8-Aminoquinoline is the 8-amino derivative of quinoline. Often abbreviated AQ, it is a pale yellow solid. It is structurally analogous to 8-hydroxyquinoline.[2]
https://en.wikipedia.org/wiki/8-Aminoquinoline
https://en.wikipedia.org/wiki/Anabaena
Common side effects include nausea, vomiting, diarrhea and upset stomach.[4] An allergic reaction, such as anaphylaxis, QT prolongation, or a type of diarrhea caused by Clostridium difficile is possible.[4] No harm has been found with its use during pregnancy.[4] Its safety during breastfeeding is not confirmed, but it is likely safe.[5] Azithromycin is an azalide, a type of macrolide antibiotic.[4] It works by decreasing the production of protein, thereby stopping bacterial growth.[4][6]
Azithromycin was discovered in 1980 by the Croatian pharmaceutical company Pliva and approved for medical use under the brand name Sumamed in 1988.[7][8] It is on the World Health Organization's List of Essential Medicines.[9] The World Health Organization classifies it as critically important for human medicine.[10] It is available as a generic medication[11]and is sold under many trade names worldwide.[1] In 2019, it was the 48th most commonly prescribed medication in the United States, with more than 15 million prescriptions.[12][13]
https://en.wikipedia.org/wiki/Azithromycin
Chloroquine is a medication primarily used to prevent and treat malaria in areas where malaria remains sensitive to its effects.[1] Certain types of malaria, resistant strains, and complicated cases typically require different or additional medication.[1] Chloroquine is also occasionally used for amebiasis that is occurring outside the intestines, rheumatoid arthritis, and lupus erythematosus.[1] While it has not been formally studied in pregnancy, it appears safe.[1][2] It was studied to treat COVID-19 early in the pandemic, but these studies were largely halted in the summer of 2020, and is not recommended for this purpose.[3] It is taken by mouth.[1]
Common side effects include muscle problems, loss of appetite, diarrhea, and skin rash.[1] Serious side effects include problems with vision, muscle damage, seizures, and low blood cell levels.[1][4] Chloroquine is a member of the drug class 4-aminoquinoline.[1] As an antimalarial, it works against the asexual form of the malaria parasite in the stage of its life cycle within the red blood cell.[1] How it works in rheumatoid arthritis and lupus erythematosus is unclear.[1]
Chloroquine was discovered in 1934 by Hans Andersag.[5][6] It is on the World Health Organization's List of Essential Medicines.[7] It is available as a generic medication.[1]
https://en.wikipedia.org/wiki/Chloroquine
https://en.wikipedia.org/wiki/Category:Antiprotozoal_agents
Antiprotozoal agents (ATC code: ATC P01) is a class of pharmaceuticals used in treatment of protozoan infection.
A paraphyletic group, protozoans have little in common with each other. For example, Entamoeba histolytica, a unikont eukaryotic organism, is more closely related to Homo sapiens (humans), which also belongs to the unikont phylogenetic group, than it is to Naegleria fowleri, a "protozoan" bikont. As a result, agents effective against one pathogen may not be effective against another.
Antiprotozoal agents can be grouped by mechanism[1] or by organism.[2] Recent papers have also proposed the use of viruses to treat infections caused by protozoa.[3][4]
https://en.wikipedia.org/wiki/Antiprotozoal
03-19-2022-2341 - Sulfadoxine
Sulfadoxine (also spelled sulphadoxine) is an ultra-long-lasting sulfonamide used in combination with pyrimethamine to treat malaria.[1]
It is also used to prevent malaria[2] but due to high levels of sulphadoxine-pyrimethamine resistance, this use has become less common.[3]
It is also used, usually in combination with other drugs, to treat or prevent various infections in livestock.[citation needed]
https://en.wikipedia.org/wiki/Sulfadoxine
sulfer diox
https://en.wikipedia.org/wiki/Organic_peroxides
https://en.wikipedia.org/wiki/Quinine
https://en.wikipedia.org/wiki/Doxycycline
https://en.wikipedia.org/wiki/Antiprotozoal
https://en.wikipedia.org/wiki/Paromomycin
https://en.wikipedia.org/wiki/Benzamidine
https://en.wikipedia.org/wiki/Fexinidazole
