Pneumocystis jirovecii (previously P. carinii) is a yeast-like fungus of the genus Pneumocystis. The causative organism of Pneumocystis pneumonia, it is an important human pathogen, particularly among immunocompromised hosts. Prior to its discovery as a human-specific pathogen, P. jirovecii was known as P. carinii.
https://en.wikipedia.org/wiki/Pneumocystis_jirovecii
Pneumocystis pneumonia (PCP), also known as Pneumocystis jirovecii pneumonia (PJP), is a form of pneumonia that is caused by the yeast-like fungus Pneumocystis jirovecii.[3][4]
Pneumocystis specimens are commonly found in the lungs of healthy people although it is usually not a cause for disease.[5] However, they are a source of opportunistic infection and can cause lung infections in people with a weak immune system or other predisposing health conditions. PCP is seen in people with HIV/AIDS (who account for 30-40% of PCP cases), those using medications that suppress the immune system, and people with cancer, autoimmune or inflammatory conditions, and chronic lung disease.[2]
https://en.wikipedia.org/wiki/Pneumocystis_pneumonia
Pentamidine is an antimicrobial medication used to treat African trypanosomiasis, leishmaniasis, Balamuthiainfections,[2] babesiosis, and to prevent and treat pneumocystis pneumonia (PCP) in people with poor immune function.[1] In African trypanosomiasis it is used for early disease before central nervous system involvement, as a second line option to suramin.[1] It is an option for both visceral leishmaniasis and cutaneous leishmaniasis.[1]Pentamidine can be given by injection into a vein or muscle or by inhalation.[1]
Common side effects of the injectable form include low blood sugar, pain at the site of injection, nausea, vomiting, low blood pressure, and kidney problems.[1] Common side effects of the inhaled form include wheezing, cough, and nausea.[1] It is unclear if doses should be changed in those with kidney or liver problems.[1] Pentamidine is not recommended in early pregnancy but may be used in later pregnancy.[1] Its safety during breastfeeding is unclear.[3]Pentamidine is in the aromatic diamidine family of medications.[4] While the way the medication works is not entirely clear, it is believed to involve decreasing the production of DNA, RNA, and protein.[1]
Pentamidine came into medical use in 1937.[5] It is on the World Health Organization's List of Essential Medicines.[6] It is available as a generic medication.[1] In regions of the world where the disease is common pentamidine is provided for free by the World Health Organization (WHO).[7]
https://en.wikipedia.org/wiki/Pentamidine
Freeze drying, also known as lyophilization or cryodesiccation, is a low temperature dehydration process[1] that involves freezing the product, lowering pressure, then removing the ice by sublimation.[2] This is in contrast to dehydration by most conventional methods that evaporate water using heat.[3]
Because of the low temperature used in processing,[1] the quality of the rehydrated product is excellent. When solid objects like strawberries are freeze dried the original shape of the product is maintained.[4] If the product to be dried is a liquid, as often seen in pharmaceutical applications, the properties of the final product are optimized by the combination of excipients (i.e., inactive ingredients). Primary applications of freeze drying include biological (e.g., bacteria and yeasts), biomedical (e.g., surgical transplants), food processing (e.g., coffee) and preservation.[1]
Freezing and annealing[edit]
During the freezing stage, the material is cooled below its triple point, the temperature at which the solid, liquid, and gas phases of the material can coexist. This ensures that sublimation rather than melting will occur in the following steps. To facilitate faster and more efficient freeze drying, larger ice crystals are preferable. The large ice crystals form a network within the product which promotes faster removal of water vapor during sublimation.[2] To produce larger crystals, the product should be frozen slowly or can be cycled up and down in temperature in a process called annealing. The freezing phase is the most critical in the whole freeze-drying process, as the freezing method can impact the speed of reconstitution, duration of freeze-drying cycle, product stability, and appropriate crystallization.[12]
Amorphous materials do not have a eutectic point, but they do have a critical point, below which the product must be maintained to prevent melt-back[further explanation needed] or collapse during primary and secondary drying.
Primary drying[edit]
During the primary drying phase, the pressure is lowered (to the range of a few millibars), and enough heat is supplied to the material for the ice to sublimate. The amount of heat necessary can be calculated using the sublimating molecules' latent heat of sublimation. In this initial drying phase, about 95% of the water in the material is sublimated. This phase may be slow (can be several days in the industry), because, if too much heat is added, the material's structure could be altered.
In this phase, pressure is controlled through the application of partial vacuum. The vacuum speeds up the sublimation, making it useful as a deliberate drying process. Furthermore, a cold condenser chamber and/or condenser plates provide a surface(s) for the water vapour to re-liquify and solidify on.
It is important to note that, in this range of pressure, the heat is brought mainly by conduction or radiation; the convection effect is negligible, due to the low air density.
Structurally sensitive goods[edit]
In the case of goods where preservation of structure is required, like food or objects with formerly-living cells, large ice crystals will break the cell walls which can result in increasingly poor texture and loss of nutritive content. In this case, the freezing is done rapidly, in order to lower the material to below its eutectic point quickly, thus avoiding the formation of large ice crystals.[2] Usually, the freezing temperatures are between −50 °C (−58 °F) and −80 °C (−112 °F).
Secondary drying[edit]
The secondary drying phase aims to remove unfrozen water molecules, since the ice was removed in the primary drying phase. This part of the freeze-drying process is governed by the material's adsorption isotherms. In this phase, the temperature is raised higher than in the primary drying phase, and can even be above 0 °C (32 °F), to break any physico-chemical interactions that have formed between the water molecules and the frozen material. Usually the pressure is also lowered in this stage to encourage desorption (typically in the range of microbars, or fractions of a pascal). However, there are products that benefit from increased pressure as well.
After the freeze-drying process is complete, the vacuum is usually broken with an inert gas, such as nitrogen, before the material is sealed.
At the end of the operation, the final residual water content in the product is extremely low, around 1% to 4%.
Examples of lyophilized biological products include many vaccines such as live measles virus vaccine, typhoid vaccine, and meningococcal polysaccharide vaccine groups A and C combined. Other freeze-dried biological products include antihemophilic factor VIII, interferon alfa, anti-blood clot medicine streptokinase, and wasp venom allergenic extract.[15]
Advanced ceramics processes sometimes use freeze-drying to create a formable powder from a sprayed slurry mist. Freeze-drying creates softer particles with a more homogeneous chemical composition than traditional hot spray drying, but it is also more expensive.
Shelf-life extension[edit]
Shelf-life extension results from low processing temperatures in conjunction with rapid transition of water through sublimation.[1] With these processing conditions, deterioration reactions, including nonenzymic browning, enzymatic browning, and protein denaturation, are minimized.[1] When the product is successfully dried, packaged properly, and placed in ideal storage conditions the foods have a shelf life of greater than 12 months.[2]
Disadvantages[edit]
Microbial growth[edit]
Since the main method of microbial decontamination for freeze drying is the low temperature dehydration process, spoilage organisms and pathogens resistant to these conditions can remain in the product. Although microbial growth is inhibited by the low moisture conditions, it can still survive in the food product.[26] An example of this is a viral hepatitis A outbreak that occurred in the United States in 2016, associated with frozen strawberries.[27] If the product is not properly packaged and/or stored, the product can absorb moisture, allowing the once inhibited pathogens to begin reproducing as well.[2]
Cost[edit]
Freeze-drying costs about five times as much as conventional drying,[4] so it is most suitable for products which increase in value with processing.[2] Costs are also variable depending on the product, the packaging material, processing capacity, etc.[4] The most energy-intensive step is sublimation.[4]
Silicone oil leakage[edit]
Silicone oil is the common fluid that is used to heat or cool shelves in the freeze-dryer. The continuous heat/cool cycle can lead to a leakage of silicone oil at weak areas that connect the shelf and hose. This can contaminate the product leading to major losses of food product. Hence, to avoid this issue, mass spectrometers are used to identify vapors released by silicone oil to immediately take corrective action and prevent contamination of the product.[28]
Products[edit]
Mammalian cells generally don't survive freeze drying even though they still can be preserved.[29][30]
Function of essential components[edit]
Chamber[edit]
The chamber is highly polished and contains insulation, internally. It is manufactured with stainless steel and contains multiple shelves for holding the product.[citation needed] A hydraulic or electric motor is in place to ensure the door is vacuum-tight when closed.
Process condenser[edit]
The process condenser consists of refrigerated coils or plates that can be external or internal to the chamber.[31] During the drying process, the condenser traps water. For increased efficiency, the condenser temperature should be 20 °C (68 °F) less than the product during primary drying[31] and have a defrosting mechanism to ensure that the maximum amount of water vapor in the air is condensed.
Shelf fluid[edit]
The amount of heat energy needed at times of the primary and secondary drying phase is regulated by an external heat exchanger.[31] Usually, silicone oil is circulated around the system with a pump.
Refrigeration system[edit]
This system works to cool shelves and the process condenser by using compressors or liquid nitrogen, which will supply energy necessary for the product to freeze.[31]
Vacuum system[edit]
During the drying process, a vacuum of 50-100 microbar is applied, by the vacuum system, to remove the solvent.[31] A two-stage rotary vacuum pump is used, however, if the chamber is large then multiple pumps are needed. This system compresses non-condensable gases through the condenser.
