendency to ulcerate.[1]:738
https://en.wikipedia.org/wiki/CD30%2B_cutaneous_T-cell_lymphoma
CD30, also known as TNFRSF8, is a cell membrane protein of the tumor necrosis factor receptor family and tumor marker.
https://en.wikipedia.org/wiki/CD30
Adult T-cell leukemia/lymphoma (ATL or ATLL) is a rare cancer of the immune system's T-cells[1][2][3] caused by human T cell leukemia/lymphotropic virus type 1 (HTLV-1).[4] All ATL cells contain integrated HTLV-1 provirus further supporting that causal role of the virus in the cause of the neoplasm.[4] A small amount of HTLV-1 individuals progress to develop ATL with a long latency period between infection and ATL development. ATL is categorized into 4 subtypes: acute, smoldering, lymphoma-type, chronic. Acute and Lymphoma-type are known to particularity be aggressive with poorer prognosis. [5]
Globally, the retrovirus HTLV-1 is estimated to infect 20 million people with the incidence of ATL approximately 0.05 per 100,000 with endemic regions such as regions of Japan, as high as 27 per 100,000.[6] However, cases have increased in non-endemic regions with highest incidence of HTLV-1 in southern/northern islands of Japan, Caribbean, Central and South America, intertropical Africa, Romania, northern Iran. ATL normally occurs around the age of 62 years but median age at diagnosis does depend on prevalence of the HTLV-1 infection in the geographic location.[7]
Current treatment regiments for ATL are based on clinical subtype and response to initial therapy. Some therapy modalities for treatment may not available in all countries therefore strategies differ across the world. All patients are referred to clinical trials if available. Beyond clinical trials, treatments are centered on multiagent chemotherapy, zidovudine plus interferon a (AZT/IFN), and allogenic hematopoietic stem cell transplantation (alloHSCT).[6]
https://en.wikipedia.org/wiki/Adult_T-cell_leukemia/lymphoma
Simian-T-lymphotropic viruses, also Simian T-cell leukemia viruses (STLVs), are retroviruses closely related to the human sexually and breastfeeding transmissible viruses HTLV. They have subtypes 1 through 4 as compared to HTLV 1 through 4, and each subtype has its own serovars.[1] Together they comprise PTLVs (primate T-lymphotropic viruses)[1] A study has shown that STLV-1 Tax and SBZ proteins have similar functions to their counterparts of HTLV-1. STLV-1 is oncogenic in Japanese macaques.[2]
In particular, the HTLV-I/STLV-I history might suggest a simian migration from Asia to Africa not much earlier than 19,500–60,000 years ago.[1]
https://en.wikipedia.org/wiki/Simian-T-lymphotropic_virus
The oral polio vaccine (OPV) AIDS hypothesis states that the AIDS pandemic originated from live polio vaccines prepared in chimpanzee tissue cultures, accidentally contaminated with SIV virus and then administered to up to one million Africans between 1957 and 1960 in experimental mass vaccination campaigns.
Data analyses in molecular biology and phylogenetic studies contradict the OPV AIDS hypothesis; consequently, scientific consensus regards the hypothesis as disproven.[1][2][3][4] The journal Nature has described the hypothesis as "refuted".[5]
https://en.wikipedia.org/wiki/Oral_polio_vaccine_AIDS_hypothesis
Lymphomatoid granulomatosis (LYG or LG) is a very rare lymphoproliferative disorder first characterized in 1972.[1]Lymphomatoid means lymphoma-like and granulomatosis denotes the microscopic characteristic of the presence of granulomaswith polymorphic lymphoid infiltrates and focal necrosis within it.