https://en.wikipedia.org/wiki/Amphotericin_B
https://en.wikipedia.org/wiki/Naphthalenesulfonate
https://en.wikipedia.org/wiki/Sodium_stibogluconate
https://en.wikipedia.org/wiki/Neomycin
https://en.wikipedia.org/wiki/Metronidazole
https://en.wikipedia.org/wiki/Benzimidazole
https://en.wikipedia.org/wiki/Nitrofuran
https://en.wikipedia.org/wiki/Tetracycline
https://en.wikipedia.org/wiki/Amoebozoa
https://en.wikipedia.org/wiki/Diloxanide
https://en.wikipedia.org/wiki/Diiodohydroxyquinoline
https://en.wikipedia.org/wiki/Category:Biocides
An amebicide (or amoebicide) is an agent that is destructive to amoeba, especially parasitic amoeba that cause amoebiasis.[1]
https://en.wikipedia.org/wiki/Amebicide
07-14-2022-0452 - Antimalarial arsenic 8-aminoquinoline quinoline quinine sulfadoxine antiprotozoal agent azobenzene benzene azide phosphor dimer organoarsenic sarin prussian blue agent blue lead white black grey iodine silver acid hydrogen acid pressure fine variance gradient proton photon phonon phosphorous phosphorus ivermectin sulfur dioxide organic peroxides polio fail anti-fungals sulfa drugs amebicide doxycycline mycin amidine zole zoal line cin sulfonate gluconate furan ine
https://en.wikipedia.org/wiki/Azinphos-methyl
https://en.wikipedia.org/wiki/4-Phenylthiosemicarbazide
https://en.wikipedia.org/wiki/Pirimiphos-methyl
https://en.wikipedia.org/wiki/Potassium_bromide
https://en.wikipedia.org/wiki/Tributyltin
https://en.wikipedia.org/wiki/Niclosamide
https://en.wikipedia.org/wiki/Vinyl_sulfone
https://en.wikipedia.org/wiki/Zyklon_B
https://en.wikipedia.org/wiki/Cryolite
https://en.wikipedia.org/wiki/Crimidine
https://en.wikipedia.org/wiki/Chlorthiophos
https://en.wikipedia.org/wiki/Chromated_copper_arsenate
https://en.wikipedia.org/wiki/Indoxacarb
https://en.wikipedia.org/wiki/Dichlofenthion
https://en.wikipedia.org/wiki/Hexachlorocyclohexane
https://en.wikipedia.org/wiki/Borate
https://en.wikipedia.org/wiki/5-Hydroxy-2(5H)-furanone
https://en.wikipedia.org/wiki/Metam_sodium
https://en.wikipedia.org/wiki/Bromine_monochloride
https://en.wikipedia.org/wiki/Acephate
https://en.wikipedia.org/wiki/Mothball
https://en.wikipedia.org/wiki/Defoliant
https://en.wikipedia.org/wiki/Category:Pesticides
https://en.wikipedia.org/wiki/Electropositive_shark_repellent
https://en.wikipedia.org/wiki/Knockdown_resistance
https://en.wikipedia.org/wiki/Methamidophos
https://en.wikipedia.org/wiki/Strychnine
https://en.wikipedia.org/wiki/Picrotoxin
https://en.wikipedia.org/wiki/Cicutoxin
https://en.wikipedia.org/wiki/Muscimol
https://en.wikipedia.org/wiki/Sulfuryl_fluoride
https://en.wikipedia.org/wiki/Cyclosarin
https://en.wikipedia.org/wiki/TBPS
https://en.wikipedia.org/wiki/Dimethylmercury
https://en.wikipedia.org/wiki/Toxopyrimidine
https://en.wikipedia.org/wiki/IDPN_(chemical)
https://en.wikipedia.org/wiki/Botulinum_toxin
https://en.wikipedia.org/wiki/Anatoxin-a
History[edit]
Anatoxin-a was first discovered by P.R. Gorham in the early 1960s, after several herds of cattle died as a result of drinking water from Saskatchewan Lake in Ontario, Canada, which contained toxic algal blooms. It was isolated in 1972 by J.P. Devlin from the cyanobacteria Anabaena flos-aquae.[2]
Occurrence[edit]
Anatoxin-a is a neurotoxin produced by multiple genera of freshwater cyanobacteria that are found in water bodies globally.[3] Some freshwater cyanobacteria are known to be salt tolerant and thus it is possible for anatoxin-a to be found in estuarine or other saline environments.[4] Blooms of cyanobacteria that produce anatoxin-a among other cyanotoxins are increasing in frequency due to increasing temperatures, stratification, and eutrophication due to nutrient runoff.[5] These expansive cyanobacterial harmful algal blooms, known as cyanoHABs, increase the amount of cyanotoxins in the surrounding water, threatening the health of both aquatic and terrestrial organisms.[6] Some species of cyanobacteria that produce anatoxin-a don't produce surface water blooms but instead form benthic mats. Many cases of anatoxin-a related animal deaths have occurred due to ingestion of detached benthic cyanobacterial mats that have washed ashore.[7]
