https://en.wikipedia.org/wiki/Category:Absent_body_parts
https://en.wikipedia.org/wiki/NSP1_(rotavirus)
https://en.wikipedia.org/wiki/Fusion_gene
https://en.wikipedia.org/wiki/Fusion_transcript
https://en.wikipedia.org/wiki/Obligate_carrier
https://en.wikipedia.org/wiki/Premutation
https://en.wikipedia.org/wiki/Protein-truncating_variants
https://en.wikipedia.org/wiki/Host-cell_reactivation
https://en.wikipedia.org/wiki/Homoplasmy
https://en.wikipedia.org/wiki/Suppressor_mutation
https://en.wikipedia.org/wiki/Allele_age
https://en.wikipedia.org/wiki/Leafy
https://en.wikipedia.org/wiki/D145E
https://en.wikipedia.org/wiki/DbSNP
https://en.wikipedia.org/wiki/Deletion_(genetics)
https://en.wikipedia.org/wiki/Mutation_bias
https://en.wikipedia.org/wiki/Template:Mutation
https://en.wikipedia.org/wiki/Mutation_Frequency_Decline
https://en.wikipedia.org/wiki/Mutation_rate
https://en.wikipedia.org/wiki/Influenza
https://en.wikipedia.org/wiki/Frameshift_mutation
https://en.wikipedia.org/wiki/Neutral_mutation
https://en.wikipedia.org/wiki/V600E
https://en.wikipedia.org/wiki/Worm_bagging
https://en.wikipedia.org/wiki/Transversion
https://en.wikipedia.org/wiki/Transition_(genetics)
https://en.wikipedia.org/wiki/Splice_site_mutation
https://en.wikipedia.org/wiki/SNPedia
https://en.wikipedia.org/wiki/Slipped_strand_mispairing
https://en.wikipedia.org/wiki/Seneca_white_deer
https://en.wikipedia.org/wiki/Selective_sweep
https://en.wikipedia.org/wiki/Resistance_mutation_(virology)
https://en.wikipedia.org/wiki/Polar_mutation
https://en.wikipedia.org/wiki/Point_accepted_mutation
https://en.wikipedia.org/wiki/Cyclopia
Tetra-amelia syndrome
Other names Autosomal recessive tetraamelia

Violetta, a 1920s sideshow performer with tetra-amelia syndrome
https://en.wikipedia.org/wiki/Tetra-amelia_syndrome
Consanguinity ("blood relation", from Latin consanguinitas) is the characteristic of having a kinship with another person (being descended from a common ancestor).
Many jurisdictions have laws prohibiting people who are related by blood from marrying or having sexual relations with each other. The degree of consanguinity that gives rise to this prohibition varies from place to place.[2] Such rules are also used to determine heirs of an estate according to statutes that govern intestate succession, which also vary from jurisdiction to jurisdiction.[3] In some places and time periods, cousin marriage is allowed or even encouraged; in others, it is taboo, and considered to be incest.
The degree of relative consanguinity can be illustrated with a consanguinity table in which each level of lineal consanguinity (generation or meiosis) appears as a row, and individuals with a collaterally consanguineous relationship share the same row.[4] The Knot System is a numerical notation that describes consanguinity using the Ahnentafel numbers of shared ancestors.[5]
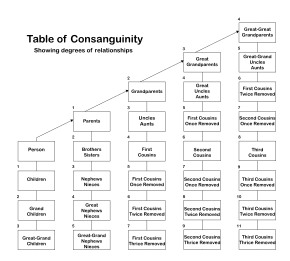
One legal definition of degrees of consanguinity.[1] The number next to each box in the table indicates the degree of relationship relative to the given person.
https://en.wikipedia.org/wiki/Consanguinity
Extracellular ligand disorders
Cytokine
EDA Hypohidrotic ectodermal dysplasia
Camurati–Engelmann disease
Ephrin
Craniofrontonasal dysplasia
WNT
Tetra-amelia syndrome
TGF
OFC 11
Fas ligand
Autoimmune lymphoproliferative syndrome 1B
Endothelin
EDN3 Waardenburg syndrome IVb
Hirschsprung's disease 4
Other
DHH (DHH XY gonadal dysgenesis)
BMP15 (Premature ovarian failure 4)
TSHB (Congenital hypothyroidism 4) See also
intercellular signaling peptides and proteins
RSPO2 and WNT3 genes
Researchers have found loss-of-function mutations in the WNT3 or the RSPO2 genes in people with tetra-amelia syndrome from several consanguineous families. These two gene encode proteins belonging to the WNT pathway which plays critical roles during development.
https://en.wikipedia.org/wiki/Tetra-amelia_syndrome
Presentation
Tetra-amelia syndrome is characterized by the complete absence of all four limbs. The syndrome causes severe malformations of various parts of the body, including the face and head, heart, nervous system, skeleton, and genitalia.[1] In many cases, the lungs are underdeveloped, which makes breathing difficult or impossible. Because children with tetra-amelia syndrome have such serious medical problems, most are stillborn or die shortly after birth
https://en.wikipedia.org/wiki/Tetra-amelia_syndrome
Tetra-amelia syndrome (tetra- + amelia), also called autosomal recessive tetraamelia,[1] is an extremely rare autosomal recessive[2] congenital disorder characterized by the absence of all four limbs. Other areas of the body are also affected by malformations, such as the face, skull, reproductive organs, anus, lungs and pelvis.[1] The disorder can be caused by recessive mutations in the WNT3 or RSPO2 genes.[2][3]
https://en.wikipedia.org/wiki/Tetra-amelia_syndrome
The Wnt signaling pathways are a group of signal transduction pathways which begin with proteins that pass signals into a cell through cell surface receptors. The name Wnt is a portmanteau created from the names Wingless and Int-1.[1] Wnt signaling pathways use either nearby cell-cell communication (paracrine) or same-cell communication (autocrine). They are highly evolutionarily conserved in animals, which means they are similar across animal species from fruit flies to humans.[2][3]
https://en.wikipedia.org/wiki/Wnt_signaling_pathway
The noncanonical planar cell polarity pathway regulates the cytoskeleton that is responsible for the shape of the cell. The noncanonical Wnt/calcium pathway regulates calcium inside the cell.
https://en.wikipedia.org/wiki/Wnt_signaling_pathway
The embryonic processes it controls include body axis patterning, cell fate specification, cell proliferation and cell migration. These processes are necessary for proper formation of important tissues including bone, heart and muscle. Its role in embryonic development was discovered when genetic mutations in Wnt pathway proteins produced abnormal fruit fly embryos. Later research found that the genes responsible for these abnormalities also influenced breast cancer development in mice. Wnt signaling also controls tissue regeneration in adult bone marrow, skin and intestine.[5]
https://en.wikipedia.org/wiki/Wnt_signaling_pathway
A segmentation gene is a generic term for a gene whose function is to specify tissue pattern in each repeated unit of a segmented organism. Animals are constructed of segments; however, Drosophila segments also contain subdivided compartments. There are five gene classes which each contribute to the segmentation and development of the embryonic drosophila. These five gene classes include the coordinate gene, gap gene, pair-rule gene, segment polarity gene, and homeotic gene. In embryonic drosophila, the pair-rule gene defines odd-skipped and even-skipped genes as parasegments, showing 7 stripes in the embryo. In the next gene class, segment polarity gene, individual segments each have their own anterior and posterior pole, resulting in 14 segments.[1][2] In the fruit fly Drosophila melanogaster, segment polarity genes help to define the anterior and posterior polarities within each embryonic parasegment by regulating the transmission of signals via the Wnt signaling pathway and Hedgehog signaling pathway. Segment polarity genes are expressed in the embryo following expression of the gap genes and pair-rule genes. The most commonly cited examples of these genes are engrailed and gooseberry in Drosophila melanogaster.[3] The segment polarity is the last step in embryonic development and a repeated pattern where each half of each segment is deleted and a mirror-image is duplicated and reversed to replace that half segment; thus, forming a pattern element.[4]
https://en.wikipedia.org/wiki/Segment_polarity_gene
Proteins
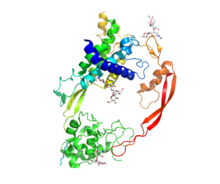
Crystal structure of Wnt8 (rainbow coloring) bound to the cysteine rich domain of Frizzled8 (green).
Wnt comprises a diverse family of secreted lipid-modified signaling glycoproteins that are 350–400 amino acids in length.[11] The lipid modification of all Wnts is palmitoleoylation of a single totally conserved serine residue.[12] Palmitoleoylation is necessary because it is required for Wnt to bind to its carrier protein Wntless (WLS) so it can be transported to the plasma membrane for secretion[13] and it allows the Wnt protein to bind its receptor Frizzled [14][15] Wnt proteins also undergo glycosylation, which attaches a carbohydrate in order to ensure proper secretion.[16] In Wnt signaling, these proteins act as ligands to activate the different Wnt pathways via paracrine and autocrine routes.[2][7]
https://en.wikipedia.org/wiki/Wnt_signaling_pathway

Figure 1. Wnt doesn't bind to the receptor. Axin, GSK and APC form a "destruction complex," and β-Cat is destroyed.
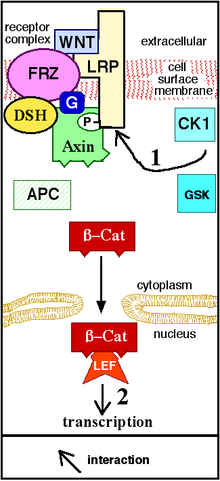
Figure 2. Wnt binds to (activates) the receptor. Axin is removed from the "destruction complex." β-Cat moves into the nucleus, binds to a transcription factor on DNA, and activates transcription of a protein. "P" represents phosphate.
https://en.wikipedia.org/wiki/Wnt_signaling_pathway
Mechanism
Foundation
Wnt signaling begins when a Wnt protein binds to the N-terminal extra-cellular cysteine-rich domain of a Frizzled (Fz) family receptor.[19] These receptors span the plasma membrane seven times and constitute a distinct family of G-protein coupled receptors (GPCRs).[20] However, to facilitate Wnt signaling, co-receptors may be required alongside the interaction between the Wnt protein and Fz receptor. Examples include lipoprotein receptor-related protein (LRP)-5/6, receptor tyrosine kinase (RTK), and ROR2.[7] Upon activation of the receptor, a signal is sent to the phosphoprotein Dishevelled (Dsh), which is located in the cytoplasm. This signal is transmitted via a direct interaction between Fz and Dsh. Dsh proteins are present in all organisms and they all share the following highly conserved protein domains: an amino-terminal DIX domain, a central PDZ domain, and a carboxy-terminal DEP domain. These different domains are important because after Dsh, the Wnt signal can branch off into multiple pathways and each pathway interacts with a different combination of the three domains.[21]
https://en.wikipedia.org/wiki/Wnt_signaling_pathway
Noncanonical pathways
The noncanonical planar cell polarity (PCP) pathway does not involve β-catenin. It does not use LRP-5/6 as its co-receptor and is thought to use NRH1, Ryk, PTK7 or ROR2. The PCP pathway is activated via the binding of Wnt to Fz and its co-receptor. The receptor then recruits Dsh, which uses its PDZ and DIX domains to form a complex with Dishevelled-associated activator of morphogenesis 1 (DAAM1). Daam1 then activates the small G-protein Rho through a guanine exchange factor. Rho activates Rho-associated kinase (ROCK), which is one of the major regulators of the cytoskeleton. Dsh also forms a complex with rac1 and mediates profilin binding to actin. Rac1 activates JNK and can also lead to actin polymerization. Profilin binding to actin can result in restructuring of the cytoskeleton and gastrulation.[7][34]
The noncanonical Wnt/calcium pathway also does not involve β-catenin. Its role is to help regulate calcium release from the endoplasmic reticulum (ER) in order to control intracellular calcium levels. Like other Wnt pathways, upon ligand binding, the activated Fz receptor directly interacts with Dsh and activates specific Dsh-protein domains. The domains involved in Wnt/calcium signaling are the PDZ and DEP domains.[7] However, unlike other Wnt pathways, the Fz receptor directly interfaces with a trimeric G-protein. This co-stimulation of Dsh and the G-protein can lead to the activation of either PLC or cGMP-specific PDE. If PLC is activated, the plasma membrane component PIP2 is cleaved into DAG and IP3. When IP3 binds its receptor on the ER, calcium is released. Increased concentrations of calcium and DAG can activate Cdc42 through PKC. Cdc42 is an important regulator of ventral patterning. Increased calcium also activates calcineurin and CaMKII. CaMKII induces activation of the transcription factor NFAT, which regulates cell adhesion, migration and tissue separation.[7] Calcineurin activates TAK1 and NLK kinase, which can interfere with TCF/β-Catenin signaling in the canonical Wnt pathway.[35] However, if PDE is activated, calcium release from the ER is inhibited. PDE mediates this through the inhibition of PKG, which subsequently causes the inhibition of calcium release.[7]
Integrated Wnt Pathway
The binary distinction of canonical and non-canonical Wnt signaling pathways has come under scrutiny and an integrated, convergent Wnt pathway has been proposed.[36] Some evidence for this was found for one Wnt ligand (Wnt5A).[37] Evidence for a convergent Wnt signaling pathway that shows integrated activation of Wnt/Ca2+ and Wnt/β-catenin signaling, for multiple Wnt ligands, was described in mammalian cell lines.[38]
Other pathways
Dsh can also interact with aPKC, Pa3, Par6 and LGl in order to control cell polarity and microtubule cytoskeleton development. While these pathways overlap with components associated with PCP and Wnt/Calcium signaling, they are considered distinct pathways because they produce different
Regulation
In order to ensure proper functioning, Wnt signaling is constantly regulated at several points along its signaling pathways.[41] For example, Wnt proteins are palmitoylated. The protein porcupine mediates this process, which means that it helps regulate when the Wnt ligand is secreted by determining when it is fully formed. Secretion is further controlled with proteins such as GPR177 (wntless) and evenness interrupted and complexes such as the retromer complex.[7][24]
Upon secretion, the ligand can be prevented from reaching its receptor through the binding of proteins such as the stabilizers Dally and glypican 3 (GPC3), which inhibit diffusion. In cancer cells, both the heparan sulfate chains[42][43] and the core protein[44][45] of GPC3 are involved in regulating Wnt binding and activation for cell proliferation.[46][47] Wnt recognizes a heparan sulfate structure on GPC3, which contains IdoA2S and GlcNS6S, and the 3-O-sulfation in GlcNS6S3S enhances the binding of Wnt to the heparan sulfate glypican.[48] A cysteine-rich domain at the N-lobe of GPC3 has been identified to form a Wnt-binding hydrophobic groove including phenylalanine-41 that interacts with Wnt.[45][49] Blocking the Wnt binding domain using a nanobody called HN3 can inhibit Wnt activation.[45]
At the Fz receptor, the binding of proteins other than Wnt can antagonize signaling. Specific antagonists include Dickkopf (Dkk), Wnt inhibitory factor 1 (WIF-1),[50][51] secreted Frizzled-related proteins (SFRP), Cerberus, Frzb, Wise, SOST, and Naked cuticle. These constitute inhibitors of Wnt signaling. However, other molecules also act as activators. Norrin and R-Spondin2 activate Wnt signaling in the absence of Wnt ligand.
Interactions between Wnt signaling pathways also regulate Wnt signaling. As previously mentioned, the Wnt/calcium pathway can inhibit TCF/β-catenin, preventing canonical Wnt pathway signaling.[7][24] Prostaglandin E2 is an essential activator of the canonical Wnt signaling pathway. Interaction of PGE2 with its receptors E2/E4 stabilizes β-catenin through cAMP/PKA mediated phosphorylation. The synthesis of PGE2 is necessary for Wnt signaling mediated processes such as tissue regeneration and control of stem cell population in zebrafish and mouse.[5] Intriguingly, the unstructured regions of several oversized Intrinsically disordered proteins play crucial roles in regulating Wnt signaling.[52]
Induced cell responses
Embryonic development
Wnt signaling plays a critical role in embryonic development. It operates in both vertebrates and invertebrates, including humans, frogs, zebrafish, C. elegans, Drosophila and others. It was first found in the segment polarity of Drosophila, where it helps to establish anterior and posterior polarities. It is implicated in other developmental processes. As its function in Drosophila suggests, it plays a key role in body axis formation, particularly the formation of the anteroposterior and dorsoventral axes. It is involved in the induction of cell differentiation to prompt formation of important organs such as lungs and ovaries. Wnt further ensures the development of these tissues through proper regulation of cell proliferation and migration. Wnt signaling functions can be divided into axis patterning, cell fate specification, cell proliferation and cell migration.[53]
Axis patterning
In early embryo development, the formation of the primary body axes is a crucial step in establishing the organism's overall body plan. The axes include the anteroposterior axis, dorsoventral axis, and right-left axis. Wnt signaling is implicated in the formation of the anteroposterior and dorsoventral (DV) axes. Wnt signaling activity in anterior-posterior development can be seen in mammals, fish and frogs. In mammals, the primitive streak and other surrounding tissues produce the morphogenic compounds Wnts, BMPs, FGFs, Nodal and retinoic acid to establish the posterior region during late gastrula. These proteins form concentration gradients. Areas of highest concentration establish the posterior region while areas of lowest concentration indicate the anterior region. In fish and frogs, β-catenin produced by canonical Wnt signaling causes the formation of organizing centers, which, alongside BMPs, elicit posterior formation. Wnt involvement in DV axis formation can be seen in the activity of the formation of the Spemann organizer, which establishes the dorsal region. Canonical Wnt signaling β-catenin production induces the formation of this organizer via the activation of the genes twin and siamois.[36][53] Similarly, in avian gastrulation, cells of the Koller's sickle express different mesodermal marker genes that allow for the differential movement of cells during the formation of the primitive streak. Wnt signaling activated by FGFs is responsible for this movement.[54][55]
Wnt signaling is also involved in the axis formation of specific body parts and organ systems later in development. In vertebrates, sonic hedgehog (Shh) and Wnt morphogenetic signaling gradients establish the dorsoventral axis of the central nervous system during neural tube axial patterning. High Wnt signaling establishes the dorsal region while high Shh signaling indicates the ventral region.[56] Wnt is involved in the DV formation of the central nervous system through its involvement in axon guidance. Wnt proteins guide the axons of the spinal cord in an anterior-posterior direction.[57] Wnt is also involved in the formation of the limb DV axis. Specifically, Wnt7a helps produce the dorsal patterning of the developing limb.[36][53]
In the embryonic differentiation waves model of development Wnt plays a critical role as part a signalling complex in competent cells ready to differentiate. Wnt reacts to the activity of the cytoskeleton, stabilizing the initial change created by a passing wave of contraction or expansion and simultaneously signals the nucleus through the use of its different signalling pathways as to which wave the individual cell has participated in. Wnt activity thereby amplifies mechanical signalling that occurs during development.[58][59]
Cell fate specification
Cell fate specification or cell differentiation is a process where undifferentiated cells can become a more specialized cell type. Wnt signaling induces differentiation of pluripotent stem cells into mesoderm and endoderm progenitor cells.[60] These progenitor cells further differentiate into cell types such as endothelial, cardiac and vascular smooth muscle lineages.[61] Wnt signaling induces blood formation from stem cells. Specifically, Wnt3 leads to mesoderm committed cells with hematopoietic potential.[62] Wnt1 antagonizes neural differentiation and is a major factor in self-renewal of neural stem cells. This allows for regeneration of nervous system cells, which is further evidence of a role in promoting neural stem cell proliferation.[60] Wnt signaling is involved in germ cell determination, gut tissue specification, hair follicle development, lung tissue development, trunk neural crest cell differentiation, nephron development, ovary development and sex determination.[53] Wnt signaling also antagonizes heart formation, and Wnt inhibition was shown to be a critical inducer of heart tissue during development,[63][64][65] and small molecule Wnt inhibitors are routinely used to produce cardiomyocytes from pluripotent stem cells.[66][67]
Cell proliferation
In order to have the mass differentiation of cells needed to form the specified cell tissues of different organisms, proliferation and growth of embryonic stem cells must take place. This process is mediated through canonical Wnt signaling, which increases nuclear and cytoplasmic β-catenin. Increased β-catenin can initiate transcriptional activation of proteins such as cyclin D1 and c-myc, which control the G1 to S phase transition in the cell cycle. Entry into the S phase causes DNA replication and ultimately mitosis, which are responsible for cell proliferation.[68] This proliferation increase is directly paired with cell differentiation because as the stem cells proliferate, they also differentiate. This allows for overall growth and development of specific tissue systems during embryonic development. This is apparent in systems such as the circulatory system where Wnt3a leads to proliferation and expansion of hematopoietic stem cells needed for red blood cell formation.[69]
The biochemistry of cancer stem cells is subtly different from that of other tumor cells. These so-called Wnt-addicted cells hijack and depend on constant stimulation of the Wnt pathway to promote their uncontrolled growth, survival and migration. In cancer, Wnt signaling can become independent of regular stimuli, through mutations in downstream oncogenes and tumor suppressor genes that become permanently activated even though the normal receptor has not received a signal. β-catenin binds to transcription factors such as the protein TCF4 and in combination the molecules activate the necessary genes. LF3 strongly inhibits this binding in vitro, in cell lines and reduced tumor growth in mouse models. It prevented replication and reduced their ability to migrate, all without affecting healthy cells. No cancer stem cells remained after treatment. The discovery was the product of "rational drug design", involving AlphaScreens and ELISA technologies.[70]
Cell migration

Diagram illustrating the epithelial-mesenchymal transition
Cell migration during embryonic development allows for the establishment of body axes, tissue formation, limb induction and several other processes. Wnt signaling helps mediate this process, particularly during convergent extension. Signaling from both the Wnt PCP pathway and canonical Wnt pathway is required for proper convergent extension during gastrulation. Convergent extension is further regulated by the Wnt/calcium pathway, which blocks convergent extension when activated. Wnt signaling also induces cell migration in later stages of development through the control of the migration behavior of neuroblasts, neural crest cells, myocytes, and tracheal cells.[71]
Wnt signaling is involved in another key migration process known as the epithelial-mesenchymal transition (EMT). This process allows epithelial cells to transform into mesenchymal cells so that they are no longer held in place at the laminin. It involves cadherin down-regulation so that cells can detach from laminin and migrate. Wnt signaling is an inducer of EMT, particularly in mammary development.[72]
Insulin sensitivity