LG most commonly affects middle aged people,[2] but has occasionally been observed in young people.[3] Males are found to be affected twice as often as females.[4]
https://en.wikipedia.org/wiki/Lymphomatoid_granulomatosis
Post-transplant lymphoproliferative disorder (PTLD) is the name given to a B-cell proliferation due to therapeutic immunosuppression after organ transplantation. These patients may develop infectious mononucleosis-like lesions or polyclonal polymorphic B-cell hyperplasia. Some of these B-cells may undergo mutations which will render them malignant, giving rise to a lymphoma.[citation needed]
In some patients, the malignant cell clone can become the dominant proliferating cell type, leading to frank lymphoma, a group of B cell lymphomas occurring in immunosuppressed patients following organ transplant.
https://en.wikipedia.org/wiki/Post-transplant_lymphoproliferative_disorder
Autoimmune lymphoproliferative syndrome (ALPS), is a form of lymphoproliferative disorder (LPDs). It affects lymphocyteapoptosis.[2]
It is a rare genetic disorder of abnormal lymphocyte survival caused by defective Fas mediated apoptosis.[3] Normally, after infectious insult, the immune system down-regulates by increasing Fas expression on activated B and T lymphocytes and Fas-ligand on activated T lymphocytes. Fas and Fas-ligand interact to trigger the caspase cascade, leading to cell apoptosis. Patients with ALPS have a defect in this apoptotic pathway, leading to chronic non-malignant lymphoproliferation, autoimmune disease, and secondary cancers.[4]
https://en.wikipedia.org/wiki/Autoimmune_lymphoproliferative_syndrome
08-11-2021-0446 - 938 - Fungal Leukemia
08-11-2021-0051 - Fungal Leukeamiaes - sp - fungus, fungal derivatives et priming by alkylation agents rads radk immunosups overvac genotox-viral gene transplant-activation of tumor gene-cell signal dysfunction/decay matter/fungai susecept/etc..
Pagetoid reticulosis (also known as "acral mycoses fungoides",[1] "localized epidermotropic reticulosis",[1] "mycosis fungoides palmaris et plantaris",[1] "unilesional mycosis fungoides",[2] and "Woringer–Kolopp disease"[1]) is a cutaneous condition, an uncommon lymphoproliferative disorder, sometimes considered a form of mycosis fungoides.[1]:734
https://en.wikipedia.org/wiki/Pagetoid_reticulosis
Mycosis fungoides, also known as Alibert-Bazin syndrome or granuloma fungoides,[1] is the most common form of cutaneous T-cell lymphoma. It generally affects the skin, but may progress internally over time. Symptoms include rash, tumors, skin lesions, and itchy skin.
While the cause remains unclear, most cases are not hereditary. Most cases are in people over 20 years of age, and it is more common in men than women. Treatment options include sunlight exposure, ultraviolet light, topical corticosteroids, chemotherapy, and radiotherapy.
sp - fungus, fungal derivatives et priming by alkylation agents rads radk immunosups overvac genotox-viral gene transplant-activation of tumor gene-cell signal dysfunction/decay matter/fungai susecept/etc..
https://en.wikipedia.org/wiki/Mycosis_fungoides
While uncommon in solid tumors, chromosomal translocations are a common cause of these diseases. This commonly leads to a different approach in diagnosis and treatment of haematological malignancies.
A subgroup of them are more severe and are known as haematological malignancies (British English)/hematological malignancies (American English) or blood cancer. They may also be referred to as liquid tumors.[3][4]
https://en.wikipedia.org/wiki/Tumors_of_the_hematopoietic_and_lymphoid_tissues
An endospore is a dormant, tough, and non-reproductive structure produced by some bacteria in the phylum Firmicutes.[1][2]
https://en.wikipedia.org/wiki/Endospore
Cry1Ac protoxin is a crystal protein produced by the gram-positive bacterium, Bacillus thuringiensis (Bt) during sporulation. Cry1Ac is one of the delta endotoxins produced by this bacterium which act as insecticides. Because of this, the genes for these have been introduced into commercially important crops by genetic engineering (such as cotton and corn) in order to confer pest resistance on those plants.[1][2][3]
Transgenic Bt cotton initially expressed a single Bt gene, which codes for Cry1Ac.[4] Subsequently, Bt cotton has added other delta endotoxins.[5] Products such as Bt cotton, Bt brinjal and genetically modified maize have received attention due to a number of issues, including genetically modified food controversies,[6][7][8] and the Séralini affair.[9][10]
Cry1Ac is also a mucosal adjuvant (an immune-response enhancer) for humans.[11][12][13] It has been used in research to develop a vaccine against the amoeba Naegleria fowleri.[14] This amoeba can invade and attack the human nervous system and brain, causing primary amoebic meningoencephalitis, which is nearly always fatal.