Anatoxin-a producing cyanobacteria have also been found in soils and aquatic plants. Anatoxin-a sorbs well to negatively charged sites in clay-like, organic-rich soils and weakly to sandy soils. One study found both bound and free anatoxin-a in 38% of aquatic plants sampled across 12 Nebraskan reservoirs, with much higher incidence of bound anatoxin-a than free.[8]
Experimental studies[edit]
In 1977, Carmichael, Gorham, and Biggs experimented with anatoxin-a. They introduced toxic cultures of A. flos-aquae into the stomachs of two young male calves, and observed that muscular fasciculations and loss of coordination occurred in a matter of minutes, while death due to respiratory failure occurred anywhere between several minutes and a few hours. They also established that extensive periods of artificial respiration did not allow for detoxification to occur and natural neuromuscular functioning to resume. From these experiments, they calculated that the oral minimum lethal dose (MLD) (of the algae, not the anatoxin molecule), for calves is roughly 420 mg/kg body weight.[9]
In the same year, Devlin and colleagues discovered the bicyclic secondary amine structure of anatoxin-a. They also performed experiments similar to those of Carmichael et al. on mice. They found that anatoxin-a kills mice 2–5 minutes after intraperitoneal injection preceded by twitching, muscle spasms, paralysis and respiratory arrest, hence the name Very Fast Death Factor.[10] They determined the LD50 for mice to be 250 µg/kg body weight.[1]
Electrophysiological experiments done by Spivak et al. (1980) on frogs showed that anatoxin-a is a potent agonist of the muscle-type (α1)2βγδ nAChR. Anatoxin-a induced depolarizing neuromuscular blockade, contracture of the frog's rectus abdominis muscle, depolarization of the frog sartorius muscle, desensitization, and alteration of the action potential. Later, Thomas et al., (1993) through his work with chicken α4β2 nAChR subunits expressed on mouse M 10 cells and chicken α7nAChR expressed in oocytes from Xenopus laevis, showed that anatoxin-a is also a potent agonist of neuronal nAChR.[1]

Cocaine, a precursor for anatoxin-a synthesis
Stability and degradation[edit]
Anatoxin-a is unstable in water and other natural conditions, and in the presence of UV light undergoes photodegradation, being converted to the less toxic products dihydroanatoxin-a and epoxyanatoxin-a. The photodegradation of anatoxin-a is dependent on pH and sunlight intensity but independent of oxygen, indicating that the degradation by light is not achieved through the process of photo-oxidation.[20]
Studies have shown that some microorganisms are capable of degrading anatoxin-a. A study done by Kiviranta and colleagues in 1991 showed that the bacterial genus Pseudomonas was capable of degrading anatoxin-a at a rate of 2–10 μg/ml per day.[29] Later experiments done by Rapala and colleagues (1994) supported these results. They compared the effects of sterilized and non-sterilized sediments on anatoxin-a degradation over the course of 22 days, and found that after that time vials with the sterilized sediments showed similar levels of anatoxin-a as at the commencement of the experiment, while vials with non-sterilized sediment showed a 25-48% decrease.[20]
https://en.wikipedia.org/wiki/Anatoxin-a
https://en.wikipedia.org/wiki/Aphanizomenon
https://en.wikipedia.org/wiki/Aphanizomenon
https://en.wikipedia.org/wiki/Microcystis
https://en.wikipedia.org/wiki/Oscillatoria
https://en.wikipedia.org/wiki/Nostoc
Planktothrix

Planktothrix rubescens
Scientific classification
Domain: Bacteria
Phylum: Cyanobacteria
Class: Cyanophyceae
Order: Oscillatoriales
Family: Microcoleaceae
Genus: Planktothrix
Anagnostidis & Komárek, 1988
https://en.wikipedia.org/wiki/Planktothrix
Spirulina is a genus of cyanobacteria.
| Spirulina | |
|---|---|
 | |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Cyanobacteria |
| Class: | Cyanophyceae |
| Order: | Spirulinales |
| Family: | Spirulinaceae |
| Genus: | Spirulina Turpin ex Gomont, 1892 |
| Species | |
See text | |
https://en.wikipedia.org/wiki/Spirulina_(genus)
https://en.wikipedia.org/wiki/Tetrathiomolybdate
Pages in category "Spirulinales"
The following 9 pages are in this category, out of 9 total. This list may not reflect recent changes (learn more).