Control system[edit]
Finally, the control system sets up controlled values for shelf temperature, pressure and time that are dependent on the product and/or the process.[32][33] The freeze-dryer can run for a few hours or days depending on the product.[31]
Contact freeze dryers[edit]
Contact freeze dryers use contact (conduction) of the food with the heating element to supply the sublimation energy. This type of freeze dryer is a basic model that is simple to set up for sample analysis. One of the major ways contact freeze dryers heat is with shelf-like platforms contacting the samples. The shelves play a major role as they behave like heat exchangers at different times of the freeze-drying process. They are connected to a silicone oil system that will remove heat energy during freezing and provide energy during drying times.[31]
Additionally, the shelf-fluid system works to provide specific temperatures to the shelves during drying by pumping a fluid (usually silicone oil) at low pressure. The downside to this type of freeze dryer is that the heat is only transferred from the heating element to the side of the sample immediately touching the heater. This problem can be minimized by maximizing the surface area of the sample touching the heating element by using a ribbed tray, slightly compressing the sample between two solid heated plates above and below, or compressing with a heated mesh from above and below.[2]
Radiant freeze dryers[edit]
Radiant freeze dryers use infrared radiation to heat the sample in the tray. This type of heating allows for simple flat trays to be used as an infrared source can be located above the flat trays to radiate downwards onto the product. Infrared radiation heating allows for a very uniform heating of the surface of the product, but has very little capacity for penetration so it is used mostly with very shallow trays and homogeneous sample matrices.[2]
Microwave-assisted freeze dryers[edit]
Microwave-assisted freeze dryers utilize microwaves to allow for deeper penetration into the sample to expedite the sublimation and heating processes in freeze-drying. This method can be very complicated to set up and run as the microwaves can create an electrical field capable of causing gases in the sample chamber to become plasma. This plasma could potentially burn the sample, so maintaining a microwave strength appropriate for the vacuum levels is imperative. The rate of sublimation in a product can affect the microwave impedance, in which power of the microwave must be changed accordingly.[2]
See also[edit]
- Chuño, ancient Incan freeze dried potatoes
- Freeze-dried food and NASA
- List of dried foods
- Supercritical drying
- Frozen mummies
- Cryofixation
https://en.wikipedia.org/wiki/Freeze-drying
Supercritical drying, also known as critical point drying, is a process to remove liquid in a precise and controlled way.[1] It is useful in the production of microelectromechanical systems (MEMS), the drying of spices, the production of aerogel, the decaffeination of coffee and in the preparation of biological specimens for scanning electron microscopy.
Phase diagram[edit]
As the substance in a liquid body crosses the boundary from liquid to gas (see green arrow in phase diagram), the liquid changes into gas at a finite rate, while the amount of liquid decreases. When this happens within a heterogeneous environment, surface tension in the liquid body pulls against any solid structures the liquid might be in contact with. Delicate structures such as cell walls, the dendrites in silica gel, and the tiny machinery of microelectromechanical devices, tend to be broken apart by this surface tension as the liquid–gas–solid junction moves by.
To avoid this, the sample can be brought via two possible alternate paths from the liquid phase to the gas phase without crossing the liquid–gas boundary on the phase diagram. In freeze-drying, this means going around to the left (low temperature, low pressure; blue arrow). However, some structures are disrupted even by the solid–gas boundary. Supercritical drying, on the other hand, goes around the line to the right, on the high-temperature, high-pressure side (red arrow). This route from liquid to gas does not cross any phase boundary, instead passing through the supercritical region, where the distinction between gas and liquid ceases to apply. Densities of the liquid phase and vapor phase become equal at critical point of drying.
Fluids[edit]
Fluids suitable for supercritical drying include carbon dioxide (critical point 304.25 K at 7.39 MPa or 31.1 °C at 1072 psi) and freon (≈300 K at 3.5–4 MPa or 25–0 °C at 500–600 psi). Nitrous oxide has similar physical behavior to carbon dioxide, but is a powerful oxidizer in its supercritical state. Supercritical water is inconvenient due to possible heat damage to a sample at its critical point temperature (647 K, 374 °C) and corrosiveness of water at such high temperatures and pressures (22.064 MPa, 3,212 psi).
In most such processes, acetone is first used to wash away all water, exploiting the complete miscibility of these two fluids. The acetone is then washed away with high pressure liquid carbon dioxide, the industry standard now that freon is unavailable. The liquid carbon dioxide is then heated until its temperature goes beyond the critical point, at which time the pressure can be gradually released, allowing the gas to escape and leaving a dried product.
See also[edit]
https://en.wikipedia.org/wiki/Supercritical_drying
Pages in category "Drying processes"
The following 7 pages are in this category, out of 7 total. This list may not reflect recent changes (learn more).
W
Torrefaction of biomass, e.g., wood or grain, is a mild form of pyrolysis at temperatures typically between 200 and 320 °C. Torrefaction changes biomass properties to provide a better fuel quality for combustion and gasification applications. Torrefaction produces a relatively dry product, which reduces or eliminates its potential for organic decomposition. Torrefaction combined with densification creates an energy-dense fuel carrier of 20 to 21 GJ/ton lower heating value(LHV).[1] Torrefaction makes the material undergo Maillard reactions. Torrefied biomass can be used as an energy carrier or as a feedstock used in the production of bio-based fuels and chemicals.[2]
Biomass can be an important energy source.[3] However, there exists a large diversity of potential biomass sources, each with its own unique characteristics. To create efficient biomass-to-energy chains, torrefaction of biomass, combined with densification (pelletisation or briquetting), is a promising step towards overcoming the logistical challenges in developing large-scale sustainable energy solutions, by making it easier to transport and store. Pellets or briquettes have higher density, contain less moisture, and are more stable in storage than the biomass they are derived from.
https://en.wikipedia.org/wiki/Torrefaction
The pyrolysis (or devolatilization) process is the thermal decomposition of materials at elevated temperatures in an inert atmosphere.[1] It involves a change of chemical composition. The word is coined from the Greek-derived elements pyro "fire", "heat", "fever" and lysis "separating".
Pyrolysis is most commonly used in the treatment of organic materials. It is one of the processes involved in charring wood.[2]In general, pyrolysis of organic substances produces volatile products and leaves char, a carbon-rich solid residue. Extreme pyrolysis, which leaves mostly carbon as the residue, is called carbonization. Pyrolysis is considered the first step in the processes of gasification or combustion.[3][4]
The process is used heavily in the chemical industry, for example, to produce ethylene, many forms of carbon, and other chemicals from petroleum, coal, and even wood, to produce coke from coal. It is used also in the conversion of natural gas(primarily methane) into non-polluting hydrogen gas and non-polluting solid carbon char, recently on an industrial scale.[5]Aspirational applications of pyrolysis would convert biomass into syngas and biochar, waste plastics back into usable oil, or waste into safely disposable substances.
https://en.wikipedia.org/wiki/Pyrolysis
Carbonization is the conversion of organic matters like plants and dead animal remains into carbon through destructive distillation.
https://en.wikipedia.org/wiki/Carbonization
Geology
Coal is composed of macerals, minerals and water.[17] Fossils and amber may be found in coal.