Diagram illustrating the interaction between the Wnt and insulin signaling pathways
Insulin is a peptide hormone involved in glucose homeostasis within certain organisms. Specifically, it leads to upregulation of glucose transporters in the cell membrane in order to increase glucose uptake from the bloodstream. This process is partially mediated by activation of Wnt/β-catenin signaling, which can increase a cell's insulin sensitivity. In particular, Wnt10b is a Wnt protein that increases this sensitivity in skeletal muscle cells.[73]
https://en.wikipedia.org/wiki/Wnt_signaling_pathway
https://en.wikipedia.org/wiki/Wingless_localisation_element_3_(WLE3)
https://en.wikipedia.org/wiki/MTOR
https://en.wikipedia.org/wiki/MYF5
https://en.wikipedia.org/wiki/Tyrosine-protein_kinase_CSK
https://en.wikipedia.org/wiki/Axon_guidance
https://en.wikipedia.org/wiki/Keratin_1
https://en.wikipedia.org/wiki/Rap1
https://en.wikipedia.org/wiki/CDC42
https://en.wikipedia.org/wiki/Protocadherin
https://en.wikipedia.org/wiki/Microtubule
https://en.wikipedia.org/wiki/Endoplasmic_reticulum
https://en.wikipedia.org/wiki/AXIN1
https://en.wikipedia.org/wiki/Adenomatous_polyposis_coli
https://en.wikipedia.org/wiki/Protein_phosphatase_2
https://en.wikipedia.org/wiki/LRP5
https://en.wikipedia.org/wiki/Ubiquitin#Ubiquitylation
https://en.wikipedia.org/wiki/PYGO2
https://en.wikipedia.org/wiki/Dishevelled
https://en.wikipedia.org/wiki/Morphogenesis
https://en.wikipedia.org/wiki/Cytoskeleton
https://en.wikipedia.org/wiki/Profilin
https://en.wikipedia.org/wiki/RAC1
https://en.wikipedia.org/wiki/Actin
https://en.wikipedia.org/wiki/C-Jun_N-terminal_kinases
https://en.wikipedia.org/wiki/Polymerization
https://en.wikipedia.org/wiki/Free-radical_reaction
https://en.wikipedia.org/wiki/Steric_effects
https://en.wikipedia.org/wiki/Electron_cloud
https://en.wikipedia.org/wiki/Ion
https://en.wikipedia.org/wiki/Magnetic_quantum_number
https://en.wikipedia.org/wiki/Spherical_harmonics#Harmonic_polynomial_representation
In science, the probability of an event is a number that indicates how likely the event is to occur. It is expressed as a number in the range from 0 and 1, or, using percentage notation, in the range from 0% to 100%. The more likely it is that the event will occur, the higher its probability. The probability of an impossible event is 0; that of an event that is certain to occur is 1.[note 1][1][2] The probabilities of two complementary events A and B – either A occurs or B occurs – add up to 1. A simple example is the tossing of a fair (unbiased) coin. If a coin is fair, the two possible outcomes ("heads" and "tails") are equally likely; since these two outcomes are complementary and the probability of "heads" equals the probability of "tails", the probability of each of the two outcomes equals 1/2 (which could also be written as 0.5 or 50%).
These concepts have been given an axiomatic mathematical formalization in probability theory, a branch of mathematics that is used in areas of study such as statistics, mathematics, science, finance, gambling, artificial intelligence, machine learning, computer science and game theory to, for example, draw inferences about the expected frequency of events. Probability theory is also used to describe the underlying mechanics and regularities of complex systems.[3]
https://en.wikipedia.org/wiki/Probability
https://en.wikipedia.org/wiki/Almost_surely
https://en.wikipedia.org/wiki/Likelihood_function
https://en.wikipedia.org/wiki/Posterior_probability
https://en.wikipedia.org/wiki/Bayesian_probability#Objective_and_subjective_Bayesian_probabilities
https://en.wikipedia.org/wiki/Propensity_probability
https://en.wikipedia.org/wiki/Bayesian_probability
https://en.wikipedia.org/wiki/Prior_probability
https://en.wikipedia.org/wiki/Aumann%27s_agreement_theorem
https://en.wikipedia.org/wiki/Glossary_of_probability_and_statistics
https://en.wikipedia.org/wiki/Reconstructive_surgery
https://en.wikipedia.org/wiki/Plastic_surgery
https://en.wikipedia.org/wiki/Microsurgery
https://en.wikipedia.org/wiki/Plastic_surgery#Cosmetic_surgery_procedures
https://en.wikipedia.org/wiki/Botox
https://en.wikipedia.org/wiki/Laser_hair_removal
https://en.wikipedia.org/wiki/Food_and_Drug_Administration
https://en.wikipedia.org/wiki/Assisted_reproductive_technology
https://en.wikipedia.org/wiki/Sperm_donation
https://en.wikipedia.org/wiki/Artificial_insemination
https://en.wikipedia.org/wiki/In_vitro_fertilisation
https://en.wikipedia.org/wiki/Fertility_tourism
Intracytoplasmic sperm injection

Oocyte cytoplasm is injected with the sperm during ICSI
MeSH D020554
[edit on Wikidata]
Intracytoplasmic sperm injection (ICSI /ˈɪksi/ IK-see) is an in vitro fertilization (IVF) procedure in which a single sperm cell is injected directly into the cytoplasm of an egg. This technique is used in order to prepare the gametes for the obtention of embryos that may be transferred to a maternal uterus. With this method, the acrosome reaction is skipped.
There are several differences between classic IVF and ICSI. However, the steps to be followed before and after insemination are the same. In terms of insemination, ICSI needs only one sperm cell per oocyte, while IVF needs 50,000–100,000. This is because the acrosome reaction has to take place and thousands of sperm cells have to be involved in IVF. Once fertilized, the egg is transformed into a pre-embryo and it has to be transferred to the uterus to continue its development.
The first human pregnancy generated by ICSI was carried out in 1991 by Gianpiero Palermo and his team.
https://en.wikipedia.org/wiki/Intracytoplasmic_sperm_injection
Testicular sperm extraction (TESE) is a surgical procedure in which a small portion of tissue is removed from the testicle and any viable sperm cells from that tissue are extracted for use in further procedures, most commonly intracytoplasmic sperm injection (ICSI) as part of in vitro fertilisation (IVF).[1] TESE is often recommended to patients who cannot produce sperm by ejaculation due to azoospermia.[2]
Testicular sperm extraction

Tissue is extracted from the seminiferous tubules during surgery in TESE
Specialty Reproductive medicine
https://en.wikipedia.org/wiki/Testicular_sperm_extraction
TESE is primarily used for non-obstructive azoospermia, where patients do not have sperm present in the ejaculate but who may produce sperm in the testis. Azoospermia in these patients could be a result of Y chromosome microdeletions, cancer of the testicles or damage to the pituitary gland or hypothalamus, which regulate sperm production. Often in these cases, TESE is used as a second option, after prior efforts to treat the azoospermia through hormone therapy have failed.[3]
https://en.wikipedia.org/wiki/Azoospermia
Semen cryopreservation (commonly called sperm banking or sperm freezing) is a procedure to preserve sperm cells. Semen can be used successfully indefinitely[citation needed] after cryopreservation. It can be used for sperm donation where the recipient wants the treatment in a different time or place, or as a means of preserving fertility for men undergoing vasectomy or treatments that may compromise their fertility, such as chemotherapy, radiation therapy or surgery. It is also often used by transgender women prior to medically transitioning in ways that affect fertility, such as feminizing hormone therapy and orchiectomies.
https://en.wikipedia.org/wiki/Semen_cryopreservation
https://en.wikipedia.org/wiki/Category:Assisted_reproductive_technology
https://en.wikipedia.org/wiki/Testicular_sperm_extraction#TESE_vs_TESA
https://en.wikipedia.org/wiki/Category:Fertility_medicine
Ejaculatory disorders include retrograde ejaculation and anejaculation; in these conditions sperm are produced but not expelled.
https://en.wikipedia.org/wiki/Azoospermia
Spermatogenesis is the process by which haploid spermatozoa develop from germ cells in the seminiferous tubules of the testis. This process starts with the mitotic division of the stem cells located close to the basement membrane of the tubules.[1] These cells are called spermatogonial stem cells. The mitotic division of these produces two types of cells. Type A cells replenish the stem cells, and type B cells differentiate into primary spermatocytes. The primary spermatocyte divides meiotically (Meiosis I) into two secondary spermatocytes; each secondary spermatocyte divides into two equal haploid spermatids by Meiosis II. The spermatids are transformed into spermatozoa (sperm) by the process of spermiogenesis. These develop into mature spermatozoa, also known as sperm cells.[2] Thus, the primary spermatocyte gives rise to two cells, the secondary spermatocytes, and the two secondary spermatocytes by their subdivision produce four spermatozoa and four haploid cells.[3]
Spermatozoa are the mature male gametes in many sexually reproducing organisms. Thus, spermatogenesis is the male version of gametogenesis, of which the female equivalent is oogenesis. In mammals it occurs in the seminiferous tubules of the male testes in a stepwise fashion. Spermatogenesis is highly dependent upon optimal conditions for the process to occur correctly, and is essential for sexual reproduction. DNA methylation and histone modification have been implicated in the regulation of this process.[4] It starts during puberty and usually continues uninterrupted until death, although a slight decrease can be discerned in the quantity of produced sperm with increase in age (see Male infertility).
Spermatogenesis starts in the bottom part of seminiferous tubes and, progressively, cells go deeper into tubes and moving along it until mature spermatozoa reaches the lumen, where mature spermatozoa are deposited. The division happens asynchronically; if the tube is cut transversally one could observe different maturation states. A group of cells with different maturation states that are being generated at the same time is called a spermatogenic wave.[5]
Purpose
Spermatogenesis produces mature male gametes, commonly called sperm but more specifically known as spermatozoa, which are able to fertilize the counterpart female gamete, the oocyte, during conception to produce a single-celled individual known as a zygote. This is the cornerstone of sexual reproduction and involves the two gametes both contributing half the normal set of chromosomes (haploid) to result in a chromosomally normal (diploid) zygote.
To preserve the number of chromosomes in the offspring – which differs between species – one of each gamete must have half the usual number of chromosomes present in other body cells. Otherwise, the offspring will have twice the normal number of chromosomes, and serious abnormalities may result. In humans, chromosomal abnormalities arising from incorrect spermatogenesis results in congenital defects and abnormal birth defects (Down syndrome, Klinefelter syndrome) and in most cases, spontaneous abortion of the developing foetus.
Location in humans
Spermatogenesis takes place within several structures of the male reproductive system. The initial stages occur within the testes and progress to the epididymis where the developing gametes mature and are stored until ejaculation. The seminiferous tubules of the testes are the starting point for the process, where spermatogonial stem cells adjacent to the inner tubule wall divide in a centripetal direction—beginning at the walls and proceeding into the innermost part, or lumen—to produce immature sperm.[2] Maturation occurs in the epididymis. The location [Testes/Scrotum] is specifically important as the process of spermatogenesis requires a lower temperature to produce viable sperm, specifically 1°-8 °C lower than normal body temperature of 37 °C (98.6 °F).[6] Clinically, small fluctuations in temperature such as from an athletic support strap, causes no impairment in sperm viability or count.[7]
Duration
For humans, the entire process of spermatogenesis is variously estimated as taking 74 days[8][9] (according to tritium-labelled biopsies) and approximately 120 days[10] (according to DNA clock measurements). Including the transport on ductal system, it takes 3 months. Testes produce 200 to 300 million spermatozoa daily.[11] However, only about half or 100 million of these become viable sperm.[12]
Stages
The entire process of spermatogenesis can be broken up into several distinct stages, each corresponding to a particular type of cell in humans. In the following table, ploidy, copy number and chromosome/chromatid counts are for one cell, generally prior to DNA synthesis and division (in G1 if applicable). The primary spermatocyte is arrested after DNA synthesis and prior to division.
Cell type ploidy/chromosomes in human DNA copy number/chromatids in human Process entered by cell
spermatogonium (types Ad, Ap and B) diploid (2N) / 46 2C / 46 spermatocytogenesis (mitosis)
primary spermatocyte diploid (2N) / 46 4C / 2x46 spermatidogenesis (meiosis I)
two secondary spermatocytes haploid (N) / 23 2C / 2x23 spermatidogenesis (meiosis II)
four spermatids haploid (N) / 23 C / 23 spermiogenesis
four functional spermatozoids haploid (N) / 23 C / 23 spermiation
Spermatocytogenesis
Main article: Spermatocytogenesis

The process of spermatogenesis as the cells progress from primary spermatocytes, to secondary spermatocytes, to spermatids, to Sperm

Cycle of the seminiferous epithelium of the testis
Spermatocytogenesis is the male form of gametocytogenesis and results in the formation of spermatocytes possessing half the normal complement of genetic material. In spermatocytogenesis, a diploid spermatogonium, which resides in the basal compartment of the seminiferous tubules, divides mitotically, producing two diploid intermediate cells called primary spermatocytes. Each primary spermatocyte then moves into the adluminal compartment of the seminiferous tubules and duplicates its DNA and subsequently undergoes meiosis I to produce two haploid secondary spermatocytes, which will later divide once more into haploid spermatids. This division implicates sources of genetic variation, such as random inclusion of either parental chromosomes, and chromosomal crossover that increases the genetic variability of the gamete. The DNA damage response (DDR) machinery plays an important role in spermatogenesis. The protein FMRP binds to meiotic chromosomes and regulates the dynamics of the DDR machinery during spermatogenesis.[13] FMRP appears to be necessary for the repair of DNA damage.
Each cell division from a spermatogonium to a spermatid is incomplete; the cells remain connected to one another by bridges of cytoplasm to allow synchronous development. Not all spermatogonia divide to produce spermatocytes; otherwise, the supply of spermatogonia would run out. Instead, spermatogonial stem cells divide mitotically to produce copies of themselves, ensuring a constant supply of spermatogonia to fuel spermatogenesis.[14]
Spermatidogenesis
Main article: Spermatidogenesis
Spermatidogenesis is the creation of spermatids from secondary spermatocytes. Secondary spermatocytes produced earlier rapidly enter meiosis II and divide to produce haploid spermatids. The brevity of this stage means that secondary spermatocytes are rarely seen in histological studies.
Spermiogenesis
Main article: Spermiogenesis
During spermiogenesis, the spermatids begin to form a tail by growing microtubules on one of the centrioles, which turns into basal body. These microtubules form an axoneme. Later the centriole is modified in the process of centrosome reduction.[15] The anterior part of the tail (called midpiece) thickens because mitochondria are arranged around the axoneme to ensure energy supply. Spermatid DNA also undergoes packaging, becoming highly condensed. The DNA is packaged firstly with specific nuclear basic proteins, which are subsequently replaced with protamines during spermatid elongation. The resultant tightly packed chromatin is transcriptionally inactive. The Golgi apparatus surrounds the now condensed nucleus, becoming the acrosome.
Maturation then takes place under the influence of testosterone, which removes the remaining unnecessary cytoplasm and organelles. The excess cytoplasm, known as residual bodies, is phagocytosed by surrounding Sertoli cells in the testes. The resulting spermatozoa are now mature but lack motility. The mature spermatozoa are released from the protective Sertoli cells into the lumen of the seminiferous tubule in a process called spermiation.
The non-motile spermatozoa are transported to the epididymis in testicular fluid secreted by the Sertoli cells with the aid of peristaltic contraction. While in the epididymis the spermatozoa gain motility and become capable of fertilization. However, transport of the mature spermatozoa through the remainder of the male reproductive system is achieved via muscle contraction rather than the spermatozoon's recently acquired motility.
Role of Sertoli cells
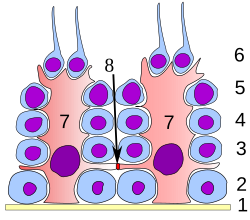
Labelled diagram of the organisation of Sertoli cells (red) and spermatocytes (blue) in the testis. Spermatids which have not yet undergone spermiation are attached to the lumenal apex of the cell
Main article: Sertoli cell
At all stages of differentiation, the spermatogenic cells are in close contact with Sertoli cells which are thought to provide structural and metabolic support to the developing sperm cells. A single Sertoli cell extends from the basement membrane to the lumen of the seminiferous tubule, although the cytoplasmic processes are difficult to distinguish at the light microscopic level.
Sertoli cells serve a number of functions during spermatogenesis, they support the developing gametes in the following ways: Maintain the environment necessary for development and maturation, via the blood-testis barrier
Secrete substances initiating meiosis
Secrete supporting testicular fluid
Secrete androgen-binding protein (ABP), which concentrates testosterone in close proximity to the developing gametes Testosterone is needed in very high quantities for maintenance of the reproductive tract, and ABP allows a much higher level of fertility
Secrete hormones affecting pituitary gland control of spermatogenesis, particularly the polypeptide hormone, inhibin
Phagocytose residual cytoplasm left over from spermiogenesis
Secretion of anti-Müllerian hormone causes deterioration of the Müllerian duct[16]
Protect spermatids from the immune system of the male, via the blood-testis barrier
Contribute to the spermatogonial stem cell niche
The intercellular adhesion molecules ICAM-1 and soluble ICAM-1 have antagonistic effects on the tight junctions forming the blood-testis barrier.[17] ICAM-2 molecules regulate spermatid adhesion on the apical side of the barrier (towards the lumen).[17]
Influencing factors
The process of spermatogenesis is highly sensitive to fluctuations in the environment, particularly hormones and temperature. Testosterone is required in large local concentrations to maintain the process, which is achieved via the binding of testosterone by androgen binding protein present in the seminiferous tubules. Testosterone is produced by interstitial cells, also known as Leydig cells, which reside adjacent to the seminiferous tubules.
Seminiferous epithelium is sensitive to elevated temperature in humans and some other species, and will be adversely affected by temperatures as high as normal body temperature. In addition, spermatogonia do not achieve maturity at body temperature in most of mammals, as β-polimerase and spermatogenic recombinase need a specific optimal temperature.[18] Consequently, the testes are located outside the body in a sack of skin called the scrotum. The optimal temperature is maintained at 2 °C (man) (8 °C mouse) below body temperature. This is achieved by regulation of blood flow[19] and positioning towards and away from the heat of the body by the cremasteric muscle and the dartos smooth muscle in the scrotum.
One important mechanism is a thermal exchange between testicular arterial and venous blood streams. Specialized anatomic arrangements consist of two zones of coiling along the internal spermatic artery. This anatomic arrangement prolongs the time of contact and the thermal exchange between the testicular arterial and venous blood streams and may, in part, explain the temperature gradient between aortic and testicular arterial blood reported in dogs and rams. Moreover, reduction in pulse pressure, occurring in the proximal one third of the coiled length of the internal spermatic artery.[clarification needed][20][21] Moreover, the activity of spermatogenic recombinase decreases, and this is supposed to be an important factor of testicles degeneration.[clarification needed][22]
Dietary deficiencies (such as vitamins B, E and A), anabolic steroids, metals (cadmium and lead), x-ray exposure, dioxin, alcohol, and infectious diseases will also adversely affect the rate of spermatogenesis.[23] In addition, the male germ line is susceptible to DNA damage caused by oxidative stress, and this damage likely has a significant impact on fertilization and pregnancy.[24] Exposure to pesticides also affects spermatogenesis.[25]
Hormonal control
Hormonal control of spermatogenesis varies among species. In humans the mechanism is not completely understood; however it is known that initiation of spermatogenesis occurs at puberty due to the interaction of the hypothalamus, pituitary gland and Leydig cells. If the pituitary gland is removed, spermatogenesis can still be initiated by follicle stimulating hormone (FSH) and testosterone.[26] In contrast to FSH, luteinizing hormone (LH) appears to have little role in spermatogenesis outside of inducing gonadal testosterone production.[26][27]
FSH stimulates both the production of androgen binding protein (ABP) by Sertoli cells, and the formation of the blood-testis barrier. ABP is essential to concentrating testosterone in levels high enough to initiate and maintain spermatogenesis. Intratesticular testosterone levels are 20–100 or 50–200 times higher than the concentration found in blood, although there is variation over a 5- to 10-fold range amongst healthy men.[28][29]Testosterone production does not remain constant throughout the day, but follows a circadian rhythm. The maximum peak of testosterone occurs at 8 a.m., which explains why men frequently suffer from morning erections. In younger men, testosterone peaks are higher.</ref> FSH may initiate the sequestering of testosterone in the testes, but once developed only testosterone is required to maintain spermatogenesis.[26] However, increasing the levels of FSH will increase the production of spermatozoa by preventing the apoptosis of type A spermatogonia. The hormone inhibin acts to decrease the levels of FSH. Studies from rodent models suggest that gonadotropins (both LH and FSH) support the process of spermatogenesis by suppressing the proapoptotic signals and therefore promote spermatogenic cell survival.[30]
The Sertoli cells themselves mediate parts of spermatogenesis through hormone production. They are capable of producing the hormones estradiol and inhibin. The Leydig cells are also capable of producing estradiol in addition to their main product testosterone. Estrogen has been found to be essential for spermatogenesis in animals.[31][32] However, a man with estrogen insensitivity syndrome (a defective ERα) was found produce sperm with a normal sperm count, albeit abnormally low sperm viability; whether he was sterile or not is unclear.[33] Levels of estrogen that are too high can be detrimental to spermatogenesis due to suppression of gonadotropin secretion and by extension intratesticular testosterone production.[34] Prolactin also appears to be important for spermatogenesis.[27]
Disorders
Disorders of spermatogenesis may cause oligospermia, which is semen with a low concentration of sperm[35] and is a common finding in male infertility.
See also
Anisogamy
Evolution of sexual reproduction
Folliculogenesis
Germ cells
Male infertility
Meiosis
Oncofertility
Oogenesis
Origin and function of meiosis
Sertoli cells
Sexual reproduction
Semen analysis
https://en.wikipedia.org/wiki/Sperm_donation
A germ cell is any cell that gives rise to the gametes of an organism that reproduces sexually. In many animals, the germ cells originate in the primitive streak and migrate via the gut of an embryo to the developing gonads. There, they undergo meiosis, followed by cellular differentiation into mature gametes, either eggs or sperm. Unlike animals, plants do not have germ cells designated in early development. Instead, germ cells can arise from somatic cells in the adult, such as the floral meristem of flowering plants.[1][2][3]
https://en.wikipedia.org/wiki/Germ_cell
Sperm bank processes
A sperm donor is usually advised not to ejaculate for two to three days before providing the sample, to increase sperm count. A sperm donor produces and collects sperm at a sperm bank or clinic by masturbation or during sexual intercourse with the use of a collection condom.[14]
Preparing the spermSperm banks and clinics may "wash" the sperm sample to extract sperm from the rest of the material in the semen. Unwashed semen may only be used for ICI (intra-cervical) inseminations, to avoid cramping, or for IVF/ICSI procedures. It may be washed after thawing for use in IUI procedures. A cryoprotectant semen extender is added if the sperm is to be placed in frozen storage in liquid nitrogen, and the sample is then frozen in a number of vials or straws. One sample will be divided into one–twenty vials or straws depending on the quantity of the ejaculate, whether the sample is washed or unwashed, or whether it is being prepared for IVF use. Following analysis of an individual donor's sperm, straws or vials may be prepared which contain differing amounts of motile sperm post-thaw. The number of sperm in a straw prepared for IVF use, for example, will be significantly less than the number of motile sperm in a straw prepared for ICI or IUI and there will therefore be more IVF straws per ejaculate. Following the necessary quarantine period, the samples are thawed and used to inseminate women through artificial insemination or other ART treatments.
https://en.wikipedia.org/wiki/Sperm_donation
https://en.wikipedia.org/wiki/Sperm_donation
https://en.wikipedia.org/wiki/Semen_extender
https://en.wikipedia.org/wiki/Conception_device
https://en.wikipedia.org/wiki/Posthumous_sperm_retrieval
https://en.wikipedia.org/wiki/Rh_disease
https://en.wikipedia.org/wiki/Plasma_membrane_transformation
https://en.wikipedia.org/wiki/Anovulation
https://en.wikipedia.org/wiki/Botany
Artificial reproduction is the creation of new life by other than the natural means available to an organism. Examples include artificial insemination, in vitro fertilization, cloning and embryonic splitting, or cleavage.
Cutting plants' stems and placing them in compost is also a form of artificial reproduction.
See alsoMale Pregnancy
Artificial Uterus
In Vitro Fertilization
Fertilization
Pregnancy
https://en.wikipedia.org/wiki/Artificial_reproduction
Cloning is the process of producing individual organisms with identical genomes, either by natural or artificial means. In nature, some organisms produce clones through asexual reproduction. In the field of biotechnology, cloning is the process of creating cloned organisms of cells and of DNA fragments.
https://en.wikipedia.org/wiki/Cloning
Natural cloning is the production of clones without the involvement of genetic engineering techniques[4]. It may occur accidentally in the case of identical twins, which are formed when a fertilized egg splits, creating two or more embryos that carry almost identical DNA. It may also be part of asexual reproduction, which is a process where a single parent organism produces genetically identical offspring by itself. [5][6]
Cloning is a natural form of reproduction that has allowed life forms to spread for hundreds of millions of years. It is a reproduction method used by plants, fungi, and bacteria, and is also the way that clonal colonies reproduce themselves.[7][8] Examples of these organisms include blueberry plants, Hazel trees, the Pando trees,[9][10] the Kentucky coffeetree, Myrica, and the American sweetgum.
https://en.wikipedia.org/wiki/Cloning
https://en.wikipedia.org/wiki/Cloning#Parthenogenesis
Molecular cloning
Main article: Molecular cloning
Molecular cloning refers to the process of making multiple molecules. Cloning is commonly used to amplify DNA fragments containing whole genes, but it can also be used to amplify any DNA sequence such as promoters, non-coding sequences and randomly fragmented DNA. It is used in a wide array of biological experiments and practical applications ranging from genetic fingerprinting to large scale protein production. Occasionally, the term cloning is misleadingly used to refer to the identification of the chromosomal location of a gene associated with a particular phenotype of interest, such as in positional cloning. In practice, localization of the gene to a chromosome or genomic region does not necessarily enable one to isolate or amplify the relevant genomic sequence. To amplify any DNA sequence in a living organism, that sequence must be linked to an origin of replication, which is a sequence of DNA capable of directing the propagation of itself and any linked sequence. However, a number of other features are needed, and a variety of specialised cloning vectors (small piece of DNA into which a foreign DNA fragment can be inserted) exist that allow protein production, affinity tagging, single-stranded RNA or DNA production and a host of other molecular biology tools.
Cloning of any DNA fragment essentially involves four steps[11] fragmentation - breaking apart a strand of DNA
ligation – gluing together pieces of DNA in a desired sequence
transfection – inserting the newly formed pieces of DNA into cells
screening/selection – selecting out the cells that were successfully transfected with the new DNA
Although these steps are invariable among cloning procedures a number of alternative routes can be selected; these are summarized as a cloning strategy.
Initially, the DNA of interest needs to be isolated to provide a DNA segment of suitable size. Subsequently, a ligation procedure is used where the amplified fragment is inserted into a vector (piece of DNA). The vector (which is frequently circular) is linearised using restriction enzymes, and incubated with the fragment of interest under appropriate conditions with an enzyme called DNA ligase. Following ligation, the vector with the insert of interest is transfected into cells. A number of alternative techniques are available, such as chemical sensitisation of cells, electroporation, optical injection and biolistics. Finally, the transfected cells are cultured. As the aforementioned procedures are of particularly low efficiency, there is a need to identify the cells that have been successfully transfected with the vector construct containing the desired insertion sequence in the required orientation. Modern cloning vectors include selectable antibiotic resistance markers, which allow only cells in which the vector has been transfected, to grow. Additionally, the cloning vectors may contain colour selection markers, which provide blue/white screening (alpha-factor complementation) on X-gal medium. Nevertheless, these selection steps do not absolutely guarantee that the DNA insert is present in the cells obtained. Further investigation of the resulting colonies must be required to confirm that cloning was successful. This may be accomplished by means of PCR, restriction fragment analysis and/or DNA sequencing.
Cell cloning
Cloning unicellular organisms