S
- Spirulina (genus)
- Spirulina abbreviata
- Spirulina agilis
- Spirulina agilissima
- Spirulina albida
- Spirulina ardissoni
- Spirulina magnifica
| Spirochaetes | |
|---|---|
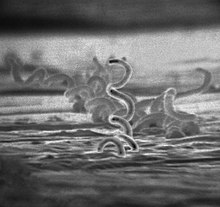 | |
| Treponema pallidum, a spirochaete which causes syphilis | |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Spirochaetota Garrity and Holt 2021[3] |
| Class: | Spirochaetia Paster 2020[1][2] |
| Orders | |
| |
| Synonyms | |
| |
| Spirochaetales | |
|---|---|
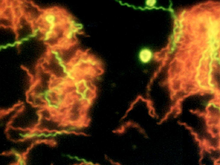 | |
| A microscopic photograph of fluorescent-stained Spirochaeta americana | |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Spirochaetota |
| Class: | Spirochaetia |
| Order: | Spirochaetales Buchanan 1917[1] (Approved Lists 1980)[2] |
| Families | |
| |
| Fusobacterium necrophorum | |
|---|---|
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Fusobacteriota |
| Class: | Fusobacteriia |
| Order: | Fusobacteriales |
| Family: | Fusobacteriaceae |
| Genus: | Fusobacterium |
| Species: | F. necrophorum |
| Binomial name | |
| Fusobacterium necrophorum (Flügge 1886) Moore and Holdeman 1969[1] | |
Treatment[edit]
F. necrophorum infection (also called F-throat[11]) usually responds to treatment with penicillin or metronidazole, but penicillin treatment for persistent pharyngitisappears anecdotally to have a higher relapse rate, although the reasons are unclear.[citation needed]
benzyl penicillinA bacteriophage (/bækˈtɪərioʊfeɪdʒ/), also known informally as a phage (/ˈfeɪdʒ/), is a virus that infects and replicates within bacteria and archaea. The term was derived from "bacteria" and the Greek φαγεῖν (phagein), meaning "to devour". Bacteriophages are composed of proteins that encapsulate a DNA or RNA genome, and may have structures that are either simple or elaborate. Their genomes may encode as few as four genes (e.g. MS2) and as many as hundreds of genes. Phages replicate within the bacterium following the injection of their genome into its cytoplasm.
Bacteriophages are among the most common and diverse entities in the biosphere.[2] Bacteriophages are ubiquitous viruses, found wherever bacteria exist. It is estimated there are more than 1031 bacteriophages on the planet, more than every other organism on Earth, including bacteria, combined.[3] Viruses are the most abundant biological entity in the water column of the world's oceans, and the second largest component of biomass after prokaryotes,[4] where up to 9x108 virions per millilitre have been found in microbial mats at the surface,[5] and up to 70% of marine bacteria may be infected by phages.[6]
Phages have been used since the late 20th century as an alternative to antibiotics in the former Soviet Union and Central Europe, as well as in France.[7][8] They are seen as a possible therapy against multi-drug-resistant strains of many bacteria (see phage therapy).[9][10][11][12] Phages are known to interact with the immune system both indirectly via bacterial expression of phage-encoded proteins and directly by influencing innate immunity and bacterial clearance.[13]
The lytic cycle (/ˈlɪtɪk/ LIT-ik) is one of the two cycles of viral reproduction (referring to bacterial viruses or bacteriophages), the other being the lysogenic cycle. The lytic cycle results in the destruction of the infected celland its membrane. Bacteriophages that only use the lytic cycle are called virulent phages (in contrast to temperatephages).
In the lytic cycle, the viral DNA exists as a separate free floating molecule within the bacterial cell, and replicates separately from the host bacterial DNA, whereas in the lysogenic cycle, the viral DNA is located within the host DNA. This is the key difference between the lytic and lysogenic (bacterio)phage cycles. However, in both cases the virus/phage replicates using the host DNA machinery.