Formation
The conversion of dead vegetation into coal is called coalification. At various times in the geologic past, the Earth had dense forests[18] in low-lying wetland areas. In these wetlands, the process of coalification began when dead plant matter was protected from biodegradation and oxidation, usually by mud or acidic water, and was converted into peat. This trapped the carbon in immense peat bogs that were eventually deeply buried by sediments. Then, over millions of years, the heat and pressure of deep burial caused the loss of water, methane and carbon dioxide and increased in the proportion of carbon.[17]The grade of coal produced depended on the maximum pressure and temperature reached, with lignite (also called "brown coal") produced under relatively mild conditions, and sub-bituminous coal, bituminous coal, or anthracite coal (also called "hard coal" or "black coal") produced in turn with increasing temperature and pressure.[2][19]
Of the factors involved in coalification, temperature is much more important than either pressure or time of burial.[20]Subbituminous coal can form at temperatures as low as 35 to 80 °C (95 to 176 °F) while anthracite requires a temperature of at least 180 to 245 °C (356 to 473 °F).[21]
Although coal is known from most geologic periods, 90% of all coal beds were deposited in the Carboniferous and Permian periods, which represent just 2% of the Earth's geologic history.[22] Paradoxically, this was during the Late Paleozoic icehouse, a time of global glaciation. However, the drop in global sea level accompanying the glaciation exposed continental shelfs that had previously been submerged, and to these were added wide river deltas produced by increased erosion due to the drop in base level. These widespread areas of wetlands provided ideal conditions for coal formation.[23] The rapid formation of coal ended with the coal gap in the Permian–Triassic extinction event, where coal is rare.[24]
Favorable geography alone does not explain the extensive Carboniferous coal beds.[25] Other factors contributing to rapid coal deposition were high oxygen levels, above 30%, that promoted intense wildfires and formation of charcoal that was all but indigestible by decomposing organisms; high carbon dioxide levels that promoted plant growth; and the nature of Carboniferous forests, which included lycophyte trees whose determinate growth meant that carbon was not tied up in heartwood of living trees for long periods.[26]
One theory suggested that about 360 million years ago, some plants evolved the ability to produce lignin, a complex polymer that made their cellulose stems much harder and more woody. The ability to produce lignin led to the evolution of the first trees. But bacteria and fungi did not immediately evolve the ability to decompose lignin, so the wood did not fully decay but became buried under sediment, eventually turning into coal. About 300 million years ago, mushrooms and other fungi developed this ability, ending the main coal-formation period of earth's history.[27][28] Although some authors pointed at some evidence of lignin degradation during the Carboniferous, and suggested that climatic and tectonic factors were a more plausible explanation,[29] reconstruction of ancestral enzymes by phylogenetic analysis corrobarated a hypothesis that lignin degrading enzymes appeared in fungi approximately 200 MYa.[30]
One likely tectonic factor was the Central Pangean Mountains, an enormous range running along the equator that reached its greatest elevation near this time. Climate modeling suggests that the Central Pangean Mountains contributed to the deposition of vast quantities of coal in the late Carboniferous. The mountains created an area of year-round heavy precipitation, with no dry season typical of a monsoon climate. This is necessary for the preservation of peat in coal swamps.[31]
Coal is known from Precambrian strata, which predate land plants. This coal is presumed to have originated from residues of algae.[32][33]
Sometimes coal seams (also known as coal beds) are interbedded with other sediments in a cyclothem. Cyclothems are thought have their origin in glacial cycles that produced fluctuations in sea level, which alternately exposed and then flooded large areas of continental shelf.[34]
Chemistry of coalification
The woody tissue of plants is composed mainly of cellulose, hemicellulose, and lignin. Modern peat is mostly lignin, with a content of cellulose and hemicellulose ranging from 5% to 40%. Various other organic compounds, such as waxes and nitrogen- and sulfur-containing compounds, are also present.[35] Lignin has a weight composition of about 54% carbon, 6% hydrogen, and 30% oxygen, while cellulose has a weight composition of about 44% carbon, 6% hydrogen, and 49% oxygen. Bituminous coal has a composition of about 84.4% carbon, 5.4% hydrogen, 6.7% oxygen, 1.7% nitrogen, and 1.8% sulfur, on a weight basis.[36] This implies that chemical processes during coalification must remove most of the oxygen and much of the hydrogen, leaving carbon, a process called carbonization.[37]
Carbonization proceeds primarily by dehydration, decarboxylation, and demethanation. Dehydration removes water molecules from the maturing coal via reactions such as[38]
- 2 R–OH → R–O–R + H2O
- 2 R-CH2-O-CH2-R → R-CH=CH-R + H2O
Decarboxylation removes carbon dioxide from the maturing coal and proceeds by reaction such as[38]
- RCOOH → RH + CO2
while demethanation proceeds by reaction such as
- 2 R-CH3 → R-CH2-R + CH4
- R-CH2-CH2-CH2-R → R-CH=CH-R + CH4
In each of these formulas, R represents the remainder of a cellulose or lignin molecule to which the reacting groups are attached.
Dehydration and decarboxylation take place early in coalification, while demethanation begins only after the coal has already reached bituminous rank.[39] The effect of decarboxylation is to reduce the percentage of oxygen, while demethanation reduces the percentage of hydrogen. Dehydration does both, and (together with demethanation) reduces the saturation of the carbon backbone (increasing the number of double bonds between carbon).
As carbonization proceeds, aliphatic compounds (carbon compounds characterized by chains of carbon atoms) are replaced by aromatic compounds (carbon compounds characterized by rings of carbon atoms) and aromatic rings begin to fuse into polyaromatic compounds (linked rings of carbon atoms).[40] The structure increasingly resembles graphene, the structural element of graphite.
Chemical changes are accompanied by physical changes, such as decrease in average pore size.[41] The macerals (organic particles) of lignite are composed of huminite, which is earthy in appearance. As the coal matures to sub-bituminous coal, huminite begins to be replaced by vitreous (shiny) vitrinite.[42] Maturation of bituminous coal is characterized by bitumenization, in which part of the coal is converted to bitumen, a hydrocarbon-rich gel.[43] Maturation to anthracite is characterized by debitumenization (from demethanation) and the increasing tendency of the anthracite to break with a conchoidal fracture, similar to the way thick glass breaks.[44]
Types
As geological processes apply pressure to dead biotic material over time, under suitable conditions, its metamorphic grade or rank increases successively into:
- Peat, a precursor of coal
- Lignite, or brown coal, the lowest rank of coal, most harmful to health,[45] used almost exclusively as fuel for electric power generation
- Jet, a compact form of lignite, sometimes polished; used as an ornamental stone since the Upper Palaeolithic
- Sub-bituminous coal, whose properties range between those of lignite and those of bituminous coal, is used primarily as fuel for steam-electric power generation.
- Bituminous coal, a dense sedimentary rock, usually black, but sometimes dark brown, often with well-defined bands of bright and dull material. It is used primarily as fuel in steam-electric power generation and to make coke. Known as steam coal in the UK, and historically used to raise steam in steam locomotives and ships
- Anthracite coal, the highest rank of coal, is a harder, glossy black coal used primarily for residential and commercial space heating.
- Graphite is difficult to ignite and not commonly used as fuel; it is most used in pencils, or powdered for lubrication.
- Cannel coal (sometimes called "candle coal") is a variety of fine-grained, high-rank coal with significant hydrogen content, which consists primarily of liptinite.
There are several international standards for coal.[46] The classification of coal is generally based on the content of volatiles. However the most important distinction is between thermal coal (also known as steam coal), which is burnt to generate electricity via steam; and metallurgical coal (also known as coking coal), which is burnt at high temperature to make steel.
Hilt's law is a geological observation that (within a small area) the deeper the coal is found, the higher its rank (or grade). It applies if the thermal gradient is entirely vertical; however, metamorphism may cause lateral changes of rank, irrespective of depth. For example, some of the coal seams of the Madrid, New Mexico coal field were partially converted to anthracite by contact metamorphism from an igneous sill while the remainder of the seams remained as bituminous coal.[47]
History
The earliest recognized use is from the Shenyang area of China where by 4000 BC Neolithic inhabitants had begun carving ornaments from black lignite.[48] Coal from the Fushun mine in northeastern China was used to smelt copper as early as 1000 BC.[49] Marco Polo, the Italian who traveled to China in the 13th century, described coal as "black stones ... which burn like logs", and said coal was so plentiful, people could take three hot baths a week.[50] In Europe, the earliest reference to the use of coal as fuel is from the geological treatise On Stones (Lap. 16) by the Greek scientist Theophrastus (c. 371–287 BC):[51][52]
Outcrop coal was used in Britain during the Bronze Age (3000–2000 BC), where it formed part of funeral pyres.[54][55] In Roman Britain, with the exception of two modern fields, "the Romans were exploiting coals in all the major coalfields in England and Wales by the end of the second century AD".[56] Evidence of trade in coal, dated to about AD 200, has been found at the Roman settlement at Heronbridge, near Chester; and in the Fenlands of East Anglia, where coal from the Midlands was transported via the Car Dyke for use in drying grain.[57] Coal cinders have been found in the hearths of villas and Roman forts, particularly in Northumberland, dated to around AD 400. In the west of England, contemporary writers described the wonder of a permanent brazier of coal on the altar of Minerva at Aquae Sulis (modern day Bath), although in fact easily accessible surface coal from what became the Somerset coalfieldwas in common use in quite lowly dwellings locally.[58] Evidence of coal's use for iron-working in the city during the Roman period has been found.[59] In Eschweiler, Rhineland, deposits of bituminous coal were used by the Romans for the smelting of iron ore.[56]
No evidence exists of coal being of great importance in Britain before about AD 1000, the High Middle Ages.[60] Coal came to be referred to as "seacoal" in the 13th century; the wharf where the material arrived in London was known as Seacoal Lane, so identified in a charter of King Henry III granted in 1253.[61] Initially, the name was given because much coal was found on the shore, having fallen from the exposed coal seams on cliffs above or washed out of underwater coal outcrops,[60] but by the time of Henry VIII, it was understood to derive from the way it was carried to London by sea.[62] In 1257–1259, coal from Newcastle upon Tyne was shipped to London for the smiths and lime-burners building Westminster Abbey.[60] Seacoal Lane and Newcastle Lane, where coal was unloaded at wharves along the River Fleet, still exist.[63]
These easily accessible sources had largely become exhausted (or could not meet the growing demand) by the 13th century, when underground extraction by shaft mining or adits was developed.[54] The alternative name was "pitcoal", because it came from mines.
Cooking and home heating with coal (in addition to firewood or instead of it) has been done in various times and places throughout human history, especially in times and places where ground-surface coal was available and firewood was scarce, but a widespread reliance on coal for home hearths probably never existed until such a switch in fuels happened in London in the late sixteenth and early seventeenth centuries.[64] Historian Ruth Goodman has traced the socioeconomic effects of that switch and its later spread throughout Britain[64] and suggested that its importance in shaping the industrial adoption of coal has been previously underappreciated.[64]: xiv–xix
The development of the Industrial Revolution led to the large-scale use of coal, as the steam engine took over from the water wheel. In 1700, five-sixths of the world's coal was mined in Britain. Britain would have run out of suitable sites for watermills by the 1830s if coal had not been available as a source of energy.[65] In 1947 there were some 750,000 miners in Britain[66] but the last deep coal mine in the UK closed in 2015.[67]
A grade between bituminous coal and anthracite was once known as "steam coal" as it was widely used as a fuel for steam locomotives. In this specialized use, it is sometimes known as "sea coal" in the United States.[68] Small "steam coal", also called dry small steam nuts (or DSSN), was used as a fuel for domestic water heating.
