Artificial digestion is a laboratory technique that reduces food to protein, fat, carbohydrates, fiber, minerals, vitamins, and non-nutrient compounds for analytical or research purposes. Digestive agents such as pepsin and hydrochloric acid are typically used to accomplish artificial digestion.
Meat inspection[edit]
Artificial digestion is used to detect the presence of encysted trichinella larvae in suspected muscle tissue. Prior to this method, a sample of muscle tissue was compressed to visually express the encysted parasite. Using artificial digestion, meat samples are dissolved by a digestive solution and the remains are examined for the presence of larvae.[1][2]
Digestion research[edit]
Artificial stomach and small intestine models are used instead of laboratory animals or human test subjects. Various models, from static one-compartment to dynamic multicompartment, exist. These models are used to study food digestion and subsequent bioavailability.[3]
https://en.wikipedia.org/wiki/Artificial_digestion
https://en.wikipedia.org/wiki/Category:Parasitology
https://online.ucpress.edu/abt/article-abstract/72/7/444/18184/How-to-Construct-an-Artificial-Stomach?redirectedFrom=fulltext
https://www.pfizer.com/news/articles/what_an_artificial_eating_machine_can_teach_us_about_medications
Simulating human digestion: developing our knowledge to create healthier and more sustainable foods
Alan Mackie  *a, Ana-Isabel Mulet-Cabero b and Amelia Torcello-Gómez
*a, Ana-Isabel Mulet-Cabero b and Amelia Torcello-Gómez  a
a
aThe School of Food Science and Nutrition, University of Leeds, Leeds, LS2 9JT, UK. E-mail: a.r.mackie@leeds.ac.uk
bQuadram Institute Bioscience, Norwich Research Park, Norwich, Norfolk NR4 7UQ, UK
First published on 22nd October 2020
https://pubs.rsc.org/en/content/articlehtml/2020/fo/d0fo01981j
ICD-10-CMICD-10-CM
Z93.4 - Other artificial openings of gastrointestinal tract status
Code
Z93.4 - Other artificial openings of gastrointestinal tract status
⑩ [Billable] [POA Exempt]
Code Tree
Z00-Z99 - Factors influencing health status and contact with health services
Z77-Z99 - Persons with potential health hazards related to family and personal history and certain conditions influencing health status
Z93 - Artificial opening status
Z93.0 - Tracheostomy status
Z93.1 - Gastrostomy status
Z93.2 - Ileostomy status
Z93.3 - Colostomy status
Z93.4 - Other artificial openings of gastrointestinal tract status
Z93.5 - Cystostomy status
Z93.6 - Other artificial openings of urinary tract status
Z93.8 - Other artificial opening status
Z93.9 - Artificial opening status, unspecified
Map to ⑨
Z93.4 converts to ICD-9-CM:
V44.4 - Status of other artificial opening of gastrointestinal tract
https://www.unboundmedicine.com/icd/view/ICD-10-CM/860668/all/Z93_4___Other_artificial_openings_of_gastrointestinal_tract_status
https://journals.lww.com/jpgn/Abstract/1985/10000/Type_of_Milk_Substitute_Influences_Growth_of_the.24.aspx
2022 ICD-10-CM Diagnosis Code Z93.4

Other artificial openings of gastrointestinal tract status
- 2016 2017 2018 2019 2020 2021 2022 Billable/Specific CodePOA Exempt
- Z93.4 is a billable/specific ICD-10-CM code that can be used to indicate a diagnosis for reimbursement purposes.
- The 2022 edition of ICD-10-CM Z93.4 became effective on October 1, 2021.
- This is the American ICD-10-CM version of Z93.4 - other international versions of ICD-10 Z93.4 may differ.
Approximate Synonyms
- History of artificial gastrointestinal tract opening
- Jejunostomy present
- Presence of artificial gastrointestinal tract opening
- Presence of enterostomy
- Presence of enterostomy (artificial opening into intestine)
- Presence of jejunostomy (artificial opening into intestine)
Present On Admission
- Z93.4 is considered exempt from POA reporting.
ICD-10-CM Z93.4 is grouped within Diagnostic Related Group(s) (MS-DRG v39.0):
- 951 Other factors influencing health status
Convert Z93.4 to ICD-9-CM
Code History
- 2016 (effective 10/1/2015): New code (first year of non-draft ICD-10-CM)
- 2017 (effective 10/1/2016): No change
- 2018 (effective 10/1/2017): No change
- 2019 (effective 10/1/2018): No change
- 2020 (effective 10/1/2019): No change
- 2021 (effective 10/1/2020): No change
- 2022 (effective 10/1/2021): No change
- Artificial
- opening status (functioning) (without complication) Z93.9
- Enterostomy
- Jejunostomy status Z93.4
- Status (post) - see also Presence (of)
- artificial opening (of) Z93.9
- enterostomy Z93.4
- jejunostomy Z93.4
Reimbursement claims with a date of service on or after October 1, 2015 require the use of ICD-10-CM codes.