Description[edit]
The lytic cycle, which is also commonly referred to as the "reproductive cycle" of the bacteriophage, is a six-stage cycle. The six stages are: attachment, penetration, transcription, biosynthesis, maturation, and lysis.
- Attachment – the phage attaches itself to the surface of the host cell in order to inject its DNA into the cell
- Penetration – the phage injects its DNA into the host cell by penetrating through the cell membrane
- Transcription – the host cell's DNA is degraded and the cell's metabolism is directed to initiate phage biosynthesis
- Biosynthesis – the phage DNA replicates inside the cell, synthesizing new phage DNA and proteins
- Maturation – the replicated material assembles into fully formed viral phages (each made up of a head, a tail and tail fibers)
- Lysis – the newly formed phages are released from the infected cell (which is itself destroyed in the process) to seek out new host cells to infect
Gene regulation biochemistry[edit]
There are three classes of genes in the phage genome that regulate whether the lytic or lysogenic cycles will emerge. The first class is the immediate early genes, the second is the delayed early genes and the third is the late genes. The following refers to the well-studied temperate phage lambda of E. coli.
- Immediate early genes: These genes are expressed from promoters recognized by the host RNA polymerase, and include Cro, cII, and N. CII is a transcription factor that stimulates expression of the main lysogenic repressor gene, cI, whereas Cro is a repressor for cI expression. The lysis-lysogeny decision is mainly influenced by the competition between Cro and CII, resulting in the determination of whether or not sufficient CI repressor is made. If so, CI represses the early promoters and the infection is shunted into the lysogenic pathway. N is an anti-termination factor that is needed for the transcription of the delayed early genes.
- Delayed early genes: These include the replication genes O and P and also Q, which encodes the anti-terminator responsible for transcription of all the late genes.
- Late genes:
Q-mediated turn-on of late transcription begins about 6–8 min after infection if the lytic pathway is chosen. More than 25 genes are expressed from the single late promoter, resulting in four parallel biosynthetic pathways. Three of the pathways are for production of the three components of the virion: the DNA-filled head, the tail, and the side tail fibers. The virions self-assemble from these components, with the first virion appearing at about 20 min after infection. The fourth pathway is for lysis. In lambda 5 proteins are involved in lysis: the holin and antiholin from gene S, the endolysin from gene R and the spanin proteins from genes Rz and Rz1. In wild-type lambda, lysis occurs at about 50 min, releasing approximately 100 completed virions. The timing of lysis is determined by the holin and antiholin proteins, with the latter inhibiting the former. In overview, the holin protein accumulates in the cytoplasmic membrane until suddenly forming micron-scale holes, which triggers lysis. The endolysin R is released to the periplasm, where it attacks the peptidoglycan. The spanin proteins Rz and Rz1 accumulate in the cytoplasmic and outer membranes, respectively, and form a complex spanning the periplasm through the meshwork of the peptidoglycan. When the endolysin degrades the peptidoglycan, the spanin complexes are liberated and cause disruption of the outer membrane. Destruction of the peptidoglycan by the endolysin and disruption of the outer membrane by the spanin complex are both required for lysis in lambda infections.