Cloning cell-line colonies using cloning rings
Cloning a cell means to derive a population of cells from a single cell. In the case of unicellular organisms such as bacteria and yeast, this process is remarkably simple and essentially only requires the inoculation of the appropriate medium. However, in the case of cell cultures from multi-cellular organisms, cell cloning is an arduous task as these cells will not readily grow in standard media.
A useful tissue culture technique used to clone distinct lineages of cell lines involves the use of cloning rings (cylinders).[12] In this technique a single-cell suspension of cells that have been exposed to a mutagenic agent or drug used to drive selection is plated at high dilution to create isolated colonies, each arising from a single and potentially clonal distinct cell. At an early growth stage when colonies consist of only a few cells, sterile polystyrene rings (cloning rings), which have been dipped in grease, are placed over an individual colony and a small amount of trypsin is added. Cloned cells are collected from inside the ring and transferred to a new vessel for further growth.
Cloning stem cells
Main article: Somatic-cell nuclear transfer
Somatic-cell nuclear transfer, popularly known as SCNT, can also be used to create embryos for research or therapeutic purposes. The most likely purpose for this is to produce embryos for use in stem cell research. This process is also called "research cloning" or "therapeutic cloning". The goal is not to create cloned human beings (called "reproductive cloning"), but rather to harvest stem cells that can be used to study human development and to potentially treat disease. While a clonal human blastocyst has been created, stem cell lines are yet to be isolated from a clonal source.[13]
Therapeutic cloning is achieved by creating embryonic stem cells in the hopes of treating diseases such as diabetes and Alzheimer's. The process begins by removing the nucleus (containing the DNA) from an egg cell and inserting a nucleus from the adult cell to be cloned.[14] In the case of someone with Alzheimer's disease, the nucleus from a skin cell of that patient is placed into an empty egg. The reprogrammed cell begins to develop into an embryo because the egg reacts with the transferred nucleus. The embryo will become genetically identical to the patient.[14] The embryo will then form a blastocyst which has the potential to form/become any cell in the body.[15]
The reason why SCNT is used for cloning is because somatic cells can be easily acquired and cultured in the lab. This process can either add or delete specific genomes of farm animals. A key point to remember is that cloning is achieved when the oocyte maintains its normal functions and instead of using sperm and egg genomes to replicate, the donor's somatic cell nucleus is inserted into the oocyte.[16] The oocyte will react to the somatic cell nucleus, the same way it would to a sperm cell's nucleus.[16]
The process of cloning a particular farm animal using SCNT is relatively the same for all animals. The first step is to collect the somatic cells from the animal that will be cloned. The somatic cells could be used immediately or stored in the laboratory for later use.[16] The hardest part of SCNT is removing maternal DNA from an oocyte at metaphase II. Once this has been done, the somatic nucleus can be inserted into an egg cytoplasm.[16] This creates a one-cell embryo. The grouped somatic cell and egg cytoplasm are then introduced to an electrical current.[16] This energy will hopefully allow the cloned embryo to begin development. The successfully developed embryos are then placed in surrogate recipients, such as a cow or sheep in the case of farm animals.[16]
SCNT is seen as a good method for producing agriculture animals for food consumption. It successfully cloned sheep, cattle, goats, and pigs. Another benefit is SCNT is seen as a solution to clone endangered species that are on the verge of going extinct.[16] However, stresses placed on both the egg cell and the introduced nucleus can be enormous, which led to a high loss in resulting cells in early research. For example, the cloned sheep Dolly was born after 277 eggs were used for SCNT, which created 29 viable embryos. Only three of these embryos survived until birth, and only one survived to adulthood.[17] As the procedure could not be automated, and had to be performed manually under a microscope, SCNT was very resource intensive. The biochemistry involved in reprogramming the differentiated somatic cell nucleus and activating the recipient egg was also far from being well understood. However, by 2014 researchers were reporting cloning success rates of seven to eight out of ten[18] and in 2016, a Korean Company Sooam Biotech was reported to be producing 500 cloned embryos per day.[19]
In SCNT, not all of the donor cell's genetic information is transferred, as the donor cell's mitochondria that contain their own mitochondrial DNA are left behind. The resulting hybrid cells retain those mitochondrial structures which originally belonged to the egg. As a consequence, clones such as Dolly that are born from SCNT are not perfect copies of the donor of the nucleus.
Organism cloning
See also: Asexual reproduction, Cuttings (plants), and vegetative reproduction
Organism cloning (also called reproductive cloning) refers to the procedure of creating a new multicellular organism, genetically identical to another. In essence this form of cloning is an asexual method of reproduction, where fertilization or inter-gamete contact does not take place. Asexual reproduction is a naturally occurring phenomenon in many species, including most plants and some insects. Scientists have made some major achievements with cloning, including the asexual reproduction of sheep and cows. There is a lot of ethical debate over whether or not cloning should be used. However, cloning, or asexual propagation,[20] has been common practice in the horticultural world for hundreds of years.
Horticultural

Propagating plants from cuttings, such as grape vines, is an ancient form of cloning.
For the use of cloning in viticulture, see Propagation of grapevines.
The term clone is used in horticulture to refer to descendants of a single plant which were produced by vegetative reproduction or apomixis. Many horticultural plant cultivars are clones, having been derived from a single individual, multiplied by some process other than sexual reproduction.[21] As an example, some European cultivars of grapes represent clones that have been propagated for over two millennia. Other examples are potato and banana.[22]
Grafting can be regarded as cloning, since all the shoots and branches coming from the graft are genetically a clone of a single individual, but this particular kind of cloning has not come under ethical scrutiny and is generally treated as an entirely different kind of operation.
Many trees, shrubs, vines, ferns and other herbaceous perennials form clonal colonies naturally. Parts of an individual plant may become detached by fragmentation and grow on to become separate clonal individuals. A common example is in the vegetative reproduction of moss and liverwort gametophyte clones by means of gemmae. Some vascular plants e.g. dandelion and certain viviparous grasses also form seeds asexually, termed apomixis, resulting in clonal populations of genetically identical individuals.
Parthenogenesis
Clonal derivation exists in nature in some animal species and is referred to as parthenogenesis (reproduction of an organism by itself without a mate). This is an asexual form of reproduction that is only found in females of some insects, crustaceans, nematodes,[23] fish (for example the hammerhead shark[24]), Cape honeybees,[25] and lizards including the Komodo dragon[24] and several whiptails. The growth and development occurs without fertilization by a male. In plants, parthenogenesis means the development of an embryo from an unfertilized egg cell, and is a component process of apomixis. In species that use the XY sex-determination system, the offspring will always be female. An example is the little fire ant (Wasmannia auropunctata), which is native to Central and South America but has spread throughout many tropical environments.
Artificial cloning of organisms
Artificial cloning of organisms may also be called reproductive cloning.
First steps
Hans Spemann, a German embryologist was awarded a Nobel Prize in Physiology or Medicine in 1935 for his discovery of the effect now known as embryonic induction, exercised by various parts of the embryo, that directs the development of groups of cells into particular tissues and organs. In 1924 he and his student, Hilde Mangold, were the first to perform somatic-cell nuclear transfer using amphibian embryos – one of the first steps towards cloning.[26]
Methods
Reproductive cloning generally uses "somatic cell nuclear transfer" (SCNT) to create animals that are genetically identical. This process entails the transfer of a nucleus from a donor adult cell (somatic cell) to an egg from which the nucleus has been removed, or to a cell from a blastocyst from which the nucleus has been removed.[27] If the egg begins to divide normally it is transferred into the uterus of the surrogate mother. Such clones are not strictly identical since the somatic cells may contain mutations in their nuclear DNA. Additionally, the mitochondria in the cytoplasm also contains DNA and during SCNT this mitochondrial DNA is wholly from the cytoplasmic donor's egg, thus the mitochondrial genome is not the same as that of the nucleus donor cell from which it was produced. This may have important implications for cross-species nuclear transfer in which nuclear-mitochondrial incompatibilities may lead to death.
Artificial embryo splitting or embryo twinning, a technique that creates monozygotic twins from a single embryo, is not considered in the same fashion as other methods of cloning. During that procedure, a donor embryo is split in two distinct embryos, that can then be transferred via embryo transfer. It is optimally performed at the 6- to 8-cell stage, where it can be used as an expansion of IVF to increase the number of available embryos.[28] If both embryos are successful, it gives rise to monozygotic (identical) twins.
Dolly the sheep
Main article: Dolly (sheep)

The taxidermied body of Dolly the sheep
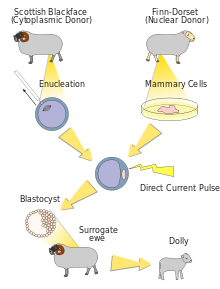
Dolly clone
Dolly, a Finn-Dorset ewe, was the first mammal to have been successfully cloned from an adult somatic cell. Dolly was formed by taking a cell from the udder of her 6-year-old biological mother.[29] Dolly's embryo was created by taking the cell and inserting it into a sheep ovum. It took 435 attempts before an embryo was successful.[30] The embryo was then placed inside a female sheep that went through a normal pregnancy.[31] She was cloned at the Roslin Institute in Scotland by British scientists Sir Ian Wilmut and Keith Campbell and lived there from her birth in 1996 until her death in 2003 when she was six. She was born on 5 July 1996 but not announced to the world until 22 February 1997.[32] Her stuffed remains were placed at Edinburgh's Royal Museum, part of the National Museums of Scotland.[33]
Dolly was publicly significant because the effort showed that genetic material from a specific adult cell, designed to express only a distinct subset of its genes, can be redesigned to grow an entirely new organism. Before this demonstration, it had been shown by John Gurdon that nuclei from differentiated cells could give rise to an entire organism after transplantation into an enucleated egg.[34] However, this concept was not yet demonstrated in a mammalian system.
The first mammalian cloning (resulting in Dolly) had a success rate of 29 embryos per 277 fertilized eggs, which produced three lambs at birth, one of which lived. In a bovine experiment involving 70 cloned calves, one-third of the calves died quite young. The first successfully cloned horse, Prometea, took 814 attempts. Notably, although the first clones were frogs, no adult cloned frog has yet been produced from a somatic adult nucleus donor cell.[35]
There were early claims that Dolly had pathologies resembling accelerated aging. Scientists speculated that Dolly's death in 2003 was related to the shortening of telomeres, DNA-protein complexes that protect the end of linear chromosomes. However, other researchers, including Ian Wilmut who led the team that successfully cloned Dolly, argue that Dolly's early death due to respiratory infection was unrelated to problems with the cloning process. This idea that the nuclei have not irreversibly aged was shown in 2013 to be true for mice.[36]
Dolly was named after performer Dolly Parton because the cells cloned to make her were from a mammary gland cell, and Parton is known for her ample cleavage.[37]
Species cloned and applications
Further information: List of animals that have been cloned
Further information: Commercial animal cloning