https://en.wikipedia.org/wiki/Cry1Ac
Toxins
enterotoxin
neurotoxin
hemotoxin
cardiotoxin
phototoxin
Activation of NF-κB: TRADD recruits TRAF2 and RIP. TRAF2 in turn recruits the multicomponent protein kinase IKK, enabling the serine-threonine kinaseRIP to activate it. An inhibitory protein, IκBα, that normally binds to NF-κB and inhibits its translocation, is phosphorylated by IKK and subsequently degraded, releasing NF-κB. NF-κB is a heterodimeric transcription factor that translocates to the nucleus and mediates the transcription of a vast array of proteins involved in cell survival and proliferation, inflammatory response, and anti-apoptotic factors.
https://en.wikipedia.org/wiki/Tumor_necrosis_factor
https://en.wikipedia.org/wiki/Thrombolysis
Plasmin
Coagulative necrosis is a type of accidental cell death typically caused by ischemia or infarction. In coagulative necrosis, the architectures of dead tissue are preserved for at least a couple of days.[1] It is believed that the injury denatures structural proteins as well as lysosomal enzymes, thus blocking the proteolysis of the damaged cells. The lack of lysosomal enzymes allows it to maintain a "coagulated" morphology for some time. Like most types of necrosis, if enough viable cells are present around the affected area, regeneration will usually occur. Coagulative necrosis occurs in most bodily organs, excluding the brain.[2] Different diseases are associated with coagulative necrosis, including acute tubular necrosis and acute myocardial infarction.[2]
Coagulative necrosis can also be induced by high local temperature; it is a desired effect of treatments such as high intensity focused ultrasound applied to cancerous cells.[3]
https://en.wikipedia.org/wiki/Coagulative_necrosis
Putrefying/decay bacteria are bacteria involved in putrefaction of living matter. Along with other decomposers, they play a critical role in recycling nitrogen from dead organisms.[1]
Nitrogen cycle[edit]
Putrefying bacteria use amino acids or urea as an energy source to decompose dead organisms. In the process, they produce ammonium ions. Nitrifying bacteria then convert this ammonium into nitrate, which can then be used by plants to create more proteins thus completing the nitrogen cycle.[2]
https://en.wikipedia.org/wiki/Putrefying_bacteria
Clostridium perfringens (formerly known as C. welchii, or Bacillus welchii) is a Gram-positive, rod-shaped, anaerobic, spore-forming pathogenic bacterium of the genus Clostridium.[1][2] C. perfringens is ever-present in nature and can be found as a normal component of decaying vegetation, marine sediment, the intestinal tract of humans and other vertebrates, insects, and soil. It has the shortest reported generation time of any organism at 6.3 minutes in thioglycolate medium.[3]
C. perfringens is one of the most common causes of food poisoning in the United States, alongside norovirus, Salmonella, Campylobacter, and Staphylococcus aureus.[4] However, it can sometimes be ingested and cause no harm.[5]
Infections due to C. perfringens show evidence of tissue necrosis, bacteremia, emphysematous cholecystitis, and gas gangrene, also known as clostridial myonecrosis. The specific name perfringens is derived from the Latin per (meaning "through") and frango ("burst"), referring to the disruption of tissue that occurs during gas gangrene.[6] The toxin involved in gas gangrene is α-toxin, which inserts into the plasma membrane of cells, producing gaps in the membrane that disrupt normal cellular function. C. perfringens can participate in polymicrobial anaerobic infections. It is commonly encountered in infections as a component of the normal flora. In this case, its role in disease is minor.