Coal played an important role in industry in the 19th and 20th century. The predecessor of the European Union, the European Coal and Steel Community, was based on the trading of this commodity.[69]
Coal continues to arrive on beaches around the world from both natural erosion of exposed coal seams and windswept spills from cargo ships. Many homes in such areas gather this coal as a significant, and sometimes primary, source of home heating fuel.[70]
Emission intensity
Emission intensity is the greenhouse gas emitted over the life of a generator per unit of electricity generated. The emission intensity of coal power stations is high, as they emit around 1000g of CO2eq for each kWh generated, while natural gas is medium-emission intensity at around 500g CO2eq per kWh. The emission intensity of coal varies with type and generator technology and exceeds 1200g per kWh in some countries.[71]
Energy density
The energy density of coal is roughly 24 megajoules per kilogram[72] (approximately 6.7 kilowatt-hours per kg). For a coal power plant with a 40% efficiency, it takes an estimated 325 kg (717 lb) of coal to power a 100 W lightbulb for one year.[73]
27.6% of world energy was supplied by coal in 2017 and Asia used almost three quarters of it.[74]
Chemistry
Composition
The composition of coal is reported either as a proximate analysis (moisture, volatile matter, fixed carbon, and ash) or an ultimate analysis (ash, carbon, hydrogen, nitrogen, oxygen, and sulfur). The "volatile matter" does not exist by itself (except for some adsorbed methane) but designates the volatile compounds that are produced and driven off by heating the coal. A typical bituminous coal may have an ultimate analysis on a dry, ash-free basis of 84.4% carbon, 5.4% hydrogen, 6.7% oxygen, 1.7% nitrogen, and 1.8% sulfur, on a weight basis.[36]
The composition of ash, given in terms of oxides, varies:[36]
| SiO 2 | 20-40 |
| Al 2O 3 | 10-35 |
| Fe 2O 3 | 5-35 |
| CaO | 1-20 |
| MgO | 0.3-4 |
| TiO 2 | 0.5-2.5 |
| Na 2O & K 2O | 1-4 |
| SO 3 | 0.1-12[75] |
Other minor components include:
| Substance | Content |
|---|---|
| Mercury (Hg) | 0.10±0.01 ppm[76] |
| Arsenic (As) | 1.4–71 ppm[77] |
| Selenium (Se) | 3 ppm[78] |
Coking coal and use of coke to smelt iron
Coke is a solid carbonaceous residue derived from coking coal (a low-ash, low-sulfur bituminous coal, also known as metallurgical coal), which is used in manufacturing steel and other iron products.[79] Coke is made from coking coal by baking in an oven without oxygen at temperatures as high as 1,000 °C, driving off the volatile constituents and fusing together the fixed carbon and residual ash. Metallurgical coke is used as a fuel and as a reducing agent in smelting iron ore in a blast furnace.[80]The carbon monoxide produced by its combustion reduces hematite (an iron oxide) to iron.
Waste carbon dioxide is also produced () together with pig iron, which is too rich in dissolved carbon so must be treated further to make steel.
Coking coal should be low in ash, sulfur, and phosphorus, so that these do not migrate to the metal.[79] The coke must be strong enough to resist the weight of overburden in the blast furnace, which is why coking coal is so important in making steel using the conventional route. Coke from coal is grey, hard, and porous and has a heating value of 29.6 MJ/kg. Some cokemaking processes produce byproducts, including coal tar, ammonia, light oils, and coal gas.
Petroleum coke (petcoke) is the solid residue obtained in oil refining, which resembles coke but contains too many impurities to be useful in metallurgical applications.
Use in foundry components
Finely ground bituminous coal, known in this application as sea coal, is a constituent of foundry sand. While the molten metal is in the mould, the coal burns slowly, releasing reducing gases at pressure, and so preventing the metal from penetrating the pores of the sand. It is also contained in 'mould wash', a paste or liquid with the same function applied to the mould before casting.[81] Sea coal can be mixed with the clay lining (the "bod") used for the bottom of a cupola furnace. When heated, the coal decomposes and the bod becomes slightly friable, easing the process of breaking open holes for tapping the molten metal.[82]
Alternatives to coke
Scrap steel can be recycled in an electric arc furnace; and an alternative to making iron by smelting is direct reduced iron, where any carbonaceous fuel can be used to make sponge or pelletised iron. To lessen carbon dioxide emissions hydrogen can be used as the reducing agent[83] and biomass or waste as the source of carbon.[84]Historically, charcoal has been used as an alternative to coke in a blast furnace, with the resultant iron being known as charcoal iron.
Gasification
Coal gasification, as part of an integrated gasification combined cycle (IGCC) coal-fired power station, is used to produce syngas, a mixture of carbon monoxide (CO) and hydrogen (H2) gas to fire gas turbines to produce electricity. Syngas can also be converted into transportation fuels, such as gasoline and diesel, through the Fischer–Tropsch process; alternatively, syngas can be converted into methanol, which can be blended into fuel directly or converted to gasoline via the methanol to gasoline process.[85] Gasification combined with Fischer–Tropsch technology was used by the Sasol chemical company of South Africa to make chemicals and motor vehicle fuels from coal.[86]
During gasification, the coal is mixed with oxygen and steam while also being heated and pressurized. During the reaction, oxygen and water molecules oxidize the coal into carbon monoxide (CO), while also releasing hydrogen gas (H2). This used to be done in underground coal mines, and also to make town gas which was piped to customers to burn for illumination, heating, and cooking.
- 3C (as Coal) + O2 + H2O → H2 + 3CO
If the refiner wants to produce gasoline, the syngas is routed into a Fischer–Tropsch reaction. This is known as indirect coal liquefaction. If hydrogen is the desired end-product, however, the syngas is fed into the water gas shift reaction, where more hydrogen is liberated:
- CO + H2O → CO2 + H2
Liquefaction
Coal can be converted directly into synthetic fuels equivalent to gasoline or diesel by hydrogenation or carbonization.[87] Coal liquefaction emits more carbon dioxide than liquid fuel production from crude oil. Mixing in biomass and using CCS would emit slightly less than the oil process but at a high cost.[88] State owned China Energy Investment runs a coal liquefaction plant and plans to build 2 more.[89]
Coal liquefaction may also refer to the cargo hazard when shipping coal.[90]
Production of chemicals
Chemicals have been produced from coal since the 1950s. Coal can be used as a feedstock in the production of a wide range of chemical fertilizers and other chemical products. The main route to these products was coal gasification to produce syngas. Primary chemicals that are produced directly from the syngas include methanol, hydrogen and carbon monoxide, which are the chemical building blocks from which a whole spectrum of derivative chemicals are manufactured, including olefins, acetic acid, formaldehyde, ammonia, urea and others. The versatility of syngas as a precursor to primary chemicals and high-value derivative products provides the option of using coal to produce a wide range of commodities. In the 21st century, however, the use of coal bed methane is becoming more important.[91]
Because the slate of chemical products that can be made via coal gasification can in general also use feedstocks derived from natural gas and petroleum, the chemical industry tends to use whatever feedstocks are most cost-effective. Therefore, interest in using coal tended to increase for higher oil and natural gas prices and during periods of high global economic growth that might have strained oil and gas production.
Coal to chemical processes require substantial quantities of water.[92] Much coal to chemical production is in China[93][94] where coal dependent provinces such as Shanxi are struggling to control its pollution.[95]
Electricity generation
Precombustion treatment
Refined coal is the product of a coal-upgrading technology that removes moisture and certain pollutants from lower-rank coals such as sub-bituminous and lignite (brown) coals. It is one form of several precombustion treatments and processes for coal that alter coal's characteristics before it is burned. Thermal efficiency improvements are achievable by improved pre-drying (especially relevant with high-moisture fuel such as lignite or biomass).[96] The goals of precombustion coal technologies are to increase efficiency and reduce emissions when the coal is burned. Precombustion technology can sometimes be used as a supplement to postcombustion technologies to control emissions from coal-fueled boilers.
Power plant combustion
Coal burnt as a solid fuel in coal power stations to generate electricity is called thermal coal. Coal is also used to produce very high temperatures through combustion. Early deaths due to air pollution have been estimated at 200 per GW-year, however they may be higher around power plants where scrubbers are not used or lower if they are far from cities.[97] Efforts around the world to reduce the use of coal have led some regions to switch to natural gas and electricity from lower carbon sources.
When coal is used for electricity generation, it is usually pulverized and then burned in a furnace with a boiler (see also Pulverized coal-fired boiler).[98] The furnace heat converts boiler water to steam, which is then used to spin turbines which turn generators and create electricity.[99] The thermodynamic efficiency of this process varies between about 25% and 50% depending on the pre-combustion treatment, turbine technology (e.g. supercritical steam generator) and the age of the plant.[100][101]
A few integrated gasification combined cycle (IGCC) power plants have been built, which burn coal more efficiently. Instead of pulverizing the coal and burning it directly as fuel in the steam-generating boiler, the coal is gasified to create syngas, which is burned in a gas turbine to produce electricity (just like natural gas is burned in a turbine). Hot exhaust gases from the turbine are used to raise steam in a heat recovery steam generator which powers a supplemental steam turbine. The overall plant efficiency when used to provide combined heat and power can reach as much as 94%.[102] IGCC power plants emit less local pollution than conventional pulverized coal-fueled plants; however the technology for carbon capture and storage after gasification and before burning has so far proved to be too expensive to use with coal.[103] Other ways to use coal are as coal-water slurry fuel (CWS), which was developed in the Soviet Union, or in an MHD topping cycle. However these are not widely used due to lack of profit.