This section needs expansion. You can help by adding to it. (March 2023)
The modern cloning techniques involving nuclear transfer have been successfully performed on several species. Notable experiments include: Tadpole: (1952) Robert Briggs and Thomas J. King had successfully cloned northern leopard frogs: thirty-five complete embryos and twenty-seven tadpoles from one-hundred and four successful nuclear transfers.[38][39]
Carp: (1963) In China, embryologist Tong Dizhou produced the world's first cloned fish by inserting the DNA from a cell of a male carp into an egg from a female carp. He published the findings in a Chinese science journal.[40]
Zebrafish: The first vertebrate cloned (1981) by George Streisinger[41]
Sheep: Marked the first mammal being cloned (1984) from early embryonic cells by Steen Willadsen. Megan and Morag[42] cloned from differentiated embryonic cells in June 1995 and Dolly from a somatic cell in 1996.[43][40]
Mice: (1986) A mouse was successfully cloned from an early embryonic cell. Soviet scientists Chaylakhyan, Veprencev, Sviridova, and Nikitin had the mouse "Masha" cloned. Research was published in the magazine Biofizika volume ХХХII, issue 5 of 1987.[clarification needed][44][45][needs update]
Rhesus monkey: Tetra (January 2000) from embryo splitting and not nuclear transfer. More akin to artificial formation of twins.[46][47]
Pig: the first cloned pigs (March 2000).[48] By 2014, BGI in China was producing 500 cloned pigs a year to test new medicines.[49]
Gaur: (2001) was the first endangered species cloned.[50]
Cattle: Alpha and Beta (males, 2001) and (2005), Brazil[51]
In 2023, Chinese scientists reported the cloning of three supercows with a milk productivity "nearly 1.7 times the amount of milk an average cow in the United States produced in 2021" and a plan for 1,000 of such super cows in the near-term. According to a news report "[i]n many countries, including the United States, farmers breed clones with conventional animals to add desirable traits, such as high milk production or disease resistance, into the gene pool".[clarification needed][when?][52]
Cat: CopyCat "CC" (female, late 2001), Little Nicky, 2004, was the first cat cloned for commercial reasons[53]
Rat: Ralph, the first cloned rat (2003)[54]
Mule: Idaho Gem, a john mule born 4 May 2003, was the first horse-family clone.[55]
Horse: Prometea, a Haflinger female born 28 May 2003, was the first horse clone.[56]
Przewalksi's Horse: An ongoing cloning program by the San Diego Zoo Wildlife Alliance and Revive & Restore attempts to reintroduce genetic diversity to this endangered species.[57] Kurt, the first cloned Przewalski's horse, was born in 2020. He was cloned from the skin tissue of a stallion which was preserved in 1980.[58]
"Trey" was born in 2023. He was cloned from the same stallion's tissue as Kurt.[59]
Dog: Snuppy, a male Afghan hound was the first cloned dog (2005).[60] In 2017, the world's first gene-editing clone dog, Apple, was created by Sinogene Biotechnology.[61] Sooam Biotech, South Korea, was reported in 2015 to have cloned 700 dogs to date for their owners, including two Yakutian Laika hunting dogs, which are seriously endangered due to crossbreeding.[62]
Cloning of super sniffer dogs was reported in 2011, four years afterwards when the dogs started working.[63] Cloning of a successful rescue dog was also reported in 2009[64] and of a similar police dog in 2019.[65] Cancer-sniffing dogs have also been cloned. A review concluded that "qualified elite working dogs can be produced by cloning a working dog that exhibits both an appropriate temperament and good health."[66]
Wolf: Snuwolf and Snuwolffy, the first two cloned female wolves (2005).[67]
Water buffalo: Samrupa was the first cloned water buffalo. It was born on 6 February 2009, at India's Karnal National Diary Research Institute but died five days later due to lung infection.[68]
Pyrenean ibex (2009) was the first extinct animal to be cloned back to life; the clone lived for seven minutes before dying of lung defects.[69][70]
Camel: (2009) Injaz, was the first cloned camel.[71]
Pashmina goat: (2012) Noori, is the first cloned pashmina goat. Scientists at the faculty of veterinary sciences and animal husbandry of Sher-e-Kashmir University of Agricultural Sciences and Technology of Kashmir successfully cloned the first Pashmina goat (Noori) using the advanced reproductive techniques under the leadership of Riaz Ahmad Shah.[72]
Goat: (2001) Scientists of Northwest A&F University successfully cloned the first goat which use the adult female cell.[73]
Gastric brooding frog: (2013) The gastric brooding frog, Rheobatrachus silus, thought to have been extinct since 1983 was cloned in Australia, although the embryos died after a few days.[74]
Macaque monkey: (2017) First successful cloning of a primate species using nuclear transfer, with the birth of two live clones named Zhong Zhong and Hua Hua. Conducted in China in 2017, and reported in January 2018.[75][76][77][78] In January 2019, scientists in China reported the creation of five identical cloned gene-edited monkeys, using the same cloning technique that was used with Zhong Zhong and Hua Hua and Dolly the sheep, and the gene-editing Crispr-Cas9 technique allegedly used by He Jiankui in creating the first ever gene-modified human babies Lulu and Nana. The monkey clones were made to study several medical diseases.[79][80]
Black-footed ferret: (2020) A team of scientists cloned a female named Willa, who died in the mid-1980s and left no living descendants. Her clone, a female named Elizabeth Ann, was born on 10 December. Scientists hope that the contribution of this individual will alleviate the effects of inbreeding and help black-footed ferrets better cope with plague. Experts estimate that this female's genome contains three times as much genetic diversity as any of the modern black-footed ferrets.[81]
First artificial parthenogenesis in mammals: (2022) Viable mice offspring was born from unfertilized eggs via targeted DNA methylation editing of seven imprinting control regions.[82]
Human cloning
Main article: Human cloning
Human cloning is the creation of a genetically identical copy of a human. The term is generally used to refer to artificial human cloning, which is the reproduction of human cells and tissues. It does not refer to the natural conception and delivery of identical twins. The possibility of human cloning has raised controversies. These ethical concerns have prompted several nations to pass legislation regarding human cloning and its legality. As of right now, scientists have no intention of trying to clone people and they believe their results should spark a wider discussion about the laws and regulations the world needs to regulate cloning.[83]
Two commonly discussed types of theoretical human cloning are therapeutic cloning and reproductive cloning. Therapeutic cloning would involve cloning cells from a human for use in medicine and transplants, and is an active area of research, but is not in medical practice anywhere in the world, as of 2021. Two common methods of therapeutic cloning that are being researched are somatic-cell nuclear transfer and, more recently, pluripotent stem cell induction. Reproductive cloning would involve making an entire cloned human, instead of just specific cells or tissues.[84]
Ethical issues of cloning
Main article: Ethics of cloning
There are a variety of ethical positions regarding the possibilities of cloning, especially human cloning. While many of these views are religious in origin, the questions raised by cloning are faced by secular perspectives as well. Perspectives on human cloning are theoretical, as human therapeutic and reproductive cloning are not commercially used; animals are currently cloned in laboratories and in livestock production.
Advocates support development of therapeutic cloning to generate tissues and whole organs to treat patients who otherwise cannot obtain transplants,[85] to avoid the need for immunosuppressive drugs,[84] and to stave off the effects of aging.[86] Advocates for reproductive cloning believe that parents who cannot otherwise procreate should have access to the technology.[87]
Opponents of cloning have concerns that technology is not yet developed enough to be safe[88] and that it could be prone to abuse (leading to the generation of humans from whom organs and tissues would be harvested),[89][90] as well as concerns about how cloned individuals could integrate with families and with society at large.[91][92] Cloning humans could lead to serious violations of human rights.[93]
Religious groups are divided, with some opposing the technology as usurping "God's place" and, to the extent embryos are used, destroying a human life; others support therapeutic cloning's potential life-saving benefits.[94][95] There is at least one religion, Raëlism, in which cloning plays a major role.[96][97][98]
Contemporary work on this topic is concerned with the ethics, adequate regulation and issues of any cloning carried out by humans, not potentially by extraterrestrials (including in the future), and largely also not replication – also described as mind cloning[99][100][101][102] – of potential whole brain emulations.
Cloning of animals is opposed by animal-groups due to the number of cloned animals that suffer from malformations before they die, and while food from cloned animals has been approved as safe by the US FDA,[103][104] its use is opposed by groups concerned about food safety.[105][106]
In practical terms, the inclusion of "licensing requirements for embryo research projects and fertility clinics, restrictions on the commodification of eggs and sperm, and measures to prevent proprietary interests from monopolizing access to stem cell lines" in international cloning regulations has been proposed, albeit e.g. effective oversight mechanisms or cloning requirements have not been described.[107]
Cloning extinct and endangered species
Main article: De-extinction
Cloning, or more precisely, the reconstruction of functional DNA from extinct species has, for decades, been a dream. Possible implications of this were dramatized in the 1984 novel Carnosaur and the 1990 novel Jurassic Park.[108][109] The best current cloning techniques have an average success rate of 9.4 percent[110] (and as high as 25 percent[36]) when working with familiar species such as mice,[note 1] while cloning wild animals is usually less than 1 percent successful.[113]
Conservation cloning
Several tissue banks have come into existence, including the "Frozen zoo" at the San Diego Zoo, to store frozen tissue from the world's rarest and most endangered species.[108][114][115][116] This is also referred to as "Conservation cloning".[117][118]
Engineers have proposed a "lunar ark" in 2021 – storing millions of seed, spore, sperm and egg samples from Earth's contemporary species in a network of lava tubes on the Moon as a genetic backup.[119][120][121] Similar proposals have been made since at least 2008.[122] These also include sending human customer DNA,[123] and a proposal for "a lunar backup record of humanity" that includes genetic information by Avi Loeb et al.[124]
Scientists at the University of Newcastle and University of New South Wales announced in March 2013 that the very recently extinct gastric-brooding frog would be the subject of a cloning attempt to resurrect the species.[125]
Many such "De-extinction" projects are described in the Long Now Foundation's Revive and Restore Project.[126]
De-extinction
One of the most anticipated targets for cloning was once the woolly mammoth, but attempts to extract DNA from frozen mammoths have been unsuccessful, though a joint Russo-Japanese team is currently working toward this goal.[when?] In January 2011, it was reported by Yomiuri Shimbun that a team of scientists headed by Akira Iritani of Kyoto University had built upon research by Dr. Wakayama, saying that they will extract DNA from a mammoth carcass that had been preserved in a Russian laboratory and insert it into the egg cells of an Asian elephant in hopes of producing a mammoth embryo. The researchers said they hoped to produce a baby mammoth within six years.[127][128] It was noted, however that the result, if possible, would be an elephant-mammoth hybrid rather than a true mammoth.[129] Another problem is the survival of the reconstructed mammoth: ruminants rely on a symbiosis with specific microbiota in their stomachs for digestion.[129]
In 2022, scientists showed major limitations and the scale of challenge of genetic-editing-based de-extinction, suggesting resources spent on more comprehensive de-extinction projects such as of the woolly mammoth may currently not be well allocated and substantially limited. Their analyses "show that even when the extremely high-quality Norway brown rat (R. norvegicus) is used as a reference, nearly 5% of the genome sequence is unrecoverable, with 1,661 genes recovered at lower than 90% completeness, and 26 completely absent", complicated further by that "distribution of regions affected is not random, but for example, if 90% completeness is used as the cutoff, genes related to immune response and olfaction are excessively affected" due to which "a reconstructed Christmas Island rat would lack attributes likely critical to surviving in its natural or natural-like environment".[130]
In a 2021 online session of the Russian Geographical Society, Russia's defense minister Sergei Shoigu mentioned using the DNA of 3,000-year-old Scythian warriors to potentially bring them back to life. The idea was described as absurd at least at this point in news reports and it was noted that Scythians likely weren't skilled warriors by default.[131][132][133]
The idea of cloning Neanderthals or bringing them back to life in general is controversial but some scientists have stated that it may be possible in the future and have outlined several issues or problems with such as well as broad rationales for doing so.[134][135][136][137][138][139]
Unsuccessful attempts
In 2001, a cow named Bessie gave birth to a cloned Asian gaur, an endangered species, but the calf died after two days. In 2003, a banteng was successfully cloned, followed by three African wildcats from a thawed frozen embryo. These successes provided hope that similar techniques (using surrogate mothers of another species) might be used to clone extinct species. Anticipating this possibility, tissue samples from the last bucardo (Pyrenean ibex) were frozen in liquid nitrogen immediately after it died in 2000. Researchers are also considering cloning endangered species such as the Giant panda and Cheetah.[140][141][142][143]
In 2002, geneticists at the Australian Museum announced that they had replicated DNA of the thylacine (Tasmanian tiger), at the time extinct for about 65 years, using polymerase chain reaction.[144] However, on 15 February 2005 the museum announced that it was stopping the project after tests showed the specimens' DNA had been too badly degraded by the (ethanol) preservative. On 15 May 2005 it was announced that the thylacine project would be revived, with new participation from researchers in New South Wales and Victoria.[145]
In 2003, for the first time, an extinct animal, the Pyrenean ibex mentioned above was cloned, at the Centre of Food Technology and Research of Aragon, using the preserved frozen cell nucleus of the skin samples from 2001 and domestic goat egg-cells. The ibex died shortly after birth due to physical defects in its lungs.[146]
Lifespan
After an eight-year project involving the use of a pioneering cloning technique, Japanese researchers created 25 generations of healthy cloned mice with normal lifespans, demonstrating that clones are not intrinsically shorter-lived than naturally born animals.[36][147] Other sources have noted that the offspring of clones tend to be healthier than the original clones and indistinguishable from animals produced naturally.[148]
Some posited that Dolly the sheep may have aged more quickly than naturally born animals, as she died relatively early for a sheep at the age of six. Ultimately, her death was attributed to a respiratory illness, and the "advanced aging" theory is disputed.[149][dubious – discuss]
A detailed study released in 2016 and less detailed studies by others suggest that once cloned animals get past the first month or two of life they are generally healthy. However, early pregnancy loss and neonatal losses are still greater with cloning than natural conception or assisted reproduction (IVF). Current research is attempting to overcome these problems.[37]
https://en.wikipedia.org/wiki/Cloning
Human cloning is the creation of a genetically identical copy of a human. The term is generally used to refer to artificial human cloning, which is the reproduction of human cells and tissue. It does not refer to the natural conception and delivery of identical twins. The possibilities of human cloning have raised controversies. These ethical concerns have prompted several nations to pass laws regarding human cloning.
Two commonly discussed types of human cloning are therapeutic cloning and reproductive cloning.
Therapeutic cloning would involve cloning cells from a human for use in medicine and transplants. It is an active area of research, but is not in medical practice anywhere in the world, as of 2023. Two common methods of therapeutic cloning that are being researched are somatic-cell nuclear transfer and (more recently) pluripotent stem cell induction.
Reproductive cloning would involve making an entire cloned human, instead of just specific cells or tissues.
History
Although the possibility of cloning humans had been the subject of speculation for much of the 20th century, scientists and policymakers began to take the prospect seriously in 1969. J. B. S. Haldane was the first to introduce the idea of human cloning, for which he used the terms "clone" and "cloning",[1] which had been used in agriculture since the early 20th century. In his speech on "Biological Possibilities for the Human Species of the Next Ten Thousand Years" at the Ciba Foundation Symposium on Man and his Future in 1963, he said:[2]
It is extremely hopeful that some human cell lines can be grown on a medium of precisely known chemical composition. Perhaps the first step will be the production of a clone from a single fertilized egg, as in Brave New World... Assuming that cloning is possible, I expect that most clones would be made from people aged at least fifty, except for athletes and dancers, who would be cloned younger. They would be made from people who were held to have excelled in a socially acceptable accomplishment...
Nobel Prize-winning geneticist Joshua Lederberg advocated cloning and genetic engineering in an article in The American Naturalist in 1966 and again, the following year, in The Washington Post.[3] He sparked a debate with conservative bioethicist Leon Kass, who wrote at the time that "the programmed reproduction of man will, in fact, dehumanize him." Another Nobel Laureate, James D. Watson, publicized the potential and the perils of cloning in his Atlantic Monthly essay, "Moving Toward the Clonal Man", in 1971.[4]
With the cloning of a sheep known as Dolly in 1996 by somatic cell nuclear transfer (SCNT), the idea of human cloning became a hot debate topic.[5] Many nations outlawed it, while a few scientists promised to make a clone within the next few years. The first hybrid human clone was created in November 1998, by Advanced Cell Technology. It was created using SCNT; a nucleus was taken from a man's leg cell and inserted into a cow's egg from which the nucleus had been removed, and the hybrid cell was cultured and developed into an embryo. The embryo was destroyed after 12 days.[6]
In 2004 and 2005, Hwang Woo-suk, a professor at Seoul National University, published two separate articles in the journal Science claiming to have successfully harvested pluripotent, embryonic stem cells from a cloned human blastocyst using SCNT techniques. Hwang claimed to have created eleven different patient-specific stem cell lines. This would have been the first major breakthrough in human cloning.[7] However, in 2006 Science retracted both of his articles on clear evidence that much of his data from the experiments was fabricated.[8]
In January 2008, Dr. Andrew French and Samuel Wood of the biotechnology company Stemagen announced that they successfully created the first five mature human embryos using SCNT. In this case, each embryo was created by taking a nucleus from a skin cell (donated by Wood and a colleague) and inserting it into a human egg from which the nucleus had been removed. The embryos were developed only to the blastocyst stage, at which point they were studied in processes that destroyed them. Members of the lab said that their next set of experiments would aim to generate embryonic stem cell lines; these are the "holy grail" that would be useful for therapeutic or reproductive cloning.[9][10]
In 2011, scientists at the New York Stem Cell Foundation announced that they had succeeded in generating embryonic stem cell lines, but their process involved leaving the oocyte's nucleus in place, resulting in triploid cells, which would not be useful for cloning.[11][12][13]
In 2013, a group of scientists led by Shoukhrat Mitalipov published the first report of embryonic stem cells created using SCNT. In this experiment, the researchers developed a protocol for using SCNT in human cells, which differs slightly from the one used in other organisms. Four embryonic stem cell lines from human fetal somatic cells were derived from those blastocysts. All four lines were derived using oocytes from the same donor, ensuring that all mitochondrial DNA inherited was identical.[11] A year later, a team led by Robert Lanza at Advanced Cell Technology reported that they had replicated Mitalipov's results and further demonstrated the effectiveness by cloning adult cells using SCNT.[5][14]
In 2018, the first successful cloning of primates using SCNT was reported with the birth of two live female clones, crab-eating macaques named Zhong Zhong and Hua Hua.[15][16] CRISPR gene editing
Methods
Somatic cell nuclear transfer (SCNT)
Main article: Somatic cell nuclear transfer

Diagram of SCNT process
In somatic cell nuclear transfer ("SCNT"), the nucleus of a somatic cell is taken from a donor and transplanted into a host egg cell, which had its own genetic material removed previously, making it an enucleated egg. After the donor somatic cell genetic material is transferred into the host oocyte with a micropipette, the somatic cell genetic material is fused with the egg using an electric current. Once the two cells have fused, the new cell can be permitted to grow in a surrogate or artificially.[17] This is the process that was used to successfully clone Dolly the sheep (see § History).[5] The technique, now refined, has indicated that it was possible to replicate cells and reestablish pluripotency, or "the potential of an embryonic cell to grow into any one of the numerous different types of mature body cells that make up a complete organism".[18]
Induced pluripotent stem cells (iPSCs)
Main article: Induced pluripotent stem cell

Overview of iPS cells
Creating induced pluripotent stem cells ("iPSCs") is a long and inefficient process. Pluripotency refers to a stem cell that has the potential to differentiate into any of the three germ layers: endoderm (interior stomach lining, gastrointestinal tract, the lungs), mesoderm (muscle, bone, blood, urogenital), or ectoderm (epidermal tissues and nervous tissue).[19] A specific set of genes, often called "reprogramming factors", are introduced into a specific adult cell type. These factors send signals in the mature cell that cause the cell to become a pluripotent stem cell. This process is highly studied and new techniques are being discovered frequently on how to improve this induction process.
Depending on the method used, reprogramming of adult cells into iPSCs for implantation could have severe limitations in humans. If a virus is used as a reprogramming factor for the cell, cancer-causing genes called oncogenes may be activated. These cells would appear as rapidly dividing cancer cells that do not respond to the body's natural cell signaling process. However, in 2008 scientists discovered a technique that could remove the presence of these oncogenes after pluripotency induction, thereby increasing the potential use of iPSC in humans.[20]
Comparing SCNT to reprogramming
Both the processes of SCNT and iPSCs have benefits and deficiencies. Historically, reprogramming methods were better studied than SCNT derived embryonic stem cells (ESCs).[11] However, more recent studies have put more emphasis on developing new procedures for SCNT-ESCs. The major advantage of SCNT over iPSCs at this time is the speed with which cells can be produced. iPSCs derivation takes several months while SCNT would take a much shorter time, which could be important for medical applications. New studies are working to improve the process of iPSC in terms of both speed and efficiency with the discovery of new reprogramming factors in oocytes.[citation needed] Another advantage SCNT could have over iPSCs is its potential to treat mitochondrial disease, as it uses a donor oocyte.[11] No other advantages are known at this time in using stem cells derived from one method over stem cells derived from the other.[21]
Uses and actual potential