Lysis inhibition: T4-like phages have two genes, rI and rIII, that inhibit the T4 holin, if the infected cell undergoes super-infection by another T4 (or closely related) virion. Repeated super-infection can cause the T4 infection to continue without lysis for hours, leading to accumulation of virions to levels 10-fold higher than normal.[4]
https://en.wikipedia.org/wiki/Lytic_cycle
https://en.wikipedia.org/wiki/X-ray
Soft and hard X-rays[edit]
X-rays with high photon energies above 5–10 keV (below 0.2–0.1 nm wavelength) are called hard X-rays, while those with lower energy (and longer wavelength) are called soft X-rays.[68] The intermediate range with photon energies of several keV is often referred to as tender X-rays. Due to their penetrating ability, hard X-rays are widely used to image the inside of objects, e.g., in medical radiography and airport security. The term X-ray is metonymically used to refer to a radiographic image produced using this method, in addition to the method itself. Since the wavelengths of hard X-rays are similar to the size of atoms, they are also useful for determining crystal structures by X-ray crystallography. By contrast, soft X-rays are easily absorbed in air; the attenuation length of 600 eV (~2 nm) X-rays in water is less than 1 micrometer.[69]
Gamma rays[edit]
There is no consensus for a definition distinguishing between X-rays and gamma rays. One common practice is to distinguish between the two types of radiation based on their source: X-rays are emitted by electrons, while gamma rays are emitted by the atomic nucleus.[70][71][72][73] This definition has several problems: other processes can also generate these high-energy photons, or sometimes the method of generation is not known. One common alternative is to distinguish X- and gamma radiation on the basis of wavelength (or, equivalently, frequency or photon energy), with radiation shorter than some arbitrary wavelength, such as 10−11 m (0.1 Å), defined as gamma radiation.[74] This criterion assigns a photon to an unambiguous category, but is only possible if wavelength is known. (Some measurement techniques do not distinguish between detected wavelengths.) However, these two definitions often coincide since the electromagnetic radiation emitted by X-ray tubes generally has a longer wavelength and lower photon energy than the radiation emitted by radioactive nuclei.[70] Occasionally, one term or the other is used in specific contexts due to historical precedent, based on measurement (detection) technique, or based on their intended use rather than their wavelength or source. Thus, gamma-rays generated for medical and industrial uses, for example radiotherapy, in the ranges of 6–20 MeV, can in this context also be referred to as X-rays.[75]
https://en.wikipedia.org/wiki/X-ray#Soft_and_hard_X-rays
X-ray welding is an experimental welding process that uses a high powered X-ray source to provide thermal energy required to weld materials.[1]
The phrase "X-ray welding" also has an older, unrelated usage in quality control. In this context, an X-ray welder is a tradesman who consistently welds at such a high proficiency that he rarely introduces defects into the weld pool, and is able to recognize and correct defects in the weld pool, during the welding process. It is assumed (or trusted) by the Quality Control Department of a fabrication or manufacturing shop that the welding work performed by an X-ray welder would pass an X-ray inspection. For example, defects like porosity, concavities, cracks, cold laps, slag and tungsten inclusions, lack of fusion & penetration, etc., are rarely seen in a radiographic X-ray inspection of a weldment performed by an X-ray welder. [2]
With the growing use of synchrotron radiation in the welding process, the older usage of the phrase "X-Ray welding" might cause confusion; but the two terms are unlikely to be used in the same work environment because synchrotron radiation (X-Ray) welding is a remotely automated and mechanized process.
https://en.wikipedia.org/wiki/X-ray_welding
Wrought iron is an iron alloy with a very low carbon content (less than 0.08%) in contrast to that of cast iron (2.1% to 4%). It is a semi-fused mass of iron with fibrous slag inclusions (up to 2% by weight), which gives it a "grain" resembling wood that is visible when it is etched, rusted, or bent to the point of failure. Wrought iron is tough, malleable, ductile, corrosion resistant, and easily forge welded, but is more difficult to weld electrically.
https://en.wikipedia.org/wiki/Wrought_iron
Zero-point energy (ZPE) is the lowest possible energy that a quantum mechanical system may have. Unlike in classical mechanics, quantum systems constantly fluctuate in their lowest energy state as described by the Heisenberg uncertainty principle.[1] Therefore, even at absolute zero, atoms and molecules retain some vibrational motion. Apart from atoms and molecules, the empty space of the vacuum also has these properties. According to quantum field theory, the universe can be thought of not as isolated particles but continuous fluctuating fields: matter fields, whose quanta are fermions (i.e., leptons and quarks), and force fields, whose quanta are bosons (e.g., photons and gluons). All these fields have zero-point energy.[2] These fluctuating zero-point fields lead to a kind of reintroduction of an aether in physics[1][3] since some systems can detect the existence of this energy. However, this aether cannot be thought of as a physical medium if it is to be Lorentz invariant such that there is no contradiction with Einstein's theory of special relativity.[1]
https://en.wikipedia.org/wiki/Zero-point_energy
09-14-2021-0315 - Zero-point energy (ZPE)
In astronomy, the zero point in a photometric system is defined as the magnitude of an object that produces 1 count per second on the detector.[1] The zero point is used to calibrate a system to the standard magnitude system, as the flux detected from stars will vary from detector to detector.[2] Traditionally, Vega is used as the calibration star for the zero point magnitude in specific pass bands (U, B, and V), although often, an average of multiple stars is used for higher accuracy.[3] It is not often practical to find Vega in the sky to calibrate the detector, so for general purposes, any star may be used in the sky that has a known apparent magnitude.[4]
https://en.wikipedia.org/wiki/Zero_point_(photometry)