In 2017 38% of the world's electricity came from coal, the same percentage as 30 years previously.[104] In 2018 global installed capacity was 2TW (of which 1TW is in China) which was 30% of total electricity generation capacity.[105] The most dependent major country is South Africa, with over 80% of its electricity generated by coal;[106] but China alone generates more than half of the world's coal-generated electricity.[107]
Maximum use of coal was reached in 2013.[108] In 2018 coal-fired power station capacity factor averaged 51%, that is they operated for about half their available operating hours.[109]
Coal industry
Mining
About 8000 Mt of coal are produced annually, about 90% of which is hard coal and 10% lignite. As of 2018 just over half is from underground mines.[110] More accidents occur during underground mining than surface mining. Not all countries publish mining accident statistics so worldwide figures are uncertain, but it is thought that most deaths occur in coal mining accidents in China: in 2017 there were 375 coal mining related deaths in China.[111] Most coal mined is thermal coal (also called steam coal as it is used to make steam to generate electricity) but metallurgical coal (also called "metcoal" or "coking coal" as it is used to make coke to make iron) accounts for 10% to 15% of global coal use.[112]
As a traded commodity
China mines almost half the world's coal, followed by India with about a tenth.[113] Australia accounts for about a third of world coal exports, followed by Indonesia and Russia; while the largest importers are Japan and India.
The price of metallurgical coal is volatile[114] and much higher than the price of thermal coal because metallurgical coal must be lower in sulfur and requires more cleaning.[115] Coal futures contracts provide coal producers and the electric power industry an important tool for hedging and risk management.
In some countries new onshore wind or solar generation already costs less than coal power from existing plants (see Cost of electricity by source).[116][117] However, for China this is forecast for the early 2020s[118] and for south-east Asia not until the late 2020s.[119] In India building new plants is uneconomic and, despite being subsidized, existing plants are losing market share to renewables.[120]
Market trends
Of the countries which produce coal China mines by far the most, almost half the world's coal, followed by less than 10% by India. China is also by far the largest consumer. Therefore, market trends depend on Chinese energy policy.[121] Although the effort to reduce pollution means that the global long-term trend is to burn less coal, the short and medium term trends may differ, in part due to Chinese financing of new coal-fired power plants in other countries.[105]
Major producers
Countries with annual production higher than 300 million tonnes are shown.
| Country | 2000 | 2005 | 2010 | 2015 | 2017 | Share (2017) |
|---|---|---|---|---|---|---|
| China | 1,384 | 2,350 | 3,235 | 3,747 | 3,523 | 46% |
| India | 335 | 429 | 574 | 678 | 716 | 9% |
| United States | 974 | 1,027 | 984 | 813 | 702 | 9% |
| Australia | 314 | 375 | 424 | 485 | 481 | 6% |
| Indonesia | 77 | 152 | 275 | 392 | 461 | 6% |
| Russia | 262 | 298 | 322 | 373 | 411 | 5% |
| Rest of World | 1380 | 1404 | 1441 | 1374 | 1433 | 19% |
| World total | 4,726 | 6,035 | 7,255 | 7,862 | 7,727 | 100% |
Major consumers
Countries with annual consumption higher than 500 million tonnes are shown. Shares are based on data expressed in tonnes oil equivalent.
| Country | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | Share |
|---|---|---|---|---|---|---|---|---|---|---|
| China | 2,691 | 2,892 | 3,352 | 3,677 | 4,538 | 4,678 | 4,539 | 3,970 coal + 441 met coke = 4,411 | 3,784 coal + 430 met coke = 4,214 | 51% |
| India | 582 | 640 | 655 | 715 | 841 | 837 | 880 | 890 coal + 33 met coke = 923 | 877 coal + 37 met coke = 914 | 11% |
| United States | 1,017 | 904 | 951 | 910 | 889 | 924 | 918 | 724 coal + 12 met coke = 736 | 663 coal + 10 met coke = 673 | 9% |
| World Total | 7,636 | 7,699 | 8,137 | 8,640 | 8,901 | 9,013 | 8,907 | 7,893 coal + 668 met coke = 8561 | 7,606 coal + 655 met coke = 8261 | 100% |
Major exporters
| Country | 2018 |
|---|---|
| Indonesia | 472 |
| Australia | 426 |
| Russia | 231 |
| United States | 115 |
| Colombia | 92 |
| South Africa | 88 |
| Mongolia | 39 |
| Canada | 37 |
| Mozambique | 16 |
Exporters are at risk of a reduction in import demand from India and China.[128]
Major importers
| Country | 2018 |
|---|---|
| China | 281 |
| India | 223 |
| Japan | 189 |
| South Korea | 149 |
| Taiwan | 76 |
| Germany | 44 |
| Netherlands | 44 |
| Turkey | 38 |
| Malaysia | 34 |
| Thailand | 25 |
Damage to human health
The use of coal as fuel causes ill health and deaths.[131] Mining and processing of coal causes air and water pollution.[132] Coal-powered plants emit nitrogen oxides, sulfur dioxide, particulate pollution and heavy metals, which adversely affect human health.[132] Coal bed methane extraction is important to avoid mining accidents.
The deadly London smog was caused primarily by the heavy use of coal. Globally coal is estimated to cause 800,000 premature deaths every year,[133] mostly in India[134] and China.[135][136][137]
Burning coal is a major emitter of sulfur dioxide, which creates PM2.5 particulates, the most dangerous form of air pollution.[138]
Coal smokestack emissions cause asthma, strokes, reduced intelligence, artery blockages, heart attacks, congestive heart failure, cardiac arrhythmias, mercury poisoning, arterial occlusion, and lung cancer.[139][140]
Annual health costs in Europe from use of coal to generate electricity are estimated at up to €43 billion.[141]
In China, improvements to air quality and human health would increase with more stringent climate policies, mainly because the country's energy is so heavily reliant on coal. And there would be a net economic benefit.[142]
A 2017 study in the Economic Journal found that for Britain during the period 1851–1860, "a one standard deviation increase in coal use raised infant mortality by 6–8% and that industrial coal use explains roughly one-third of the urban mortality penalty observed during this period."[143]
Breathing in coal dust causes coalworker's pneumoconiosis or "black lung", so-called because the coal dust literally turns the lungs black from their usual pink color.[144]In the United States alone, it is estimated that 1,500 former employees of the coal industry die every year from the effects of breathing in coal mine dust.[145]
Huge amounts of coal ash and other waste is produced annually. Use of coal generates hundreds of millions of tons of ash and other waste products every year. These include fly ash, bottom ash, and flue-gas desulfurization sludge, that contain mercury, uranium, thorium, arsenic, and other heavy metals, along with non-metals such as selenium.[146]
Around 10% of coal is ash:[147] coal ash is hazardous and toxic to human beings and some other living things.[148] Coal ash contains the radioactive elements uraniumand thorium. Coal ash and other solid combustion byproducts are stored locally and escape in various ways that expose those living near coal plants to radiation and environmental toxics.[149]
Damage to the environment
Coal mining and coal fueling of power stations and industrial processes can cause major environmental damage.[150]
Water systems are affected by coal mining.[151] For example, mining affects groundwater and water table levels and acidity. Spills of fly ash, such as the Kingston Fossil Plant coal fly ash slurry spill, can also contaminate land and waterways, and destroy homes. Power stations that burn coal also consume large quantities of water. This can affect the flows of rivers, and has consequential impacts on other land uses. In areas of water scarcity, such as the Thar Desert in Pakistan, coal mining and coal power plants would use significant quantities of water.[152]
One of the earliest known impacts of coal on the water cycle was acid rain. In 2014 approximately 100 Tg/S of sulfur dioxide(SO2) was released, over half of which was from burning coal.[153] After release, the sulfur dioxide is oxidized to H2SO4 which scatters solar radiation, hence its increase in the atmosphere exerts a cooling effect on climate. This beneficially masks some of the warming caused by increased greenhouse gases. However, the sulfur is precipitated out of the atmosphere as acid rain in a matter of weeks,[154] whereas carbon dioxide remains in the atmosphere for hundreds of years. Release of SO2 also contributes to the widespread acidification of ecosystems.[155]
Disused coal mines can also cause issues. Subsidence can occur above tunnels, causing damage to infrastructure or cropland. Coal mining can also cause long lasting fires, and it has been estimated that thousands of coal seam fires are burning at any given time.[156] For example, Brennender Berg has been burning since 1668 and is still burning in the 21st century.[157]
The production of coke from coal produces ammonia, coal tar, and gaseous compounds as by-products which if discharged to land, air or waterways can pollute the environment.[158] The Whyalla steelworks is one example of a coke producing facility where liquid ammonia was discharged to the marine environment.[159]
Underground fires
Thousands of coal fires are burning around the world.[160] Those burning underground can be difficult to locate and many cannot be extinguished. Fires can cause the ground above to subside, their combustion gases are dangerous to life, and breaking out to the surface can initiate surface wildfires. Coal seams can be set on fire by spontaneous combustion or contact with a mine fire or surface fire. Lightning strikes are an important source of ignition. The coal continues to burn slowly back into the seam until oxygen (air) can no longer reach the flame front. A grass fire in a coal area can set dozens of coal seams on fire.[161][162] Coal fires in China burn an estimated 120 million tons of coal a year, emitting 360 million metric tons of CO2, amounting to 2–3% of the annual worldwide production of CO2 from fossil fuels.[163][164] In Centralia, Pennsylvania (a borough located in the Coal Region of the United States), an exposed vein of anthracite ignited in 1962 due to a trash fire in the borough landfill, located in an abandoned anthracite strip mine pit. Attempts to extinguish the fire were unsuccessful, and it continues to burn underground to this day. The Australian Burning Mountain was originally believed to be a volcano, but the smoke and ash come from a coal fire that has been burning for some 6,000 years.[165]
At Kuh i Malik in Yagnob Valley, Tajikistan, coal deposits have been burning for thousands of years, creating vast underground labyrinths full of unique minerals, some of them very beautiful.