Stem cell treatments
Work on cloning techniques has advanced our understanding of developmental biology in humans. Observing human pluripotent stem cells grown in culture provides great insight into human embryo development, which otherwise cannot be seen. Scientists are now able to better define steps of early human development. Studying signal transduction along with genetic manipulation within the early human embryo has the potential to provide answers to many developmental diseases and defects. Many human-specific signaling pathways have been discovered by studying human embryonic stem cells. Studying developmental pathways in humans has given developmental biologists more evidence toward the hypothesis that developmental pathways are conserved throughout species.[22]
iPSCs and cells created by SCNT are useful for research into the causes of disease, and as model systems used in drug discovery.[23][24]
Cells produced with SCNT, or iPSCs could eventually be used in stem cell therapy,[25] or to create organs to be used in transplantation, known as regenerative medicine. Stem cell therapy is the use of stem cells to treat or prevent a disease or condition. Bone marrow transplantation is a widely used form of stem cell therapy.[26] No other forms of stem cell therapy are in clinical use at this time. Research is underway to potentially use stem cell therapy to treat heart disease, diabetes, and spinal cord injuries.[27][28] Regenerative medicine is not in clinical practice, but is heavily researched for its potential uses. This type of medicine would allow for autologous transplantation, thus removing the risk of organ transplant rejection by the recipient.[29] For instance, a person with liver disease could potentially have a new liver grown using their same genetic material and transplanted to remove the damaged liver.[30] In current research, human pluripotent stem cells have been promised as a reliable source for generating human neurons, showing the potential for regenerative medicine in brain and neural injuries.[31]
Ethical implications
Main article: Ethics of cloning
In bioethics, the ethics of cloning refers to a variety of ethical positions regarding the practice and possibilities of cloning, especially human cloning. While many of these views are religious in origin, for instance relating to Christian views of procreation and personhood,[32] the questions raised by cloning engage secular perspectives as well, particularly the concept of identity.[33]
Advocates support development of therapeutic cloning in order to generate tissues and whole organs to treat patients who otherwise cannot obtain transplants,[34] to avoid the need for immunosuppressive drugs,[35] and to stave off the effects of aging.[36] Advocates for reproductive cloning believe that parents who cannot otherwise procreate should have access to the technology.[37]
Opposition to therapeutic cloning mainly centers around the status of embryonic stem cells, which has connections with the abortion debate.[38]
Some opponents of reproductive cloning have concerns that technology is not yet developed enough to be safe – for example, the position of the American Association for the Advancement of Science as of 2014,[39] while others emphasize that reproductive cloning could be prone to abuse (leading to the generation of humans whose organs and tissues would be harvested),[40][41] and have concerns about how cloned individuals could integrate with families and with society at large.[42][43]
Members of religious groups are divided. Some Christian theologians perceive the technology as usurping God's role in creation and, to the extent embryos are used, destroying a human life;[32] others see no inconsistency between Christian tenets and cloning's positive and potentially life-saving benefits.[44][45]
https://en.wikipedia.org/wiki/Human_cloning
Cytoplasmic transfer
Cytoplasmic transfer was originally developed in the 1980s in the course of basic research conducted with mice to study the role that parts of the cell outside of the nucleus played in embryonic development.[2] In this technique, cytoplasm, including proteins, messenger RNA (mRNA), mitochondria and other organelles, is taken from a donor egg and injected into the recipient egg, resulting in a mixture of mitochondrial genetic material.[2] This technique started to be used in the late 1990s to "boost" the eggs of older women who were having problems conceiving and led to the birth of about 30 babies.[2] Concerns were raised that the mixture of genetic material and proteins could cause problems with respect to epigenetic clashes, or differences in the ability of the recipient and donor materials to effect the development process, or due to the injection of the donor material.[2] After three children born through the technique were found to have developmental disorders (two cases of Turner's syndrome and one case of pervasive developmental disorder (an autism spectrum disorder), the FDA banned the procedure until a clinical trial could prove its safety.[2] As of 2015 that study had not been conducted, but the procedure was in use in other countries.[2]
A related approach uses autologous mitochondria taken from healthy tissue to replace the mitochondria in damaged tissue. Transfer techniques include direct injection into damaged tissue and injection into vessels that supply blood to the tissue.[3]
https://en.wikipedia.org/wiki/Mitochondrial_replacement_therapy#Cytoplasmic_transfer
Zygote intra fallopian transfer (ZIFT) is an infertility treatment used when a blockage in the fallopian tubes prevents the normal binding of sperm to the egg. Egg cells are removed from a woman's ovaries, and in vitro fertilised. The resulting zygote is placed into the fallopian tube by the use of laparoscopy. The procedure is a spin-off of the gamete intrafallopian transfer (GIFT) procedure. The pregnancy and implantation rates in ZIFT cycles are 52.3 and 23.2% which were higher than what was observed in IVF cycles which were 17.5 and 9.7%.[1]
https://en.wikipedia.org/wiki/Zygote_intrafallopian_transfer
Embryo splitting may refer to: when spontaneous, the natural way in which identical twins are formed.
when artificially induced, a method of cloning. See Cloning#Methods
https://en.wikipedia.org/wiki/Embryo_splitting
Sperm sorting is a means of choosing what type of sperm cell is to fertilize the egg cell. Several conventional techniques of centrifugation or swim-up. Newly applied methods such as flow cytometry expand the possibilities of sperm sorting and new techniques of sperm sorting are being developed.
It can be used to sort out sperm that are most healthy, as well as for determination of more specific traits, such as sex selection in which spermatozoa are separated into X- (female) and Y- (male) chromosome bearing populations based on their difference in DNA content. The resultant 'sex-sorted' spermatozoa are then able to be used in conjunction with other assisted reproductive technologies such as artificial insemination or in-vitro fertilization (IVF) to produce offspring of the desired sex - in farming animals but also in human medical practice.
Methods
Conventional techniques
Several methods have been used to sort sperm before the advent of flow cytometry. Density gradient centrifugation (in a continuous or discontinuous gradient) can concentrate semen samples with low concentration of sperm, using the density of sperm as a measure of their quality.[1][2] Similarly, so-called swim-up techniques apply a centrifugation step and then sperm is allowed to swim up into a medium, thus enriching a subpopulation of motile sperm. However, use of sperm centrifugation is detrimental to the sperm viability and elicits production of reactive oxygen species.[1] Conventional techniques are routinely used in assisted reproductive technology.[3]
Flow cytometry
Flow cytometry is another method used to sort sperm and adaptations of this technique opens new opportunities in sperm sorting. However, because flow cytometry-based sperm sorting often uses fluorescent dyes that often stain DNA, the safety of this technique in human reproductive medicine is a matter of scientific discussion.[4][5]
However, flow cytometry is the only currently used technique able to determine the sex of future progeny by measuring DNA content of individual sperm cells. It evaluates if they contain the larger X chromosome (giving rise to a female offspring) or smaller Y chromosome (leading to male progeny). It then allows separation of X and Y sperm.[6] The so-called Beltsfield Sperm Sexing Technology was developed by USDA in conjunction with Lawrence Livermore National Laboratories, relying on the DNA difference between the X- and Y- chromosomes.[7] Prior to flow cytometric sorting, semen is labeled with a fluorescent dye called Hoechst 33342 which binds to the DNA of each spermatozoon. As the X chromosome is larger (i.e. has more DNA) than the Y chromosome, the "female" (X-chromosome bearing) spermatozoa will absorb a greater amount of dye than its male (Y-chromosome bearing) counterpart. As a consequence, when exposed to UV light during flow cytometry, X spermatozoa fluoresce brighter than Y- spermatozoa. As the spermatozoa pass through the flow cytometer in single file, each spermatozoon is encased by a single droplet of fluid and assigned an electric charge corresponding to its chromosome status (e.g. X-positive charge, Y-negative charge). The stream of X- and Y- droplets is then separated by means of electrostatic deflection and collected into separate collection tubes for subsequent processing.[8]
Another cytometric technique used in sperm sorting is magnetic-activated cell sorting (MACS) which is routinely applied in assisted reproduction hospitals to sort out sperm with fragmented DNA. This is achieved using antibodies to surface markers of programmed cell death (apoptosis) such as annexin V, coupled with magnetic beads. Following the binding of these antibodies, spermatozoa which undergo apoptosis are sorted by applying magnetic field to the sperm suspension.[9] MACS obviates the need for fluorescent DNA binding molecules.
Other techniques
DNA damage in sperm cells may be detected by using Raman spectroscopy.[10] It is not specific enough to detect individual traits, however.[10] The sperm cells having least DNA damage may subsequently be injected into the egg cell by intracytoplasmic sperm injection (ICSI).[10] Many other methods for sperm sorting have been proposed or are currently tested.[1][3]
To select spermatozoa with low DNA damage index the population of sperm could be enriched with spermatozoa with non-fragmented DNA, with techniques like electrophoresis,[11] Z method[12] and MACS (Magnetic Activating Cell Sorting), which in combination with density gradient centrifugation in single sperm preparation protocols results in spermatozoa with superior quality.[13]
Hyaluronic acid (HA) binding sites on the sperm plasma membrane are an indicator of sperm maturity (Huszar et al., 2003, Yudin et al.,1999). There are two methods based on this fact: physiological intracytoplasmic sperm injection (PICSI), and a sperm slow procedure; both methods require sperm preparation via sperm washing or centrifugation.
Applications
Sperm undergoes a process of natural selection when millions of sperm enter the vagina but only few reach the egg cell and then only one is usually allowed to fertilize it. The sperm is selected not only by its highest motility but also by other factors such as DNA integrity, production of reactive oxygen species and viability. This selection is largely circumvented in case of in-vitro fertilization which leads to higher incidence of birth defects associated with assisted reproductive techniques. Egg cells are often fertilized by sperm which would have low chance of fertilizing it in natural conditions.[1] Sperm sorting could thus be used to decrease risks associated with assisted reproduction. Additionally, there is ongoing debate about using sperm sorting for choosing the child's sex.
For general health
Conventional methods of sperm sorting have been widely used to assess quality of sperm before subsequent artificial insemination or in-vitro fertilization. It has been verified that sperm sorted using these techniques is of superior quality than unsorted.[14][15] However, important characteristics of sperm such as DNA integrity remain untested by these conventional methods. New flow-cytometry based techniques such as YO-PRO staining can discriminate apoptotic and dead spermatozoa from the viable ones.[2] For example, annexin V staining followed by MACS can significantly improve pregnancy rates in couples with previous assisted reproduction failure.[9]
For sex selection
In farming
Sperm sorting by flow cytometry is an established technique in veterinary practice, and in the dairy industry most female cows are artificially inseminated with sorted semen to increase the number of female calves (using sperm sorting is less common in other species of farm animals, however artificial insemination is common).[16] Artificial insemination of farm animals with sorted sperm is recognized by the Food and Agriculture Organization (FAO) as a promising way of increasing efficiency of agriculture needed to produce enough food for the growing human population. Utilizing artificial insemination with sorted sperm is seen as a way to create an optimal ratio of male and female calves to increase dairy milk production.[16]
In humans
Further information: Sex selection § Ethical concerns
Choosing the sex of children might help prevent sex-associated heritable diseases such as Duchene muscular dystrophy or haemophilia in families with a history of these diseases. On the other hand, sperm sorting in humans raises the ethical concerns implicit to the idea of sex selection. If applied large-scale, it has a potential to elicit a sex-ratio imbalance. It could also have implications on gender equality if parents consistently choose to have a boy as their first-born (first-borns were shown to be more likely to succeed in life).[17]
There is no country in the world which explicitly permits sex selection for non-medical purposes. There were 31 countries in 2009 which allowed sex selection in case of sex-linked disease risk or other medical purpose.[18] In the US, for humans, the application of sperm sorting in sex selection is tightly regulated by the FDA. After the establishment of the MicroSort technique, it was offered to parents as a part of a clinical trial. The procedure was made available to a limited number of participants each month, in addition to fulfilling certain criteria, such as having a disease with sex linkage or having at least one child (for family balancing).[19] There are currently MicroSort laboratories and collaborating physicians in several countries (some for general purposes, some only offering service in case of genetic disease risks associated with one sex).[20]
While highly accurate, sperm sorting by flow cytometry will not produce two completely separate populations. That is to say, there will always be some "male" sperm among the "female" sperm and vice versa. The exact percentage purity of each population is dependent on the species being sorted and the 'gates' which the operator places around the total population visible to the machine. In general, the larger the DNA difference between the X and Y chromosome of a species, the easier it is to produce a highly pure population. In sheep and cattle, purities for each sex will usually remain above 90% depending on 'gating', while for humans these may be reduced to 90% for "female" spermatozoa and 70% for "male" spermatozoa.[19]
See alsoSex selection
Preimplantation genetic diagnosis
https://en.wikipedia.org/wiki/Sperm_sorting
Others
Other assisted reproduction techniques include: Mitochondrial replacement therapy (MRT, sometimes called mitochondrial donation) is the replacement of mitochondria in one or more cells to prevent or ameliorate disease. MRT originated as a special form of IVF in which some or all of the future baby's mitochondrial DNA comes from a third party. This technique is used in cases when mothers carry genes for mitochondrial diseases. The therapy is approved for use in the United Kingdom.[10][11]
In gamete intrafallopian transfer (GIFT) a mixture of sperm and eggs is placed directly into a woman's fallopian tubes using laparoscopy following a transvaginal ovum retrieval.
Reproductive surgery, treating e.g. fallopian tube obstruction and vas deferens obstruction, or reversing a vasectomy by a reverse vasectomy. In surgical sperm retrieval (SSR) the reproductive urologist obtains sperm from the vas deferens, epididymis or directly from the testis in a short outpatient procedure.
By cryopreservation, eggs, sperm and reproductive tissue can be preserved for later IVF.
https://en.wikipedia.org/wiki/Assisted_reproductive_technology
Risks
The majority of IVF-conceived infants do not have birth defects.[12] However, some studies have suggested that assisted reproductive technology is associated with an increased risk of birth defects.[13][14] Artificial reproductive technology is becoming more available. Early studies suggest that there could be an increased risk for medical complications with both the mother and baby. Some of these include low birth weight, placental insufficiency, chromosomal disorders, preterm deliveries, gestational diabetes, and pre-eclampsia (Aiken and Brockelsby).[15]
In the largest U.S. study, which used data from a statewide registry of birth defects,[16] 6.2% of IVF-conceived children had major defects, as compared with 4.4% of naturally conceived children matched for maternal age and other factors (odds ratio, 1.3; 95% confidence interval, 1.00 to 1.67).[12] ART carries with it a risk for heterotopic pregnancy (simultaneous intrauterine and extrauterine pregnancy).[17] The main risks are: Genetic disorders
Low birth weight.[18] In IVF and ICSI, a risk factor is the decreased expression of proteins in energy metabolism; Ferritin light chain and ATP5A1.[19]
Preterm birth. Low birth weight and preterm birth are strongly associated with many health problems, such as visual impairment and cerebral palsy. Children born after IVF are roughly twice as likely to have cerebral palsy.[20]
Sperm donation is an exception, with a birth defect rate of almost a fifth compared to the general population. It may be explained by that sperm banks accept only people with high sperm count.
Germ cells of the mouse normally have a frequency of spontaneous point mutations that is 5 to 10-fold lower than that in somatic cells from the same individual.[21] This low frequency in the germline leads to embryos that have a low frequency of point mutations in the next generation. No significant differences were observed in the frequency or spectrum of mutations between naturally conceived fetuses and assisted-conception fetuses.[21] This suggests that with respect to the maintenance of genetic integrity assisted conception is safe.[21]
Current data indicate little or no increased risk for postpartum depression among women who use ART.[22]
Usage of assisted reproductive technology including ovarian stimulation and in vitro fertilization have been associated with an increased overall risk of childhood cancer in the offspring, which may be caused by the same original disease or condition that caused the infertility or subfertility in the mother or father.[23]
That said, In a landmark paper by Jacques Balayla et al. it was determined that infants born after ART have similar neurodevelopment than infants born after natural conception.[24]
ART may also pose risks to the mother. A large US database study compared pregnancy outcomes among 106,000 assisted conception pregnancies with 34 million natural conception pregnancies. It found that assisted conception pregnancies were associated with an increased risk of cardiovascular diseases, including acute kidney injury and arrhythmia. Assisted conception pregnancies were also associated with a higher risk of caesarean delivery and premature birth.[25][26]
In theory, ART can solve almost all reproductive problems, except for severe pathology or the absence of a uterus (or womb), using specific gamete or embryo donation techniques. However, this does not mean that all women can be treated with assisted reproductive techniques, or that all women who are treated will achieve pregnancy.
https://en.wikipedia.org/wiki/Assisted_reproductive_technology
See also

Wikimedia Commons has media related to Assisted reproductive technology. Artificial uterus
Artificial insemination
Diethylstilbestrol
Embryo
Fertility fraud
Human cloning
Religious response to ART
Ova bank
Sperm bank
Sperm donation
Spontaneous conception, the unassisted conception of a subsequent child after prior use of assisted reproductive technology
Egg donation
Ralph L. Brinster
https://en.wikipedia.org/wiki/Assisted_reproductive_technology
Spontaneous conception is the conception and birth of a subsequent child, after the birth of a child conceived through in vitro fertilisation or other forms of assisted reproductive technology. There is an overall 18% chance of spontaneous conception after an in vitro fertilization (IVF) treatment, but that chance rose to 37% among younger women (less than 27 years). The likelihood also depends on the sperm performance of the man and the egg count of the woman.[1]
https://en.wikipedia.org/wiki/Spontaneous_conception
An artificial womb or artificial uterus is a device that would allow for extracorporeal pregnancy[2] by growing a fetus outside the body of an organism that would normally carry the fetus to term.
An artificial uterus, as a replacement organ, would have many applications. It could be used to assist male or female couples in the development of a fetus.[2] This can potentially be performed as a switch from a natural uterus to an artificial uterus, thereby moving the threshold of fetal viability to a much earlier stage of pregnancy.[2] In this sense, it can be regarded as a neonatal incubator with very extended functions. It could also be used for the initiation of fetal development.[2] An artificial uterus could also help make fetal surgery procedures at an early stage an option instead of having to postpone them until term of pregnancy.[2]
In 2016, scientists published two studies regarding human embryos developing for thirteen days within an ecto-uterine environment.[3][4] Currently, a 14-day rule prevents human embryos from being kept in artificial wombs longer than 14 days. This rule has been codified into law in twelve countries.[5] "Last year, the International Society for Stem Cell Research relaxed a historical “14-day rule” that said researchers could grow natural embryos for only 14 days in the laboratory, allowing researchers to seek approval for longer studies. Human embryo models are banned from being implanted into a uterus," claims The Washington Post.[6]
In 2017, fetal researchers at the Children's Hospital of Philadelphia published a study showing they had grown premature lamb fetuses for four weeks in an extra-uterine life support system.[1][7][8]
Components
An artificial uterus, sometimes referred to as an 'exowomb[9]', would have to provide nutrients and oxygen to nurture a fetus, as well as dispose of waste material. The scope of an artificial uterus (or "artificial uterus system" to emphasize a broader scope) may also include the interface serving the function otherwise provided by the placenta, an amniotic tank functioning as the amniotic sac, as well as an umbilical cord.
Nutrition, oxygen supply and waste disposal
A woman may still supply nutrients and dispose of waste products if the artificial uterus is connected to her.[2] She may also provide immune protection against diseases by passing of IgG antibodies to the embryo or fetus.[2]
Artificial supply and disposal have the potential advantage of allowing the fetus to develop in an environment that is not influenced by the presence of disease, environmental pollutants, alcohol, or drugs which a human may have in the circulatory system.[2] There is no risk of an immune reaction towards the embryo or fetus that could otherwise arise from insufficient gestational immune tolerance.[2] Some individual functions of an artificial supplier and disposer include: Waste disposal may be performed through dialysis.[2]
For oxygenation of the embryo or fetus, and removal of carbon dioxide, extracorporeal membrane oxygenation (ECMO) is a functioning technique, having successfully kept goat fetuses alive for up to 237 hours in amniotic tanks.[10] ECMO is currently a technique used in selected neonatal intensive care units to treat term infants with selected medical problems that result in the infant's inability to survive through gas exchange using the lungs.[11] However, the cerebral vasculature and germinal matrix are poorly developed in fetuses, and subsequently, there is an unacceptably high risk for intraventricular hemorrhage (IVH) if administering ECMO at a gestational age less than 32 weeks.[12] Liquid ventilation has been suggested as an alternative method of oxygenation, or at least providing an intermediate stage between the womb and breathing in open air.[2]
For artificial nutrition, current techniques are problematic.[2] Total parenteral nutrition, as studied on infants with severe short bowel syndrome, has a 5-year survival of approximately 20%.[2][13]
Issues related to hormonal stability also remain to be addressed.[2]
Theoretically, animal suppliers and disposers may be used, but when involving an animal's uterus the technique may rather be in the scope of interspecific pregnancy.[original research?]
Uterine wall
In a normal uterus, the myometrium of the uterine wall functions to expel the fetus at the end of a pregnancy, and the endometrium plays a role in forming the placenta. An artificial uterus may include components of equivalent function. Methods have been considered to connect an artificial placenta and other "inner" components directly to an external circulation.[2]
Interface (artificial placenta)
An interface between the supplier and the embryo or fetus may be entirely artificial, e.g. by using one or more semipermeable membranes such as is used in extracorporeal membrane oxygenation (ECMO).[10]
There is also potential to grow a placenta using human endometrial cells. In 2002, it was announced that tissue samples from cultured endometrial cells removed from a human donor had successfully grown.[14][15] The tissue sample was then engineered to form the shape of a natural uterus, and human embryos were then implanted into the tissue. The embryos correctly implanted into the artificial uterus' lining and started to grow. However, the experiments were halted after six days to stay within the permitted legal limits of in vitro fertilisation (IVF) legislation in the United States.[2]
A human placenta may theoretically be transplanted inside an artificial uterus, but the passage of nutrients across this artificial uterus remains an unsolved issue.[2]
Amniotic tank (artificial amniotic sac)
The main function of an amniotic tank would be to fill the function of the amniotic sac in physically protecting the embryo or fetus, optimally allowing it to move freely. It should also be able to maintain an optimal temperature. Lactated Ringer's solution can be used as a substitute for amniotic fluid.[10]
Umbilical cord
Theoretically, in case of premature removal of the fetus from the natural uterus, the natural umbilical cord could be used, kept open either by medical inhibition of physiological occlusion, by anti-coagulation as well as by stenting or creating a bypass for sustaining blood flow between the mother and fetus.[2]
Research and development
The use of artificial wombs was first termed ectogenesis by JBS Haldane in 1923.[16][17][18][19]
Emanuel M. Greenberg (USA)
Emanuel M. Greenberg wrote various papers on the topic of the artificial womb and its potential use in the future.[citation needed]
On 22 July 1954 Emanuel M. Greenberg filed a patent on the design for an artificial womb.[20] The patent included two images of the design for an artificial womb. The design itself included a tank to place the fetus filled with amniotic fluid, a machine connecting to the umbilical cord, blood pumps, an artificial kidney, and a water heater. He was granted the patent on 15 November 1955.[20]
On 11 May 1960, Greenberg wrote to the editors of the American Journal of Obstetrics and Gynecology. Greenberg claimed that the journal had published the article "Attempts to Make an 'Artificial Uterus'", which failed to include any citations on the topic of the artificial uterus.[citation needed] According to Greenberg, this suggested that the idea of the artificial uterus was a new one although he himself had published several papers on the topic.[citation needed]
Juntendo University (Japan)
In 1996, Juntendo University in Tokyo developed the extra-uterine fetal incubation (EUFI).[21] The project was led by Yoshinori Kuwabara, who was interested in the development of immature newborns. The system was developed using fourteen goat fetuses that were then placed into artificial amniotic fluid under the same conditions of a mother goat.[21][22] Kuwabara and his team succeeded in keeping the goat fetuses in the system for three weeks.[21][22] The system, however, ran into several problems and was not ready for human testing.[21] Kuwabara remained hopeful that the system would be improved and would later be used on human fetuses.[21][22]
Children's Hospital of Philadelphia
In 2017, researchers at the Children's Hospital of Philadelphia were able to further develop the extra-uterine system. The study uses fetal lambs which are then placed in a plastic bag filled with artificial amniotic fluid.[1][8] The system consist in 3 main components: a pumpless arteriovenous circuit, a closed sterile fluid environment and an umbilical vascular access. Regarding the pumpless arteriovenous circuit, the blood flow is driven exclusively by the fetal heart, combined with a very low resistance oxygenator to most closely mimic the normal fetal/placental circulation. The closed sterile fluid environment is important to ensure sterility. Scientists developed a technique for umbilical cord vessel cannulation that maintains a length of native umbilical cord (5–10 cm) between the cannula tips and the abdominal wall, to minimize decannulation events and the risk of mechanical obstruction.[23] The umbilical cord of the lambs are attached to a machine outside of the bag designed to act like a placenta and provide oxygen and nutrients and also remove any waste.[1][8] The researchers kept the machine "in a dark, warm room where researchers can play the sounds of the mother's heart for the lamb fetus."[8] The system succeeded in helping the premature lamb fetuses develop normally for a month.[8] Indeed, scientists have run 8 lambs with maintenance of stable levels of circuit flow equivalent to the normal flow to the placenta. Specifically, they have run 5 fetuses from 105 to 108 days of gestation for 25–28 days, and 3 fetuses from 115 to 120 days of gestation for 20–28 days. The longest runs were terminated at 28 days due to animal protocol limitations rather than any instability, suggesting that support of these early gestational animals could be maintained beyond 4 weeks.[23] Alan Flake, a fetal surgeon at the Children's Hospital of Philadelphia hopes to move testing to premature human fetuses, but this could take anywhere from three to five years to become a reality.[8] Flake, who led the study, calls the possibility of their technology recreating a full pregnancy a "pipe dream at this point" and does not personally intend to create the technology to do so.[8]
Eindhoven University of Technology (NL)
Since 2016, researchers of TU/e and partners aim to develop an artificial womb, which is an adequate substitute for the protective environment of the maternal womb in case of premature birth, preventing health complications. The artificial womb and placenta will provide a natural environment for the baby with the goal to ease the transition to newborn life. The perinatal life support (PLS) system will be developed using breakthrough technology: a manikin will mimic the infant during testing and training, advanced monitoring and computational modeling will provide clinical guidance.[24]
The consortium of 3 European universities working on the project consists out of Aachen, Milaan and Eindhoven. In 2019 this consortium was granted a subsidy of 3 million euro, and a second grant of 10 million is in progress. Together, the PLS partners provide joint medical, engineering, and mathematical expertise to develop and validate the Perinatal Life Support system using breakthrough simulation technologies. The interdisciplinary consortium will push the development of these technologies forward and combine them to establish the first ex vivo fetal maturation system for clinical use. This project, coordinated by the Eindhoven University of Technology brings together world-leading experts in obstetrics, neonatology, industrial design, mathematical modelling, ex vivo organ support, and non-invasive fetal monitoring. This consortium is led by professor Frans van de Vosse and Professor and doctor Guid Oei. in 2020 the spin off Juno Perinatal Healthcare has been set up by engineers Jasmijn Kok and Lyla Kok, assuring valorisation of the research done. More information about the spin off can be found here;[25]
More information about the project of the technical universities and its researchers can be found here:[26]
Weizmann Institute of Science (Israel)
Further information: Ectogenesis § Synthetic embryo

Electronically controlled ex utero roller culture system (technical steps during sEmbryo culture protocol)[27]
In 2021, the Weizmann Institute of Science in Israel built a mechanical uterus and grew mouse embryos outside the uterus for several days.[27] This device was also used in 2022 to nurture mouse stem cells for over a week and grow synthetic embryos from stem cells.[28][29]
Philosophical considerations
Bioethics
The development of artificial uteri and ectogenesis raises bioethical and legal considerations, and also has important implications for reproductive rights and the abortion debate.
Artificial uteri may expand the range of fetal viability, raising questions about the role that fetal viability plays within abortion law. Within severance theory, for example, abortion rights only include the right to remove the fetus, and do not always extend to the termination of the fetus. If transferring the fetus from a woman's womb to an artificial uterus is possible, the choice to terminate a pregnancy in this way could provide an alternative to aborting the fetus.[30][31]
There are also theoretical concerns that children who develop in an artificial uterus may lack "some essential bond with their mothers that other children have".[32]
Gender equality and LGBT
In the 1970 book The Dialectic of Sex, feminist Shulamith Firestone wrote that differences in biological reproductive roles are a source of gender inequality. Firestone singled out pregnancy and childbirth, making the argument that an artificial womb would free "women from the tyranny of their reproductive biology."[33][34]
Arathi Prasad argues in her column on The Guardian in her article "How artificial wombs will change our ideas of gender, family and equality" that "It will [...] give men an essential tool to have a child entirely without a woman, should they choose. It will ask us to question concepts of gender and parenthood." She furthermore argues for the benefits for same-sex couples: "It might also mean that the divide between mother and father can be dispensed with: a womb outside a woman’s body would serve women, trans women and male same-sex couples equally without prejudice."[35]
See alsoAmniotic fluid
Apheresis
Brave New World
Ectogenesis
Embryo space colonization
Extracorporeal membrane oxygenation
Hemodialysis
In vitro fertilisation
Male pregnancy
Postgenderism
Tissue engineering
https://en.wikipedia.org/wiki/Artificial_womb
https://en.wikipedia.org/wiki/Artificial_womb
Tissue engineering is a biomedical engineering discipline that uses a combination of cells, engineering, materials methods, and suitable biochemical and physicochemical factors to restore, maintain, improve, or replace different types of biological tissues. Tissue engineering often involves the use of cells placed on tissue scaffolds in the formation of new viable tissue for a medical purpose but is not limited to applications involving cells and tissue scaffolds. While it was once categorized as a sub-field of biomaterials, having grown in scope and importance it can be considered as a field of its own.
What tissue engineering is and how it works
While most definitions of tissue engineering cover a broad range of applications, in practice the term is closely associated with applications that repair or replace portions of or whole tissues (i.e. bone, cartilage,[1] blood vessels, bladder, skin, muscle etc.). Often, the tissues involved require certain mechanical and structural properties for proper functioning. The term has also been applied to efforts to perform specific biochemical functions using cells within an artificially-created support system (e.g. an artificial pancreas, or a bio artificial liver). The term regenerative medicine is often used synonymously with tissue engineering, although those involved in regenerative medicine place more emphasis on the use of stem cells or progenitor cells to produce tissues.
Overview