The action of C. perfringens on dead bodies is called tissue gas. It causes extremely accelerated decomposition, and cannot be stopped by normal embalming measures. These bacteria are resistant to the presence of formaldehyde in normal concentrations.
https://en.wikipedia.org/wiki/Clostridium_perfringens
Lysinibacillus fusiformis (commonly abbreviated L. fusiformis) is a gram-positive, rod-shaped bacterium of the genus Lysinibacillus.[1] Scientists have yet to completely characterize this microbe's pathogenic nature.[2][3]Though little is known about this organism, several genome sequencing projects for various strains of L. fusiformis are currently underway.[4]
https://en.wikipedia.org/wiki/Lysinibacillus_fusiformis
This practice largely died out after the introduction of antibiotics, acetonitrile,[citation needed] and enzyme to the range of treatments for wounds.
https://en.wikipedia.org/wiki/Gangrene
Penicillins (P, PCN or PEN) are a group of antibiotics originally obtained from Penicillium moulds, principally P. chrysogenum and P. rubens. Most penicillins in clinical use are chemically synthesised from naturally-produced penicillins. A number of natural penicillins have been discovered, but only two purified compounds are in clinical use: penicillin G (intravenous use) and penicillin V (given by mouth). Penicillins were among the first medications to be effective against many bacterial infections caused by staphylococciand streptococci. They are members of the β-lactam antibiotics.[2] They are still widely used today for different bacterial infections, though many types of bacteria have developed resistance following extensive use.
About 10% of people report that they are allergic to penicillin; however, up to 90% of this group may not actually be allergic.[3] Serious allergies only occur in about 0.03%.[for whom?][3] Those who are allergic to penicillin are most often given cephalosporin C (another β-lactam antibiotic) because there is only 10% crossover in allergy between the penicillins and cephalosporins.[2]
Penicillin was discovered in 1928 by Scottish scientist Alexander Fleming as a crude extract of P. rubens.[4] Fleming's student Cecil George Paine was the first to successfully use penicillin to treat eye infection (ophthalmia neonatorum) in 1930. The purified compound (penicillin F) was isolated in 1940 by a research team led by Howard Florey and Ernst Boris Chain at the University of Oxford. Fleming first used the purified penicillin to treat streptococcal meningitis in 1942.[5] For the discovery, Fleming shared the 1945 Nobel Prize in Physiology or Medicine with Florey and Chain.
Several semisynthetic penicillins are effective against a broader spectrum of bacteria: these include the antistaphylococcal penicillins, aminopenicillins and the antipseudomonal penicillins.
https://en.wikipedia.org/wiki/Penicillin
08-03-2021-1538 radioactive comp salt potas chlor trihydro cat distance incap wep fusor maser etc.
08-03-2021-1538 radioactive comp salt potas chlor trihydro cat distance incap wep fusor maser etc.
08-04-2021-1932 - Intercalation Alkylation Radioactive Potas Trihydrocat OxygenTri/ozone/etc. genotox rad DM DVM
The genotoxic substances induce damage to the genetic material in the cells through interactions with the DNA sequence and structure. For example, the transition metal chromium interacts with DNA in its high-valent oxidation state so to incur DNA lesions leading to carcinogenesis. The metastable oxidation state Cr(V) is achieved through reductive activation. The researchers performed an experiment to study the interaction between DNA with the carcinogenic chromium by using a Cr(V)-Salen complex at the specific oxidation state.[3] The interaction was specific to the guanine nucleotide in the genetic sequence. In order to narrow the interaction between the Cr(V)-Salen complex with the guanine base, the researchers modified the bases to 8-oxo-G so to have site specific oxidation. The reaction between the two molecules caused DNA lesions; the two lesions observed at the modified base site were guanidinohydantoin and spiroiminodihydantoin. To further analyze the site of lesion, it was observed that polymerase stopped at the site and adenine was inappropriately incorporated into the DNA sequence opposite of the 8-oxo-G base.
https://en.wikipedia.org/wiki/Genotoxicity
Acute lymphoblastic leukemia
https://en.wikipedia.org/wiki/Acute_lymphoblastic_leukemia
http://www.thisdayinquotes.com/2019/05/luke-i-am-your-father-most-famous-movie.html
https://memegenerator.net/instance/69083853/luke-skywalker-and-darth-vader-luke-i-am-your-father
https://nikiyaantonbettey.blogspot.com/2021/08/08-04-2021-1932-intercalation.html
Subscribe to:
Comments (Atom)