The reddish siltstone rock that caps many ridges and buttes in the Powder River Basin in Wyoming and in western North Dakota is called porcelanite, which resembles the coal burning waste "clinker" or volcanic "scoria".[166] Clinker is rock that has been fused by the natural burning of coal. In the Powder River Basin approximately 27 to 54 billion tons of coal burned within the past three million years.[167] Wild coal fires in the area were reported by the Lewis and Clark Expedition as well as explorers and settlers in the area.[168]
Climate change
The largest and most long-term effect of coal use is the release of carbon dioxide, a greenhouse gas that causes climate change. Coal-fired power plants were the single largest contributor to the growth in global CO2 emissions in 2018,[169] 40% of the total fossil fuel emissions,[8] and more than a quarter of total emissions.[170][note 1] Coal mining can emit methane, another greenhouse gas.[171][172]
In 2016 world gross carbon dioxide emissions from coal usage were 14.5 gigatonnes.[173] For every megawatt-hour generated, coal-fired electric power generation emits around a tonne of carbon dioxide, which is double the approximately 500 kg of carbon dioxide released by a natural gas-fired electric plant.[174] In 2013, the head of the UN climate agency advised that most of the world's coal reserves should be left in the ground to avoid catastrophic global warming.[175] To keep global warming below 1.5 °C or 2 °C hundreds, or possibly thousands, of coal-fired power plants will need to be retired early.[176]
Pollution mitigation
Coal pollution mitigation, sometimes called clean coal, is a series of systems and technologies that seek to mitigate the health and environmental impact of coal;[177] in particular air pollution from coal-fired power stations, and from coal burnt by heavy industry.
The primary focus is on sulfur dioxide (SO2) and nitrogen oxides (NOx), the most important gases which caused acid rain; and particulates which cause visible air pollution, illness and premature deaths. SO2 can be removed by flue-gas desulfurization and NO2 by selective catalytic reduction (SCR). Particulates can be removed with electrostatic precipitators. Although perhaps less efficient, wet scrubbers can remove both gases and particulates. Reducing fly ash reduces emissions of radioactive materials. Mercury emissions can be reduced up to 95%.[178] However capturing carbon dioxide emissions from coal is generally not economically viable.Standards
Local pollution standards include GB13223-2011 (China), India,[179] the Industrial Emissions Directive (EU) and the Clean Air Act (United States).
Satellite monitoring
Satellite monitoring is now used to crosscheck national data, for example Sentinel-5 Precursor has shown that Chinese control of SO2 has only been partially successful.[180] It has also revealed that low use of technology such as SCR has resulted in high NO2 emissions in South Africa and India.[181]
Combined cycle power plants
A few Integrated gasification combined cycle (IGCC) coal-fired power plants have been built with coal gasification. Although they burn coal more efficiently and therefore emit less pollution, the technology has not generally proved economically viable for coal, except possibly in Japan although this is controversial.[182][183]
Carbon capture and storage
Although still being intensively researched and considered economically viable for some uses other than with coal; carbon capture and storage has been tested at the Petra Nova and Boundary Dam coal-fired power plants and has been found to be technically feasible but not economically viable for use with coal, due to reductions in the cost of solar PV technology.[184]
Economics
In 2018 US$80 billion was invested in coal supply but almost all for sustaining production levels rather than opening new mines.[185] In the long term coal and oil could cost the world trillions of dollars per year.[186][187] Coal alone may cost Australia billions,[188] whereas costs to some smaller companies or cities could be on the scale of millions of dollars.[189] The economies most damaged by coal (via climate change) may be India and the US as they are the countries with the highest social cost of carbon.[190] Bank loans to finance coal are a risk to the Indian economy.[134]
China is the largest producer of coal in the world. It is the world's largest energy consumer, and coal in China supplies 60% of its primary energy. However two fifths of China's coal power stations are estimated to be loss-making.[118]
Air pollution from coal storage and handling costs the USA almost 200 dollars for every extra ton stored, due to PM2.5.[191] Coal pollution costs the €43 billion each year.[192] Measures to cut air pollution benefit individuals financially and the economies of countries[193][194] such as China.[195]
Subsidies
Broadly defined total subsidies for coal in 2015 have been estimated at around US$2.5 trillion, about 3% of global GDP.[196] As of 2019 G20 countries provide at least US$63.9 billion[169] of government support per year for the production of coal, including coal-fired power: many subsidies are impossible to quantify[197] but they include US$27.6 billion in domestic and international public finance, US$15.4 billion in fiscal support, and US$20.9 billion in state-owned enterprise (SOE) investments per year.[169] In the EU state aid to new coal-fired plants is banned from 2020, and to existing coal-fired plants from 2025.[198] As pf 2018, government funding for new coal power plants was supplied by Exim Bank of China,[199] the Japan Bank for International Cooperation and Indian public sector banks.[200] Coal in Kazakhstan was the main recipient of coal consumption subsidies totalling US$2 billion in 2017.[201] Coal in Turkey benefited from substantial subsidies in 2021.[202]
Stranded assets
Some coal-fired power stations could become stranded assets, for example China Energy Investment, the world's largest power company, risks losing half its capital.[118] However, state-owned electricity utilities such as Eskom in South Africa, Perusahaan Listrik Negara in Indonesia, Sarawak Energy in Malaysia, Taipower in Taiwan, EGAT in Thailand, Vietnam Electricity and EÜAŞ in Turkey are building or planning new plants.[203] As of 2021 this may be helping to cause a carbon bubblewhich could cause financial instability if it bursts.[204][205][206]
Politics
Countries building or financing new coal-fired power stations, such as China, India, Indonesia, Vietnam, Turkey and Bangladesh, face mounting international criticism for obstructing the aims of the Paris Agreement.[105][207][208] In 2019, the Pacific Island nations (in particular Vanuatu and Fiji) criticized Australia for failing to cut their emissions at a faster rate than they were, citing concerns about coastal inundation and erosion.[209] In May 2021, the G7 members agreed to end new direct government support for international coal power generation.[210]
Opposition to coal
Opposition to coal pollution was one of the main reasons the modern environmental movement started in the 19th century.
Transition away from coal
In order to meet global climate goals and provide power to those that don't currently have it coal power must be reduced from nearly 10,000 TWh to less than 2,000 TWh by 2040.[211] Phasing out coal has short-term health and environmental benefits which exceed the costs,[212] but some countries still favor coal,[213] and there is much disagreement about how quickly it should be phased out.[214][215] However many countries, such as the Powering Past Coal Alliance, have already or are transitioned away from coal;[216] the largest transition announced so far being Germany, which is due to shut down its last coal-fired power station between 2035 and 2038.[217] Some countries use the ideas of a "Just Transition", for example to use some of the benefits of transition to provide early pensions for coal miners.[218] However low-lying Pacific Islands are concerned the transition is not fast enough and that they will be inundated by sea level rise; so they have called for OECD countries to completely phase out coal by 2030 and other countries by 2040.[209] In 2020, although China built some plants, globally more coal power was retired than built: the UN Secretary General has also said that OECD countries should stop generating electricity from coal by 2030 and the rest of the world by 2040.[219] Phasing down coal was agreed at COP26 in the Glasgow Climate Pact.
Peak coal
Peak coal is the peak consumption or production of coal by a human community. Global coal consumption peaked in 2013, and had dropped slightly by the end of the 2010s.[220][221] The peak of coal's share in the global energy mix was in 2008, when coal accounted for 30% of global energy production.[220] The decline in coal use is largely driven by consumption declines in the United States and Europe, as well as developed economies in Asia.[220] In 2019, production increases in countries such as China, Indonesia, India, Russia and Australia compensated for the falls in the United States and Europe.[221] However, coal's structural decline continued in the 2020s.[222]
Peak coal can be driven by peak demand or peak supply. Historically, it was widely believed that the supply-side would eventually drive peak coal due to the depletion of coal reserves. However, since the increasing global efforts to limit climate change, peak coal has been driven by demand, which has stayed below the 2013 peak consumption.[220] This is due in large part to the rapid expansion of natural gas and renewable energy.[220] Many countries have pledged to phase-out coal, despite estimates that project coal reserves to have the capacity to last for centuries at current consumption levels. In some countries[which?] coal consumption may still increase in the early 2020s.[223]Switch to cleaner fuels and lower carbon electricity generation
Coal-fired generation puts out about twice as much carbon dioxide—around a tonne for every megawatt hour generated—as electricity generated by burning natural gas at 500 kg of greenhouse gas per megawatt hour.[224] In addition to generating electricity, natural gas is also popular in some countries for heating and as an automotive fuel.