Micro-mass cultures of C3H-10T1/2 cells at varied oxygen tensions stained with Alcian blue
A commonly applied definition of tissue engineering, as stated by Langer[2] and Vacanti,[3] is "an interdisciplinary field that applies the principles of engineering and life sciences toward the development of biological substitutes that restore, maintain, or improve [Biological tissue] function or a whole organ".[4] In addition, Langer and Vacanti also state that there are three main types of tissue engineering: cells, tissue-inducing substances, and a cells + matrix approach (often referred to as a scaffold). Tissue engineering has also been defined as "understanding the principles of tissue growth, and applying this to produce functional replacement tissue for clinical use".[5] A further description goes on to say that an "underlying supposition of tissue engineering is that the employment of natural biology of the system will allow for greater success in developing therapeutic strategies aimed at the replacement, repair, maintenance, or enhancement of tissue function".[5]
Developments in the multidisciplinary field of tissue engineering have yielded a novel set of tissue replacement parts and implementation strategies. Scientific advances in biomaterials, stem cells, growth and differentiation factors, and biomimetic environments have created unique opportunities to fabricate or improve existing tissues in the laboratory from combinations of engineered extracellular matrices ("scaffolds"), cells, and biologically active molecules. Among the major challenges now facing tissue engineering is the need for more complex functionality, biomechanical stability, and vascularization in laboratory-grown tissues destined for transplantation.[6]
Etymology
The historic origin of the term is unclear as the definition of the word has changed throughout the past few decades. The term first appeared in a 1984 publication that described the organization of an endothelium-like membrane on the surface of a long-implanted, synthetic ophthalmic prosthesis.[7]
The first modern use of the term as recognized today was in 1985 by the researcher, physiologist and bioengineer Yuan-Cheng Fung of the Engineering Research Center. He proposed the joining of the terms tissue (in reference to the fundamental relationship between cells and organs) and engineering (in reference to the field of modification of said tissues). The term was officially adopted in 1987.[7]
History
Ancient era (pre-17th century)
A rudimentary understanding of the inner workings of human tissues may date back further than most would expect. As early as the Neolithic period, sutures were being used to close wounds and aid in healing. Later on, societies such as ancient Egypt developed better materials for sewing up wounds such as linen sutures. Around 2500 BC in ancient India, skin grafts were developed by cutting skin from the buttock and suturing it to wound sites in the ear, nose, or lips. Ancient Egyptians often would graft skin from corpses onto living humans and even attempted to use honey as a type of antibiotic and grease as a protective barrier to prevent infection. In the 1st and 2nd centuries AD, Gallo-Romans developed wrought iron implants and dental implants could be found in ancient Mayans.
Enlightenment (17th century–19th century)
While these ancient societies had developed techniques that were way ahead of their time, they still lacked a mechanistic understanding of how the body was reacting to these procedures. This mechanistic approach came along in tandem with the development of the empirical method of science pioneered by René Descartes. Sir Isaac Newton began to describe the body as a "physiochemical machine" and postured that disease was a breakdown in the machine. In the 17th century, Robert Hooke discovered the cell and a letter from Benedict de Spinoza brought forward the idea of the homeostasis between the dynamic processes in the body. Hydra experiments performed by Abraham Trembley in the 18th century began to delve into the regenerative capabilities of cells. During the 19th century, a better understanding of how different metals reacted with the body led to the development of better sutures and a shift towards screw and plate implants in bone fixation. Further, it was first hypothesized in the mid-1800s that cell-environment interactions and cell proliferation were vital for tissue regeneration.
Modern era (20th and 21st centuries)
As time progresses and technology advances, there is a constant need for change in the approach researchers take in their studies. Tissue engineering has continued to evolve over centuries. In the beginning people used to look at and use samples directly from human or animal cadavers. Now, tissue engineers have the ability to remake many of the tissues in the body through the use of modern techniques such as microfabrication and three-dimensional bioprinting in conjunction with native tissue cells/stem cells. These advances have allowed researchers to generate new tissues in a much more efficient manner. For example, these techniques allow for more personalization which allow for better biocompatibility, decreased immune response, cellular integration, and longevity. There is no doubt that these techniques will continue to evolve, as we have continued to see microfabrication and bioprinting evolve over the past decade.
In 1960, Wichterle and Lim were the first to publish experiments on hydrogels for biomedical applications by using them in contact lens construction. Work on the field developed slowly over the next two decades, but later found traction when hydrogels were repurposed for drug delivery. In 1984, Charles Hull developed bioprinting by converting a Hewlett-Packard inkjet printer into a device capable of depositing cells in 2-D. Three dimensional (3-D) printing is a type of additive manufacturing which has since found various applications in medical engineering, due to its high precision and efficiency. With biologist James Thompson's development of first human stem cell lines in 1998 followed by transplantation of first laboratory-grown internal organs in 1999 and creation of the first bioprinter in 2003 by the University of Missouri when they printed spheroids without the need of scaffolds, 3-D bioprinting became more conventionally used in medical field than ever before. So far, scientists have been able to print mini organoids and organs-on-chips that have rendered practical insights into the functions of a human body. Pharmaceutical companies are using these models to test drugs before moving on to animal studies. However, a fully functional and structurally similar organ hasn't been printed yet. A team at University of Utah has reportedly printed ears and successfully transplanted those onto children born with defects that left their ears partially developed.
Today hydrogels are considered the preferred choice of bio-inks for 3-D bioprinting since they mimic cells' natural ECM while also containing strong mechanical properties capable of sustaining 3-D structures. Furthermore, hydrogels in conjunction with 3-D bioprinting allow researchers to produce different scaffolds which can be used to form new tissues or organs. 3-D printed tissues still face many challenges such as adding vasculature. Meanwhile, 3-D printing parts of tissues definitely will improve our understanding of the human body, thus accelerating both basic and clinical research.
Examples

Regenerating a human ear using a scaffold
As defined by Langer and Vacanti,[4] examples of tissue engineering fall into one or more of three categories: "just cells," "cells and scaffold," or "tissue-inducing factors." In vitro meat: Edible artificial animal muscle tissue cultured in vitro.
Bioartificial liver device, "Temporary Liver", Extracorporeal Liver Assist Device (ELAD): The human hepatocyte cell line (C3A line) in a hollow fiber bioreactor can mimic the hepatic function of the liver for acute instances of liver failure. A fully capable ELAD would temporarily function as an individual's liver, thus avoiding transplantation and allowing regeneration of their own liver.
Artificial pancreas: Research involves using islet cells to regulate the body's blood sugar, particularly in cases of diabetes . Biochemical factors may be used to cause human pluripotent stem cells to differentiate (turn into) cells that function similarly to beta cells, which are in an islet cell in charge of producing insulin.
Artificial bladders: Anthony Atala[8] (Wake Forest University) has successfully implanted artificial bladders, constructed of cultured cells seeded onto a bladder-shaped scaffold, into seven out of approximately 20 human test subjects as part of a long-term experiment.[9]
Cartilage: lab-grown cartilage, cultured in vitro on a scaffold, was successfully used as an autologous transplant to repair patients' knees.[10]
Scaffold-free cartilage: Cartilage generated without the use of exogenous scaffold material. In this methodology, all material in the construct is cellular produced directly by the cells.[11]
Bioartificial heart: Doris Taylor's lab constructed a biocompatible rat heart by re-cellularising a de-cellularised rat heart. This scaffold and cells were placed in a bioreactor, where it matured to become a partially or fully transplantable organ.[12] the work was called a "landmark". The lab first stripped the cells away from a rat heart (a process called "decellularization") and then injected rat stem cells into the decellularized rat heart.[13]
Tissue-engineered blood vessels:[14] Blood vessels that have been grown in a lab and can be used to repair damaged blood vessels without eliciting an immune response.
Artificial skin constructed from human skin cells embedded in a hydrogel, such as in the case of bio-printed constructs for battlefield burn repairs.[15]
Artificial bone marrow: Bone marrow cultured in vitro to be transplanted serves as a "just cells" approach to tissue engineering.[16]
Tissue engineered bone: A structural matrix can be composed of metals such as titanium, polymers of varying degradation rates, or certain types of ceramics.[17] Materials are often chosen to recruit osteoblasts to aid in reforming the bone and returning biological function.[18] Various types of cells can be added directly into the matrix to expedite the process.[17]
Laboratory-grown penis: Decellularized scaffolds of rabbit penises were recellularised with smooth muscle and endothelial cells. The organ was then transplanted to live rabbits and functioned comparably to the native organ, suggesting potential as treatment for genital trauma.[19]
Oral mucosa tissue engineering uses a cells and scaffold approach to replicate the 3 dimensional structure and function of oral mucosa.
Cells as building blocks
Stained cells in culture
Cells are one of the main components for the success of tissue engineering approaches. Tissue engineering uses cells as strategies for creation/replacement of new tissue. Examples include fibroblasts used for skin repair or renewal,[20] chondrocytes used for cartilage repair (MACI–FDA approved product), and hepatocytes used in liver support systems
Cells can be used alone or with support matrices for tissue engineering applications. An adequate environment for promoting cell growth, differentiation, and integration with the existing tissue is a critical factor for cell-based building blocks.[21] Manipulation of any of these cell processes create alternative avenues for the development of new tissue (e.g., reprogramming of somatic cells, vascularization).
Isolation
Techniques for cell isolation depend on the cell source. Centrifugation and apheresis are techniques used for extracting cells from biofluids (e.g., blood). Whereas digestion processes, typically using enzymes to remove the extracellular matrix (ECM), are required prior to centrifugation or apheresis techniques to extract cells from tissues/organs. Trypsin and collagenase are the most common enzymes used for tissue digestion. While trypsin is temperature dependent, collagenase is less sensitive to changes in temperature.
Cell sources

Mouse embryonic stem cells
Primary cells are those directly isolated from host tissue. These cells provide an ex-vivo model of cell behavior without any genetic, epigenetic, or developmental changes; making them a closer replication of in-vivo conditions than cells derived from other methods.[22] This constraint however, can also make studying them difficult. These are mature cells, often terminally differentiated, meaning that for many cell types proliferation is difficult or impossible. Additionally, the microenvironments these cells exist in are highly specialized, often making replication of these conditions difficult.[23]
Secondary cells A portion of cells from a primary culture is moved to a new repository/vessel to continue being cultured. Medium from the primary culture is removed, the cells that are desired to be transferred are obtained, and then cultured in a new vessel with fresh growth medium.[citation needed] A secondary cell culture is useful in order to ensure that cells have both the room and nutrients that they require to grow. Secondary cultures are most notably used in any scenario in which a larger quantity of cells than can be found in the primary culture is desired. Secondary cells share the constraints of primary cells (see above) but have an added risk of contamination when transferring to a new vessel.
Genetic classifications of cells
Autologous: The donor and the recipient of the cells are the same individual. Cells are harvested, cultured or stored, and then reintroduced to the host. As a result of the host's own cells being reintroduced, an antigenic response is not elicited. The body's immune system recognizes these re-implanted cells as its own, and does not target them for attack. Autologous cell dependence on host cell health and donor site morbidity may be deterrents to their use. Adipose-derived and bone marrow-derived mesenchymal stem cells are commonly autologous in nature, and can be used in a myriad of ways, from helping repair skeletal tissue to replenishing beta cells in diabetic patients.[24][25][26][27]
Allogenic: Cells are obtained from the body of a donor of the same species as the recipient. While there are some ethical constraints to the use of human cells for in vitro studies (i.e. human brain tissue chimera development[28]), the employment of dermal fibroblasts from human foreskin demonstrates an immunologically safe and thus a viable choice for allogenic tissue engineering of the skin.
Xenogenic: These cells are derived isolated cells from alternate species from the recipient. A notable example of xenogeneic tissue utilization is cardiovascular implant construction via animal cells. Chimeric human-animal farming raises ethical concerns around the potential for improved consciousness from implanting human organs in animals.[29]
Syngeneic or isogenic: These cells describe those borne from identical genetic code. This imparts an immunologic benefit similar to autologous cell lines (see above).[30] Autologous cells can be considered syngenic, but the classification also extends to non-autologously derived cells such as those from an identical twin, from genetically identical (cloned) research models, or induced stem cells (iSC)[31] as related to the donor.
Stem cells
Stem cells are undifferentiated cells with the ability to divide in culture and give rise to different forms of specialized cells. Stem cells are divided into "adult" and "embryonic" stem cells according to their source. While there is still a large ethical debate related to the use of embryonic stem cells, it is thought that another alternative source – induced pluripotent stem cells – may be useful for the repair of diseased or damaged tissues, or may be used to grow new organs.
Totipotent cells are stem cells which can divide into further stem cells or differentiate into any cell type in the body, including extra-embryonic tissue.
Pluripotent cells are stem cells which can differentiate into any cell type in the body except extra-embryonic tissue. induced pluripotent stem cells (iPSCs) are subclass of pluripotent stem cells resembling embryonic stem cells (ESCs) that have been derived from adult differentiated cells. iPSCs are created by altering the expression of transcriptional factors in adult cells until they become like embryonic stem cells.[citation needed]
Multipotent stem cells can be differentiated into any cell within the same class, such as blood or bone. A common example of multipotent cells is Mesenchymal stem cells (MSCs).
Scaffolds
Scaffolds are materials that have been engineered to cause desirable cellular interactions to contribute to the formation of new functional tissues for medical purposes. Cells are often 'seeded' into these structures capable of supporting three-dimensional tissue formation. Scaffolds mimic the extracellular matrix of the native tissue, recapitulating the in vivo milieu and allowing cells to influence their own microenvironments. They usually serve at least one of the following purposes: allowing cell attachment and migration, delivering and retaining cells and biochemical factors, enabling diffusion of vital cell nutrients and expressed products, and exerting certain mechanical and biological influences to modify the behaviour of the cell phase.
In 2009, an interdisciplinary team led by the thoracic surgeon Thorsten Walles implanted the first bioartificial transplant that provides an innate vascular network for post-transplant graft supply successfully into a patient awaiting tracheal reconstruction.[32]

This animation of a rotating carbon nanotube shows its 3D structure. Carbon nanotubes are among the numerous candidates for tissue engineering scaffolds since they are biocompatible, resistant to biodegradation and can be functionalized with biomolecules. However, the possibility of toxicity with non-biodegradable nano-materials is not fully understood.[33]
To achieve the goal of tissue reconstruction, scaffolds must meet some specific requirements. High porosity and adequate pore size are necessary to facilitate cell seeding and diffusion throughout the whole structure of both cells and nutrients. Biodegradability is often an essential factor since scaffolds should preferably be absorbed by the surrounding tissues without the necessity of surgical removal. The rate at which degradation occurs has to coincide as much as possible with the rate of tissue formation: this means that while cells are fabricating their own natural matrix structure around themselves, the scaffold is able to provide structural integrity within the body and eventually it will break down leaving the newly formed tissue which will take over the mechanical load. Injectability is also important for clinical uses. Recent research on organ printing is showing how crucial a good control of the 3D environment is to ensure reproducibility of experiments and offer better results.
Materials
Material selection is an essential aspect of producing a scaffold. The materials utilized can be natural or synthetic and can be biodegradable or non-biodegradable. Additionally, they must be biocompatible, meaning that they don't cause any adverse effects to cells.[34] Silicone, for example, is a synthetic, non-biodegradable material commonly used as a drug delivery material,[35][36] while gelatin is a biodegradable, natural material commonly used in cell-culture scaffolds[37][38][39]
The material needed for each application is different, and dependent on the desired mechanical properties of the material. Tissue engineering of long bone defects for example, will require a rigid scaffold with a compressive strength similar to that of cortical bone (100-150 MPa), which is much higher compared to a scaffold for skin regeneration.[40][41]
There are a few versatile synthetic materials used for many different scaffold applications. One of these commonly used materials is polylactic acid (PLA), a synthetic polymer. PLA – polylactic acid. This is a polyester which degrades within the human body to form lactic acid, a naturally occurring chemical which is easily removed from the body. Similar materials are polyglycolic acid (PGA) and polycaprolactone (PCL): their degradation mechanism is similar to that of PLA, but PCL degrades slower and PGA degrades faster.[citation needed] PLA is commonly combined with PGA to create poly-lactic-co-glycolic acid (PLGA). This is especially useful because the degradation of PLGA can be tailored by altering the weight percentages of PLA and PGA: More PLA – slower degradation, more PGA – faster degradation. This tunability, along with its biocompatibility, makes it an extremely useful material for scaffold creation.[42]
Scaffolds may also be constructed from natural materials: in particular different derivatives of the extracellular matrix have been studied to evaluate their ability to support cell growth. Protein based materials – such as collagen, or fibrin, and polysaccharidic materials- like chitosan[43] or glycosaminoglycans (GAGs), have all proved suitable in terms of cell compatibility. Among GAGs, hyaluronic acid, possibly in combination with cross linking agents (e.g. glutaraldehyde, water-soluble carbodiimide, etc.), is one of the possible choices as scaffold material. Due to the covalent attachment of thiol groups to these polymers, they can crosslink via disulfide bond formation.[44] The use of thiolated polymers (thiomers) as scaffold material for tissue engineering was initially introduced at the 4th Central European Symposium on Pharmaceutical Technology in Vienna 2001.[45] As thiomers are biocompatible, exhibit cellular mimicking properties and efficiently support proliferation and differentiation of various cell types, they are extensively used as scaffolds for tissue engineering.[46][47][48] Furthermore thiomers such as thiolated hyaluronic acid[49] and thiolated chitosan[50] were shown to exhibit wound healing properties and are subject of numerous clinical trials.[51] Additionally, a fragment of an extracellular matrix protein, such as the RGD peptide, can be coupled to a non-bioactive material to promote cell attachment.[52] Another form of scaffold is decellularized tissue. This is a process where chemicals are used to extracts cells from tissues, leaving just the extracellular matrix. This has the benefit of a fully formed matrix specific to the desired tissue type. However, the decellurised scaffold may present immune problems with future introduced cells.
Synthesis