The use of coal in the United Kingdom declined as a result of the development of North Sea oil and the subsequent dash for gas during the 1990s. In Canada some coal power plants, such as the Hearn Generating Station, switched from coal to natural gas. In 2017, coal power in the United States provided 30% of the electricity, down from approximately 49% in 2008,[225][226][227] due to plentiful supplies of low cost natural gas obtained by hydraulic fracturing of tight shale formations.[228]
Coal regions in transition
Some coal-mining regions are highly dependent on coal.[229]
Employment
Some coal miners are concerned their jobs may be lost in the transition.[230] A just transition from coal is supported by the European Bank for Reconstruction and Development.[231]
Bioremediation
The white rot fungus Trametes versicolor can grow on and metabolize naturally occurring coal.[232] The bacteria Diplococcus has been found to degrade coal, raising its temperature.[233]
Cultural usage
Coal is the official state mineral of Kentucky[234] and the official state rock of Utah;[235] both U.S. states have a historic link to coal mining.
Some cultures hold that children who misbehave will receive only a lump of coal from Santa Claus for Christmas in their christmas stockings instead of presents.
It is also customary and considered lucky in Scotland and the North of England to give coal as a gift on New Year's Day. This occurs as part of First-Footing and represents warmth for the year to come.
See also
- Biochar – Lightweight black residue, made of carbon and ashes, after pyrolysis of biomass
- Carbochemistry
- Coal pollution mitigation – Series of systems and technologies to mitigate the pollution associated with the burning of coal
- Coal assay
- Coal blending
- Coal homogenization
- Coal measures (stratigraphic unit)
- Coal phase out
- Coal-tar
- Environmental issues with coal
- Fluidized bed combustion – Technology used to burn solid fuels
- Fossil fuel – Fuel formed over millions of years from dead plants and animals
- Fossil fuel phase-out – Gradual reduction of fossil fuel use to zero
- Gytta
- Major coal producing regions
- Mountaintop removal mining – Type of surface mining
- The Coal Question
- Tonstein – Type of sedimentary rock
- World Coal Association
Coal tar is a thick dark liquid which is a by-product of the production of coke and coal gas from coal.[2][3] It has both medical and industrial uses.[2][4] Medicinally it is a topical medication applied to skin to treat psoriasis and seborrheic dermatitis (dandruff).[5] It may be used in combination with ultraviolet light therapy.[5] Industrially it is a railroad tiepreservative and used in the surfacing of roads.[6] Coal tar was listed as a known human carcinogen in the first Report on Carcinogens from the U.S. Federal Government.[7]
Coal tar was discovered circa 1665 and used for medical purposes as early as the 1800s.[6][8] Circa 1850, the discovery that it could be used as the main ingredient in synthetic dyes engendered an entire industry.[9] It is on the World Health Organization's List of Essential Medicines.[10] Coal tar is available as a generic medication and over the counter.[4]
Side effects include skin irritation, sun sensitivity, allergic reactions, and skin discoloration.[5] It is unclear if use during pregnancy is safe for the baby and use during breastfeeding is not typically recommended.[11] The exact mechanism of action is unknown.[12] It is a complex mixture of phenols, polycyclic aromatic hydrocarbons (PAHs), and heterocyclic compounds.[2] It demonstrates antifungal, anti-inflammatory, anti-itch, and antiparasitic properties.[12]
| Clinical data | |
|---|---|
| Trade names | Balnetar, Cutar, others |
| Other names | liquor carbonis detergens (LCD) liquor picis carbonis (LPC)[1] |
| AHFS/Drugs.com | Multum Consumer Information |
| Routes of administration | Topical |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| ChemSpider |
|
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.029.417 |
Mechanism of action[edit]
The exact mechanism of action is unknown.[12] Coal tar is a complex mixture of phenols, polycyclic aromatic hydrocarbons (PAHs), and heterocyclic compounds.[2]
It is a keratolytic agent, which reduces the growth rate of skin cells and softens the skin's keratin.[45][38]
Composition[edit]
Coal tar is produced through thermal destruction (pyrolysis) of coal. Its composition varies with the process and type of coal used – lignite, bituminous or anthracite.[38]
Coal tar contains approximately 10,000 chemicals, of which only about 50% have been identified.[46][better source needed] Components include polycyclic aromatic hydrocarbons (4-rings: chrysene, fluoranthene, pyrene, triphenylene, naphthacene, benzanthracene, 5-rings: picene, benzo[a]pyrene, benzo[e]pyrene, benzofluoranthenes, perylene, 6-rings: dibenzopyrenes, dibenzofluoranthenes, benzoperylenes, 7-rings: coronene), as well as methylated and polymethylated derivatives, mono- and polyhydroxylated derivatives, and heterocyclic compounds.[34][47] Others include benzene, toluene, xylenes, cumenes, coumarone, indene, benzofuran, naphthalene and methyl-naphthalenes, acenaphthene, fluorene, phenol, cresols, pyridine, picolines, phenanthracene, carbazole, quinolines, fluoranthene.[38] Many of these constituents are known carcinogens.[48][35]
Derivatives[edit]
Various phenolic coal tar derivatives have analgesic (pain-killer) properties. These included acetanilide, phenacetin, and paracetamol aka acetaminophen.[49]Paracetamol may be the only coal-tar derived analgesic still in use today.[50] Industrial phenol is now usually synthesized from crude oil rather than coal tar.[51]
Coal tar derivatives are contra-indicated for people with the inherited red cell blood disorder glucose-6-phosphate dehydrogenase deficiency (G6PD deficiency), as they can cause oxidative stress leading to red blood cell breakdown.[52]
Society and culture[edit]
Coal tar is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system.[10] Coal tar is generally available as a generic medication and over the counter.[4]
Regulation[edit]
Exposure to coal tar pitch volatiles can occur in the workplace by breathing, skin contact, or eye contact. The Occupational Safety and Health Administration (OSHA) has set the permissible exposure limit) to 0.2 mg/m3 benzene-soluble fraction over an 8-hour workday. The National Institute for Occupational Safety and Health(NIOSH) has set a recommended exposure limit (REL) of 0.1 mg/m3 cyclohexane-extractable fraction over an 8-hour workday. At levels of 80 mg/m3, coal tar pitch volatiles are immediately dangerous to life and health.[53]
When used as a medication in the United States, coal tar preparations are considered over-the-counter drug pharmaceuticals and are subject to regulation by the Food and Drug Administration (FDA).
See also[edit]
https://en.wikipedia.org/wiki/Coal_tar
Mineral products resembling tar can be produced from fossil hydrocarbons, such as petroleum. Coal tar is produced from coal as a byproduct of coke production.
Terminology[edit]
"Tar" and "pitch" can be used interchangeably; asphalt (naturally occurring pitch) may also be called either "mineral tar" or "mineral pitch". There is a tendency to use "tar" for more liquid substances and "pitch" for more solid (viscoelastic) substances.[2] Both "tar" and "pitch" are applied to viscous forms of asphalt, such as the asphalt found in naturally occurring tar pits (e.g., the La Brea Tar Pits in Los Angeles). "Rangoon tar", also known as "Burmese oil" or "Burmese naphtha", is also a form of petroleum.[citation needed] Oil sands, almost exclusively produced in Alberta, Canada, are colloquially referred to as "tar sands" but are in fact composed of asphalt, also called bitumen.[3][4]
Wood tar[edit]
For at least 600 years, wood tar has been used as a water repellent coating for boats, ships, and roofs. In Scandinavia, it was produced as a cash crop. "Peasant Tar" might be named for the district of its production.[5]
Wood tar is still used as an additive in the flavoring of candy, alcohol, and other foods. Wood tar is microbicidal. Producing tar from wood was known in ancient Greece and has probably been used in Scandinavia since the Iron Age. Production and trade in pine-derived tar was a major contributor in the economies of Northern Europe[6] and Colonial America. Its main use was in preserving wooden sailing vessels against rot. For centuries, dating back at least to the 14th century, tar was among Sweden's most important exports. Sweden exported 13,000 barrels of tar in 1615 and 227,000 barrels in the peak year of 1863. The largest user was the Royal Navy of the United Kingdom. Demand for tar declined with the advent of iron and steel ships. Production nearly stopped in the early 20th century, when other chemicals replaced tar, and wooden ships were replaced by steel ships. Traditional wooden boats are still sometimes tarred.
The heating (dry distilling) of pine wood causes tar and pitch to drip away from the wood[citation needed] and leave behind charcoal. Birch bark is used to make particularly fine tar, known as "Russian oil", suitable for leather protection. The by-products of wood tar are turpentine and charcoal. When deciduous tree woods are subjected to destructive distillation, the products are methanol (wood alcohol) and charcoal.
Tar kilns (Swedish: tjärmila, Danish: tjæremile, Norwegian: tjæremile, Finnish: tervahauta) are dry distillation ovens, historically used in Scandinavia for producing tar from wood. They were built close to the forest, from limestone or from more primitive holes in the ground. The bottom is sloped into an outlet hole to allow the tar to pour out. The wood is split into dimensions of a finger, stacked densely, and finally covered tight with earth and moss. If oxygen can enter, the wood might catch fire, and the production would be ruined. On top of this, a fire is stacked and lit. After a few hours, the tar starts to pour out and continues to do so for a few days.
Uses[edit]
Tar was used as seal for roofing shingles and tar paper and to seal the hulls of ships and boats. For millennia, wood tar was used to waterproof sails and boats, but today, sails made from inherently waterproof synthetic substances have reduced the demand for tar. Wood tar is still used to seal traditional wooden boats and the roofs of historic, shingle-roofed churches, as well as painting exterior walls of log buildings. Tar is also a general disinfectant. Pine tar oil, or wood tar oil, is used for the surface treatment of wooden shingle roofs, boats, buckets, and tubs and in the medicine, soap, and rubber industries. Pine tar has good penetration on the rough wood. An old wood tar oil recipe for the treatment of wood is one-third each genuine wood tar, balsam turpentine, and boiled or raw linseed oil or Chinese tung oil.
In Finland, wood tar was once considered a panacea reputed to heal "even those cut in twain through their midriff". A Finnish proverb states that "if sauna, vodka and tar won't help, the disease is fatal."[7] Wood tar is used in traditional Finnish medicine because of its microbicidal properties.
Wood tar is also available diluted as tar water, which has numerous uses:
- As a flavoring for candies (e.g., Terva Leijona) and alcohol (Terva Viina).
- As a spice for food, like meat.
- As a scent for saunas. Tar water is mixed into water, which is turned into steam in the sauna.
- As an anti-dandruff agent in shampoo.
- As a component of cosmetics.
Mixing tar with linseed oil varnish produces tar paint. Tar paint has a translucent brownish hue and can be used to saturate and tone wood and protect it from weather. Tar paint can also be toned with various pigments, producing translucent colors and preserving the wood texture.
Tar was once used for public humiliation, known as tarring and feathering. By pouring hot wood tar onto somebody's bare skin and waiting for it to cool, they would remain stuck in one position. From there, people would attach feathers to the tar, which would remain stuck on the tarred person for the duration of the punishment. That person would then become a public example for the rest of the day.[8]
Tar was used in 9th-century Baghdad, which had streets paved with tar, derived from petroleum that became accessible from natural fields in the region.[9]
Coal tar[edit]
Coal tar was formerly one of the products of gasworks. Tar made from coal or petroleum is considered toxic and carcinogenicbecause of its high benzene content, though coal tar in low concentrations is used as a topical medicine. Coal and petroleum tar has a pungent odour.
Coal tar is listed at number 1999 in the United Nations list of dangerous goods.
See also[edit]
The 10 μm process is the level of MOSFET semiconductor process technology that was commercially reached around 1971,[1][2] by leading semiconductor companies such as RCA and Intel.
In 1960, Egyptian-American engineer Mohamed M. Atalla and Korean-American engineer Dawon Kahng, while working at Bell Labs, demonstrated the first MOSFET transistors with 20 μm and then 10 μm gate lengths.[3][4] In 1969, Intel introduced the 1101 MOS SRAM chip with a 12 μm process.[5][6][7]
https://en.wikipedia.org/wiki/10_µm_process
Subcategories
This category has the following 11 subcategories, out of 11 total.
A
- Aflatoxins (7 P)
B
C
- Chewing tobacco brands (9 P)
H
- Helicobacter pylori (11 P)
P
- Pipe tobacco brands (4 P)
U
Pages in category "IARC Group 1 carcinogens"
The following 112 pages are in this category, out of 112 total. This list may not reflect recent changes (learn more).
A
B
C
N
P
S
T
https://en.wikipedia.org/wiki/Category:IARC_Group_1_carcinogens
Schistosoma haematobium (urinary blood fluke) is a species of digenetic trematode, belonging to a group (genus) of blood flukes (Schistosoma). It is found in Africa and the Middle East. It is the major agent of schistosomiasis, the most prevalent parasitic infection in humans.[1] It is the only blood fluke that infects the urinary tract, causing urinary schistosomiasis, and is the leading cause of bladder cancer (only next to tobacco smoking).[2][3] The diseases are caused by the eggs.
Adults are found in the venous plexuses around the urinary bladder and the released eggs travels to the wall of the urine bladder causing haematuria and fibrosis of the bladder. The bladder becomes calcified, and there is increased pressure on ureters and kidneys otherwise known as hydronephrosis. Inflammation of the genitals due to S. haematobium may contribute to the propagation of HIV.[4]
S. haematobium was the first blood fluke discovered. Theodor Bilharz, a German surgeon working in Cairo, identified the parasite as a causative agent of urinary infection in 1851. After the discoverer, the infection (generally including all schistosome infections) was called bilharzia or bilharziasis.[5] Along with other helminth parasites Clonorchis sinensis and Opisthorchis viverrini, S. haematobium was declared as Group 1 (extensively proven) carcinogens by the WHO International Agency for Research on Cancer (IARC) Working Group on the Evaluation of Carcinogenic Risks to Humans in 2009.[6]
https://en.wikipedia.org/wiki/Schistosoma_haematobium
Opisthorchis viverrini, common name Southeast Asian liver fluke, is a food-borne trematode parasite from the family Opisthorchiidae that infects the bile duct. People are infected after eating raw or undercooked fish.[2] Infection with the parasite is called opisthorchiasis. O. viverrini infection also increases the risk of cholangiocarcinoma, a cancer of the bile ducts.[3]
A small, leaf-like fluke, O. viverrini completes its lifecycle in three different animals. Snails of the species Bithynia are the first intermediate hosts, fish belonging to the family Cyprinidae are the second intermediate host, and the definitive hosts are humans and other mammals such as dogs, cats, rats, and pigs. It was first discovered in the Indian fishing cat (Prionailurus viverrus) by M.J. Poirier in 1886. The first human case was discovered by Robert Thomson Leiper in 1915.
O. viverrini (together with Clonorchis sinensis and Opisthorchis felineus) is one of the three most medically important species in the family Opisthorchiidae.[4] In fact O. viverrini and C. sinensis are capable of causing cancer in humans, and are classified by the International Agency for Research on Cancer as a group 1 biological carcinogen in 2009.[5][6][7] O. viverrini is found in Thailand, the Laos, Vietnam, and Cambodia.[8] It is most widely distributed in northern Thailand, with high prevalence in humans, while central Thailand has a low rate of prevalence.[9][10]
https://en.wikipedia.org/wiki/Opisthorchis_viverrini
https://en.wikipedia.org/wiki/Opisthorchis
https://en.wikipedia.org/wiki/Opisthorchiasis
Paragonimiasis is a food-borne parasitic infection caused by the lung fluke, most commonly Paragonimus westermani. It infects an estimated 22 million people yearly worldwide.[4] It is particularly common in East Asia. More than 30 species of trematodes (flukes) of the genus Paragonimus have been reported; among the more than 10 species reported to infect humans, and only 8 bringing about infections in humans,[5] the most common is P. westermani, the oriental lung fluke.[6]
https://en.wikipedia.org/wiki/Paragonimiasis
https://en.wikipedia.org/wiki/Bithionol
https://en.wikipedia.org/wiki/Praziquantel
Drug interactions
[edit]
The antibiotic rifampicin decreases plasma concentrations of praziquantel.[17]
Carbamazepine and phenytoin are reported to reduce the bioavailability of praziquantel.[18] Chloroquine also reduces its bioavailability.[19]
The drug cimetidine heightens praziquantel bioavailability.[20][21]
https://en.wikipedia.org/wiki/Praziquantel
https://en.wikipedia.org/wiki/Rifampicin
https://en.wikipedia.org/wiki/Chloroquine
Haemozoin is a disposal product formed from the digestion of blood by some blood-feeding parasites. These hematophagous organisms such as malaria parasites (Plasmodium spp.), Rhodnius and Schistosoma digest haemoglobinand release high quantities of free heme, which is the non-protein component of haemoglobin. Heme is a prosthetic groupconsisting of an iron atom contained in the center of a heterocyclic porphyrin ring. Free heme is toxic to cells, so the parasites convert it into an insoluble crystalline form called hemozoin. In malaria parasites, hemozoin is often called malaria pigment.
Since the formation of hemozoin is essential to the survival of these parasites, it is an attractive target for developing drugsand is much-studied in Plasmodium as a way to find drugs to treat malaria (malaria's Achilles' heel). Several currently used antimalarial drugs, such as chloroquine and mefloquine, are thought to kill malaria parasites by inhibiting haemozoin biocrystallization.
https://en.wikipedia.org/wiki/Hemozoin
Biocrystallization is the formation of crystals from organic macromolecules by living organisms.[1] This may be a stress response, a normal part of metabolism such as processes that dispose of waste compounds, or a pathology. Template mediated crystallization is qualitatively different from in vitro crystallization. Inhibitors of biocrystallization are of interest in drug design efforts against lithiasis and against pathogens that feed on blood, since many of these organisms use this process to safely dispose of heme.
Part of a series related toBiomineralization
https://en.wikipedia.org/wiki/Biocrystallization


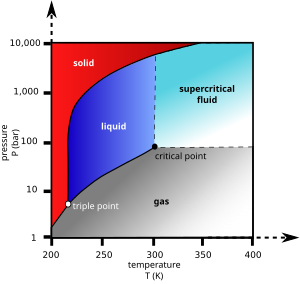
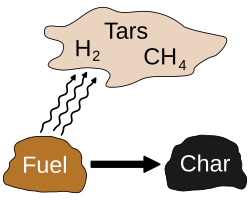


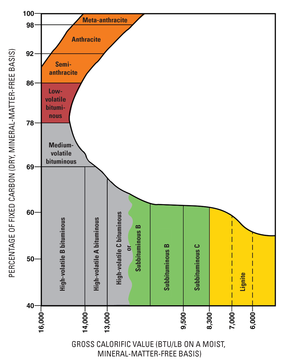



















No comments:
Post a Comment