Tissue engineered vascular graft

Tissue engineered heart valve
A number of different methods have been described in the literature for preparing porous structures to be employed as tissue engineering scaffolds. Each of these techniques presents its own advantages, but none are free of drawbacks.
Nanofiber self-assembly
Molecular self-assembly is one of the few methods for creating biomaterials with properties similar in scale and chemistry to that of the natural in vivo extracellular matrix (ECM), a crucial step toward tissue engineering of complex tissues.[53] Moreover, these hydrogel scaffolds have shown superiority in in vivo toxicology and biocompatibility compared to traditional macro-scaffolds and animal-derived materials.
Textile technologies
These techniques include all the approaches that have been successfully employed for the preparation of non-woven meshes of different polymers. In particular, non-woven polyglycolide structures have been tested for tissue engineering applications: such fibrous structures have been found useful to grow different types of cells. The principal drawbacks are related to the difficulties in obtaining high porosity and regular pore size.
Solvent casting and particulate leaching
Solvent casting and particulate leaching (SCPL) allows for the preparation of structures with regular porosity, but with limited thickness. First, the polymer is dissolved into a suitable organic solvent (e.g. polylactic acid could be dissolved into dichloromethane), then the solution is cast into a mold filled with porogen particles. Such porogen can be an inorganic salt like sodium chloride, crystals of saccharose, gelatin spheres or paraffin spheres. The size of the porogen particles will affect the size of the scaffold pores, while the polymer to porogen ratio is directly correlated to the amount of porosity of the final structure. After the polymer solution has been cast the solvent is allowed to fully evaporate, then the composite structure in the mold is immersed in a bath of a liquid suitable for dissolving the porogen: water in the case of sodium chloride, saccharose and gelatin or an aliphatic solvent like hexane for use with paraffin. Once the porogen has been fully dissolved, a porous structure is obtained. Other than the small thickness range that can be obtained, another drawback of SCPL lies in its use of organic solvents which must be fully removed to avoid any possible damage to the cells seeded on the scaffold.
Gas foaming
To overcome the need to use organic solvents and solid porogens, a technique using gas as a porogen has been developed. First, disc-shaped structures made of the desired polymer are prepared by means of compression molding using a heated mold. The discs are then placed in a chamber where they are exposed to high pressure CO2 for several days. The pressure inside the chamber is gradually restored to atmospheric levels. During this procedure the pores are formed by the carbon dioxide molecules that abandon the polymer, resulting in a sponge-like structure. The main problems resulting from such a technique are caused by the excessive heat used during compression molding (which prohibits the incorporation of any temperature labile material into the polymer matrix) and by the fact that the pores do not form an interconnected structure.
Emulsification freeze-drying
This technique does not require the use of a solid porogen like SCPL. First, a synthetic polymer is dissolved into a suitable solvent (e.g. polylactic acid in dichloromethane) then water is added to the polymeric solution and the two liquids are mixed in order to obtain an emulsion. Before the two phases can separate, the emulsion is cast into a mold and quickly frozen by means of immersion into liquid nitrogen. The frozen emulsion is subsequently freeze-dried to remove the dispersed water and the solvent, thus leaving a solidified, porous polymeric structure. While emulsification and freeze-drying allow for a faster preparation when compared to SCPL (since it does not require a time-consuming leaching step), it still requires the use of solvents. Moreover, pore size is relatively small and porosity is often irregular. Freeze-drying by itself is also a commonly employed technique for the fabrication of scaffolds. In particular, it is used to prepare collagen sponges: collagen is dissolved into acidic solutions of acetic acid or hydrochloric acid that are cast into a mold, frozen with liquid nitrogen and then lyophilized.
Thermally induced phase separation
Similar to the previous technique, the TIPS phase separation procedure requires the use of a solvent with a low melting point that is easy to sublime. For example, dioxane could be used to dissolve polylactic acid, then phase separation is induced through the addition of a small quantity of water: a polymer-rich and a polymer-poor phase are formed. Following cooling below the solvent melting point and some days of vacuum-drying to sublime the solvent, a porous scaffold is obtained. Liquid-liquid phase separation presents the same drawbacks of emulsification/freeze-drying.[54]
Electrospinning
Electrospinning is a highly versatile technique that can be used to produce continuous fibers ranging in diameter from a few microns to a few nanometers. In a typical electrospinning set-up, the desired scaffold material is dissolved within a solvent and placed within a syringe. This solution is fed through a needle and a high voltage is applied to the tip and to a conductive collection surface. The buildup of electrostatic forces within the solution causes it to eject a thin fibrous stream towards the oppositely charged or grounded collection surface. During this process the solvent evaporates, leaving solid fibers leaving a highly porous network. This technique is highly tunable, with variation to solvent, voltage, working distance (distance from the needle to collection surface), flow rate of solution, solute concentration, and collection surface. This allows for precise control of fiber morphology.
On a commercial level however, due to scalability reasons, there are 40 or sometimes 96 needles involved operating at once. The bottle-necks in such set-ups are: 1) Maintaining the aforementioned variables uniformly for all of the needles and 2) formation of "beads" in single fibers that we as engineers, want to be of a uniform diameter. By modifying variables such as the distance to collector, magnitude of applied voltage, or solution flow rate – researchers can dramatically change the overall scaffold architecture.
Historically, research on electrospun fibrous scaffolds dates back to at least the late 1980s when Simon showed that electrospinning could be used to produced nano- and submicron-scale fibrous scaffolds from polymer solutions specifically intended for use as in vitro cell and tissue substrates. This early use of electrospun lattices for cell culture and tissue engineering showed that various cell types would adhere to and proliferate upon polycarbonate fibers. It was noted that as opposed to the flattened morphology typically seen in 2D culture, cells grown on the electrospun fibers exhibited a more rounded 3-dimensional morphology generally observed of tissues in vivo.[55]
CAD/CAM technologies
Because most of the above techniques are limited when it comes to the control of porosity and pore size, computer assisted design and manufacturing techniques have been introduced to tissue engineering. First, a three-dimensional structure is designed using CAD software. The porosity can be tailored using algorithms within the software.[56] The scaffold is then realized by using ink-jet printing of polymer powders or through Fused Deposition Modeling of a polymer melt.[57]
A 2011 study by El-Ayoubi et al. investigated "3D-plotting technique to produce (biocompatible and biodegradable) poly-L-Lactide macroporous scaffolds with two different pore sizes" via solid free-form fabrication (SSF) with computer-aided-design (CAD), to explore therapeutic articular cartilage replacement as an "alternative to conventional tissue repair".[58] The study found the smaller the pore size paired with mechanical stress in a bioreactor (to induce in vivo-like conditions), the higher the cell viability in potential therapeutic functionality via decreasing recovery time and increasing transplant effectiveness.[58]
Laser-assisted bioprinting
In a 2012 study,[59] Koch et al. focused on whether Laser-assisted BioPrinting (LaBP) can be used to build multicellular 3D patterns in natural matrix, and whether the generated constructs are functioning and forming tissue. LaBP arranges small volumes of living cell suspensions in set high-resolution patterns.[59] The investigation was successful, the researchers foresee that "generated tissue constructs might be used for in vivo testing by implanting them into animal models" (14). As of this study, only human skin tissue has been synthesized, though researchers project that "by integrating further cell types (e.g. melanocytes, Schwann cells, hair follicle cells) into the printed cell construct, the behavior of these cells in a 3D in vitro microenvironment similar to their natural one can be analyzed", which is useful for drug discovery and toxicology studies.[59]
Self-assembled recombinant spider silk nanomembranes
Gustafsson et al.[60] demonstrated free‐standing, bioactive membranes of cm-sized area, but only 250 nm thin, that were formed by self‐assembly of spider silk at the interface of an aqueous solution. The membranes uniquely combine nanoscale thickness, biodegradability, ultrahigh strain and strength, permeability to proteins and promote rapid cell adherence and proliferation. They demonstrated growing a coherent layer of keratinocytes. These spider silk nanomembranes have also been used to create a static in-vitro model of a blood vessel.[61]
Assembly methods
A persistent problem within tissue engineering is mass transport limitations. Engineered tissues generally lack an initial blood supply, thus making it difficult for any implanted cells to obtain sufficient oxygen and nutrients to survive, or function properly.
Self-assembly
Self-assembly methods have been shown to be promising methods for tissue engineering. Self-assembly methods have the advantage of allowing tissues to develop their own extracellular matrix, resulting in tissue that better recapitulates biochemical and biomechanical properties of native tissue. Self-assembling engineered articular cartilage was introduced by Jerry Hu and Kyriacos A. Athanasiou in 2006[62] and applications of the process have resulted in engineered cartilage approaching the strength of native tissue.[63] Self-assembly is a prime technology to get cells grown in a lab to assemble into three-dimensional shapes. To break down tissues into cells, researchers first have to dissolve the extracellular matrix that normally binds them together. Once cells are isolated, they must form the complex structures that make up our natural tissues.
Liquid-based template assembly
The air-liquid surface established by Faraday waves is explored as a template to assemble biological entities for bottom-up tissue engineering. This liquid-based template can be dynamically reconfigured in a few seconds, and the assembly on the template can be achieved in a scalable and parallel manner. Assembly of microscale hydrogels, cells, neuron-seeded micro-carrier beads, cell spheroids into various symmetrical and periodic structures was demonstrated with good cell viability. Formation of 3-D neural network was achieved after 14-day tissue culture.[64]
Additive manufacturing
Main article: Organ printing
It might be possible to print organs, or possibly entire organisms using additive manufacturing techniques. A recent innovative method of construction uses an ink-jet mechanism to print precise layers of cells in a matrix of thermo-reversible gel. Endothelial cells, the cells that line blood vessels, have been printed in a set of stacked rings. When incubated, these fused into a tube.[57][65] This technique has been referred to as "bioprinting" within the field as it involves the printing of biological components in a structure resembling the organ of focus.
The field of three-dimensional and highly accurate models of biological systems is pioneered by multiple projects and technologies including a rapid method for creating tissues and even whole organs involve a 3-D printer that can bio-print the scaffolding and cells layer by layer into a working tissue sample or organ. The device is presented in a TED talk by Dr. Anthony Atala, M.D. the Director of the Wake Forest Institute for Regenerative Medicine, and the W.H. Boyce Professor and Chair of the Department of Urology at Wake Forest University, in which a kidney is printed on stage during the seminar and then presented to the crowd.[66][67][68] It is anticipated that this technology will enable the production of livers in the future for transplantation and theoretically for toxicology and other biological studies as well.
Recently Multi-Photon Processing (MPP) was employed for in vivo experiments by engineering artificial cartilage constructs. An ex vivo histological examination showed that certain pore geometry and the pre-growing of chondrocytes (Cho) prior to implantation significantly improves the performance of the created 3-D scaffolds. The achieved biocompatibility was comparable to the commercially available collagen membranes. The successful outcome of this study supports the idea that hexagonal-pore-shaped hybrid organic-inorganic micro-structured scaffolds in combination with Cho seeding may be successfully implemented for cartilage tissue engineering.[69]
Scaffolding
In 2013, using a 3-D scaffolding of Matrigel in various configurations, substantial pancreatic organoids was produced in vitro. Clusters of small numbers of cells proliferated into 40,000 cells within one week. The clusters transform into cells that make either digestive enzymes or hormones like insulin, self-organizing into branched pancreatic organoids that resemble the pancreas.[70]
The cells are sensitive to the environment, such as gel stiffness and contact with other cells. Individual cells do not thrive; a minimum of four proximate cells was required for subsequent organoid development. Modifications to the medium composition produced either hollow spheres mainly composed of pancreatic progenitors, or complex organoids that spontaneously undergo pancreatic morphogenesis and differentiation. Maintenance and expansion of pancreatic progenitors require active Notch and FGF signaling, recapitulating in vivo niche signaling interactions.[70]
The organoids were seen as potentially offering mini-organs for drug testing and for spare insulin-producing cells.[70]
Aside from Matrigel 3-D scaffolds, other collagen gel systems have been developed. Collagen/hyaluronic acid scaffolds have been used for modeling the mammary gland In Vitro while co-coculturing epithelial and adipocyte cells. The HyStem kit is another 3-D platform containing ECM components and hyaluronic acid that has been used for cancer research. Additionally, hydrogel constituents can be chemically modified to assist in crosslinking and enhance their mechanical properties.
Tissue culture
In many cases, creation of functional tissues and biological structures in vitro requires extensive culturing to promote survival, growth and inducement of functionality. In general, the basic requirements of cells must be maintained in culture, which include oxygen, pH, humidity, temperature, nutrients and osmotic pressure maintenance.
Tissue engineered cultures also present additional problems in maintaining culture conditions. In standard cell culture, diffusion is often the sole means of nutrient and metabolite transport. However, as a culture becomes larger and more complex, such as the case with engineered organs and whole tissues, other mechanisms must be employed to maintain the culture, such as the creation of capillary networks within the tissue.

Bioreactor for cultivation of vascular grafts
Another issue with tissue culture is introducing the proper factors or stimuli required to induce functionality. In many cases, simple maintenance culture is not sufficient. Growth factors, hormones, specific metabolites or nutrients, chemical and physical stimuli are sometimes required. For example, certain cells respond to changes in oxygen tension as part of their normal development, such as chondrocytes, which must adapt to low oxygen conditions or hypoxia during skeletal development. Others, such as endothelial cells, respond to shear stress from fluid flow, which is encountered in blood vessels. Mechanical stimuli, such as pressure pulses seem to be beneficial to all kind of cardiovascular tissue such as heart valves, blood vessels or pericardium.
Bioreactors
Main article: Bioreactor
In tissue engineering, a bioreactor is a device that attempts to simulate a physiological environment in order to promote cell or tissue growth in vitro. A physiological environment can consist of many different parameters such as temperature, pressure, oxygen or carbon dioxide concentration, or osmolality of fluid environment, and it can extend to all kinds of biological, chemical or mechanical stimuli. Therefore, there are systems that may include the application of forces such as electromagnetic forces, mechanical pressures, or fluid pressures to the tissue. These systems can be two- or three-dimensional setups. Bioreactors can be used in both academic and industry applications. General-use and application-specific bioreactors are also commercially available, which may provide static chemical stimulation or a combination of chemical and mechanical stimulation.
Cell proliferation and differentiation are largely influenced by mechanical[71] and biochemical[72] cues in the surrounding extracellular matrix environment. Bioreactors are typically developed to replicate the specific physiological environment of the tissue being grown (e.g., flex and fluid shearing for heart tissue growth).[73] This can allow specialized cell lines to thrive in cultures replicating their native environments, but it also makes bioreactors attractive tools for culturing stem cells. A successful stem-cell-based bioreactor is effective at expanding stem cells with uniform properties and/or promoting controlled, reproducible differentiation into selected mature cell types.[74]
There are a variety of bioreactors designed for 3D cell cultures. There are small plastic cylindrical chambers, as well as glass chambers, with regulated internal humidity and moisture specifically engineered for the purpose of growing cells in three dimensions.[75] The bioreactor uses bioactive synthetic materials such as polyethylene terephthalate membranes to surround the spheroid cells in an environment that maintains high levels of nutrients.[76][77] They are easy to open and close, so that cell spheroids can be removed for testing, yet the chamber is able to maintain 100% humidity throughout.[78] This humidity is important to achieve maximum cell growth and function. The bioreactor chamber is part of a larger device that rotates to ensure equal cell growth in each direction across three dimensions.[78]
QuinXell Technologies now under Quintech Life Sciences from Singapore has developed a bioreactor known as the TisXell Biaxial Bioreactor which is specially designed for the purpose of tissue engineering. It is the first bioreactor in the world to have a spherical glass chamber with biaxial rotation; specifically to mimic the rotation of the fetus in the womb; which provides a conducive environment for the growth of tissues.[79]
Multiple forms of mechanical stimulation have also been combined into a single bioreactor. Using gene expression analysis, one academic study found that applying a combination of cyclic strain and ultrasound stimulation to pre-osteoblast cells in a bioreactor accelerated matrix maturation and differentiation.[80] The technology of this combined stimulation bioreactor could be used to grow bone cells more quickly and effectively in future clinical stem cell therapies.[81]
MC2 Biotek has also developed a bioreactor known as ProtoTissue[75] that uses gas exchange to maintain high oxygen levels within the cell chamber; improving upon previous bioreactors, since the higher oxygen levels help the cell grow and undergo normal cell respiration.[82]
Active areas of research on bioreactors includes increasing production scale and refining the physiological environment, both of which could improve the efficiency and efficacy of bioreactors in research or clinical use. Bioreactors are currently used to study, among other things, cell and tissue level therapies, cell and tissue response to specific physiological environment changes, and development of disease and injury.
Long fiber generation
In 2013, a group from the University of Tokyo developed cell laden fibers up to a meter in length and on the order of 100 µm in size.[83] These fibers were created using a microfluidic device that forms a double coaxial laminar flow. Each 'layer' of the microfluidic device (cells seeded in ECM, a hydrogel sheath, and finally a calcium chloride solution). The seeded cells culture within the hydrogel sheath for several days, and then the sheath is removed with viable cell fibers. Various cell types were inserted into the ECM core, including myocytes, endothelial cells, nerve cell fibers, and epithelial cell fibers. This group then showed that these fibers can be woven together to fabricate tissues or organs in a mechanism similar to textile weaving. Fibrous morphologies are advantageous in that they provide an alternative to traditional scaffold design, and many organs (such as muscle) are composed of fibrous cells.
Bioartificial organs

This section's tone or style may not reflect the encyclopedic tone used on Wikipedia. See Wikipedia's guide to writing better articles for suggestions. (April 2014) (Learn how and when to remove this template message)
Main article: Bioartificial organ
An artificial organ is an engineered device that can be extra corporeal or implanted to support impaired or failing organ systems.[84] Bioartificial organs are typically created with the intent to restore critical biological functions like in the replacement of diseased hearts and lungs, or provide drastic quality of life improvements like in the use of engineered skin on burn victims.[84] While some examples of bioartificial organs are still in the research stage of development due to the limitations involved with creating functional organs, others are currently being used in clinical settings experimentally and commercially.[85]
Lung
Extracorporeal membrane oxygenation (ECMO) machines, otherwise known as heart and lung machines, are an adaptation of cardiopulmonary bypass techniques that provide heart and lung support.[86] It is used primarily to support the lungs for a prolonged but still temporary timeframe (1–30 days) and allow for recovery from reversible diseases.[86] Robert Bartlett is known as the father of ECMO and performed the first treatment of a newborn using an EMCO machine in 1975.[87]
Skin
Tissue-engineered skin is a type of bioartificial organ that is often used to treat burns, diabetic foot ulcers, or other large wounds that cannot heal well on their own. Artificial skin can be made from autografts, allografts, and xenografts. Autografted skin comes from a patient's own skin, which allows the dermis to have a faster healing rate, and the donor site can be re-harvested a few times. Allograft skin often comes from cadaver skin and is mostly used to treat burn victims. Lastly, xenografted skin comes from animals and provides a temporary healing structure for the skin. They assist in dermal regeneration, but cannot become part of the host skin.[88] Tissue-engineered skin is now available in commercial products. Integra, originally used to only treat burns, consists of a collagen matrix and chondroitin sulfate that can be used as a skin replacement. The chondroitin sulfate functions as a component of proteoglycans, which helps to form the extracellular matrix.[89] Integra can be repopulated and revascularized while maintaining its dermal collagen architecture, making it a bioartificial organ[90] Dermagraft, another commercial-made tissue-engineered skin product, is made out of living fibroblasts. These fibroblasts proliferate and produce growth factors, collagen, and ECM proteins, that help build granulation tissue.[91]
Heart
Since the number of patients awaiting a heart transplant is continuously increasing over time, and the number of patients on the waiting list surpasses the organ availability,[92] artificial organs used as replacement therapy for terminal heart failure would help alleviate this difficulty. Artificial hearts are usually used to bridge the heart transplantation or can be applied as replacement therapy for terminal heart malfunction.[93] The total artificial heart (TAH), first introduced by Dr. Vladimir P. Demikhov in 1937,[94] emerged as an ideal alternative. Since then it has been developed and improved as a mechanical pump that provides long-term circulatory support and replaces diseased or damaged heart ventricles that cannot properly pump the blood, restoring thus the pulmonary and systemic flow.[95] Some of the current TAHs include AbioCor, an FDA-approved device that comprises two artificial ventricles and their valves, and does not require subcutaneous connections, and is indicated for patients with biventricular heart failure. In 2010 SynCardia released the portable freedom driver that allows patients to have a portable device without being confined to the hospital.[96]
Kidney
While kidney transplants are possible, renal failure is more often treated using an artificial kidney.[97] The first artificial kidneys and the majority of those currently in use are extracorporeal, such as with hemodialysis, which filters blood directly, or peritoneal dialysis, which filters via a fluid in the abdomen.[97][98] In order to contribute to the biological functions of a kidney such as producing metabolic factors or hormones, some artificial kidneys incorporate renal cells.[97][98] There has been progress in the way of making these devices smaller and more transportable, or even implantable . One challenge still to be faced in these smaller devices is countering the limited volume and therefore limited filtering capabilities.[97]
Bioscaffolds have also been introduced to provide a framework upon which normal kidney tissue can be regenerated. These scaffolds encompass natural scaffolds (e.g., decellularized kidneys,[99] collagen hydrogel,[100][101] or silk fibroin[102]), synthetic scaffolds (e.g., poly[lactic-co-glycolic acid][103][104] or other polymers), or a combination of two or more natural and synthetic scaffolds. These scaffolds can be implanted into the body either without cell treatment or after a period of stem cell seeding and incubation. In vitro and In vivo studies are being conducted to compare and optimize the type of scaffold and to assess whether cell seeding prior to implantation adds to the viability, regeneration and effective function of the kidneys. A recent systematic review and meta-analysis compared the results of published animal studies and identified that improved outcomes are reported with the use of hybrid (mixed) scaffolds and cell seeding;[105] however, the meta-analysis of these results were not in agreeement with the evaluation of descriptive results from the review. Therefore, further studies involving larger animals and novel scaffolds, and more transparent reproduction of previous studies are advisable.
Biomimetics

This section's tone or style may not reflect the encyclopedic tone used on Wikipedia. See Wikipedia's guide to writing better articles for suggestions. (December 2019) (Learn how and when to remove this template message)
Main article: Biomimetics
Biomimetics is a field that aims to produce materials and systems that replicate those present in nature.[106] In the context of tissue engineering, this is a common approach used by engineers to create materials for these applications that are comparable to native tissues in terms of their structure, properties, and biocompatibility. Material properties are largely dependent on physical, structural, and chemical characteristics of that material. Subsequently, a biomimetic approach to system design will become significant in material integration, and a sufficient understanding of biological processes and interactions will be necessary. Replication of biological systems and processes may also be used in the synthesis of bio-inspired materials to achieve conditions that produce the desired biological material. Therefore, if a material is synthesized having the same characteristics of biological tissues both structurally and chemically, then ideally the synthesized material will have similar properties. This technique has an extensive history originating from the idea of using natural phenomenon as design inspiration for solutions to human problems. Many modern advancements in technology have been inspired by nature and natural systems, including aircraft, automobiles, architecture, and even industrial systems. Advancements in nanotechnology initiated the application of this technique to micro- and nano-scale problems, including tissue engineering. This technique has been used to develop synthetic bone tissues, vascular technologies, scaffolding materials and integration techniques, and functionalized nanoparticles.[106]
Constructing neural networks in soft material
In 2018, scientists at Brandeis University reported their research on soft material embedded with chemical networks which can mimic the smooth and coordinated behavior of neural tissue. This research was funded by the U.S. Army Research Laboratory.[107] The researchers presented an experimental system of neural networks, theoretically modeled as reaction-diffusion systems. Within the networks was an array of patterned reactors, each performing the Belousov-Zhabotinsky (BZ) reaction. These reactors could function on a nanoliter scale.[108]
The researchers state that the inspiration for their project was the movement of the blue ribbon eel. The eel's movements are controlled by electrical impulses determined by a class of neural networks called the central pattern generator. Central Pattern Generators function within the autonomic nervous system to control bodily functions such as respiration, movement, and peristalsis.[109]
Qualities of the reactor that were designed were the network topology, boundary conditions, initial conditions, reactor volume, coupling strength, and the synaptic polarity of the reactor (whether its behavior is inhibitory or excitatory).[109] A BZ emulsion system with a solid elastomer polydimethylsiloxane (PDMS) was designed. Both light and bromine permeable PDMS have been reported as viable methods to create a pacemaker for neural networks.[108]
Market
The history of the tissue engineering market can be divided into three major parts. The time before the crash of the biotech market in the early 2000s, the crash and the time afterward.
Beginning
Most early progress in tissue engineering research was done in the US. This is due to less strict regulations regarding stem cell research and more available funding than in other countries. This leads to the creation of academic startups many of them coming from Harvard or MIT. Examples are BioHybrid Technologies whose founder, Bill Chick, went to Harvard Medical School and focused on the creation of artificial pancreas. Another example would be Organogenesis Inc. whose founder went to MIT and worked on skin engineering products. Other companies with links to the MIT are TEI Biosciences, Therics and Guilford Pharmaceuticals.[7] The renewed interest in biotechnologies in the 1980s leads to many private investors investing in these new technologies even though the business models of these early startups were often not very clear and did not present a path to long term profitability.[110] Government sponsors were more restrained in their funding as tissue engineering was considered a high-risk investment.[7]
In the UK the market got off to a slower start even though the regulations on stem cell research were not strict as well. This is mainly due to more investors being less willing to invest in these new technologies which were considered to be high-risk investments.[110] Another problem faced by British companies was getting the NHS to pay for their products. This especially because the NHS runs a cost-effectiveness analysis on all supported products. Novel technologies often do not do well in this respect.[110]
In Japan, the regulatory situation was quite different. First cell cultivation was only allowed in a hospital setting and second academic scientists employed by state-owned universities were not allowed outside employment until 1998. Moreover, the Japanese authorities took longer to approve new drugs and treatments than there US and European counterparts.[110]
For these reasons in the early days of the Japanese market, the focus was mainly on getting products that were already approved elsewhere in Japan and selling them. Contrary to the US market the early actors in Japan were mainly big firms or sub-companies of such big firms, such as J-TEC, Menicon and Terumo, and not small startups.[110] After regulatory changes in 2014, which allowed cell cultivation outside of a hospital setting, the speed of research in Japan increased and Japanese companies also started to develop their own products.[110]
Crash
Soon after the big boom, the first problems started to appear. There were problems getting products approved by the FDA and if they got approved there were often difficulties in getting insurance providers to pay for the products and getting it accepted by health care providers.[110][111]
For example, organogenesis ran into problems marketing its product and integrating its product in the health system. This partially due to the difficulties of handling living cells and the increased difficulties faced by physicians in using these products over conventional methods.[110]
Another example would be Advanced Tissue Sciences Dermagraft skin product which could not create a high enough demand without reimbursements from insurance providers. Reasons for this were $4000 price-tag and the circumstance that Additionally Advanced Tissue Sciences struggled to get their product known by physicians.[110]
The above examples demonstrate how companies struggled to make profit. This, in turn, lead investors to lose patience and stopping further funding. In consequence, several Tissue Engineering companies such as Organogenesis and Advanced Tissue Sciences filed for bankruptcy in the early 2000s. At this time, these were the only ones having commercial skin products on the market.[111]
Reemergence
The technologies of the bankrupt or struggling companies were often bought by other companies which continued the development under more conservative business models.[111] Examples of companies who sold their products after folding were Curis[111] and Intercytex.[110]
Many of the companies abandoned their long-term goals of developing fully functional organs in favor of products and technologies that could turn a profit in the short run.[110] Examples of these kinds of products are products in the cosmetic and testing industry.
In other cases such as in the case of Advanced Tissue Sciences, the founders started new companies.[110]
In the 2010s the regulatory framework also started to facilitate faster time to market especially in the US as new centres and pathways were created by the FDA specifically aimed at products coming from living cells such as the Center for Biologics Evaluation and Research.[110]
The first tissue engineering products started to get commercially profitable in the 2010s.[111]
Regulation
In Europe, regulation is currently split into three areas of regulation: medical devices, medicinal products, and biologics. Tissue engineering products are often of hybrid nature, as they are often composed of cells and a supporting structure. While some products can be approved as medicinal products, others need to gain approval as medical devices.[112] Derksen explains in her thesis that tissue engineering researchers are sometimes confronted with regulation that does not fit the characteristics of tissue engineering.[113]
New regulatory regimes have been observed in Europe that tackle these issues.[114] An explanation for the difficulties in finding regulatory consensus in this matter is given by a survey conducted in the UK.[112] The authors attribute these problems to the close relatedness and overlap with other technologies such as xenotransplantation. It can therefore not be handled separately by regulatory bodies.[112] Regulation is further complicated by the ethical controversies associated with this and related fields of research (e.g. stem cells controversy, ethics of organ transplantation). The same survey as mentioned above[112] shows on the example of autologous cartilage transplantation that a specific technology can be regarded as 'pure' or 'polluted' by the same social actor.
Two regulatory movements are most relevant to tissue engineering in the European Union. These are Directive 2004/23/EC on standards of quality and safety for the sourcing and processing of human tissues[115] which was adopted by the European Parliament in 2004 and a proposed Human Tissue-Engineered Products regulation. The latter was developed under the auspices of the European Commission DG Enterprise and presented in Brussels in 2004.[116]
See also
 Biology portal
Biology portal Engineering portal
Engineering portal Biomedical engineering
Biological engineering
Biomolecular engineering
Biochemical engineering
Cell engineering
Chemical engineering
ECM Biomaterial
In vivo bioreactor
Induced stem cells
Molecular processor
Molecular self-assembly
Muscle tissue engineering
National Institutes of Health
National Science Foundation
Quality control in tissue engineering
Regeneration in humans
Soft tissues
Thiomers
Tissue Engineering and Regenerative Medicine International Society
Tissue engineering of heart valves
Xenotransplantation
https://en.wikipedia.org/wiki/Tissue_engineering
Ectogenesis (from the Greek ἐκτός, "outside," and genesis) is the growth of an organism in an artificial environment[1] outside the body in which it would normally be found, such as the growth of an embryo or fetus outside the mother's body, or the growth of bacteria outside the body of a host.[2] The term was coined by British scientist J.B.S. Haldane in 1924.[3][4]
https://en.wikipedia.org/wiki/Ectogenesis
Extracorporeal membrane oxygenation (ECMO), also known as extracorporeal life support (ECLS), is an extracorporeal technique of providing prolonged cardiac and respiratory support to persons whose heart and lungs are unable to provide an adequate amount of gas exchange or perfusion to sustain life. The technology for ECMO is largely derived from cardiopulmonary bypass, which provides shorter-term support with arrested native circulation. The device used is a membrane oxygenator, also known as an artificial lung.
ECMO works by temporarily drawing blood from the body to allow artificial oxygenation of the red blood cells and removal of carbon dioxide. Generally, it is used either post-cardiopulmonary bypass or in late-stage treatment of a person with profound heart and/or lung failure, although it is now seeing use as a treatment for cardiac arrest in certain centers, allowing treatment of the underlying cause of arrest while circulation and oxygenation are supported. ECMO is also used to support patients with the acute viral pneumonia associated with COVID-19 in cases where artificial ventilation alone is not sufficient to sustain blood oxygenation levels.
https://en.wikipedia.org/wiki/Extracorporeal_membrane_oxygenation
Apheresis (ἀφαίρεσις (aphairesis, "a taking away")) is a medical technology in which the blood of a person is passed through an apparatus that separates out one particular constituent and returns the remainder to the circulation. It is thus an extracorporeal therapy.
One of the uses of apheresis is for collecting stem cells.[1]
| Apheresis | |
|---|---|
 Whole
blood enters the centrifuge (1) and separates into plasma (2),
leukocytes (3), and erythrocytes (4). Selected components are then drawn
off (5). | |
| MeSH | D016238 |
https://en.wikipedia.org/wiki/Apheresis
Fetal viability is the ability of a human fetus to survive outside the uterus. Medical viability is generally considered to be between 23 and 24 weeks gestational age.[1][2] Viability depends upon factors such as birth weight, gestational age, and the availability of advanced medical care. In low-income countries, half of newborns born at or below 32 weeks gestational age died due to a lack of medical access; in high-income countries, the vast majority of newborns born above 24 weeks gestational age survive.[3]
As of 2022, the world record for the lowest gestational age newborn to survive is held by Curtis Zy-Keith Means, who was born on 5 July 2020 in the United States, at 21 weeks and 1 day gestational age, weighing 420 grams.[4]
https://en.wikipedia.org/wiki/Fetal_viability
Artificial neural networks (ANNs), usually simply called neural networks (NNs) or neural nets,[1] are computing systems inspired by the biological neural networks that constitute animal brains.[2]
An ANN is based on a collection of connected units or nodes called artificial neurons, which loosely model the neurons in a biological brain. Each connection, like the synapses in a biological brain, can transmit a signal to other neurons. An artificial neuron receives signals then processes them and can signal neurons connected to it. The "signal" at a connection is a real number, and the output of each neuron is computed by some non-linear function of the sum of its inputs. The connections are called edges. Neurons and edges typically have a weight that adjusts as learning proceeds. The weight increases or decreases the strength of the signal at a connection. Neurons may have a threshold such that a signal is sent only if the aggregate signal crosses that threshold.
Typically, neurons are aggregated into layers. Different layers may perform different transformations on their inputs. Signals travel from the first layer (the input layer), to the last layer (the output layer), possibly after traversing the layers multiple times.
https://en.wikipedia.org/wiki/Artificial_neural_network
An artificial neuron is a mathematical function conceived as a model of biological neurons, a neural network. Artificial neurons are elementary units in an artificial neural network.[1] The artificial neuron receives one or more inputs (representing excitatory postsynaptic potentials and inhibitory postsynaptic potentials at neural dendrites) and sums them to produce an output (or activation, representing a neuron's action potential which is transmitted along its axon). Usually each input is separately weighted, and the sum is passed through a non-linear function known as an activation function or transfer function[clarification needed]. The transfer functions usually have a sigmoid shape, but they may also take the form of other non-linear functions, piecewise linear functions, or step functions. They are also often monotonically increasing, continuous, differentiable and bounded. Non-monotonic, unbounded and oscillating activation functions with multiple zeros that outperform sigmoidal and ReLU like activation functions on many tasks have also been recently explored. The thresholding function has inspired building logic gates referred to as threshold logic; applicable to building logic circuits resembling brain processing. For example, new devices such as memristors have been extensively used to develop such logic in recent times.[2]
The artificial neuron transfer function should not be confused with a linear system's transfer function.
Artificial neurons can also refer to artificial cells in neuromorphic engineering that are similar to natural physical neurons.
https://en.wikipedia.org/wiki/Artificial_neuron
A neural circuit (also known as a biological neural network) is a population of neurons interconnected by synapses to carry out a specific function when activated.[1] Multiple neural circuits interconnect with one another to form large scale brain networks.[2]
Neural circuits have inspired the design of artificial neural networks, though there are significant differences.
https://en.wikipedia.org/wiki/Neural_circuit
A memristor (/ˈmɛmrɪstər/; a portmanteau of memory resistor) is a non-linear two-terminal electrical component relating electric charge and magnetic flux linkage. It was described and named in 1971 by Leon Chua, completing a theoretical quartet of fundamental electrical components which comprises also the resistor, capacitor and inductor.[1]
Chua and Kang later generalized the concept to memristive systems.[2] Such a system comprises a circuit, of multiple conventional components, which mimics key properties of the ideal memristor component and is also commonly referred to as a memristor. Several such memristor system technologies have been developed, notably ReRAM.
The identification of memristive properties in electronic devices has attracted controversy. Experimentally, the ideal memristor has yet to be demonstrated.[3][4]
 Memristor developed by the University of Illinois at Urbana-Champaign and National Energy Technology Laboratory | |
| Invented | Leon Chua (1971) |
|---|---|
| Electronic symbol | |
A memristor (/ˈmɛmrɪstər/; a portmanteau of memory resistor) is a non-linear two-terminal electrical component relating electric charge and magnetic flux linkage. It was described and named in 1971 by Leon Chua, completing a theoretical quartet of fundamental electrical components which comprises also the resistor, capacitor and inductor.[1]
Chua and Kang later generalized the concept to memristive systems.[2] Such a system comprises a circuit, of multiple conventional components, which mimics key properties of the ideal memristor component and is also commonly referred to as a memristor. Several such memristor system technologies have been developed, notably ReRAM.
The identification of memristive properties in electronic devices has attracted controversy. Experimentally, the ideal memristor has yet to be demonstrated.[3][4]
As a fundamental electrical component
Conceptual symmetries of resistor, capacitor, inductor, and memristorhttps://en.wikipedia.org/wiki/Memristor
https://en.wikipedia.org/wiki/Phasor
https://en.wikipedia.org/wiki/fusor
https://en.wikipedia.org/wiki/taser
https://en.wikipedia.org/wiki/Maser
An artificial organ is a human made organ device or tissue that is implanted or integrated into a human — interfacing with living tissue — to replace a natural organ, to duplicate or augment a specific function or functions so the patient may return to a normal life as soon as possible.[1] The replaced function does not have to be related to life support, but it often is. For example, replacement bones and joints, such as those found in hip replacements, could also be considered artificial organs.[2][3]
Implied by definition, is that the device must not be continuously tethered to a stationary power supply or other stationary resources such as filters or chemical processing units. (Periodic rapid recharging of batteries, refilling of chemicals, and/or cleaning/replacing of filters would exclude a device from being called an artificial organ.)[4] Thus, a dialysis machine, while a very successful and critically important life support device that almost completely replaces the duties of a kidney, is not an artificial organ.
Enhancement
It is also possible to construct and install an artificial organ to give its possessor abilities that are not naturally occurring. Research is proceeding in areas of vision, memory, and information processing. Some current research focuses on restoring short-term memory in accident victims and long-term memory in dementia patients.
One area of success was achieved when Kevin Warwick carried out a series of experiments extending his nervous system over the internet to control a robotic hand and the first direct electronic communication between the nervous systems of two humans.[49]
This might also include the existing practice of implanting subcutaneous chips for identification and location purposes (ex. RFID tags).[50]
Microchips
Organ chips are devices containing hollow microvessels filled with cells simulating tissue and/or organs as a microfluidic system that can provide key chemical and electrical signal information.[51] This is distinct from an alternative use of the term microchip, which refers to small, electronic chips that are commonly used as an identifier and can also contain a transponder.
This information can create various applications such as creating "human in vitro models" for both healthy and diseased organs, drug advancements in toxicity screening as well as replacing animal testing.[51]
Using 3D cell culture techniques enables scientists to recreate the complex extracellular matrix, ECM, found in in vivo to mimic human response to drugs and human diseases.[52] Organs on chips are used to reduce the failure rate in new drug development; microengineering these allows for a microenvironment to be modeled as an organ.
See also
- Artificial bone, skin, uterus, kidney
- Biomechatronics
- Biomedical Engineering
- Decellularization
- Organ transplant
- Organ culture
- Tissue scaffold
- Xenotransplant
- Organoid
https://en.wikipedia.org/wiki/Artificial_organ
An artificial ovary is a potential fertility preservation treatment that aims to mimic the function of the natural ovary.
Conventional fertility preservation for females involves oocyte cryopreservation or ovarian tissue cryopreservation. However, there are drawbacks to these treatments. Oocyte cryopreservation is not possible for those with pre-pubertal cancer or premature ovarian insufficiency. Ovarian tissue cryopreservation also poses a risk of reintroducing malignant cells after cancer recovery, particular in those with previous leukaemia.[1]
Artificial ovaries could be an effective alternative in fertility preservation. The artificial ovary aims to replicate its natural counterpart by producing oocytes and releasing steroid hormones. To date, no human oocytes have been fertilised or used to produce offspring using an artificial ovary and it is unlikely that this will occur until further research has been completed and bioethical concerns have been considered.[2]
Ideally, the artificial ovary should contain follicles or oocytes obtained from ovarian tissue cryopreservation, as well as other ovarian cells to provide growth factors.[3] Isolated follicles are then transplanted (either at the normal site of the ovary or elsewhere in the body) in a delivery scaffold.[4] An ideal biocompatible scaffold would cause minimal inflammation, be suitable for neo‐angiogenesis, and degrade after transplantation.[5]
There are some limitations to artificial ovaries. From an ethical perspective, there is the issue of justice of who would qualify to receive artificial ovaries (except in autologous transplant) as there is limited availability.[5] There is also a bioethical concerns around pre-implantation diagnosis and genetic manipulation of artificial ovaries.[5] If a patient's own ovarian tissue is used for generating artificial ovaries, the risk of reintroducing malignancy is still present, although this risk would be lowered if only oocytes were used.[5]
One area of future research in this field will look at the source of oocytes for artificial ovaries. There is potential for induced pluripotent stem cells (iPSCs) to be used as an alternative source to a patient's own gametes. Although this has not yet been tested with human stem cells, mice transplanted with these cells were able to successfully reproduce through in vitro maturation and fertilisation.[5] However, human iPSCs are known to have mitochondrial DNA mutations even when isolated from healthy donors, therefore there is still more work to be done with this area.[6]
How they are made
The ovarian tissue will undergo sequential culture steps to (hopefully) produce fertilisable mature oocytes:[2]
- Culture the cortical ovarian tissue to enhance primordial follicle (immature follicle) growth and isolate the primordial and primary follicles
- Culture the growing ovarian follicles within a 3D microenvironment
- Isolate and culture the immature oocytes in an attempt to produce mature oocytes which are ready for IVF or cryopreservation
Culturing of cortical ovarian tissue and isolation of follicles
A common source of ovarian tissue used comes from tissue excised from the patient prior to cancer treatment, which is then cryopreserved.[7] The tissue is then cultured to activate the primordial follicles and allow them to develop.[2] To isolate the follicles, a combination of enzymatic and mechanical tissue digestion has shown to be the most effective method to yield a high quantity of follicles whilst maintaining their quality.[8] The enzymes used, liberase DH and DNase, are produced by good manufacturing practice (GMP) to fully comply with GMP guidelines to ensure future application to patients. The enzymatic digestion process is inactivated every 30 minutes and the suspension is filtered to allow fully isolated follicles to be removed and reduce unnecessary enzyme exposure which may lead to damage of their basement membrane and their death.[8]
When recovering the isolated follicles, malignant cells may be inadvertently retrieved, which poses the risk of re-introducing malignant cells into the patient.[8] To minimise the risk of contamination, the isolated follicles undergo a washing step which involves rinsing the follicles with fresh dissecting media, three times, to separate them from surrounding isolated cells.[2][8]
Culturing the growing follicles within a 3D microenvironment
The isolated follicles are then encapsulated within a 3D matrix and cultured for up to 4 weeks.[7] The material used has to meet biosafety and clinically compatible standards, such as adequate protection and support of the follicles and adaptability to human body temperature, if artificial ovaries are to be transplanted into a patient.[5] Potential materials are divided into synthetic polymers and natural polymers.[8] Synthetic polymers tend to be more predictable than natural polymers in terms of their rate of degradation and their mechanical properties can be tailored to the specific clinical requirements.[8] Although they contain no essential molecules for cell adhesion, bioactive factors can be incorporated to stimulate this.[8] The only synthetic polymer utilised so far has been poly(ethylene glycol), which developed immature mouse follicles into antral follicles and corpora lutea.[8][5]
Natural polymers have bioactive molecules which play a role in cell adhesion, migration, proliferation and differentiation.[8] However, they lack mechanical strength and the adaptability that synthetic polymers have.[8] Unlike synthetic polymers, there has been a success with a wider range of natural polymers: collagen, plasma clots, fibrin, alginate and decellularized ovarian tissue.[8][5]
The microenvironment of the structure should mimic that of the natural ovary, so the artificial ovary should support the follicles structurally, but also cellularly.[8] Ovarian stromal cells are integrated into the microenvironment as they play an important role in early development of the follicles.[8] They release various factors which positively regulate the transition of primordial follicles to primary follicles, but also release other cells which will differentiate into theca cells; those that play a supportive role for growing follicles and produce sex steroids such as androstenedione and testosterone.[8] This can be achieved by isolating them from a second fresh ovarian biopsy once the patient has completed their cancer treatment, thus avoiding potential contamination.[8] Endothelial cells should also be co-transported as they are key to promoting angiogenesis of the artificial ovary.[8]
Oocyte culture
The immature oocytes are retrieved from the artificial ovary and cultured in vitro for a further 24–48 hours, allowing them to mature oocytes which are ready for IVF or vitrification (cryopreservation).[7]
Mouse models
Initial experiments
The majority of knowledge we have about the artificial ovary has been discovered through the use of mouse models. Initial experiments in the 1990s were performed on mice that saw the grafting of preantral follicles onto an artificial ovary made with collagen.[8] The preantral follicles were shown to undergo in vitro growth (IVG) therefore suggesting that a collagen matrix could be of good use for an artificial ovary.[8] Despite the positive results, the growth was accompanied by atresia of antral follicles meaning that it was necessary to look for other alternatives to collagen that allowed follicle growth when the artificial ovary was implanted back into the mouse.[8]
Natural matrices in the mouse model
Since then, a range of different natural matrices have been tested for their usefulness as an artificial ovary. Included in these are fibrin, alginate and decellularized human ovary which have shown in vitro maturation, ovary-like structure production and the production of offspring when transplanted into mice.[8][1] In addition to these events being observed separately, the full process of development from the grafting of preantral follicles to the ovary through to the birth of live offspring has been demonstrated in the mouse model.[1]
Synthetic matrices in the mouse model
In addition to these natural matrices a range of synthetic matrices have also been tested in mice. Synthetic matrices have the advantage that they can be made in bulk quantities and kept for a long time.[1] However they do not contain biological factors needed for cell adhesion, therefore adding another layer of complexity to their creation.[8] It is hoped that the knowledge we have gained using mouse models may one day be applied clinically, whether that be through the use of natural or synthetic matrices.
Restoration of puberty in mice
Not only have artificial ovaries shown the ability to restore fertility, they have further been linked to the complete restoration of hormone production leading to puberty. Transplantation of a human decellularized artificial ovary containing murine primary follicles has been shown to induce puberty in mice without oocytes by promoting oestradiol and inhibin B production.[9] Mice were then shown to be able to produce viable offspring suggesting that artificial ovaries could be useful in women who have not undergone puberty.[10]
Human models
There could be many possible applications of human artificial ovaries.
In vitro matured oocytes in IVF and cryopreservation
One emerging application of human artificial ovaries would be the use of oocytes which have undergone in vitro maturation (IVM) in IVF or cryopreservation. Oocyte retrieval followed by IVM does not require hormonal stimulation and can be a quick procedure, therefore would be advantageous in fertility preservation of cancer patients – especially where chemotherapy must start as soon as possible.[11]
Re-transplantation of ovarian follicles grown in vitro
Another possible clinical application of human artificial ovaries is re-transplanting ovarian follicles which have been grown in vitro. In animal models, pre-antral ovarian follicles have been grown in vitro, then isolated and implanted into a biodegradable 3D artificial ovary for re-transplantation back into the animal ovary.[2] This method has shown potential success in animal models, but in humans remains a theoretical concept for now.
Re-transplantation of ovarian tissue activated in vitro
A third possible clinical application is the re-transplantation of in vitro activated ovarian tissue. This would enable ovarian tissue to be removed from a patient, activated in vitro and then auto-transplanted into the same patient. However, this treatment is not advised for patients who have cancers that may metastasise in the ovaries (e.g. leukaemia) or those with ovarian carcinomas, due to concerns that the cancer cells may be re-implanted back into the patient. Auto-transplantation of the activated ovarian tissue into the broad ligament of the uterus, ovarian fossa or the remaining ovary can be completed by laparoscopy or mini-laparoscopy procedures. This procedure has resulted in healthy offspring being born to patients who suffered from premature ovarian insufficiency.[2][1]
Future possibilities
Further research is needed to enable the procedures outlined above to become more successful. One area research is progressing in is that of the 3D printed ovary. A 3D printed microporous hydrogel scaffold could be created, into which isolated ovarian follicles could be implanted. This would support further follicular growth in vivo after transplantation. Full endocrine and reproductive ovarian function was restored in sterilised mice using this method.[2]
See also
https://en.wikipedia.org/wiki/Artificial_ovary
An organ-on-a-chip (OOC) is a multi-channel 3-D microfluidic cell culture, integrated circuit (chip) that simulates the activities, mechanics and physiological response of an entire organ or an organ system.[1][2] It constitutes the subject matter of significant biomedical engineering research, more precisely in bio-MEMS. The convergence of labs-on-chips (LOCs) and cell biology has permitted the study of human physiology in an organ-specific context. By acting as a more sophisticated in vitro approximation of complex tissues than standard cell culture, they provide the potential as an alternative to animal models for drug development and toxin testing.
Although multiple publications claim to have translated organ functions onto this interface, the development of these microfluidic applications is still in its infancy. Organs-on-chips vary in design and approach between different researchers. Organs that have been simulated by microfluidic devices include brain, lung, heart, kidney, liver, prostate, vessel (artery), skin, bone, cartilage and more.[citation needed]
A limitation of the early organ-on-a-chip approach is that simulation of an isolated organ may miss significant biological phenomena that occur in the body's complex network of physiological processes, and that this oversimplification limits the inferences that can be drawn. Many aspects of subsequent microphysiometry aim to address these constraints by modeling more sophisticated physiological responses under accurately simulated conditions via microfabrication, microelectronics and microfluidics.[3]
The development of organ chips has enabled the study of the complex pathophysiology of human viral infections. An example is the liver chip platform that has enabled studies of viral hepatitis.[4]
https://en.wikipedia.org/wiki/Organ-on-a-chip