https://www.icd10data.com/ICD10CM/Codes/Z00-Z99/Z77-Z99/Z93-/Z93.4
Cellulose is an organic compound with the formula (C
6H
10O
5)
n, a polysaccharide consisting of a linear chain of several hundred to many thousands of β(1→4) linked D-glucose units.[3][4] Cellulose is an important structural component of the primary cell wall of green plants, many forms of algae and the oomycetes. Some species of bacteria secrete it to form biofilms.[5] Cellulose is the most abundant organic polymer on Earth.[6] The cellulose content of cotton fiber is 90%, that of wood is 40–50%, and that of dried hemp is approximately 57%.[7][8][9]
Cellulose is mainly used to produce paperboard and paper. Smaller quantities are converted into a wide variety of derivative products such as cellophane and rayon. Conversion of cellulose from energy crops into biofuels such as cellulosic ethanol is under development as a renewable fuel source. Cellulose for industrial use is mainly obtained from wood pulp and cotton.[6]
Some animals, particularly ruminants and termites, can digest cellulose with the help of symbiotic micro-organisms that live in their guts, such as Trichonympha. In human nutrition, cellulose is a non-digestible constituent of insoluble dietary fiber, acting as a hydrophilic bulking agent for feces and potentially aiding in defecation.
https://en.wikipedia.org/wiki/Cellulose
Equine nutrition is the feeding of horses, ponies, mules, donkeys, and other equines. Correct and balanced nutrition is a critical component of proper horse care.
Horses are non-ruminant herbivores of a type known as a "hindgut fermenter." Horses have only one stomach, as do humans. However, unlike humans, they also need to digest plant fiber (largely cellulose) that comes from grass or hay. Ruminants like cattle are foregut fermenters, and digest fiber in plant matter by use of a multi-chambered stomach, whereas horses use microbial fermentation in a part of the digestive system known as the cecum (or caecum) to break down the cellulose.[1]
In practical terms, horses prefer to eat small amounts of food steadily throughout the day, as they do in nature when grazing on pasture lands.[2] Although this is not always possible with modern stabling practices and human schedules that favor feeding horses twice a day, it is important to remember the underlying biology of the animal when determining what to feed, how often, and in what quantities.
The digestive system of the horse is somewhat delicate. Horses are unable to regurgitate food, except from the esophagus. Thus, if they overeat or eat something poisonous, vomiting is not an option.[3] They also have a long, complex large intestine and a balance of beneficial microbes in their cecum that can be upset by rapid changes in feed. Because of these factors, they are very susceptible to colic, which is a leading cause of death in horses.[4] Therefore, horses require clean, high-quality feed, provided at regular intervals, plus water or they may become ill if subjected to abrupt changes in their diets.[5] Horses are also sensitive to molds and toxins. For this reason, they must never be fed contaminated fermentable materials such as lawn clippings.[6] Fermented silage or "haylage" is fed to horses in some places; however, contamination or failure of the fermentation process that allows any mold or spoilage may be toxic.[7][8]
https://en.wikipedia.org/wiki/Equine_nutrition
Hindgut fermentation is a digestive process seen in monogastric herbivores, animals with a simple, single-chambered stomach. Cellulose is digested with the aid of symbiotic bacteria.[1] The microbial fermentation occurs in the digestive organs that follow the small intestine: the large intestine and cecum. Examples of hindgutfermenters include proboscideans and large odd-toed ungulates such as horses and rhinos, as well as small animals such as rodents, rabbits and koalas.[2] In contrast, foregut fermentation is the form of cellulose digestion seen in ruminants such as cattle which have a four-chambered stomach,[3] as well as in sloths, macropodids, some monkeys, and one bird, the hoatzin.[4]
https://en.wikipedia.org/wiki/Hindgut_fermentation
Nuclear reactor physics is the field of physics that studies and deals with the applied study and engineering applications of chain reaction to induce a controlled rate of fission in a nuclear reactor for the production of energy.[1] Most nuclear reactors use a chain reaction to induce a controlled rate of nuclear fission in fissile material, releasing both energy and free neutrons. A reactor consists of an assembly of nuclear fuel (a reactor core), usually surrounded by a neutron moderator such as regular water, heavy water, graphite, or zirconium hydride, and fitted with mechanisms such as control rods that control the rate of the reaction.
The physics of nuclear fission has several quirks that affect the design and behavior of nuclear reactors. This article presents a general overview of the physics of nuclear reactors and their behavior.
https://en.wikipedia.org/wiki/Nuclear_reactor_physics
A chemical reactor is an enclosed volume in which a chemical reaction takes place.[1][2][3][4] In chemical engineering, it is generally understood to be a process vessel used to carry out a chemical reaction,[5] which is one of the classic unit operations in chemical process analysis. The design of a chemical reactor deals with multiple aspects of chemical engineering. Chemical engineers design reactors to maximize net present value for the given reaction. Designers ensure that the reaction proceeds with the highest efficiency towards the desired output product, producing the highest yield of product while requiring the least amount of money to purchase and operate. Normal operating expenses include energy input, energy removal, raw material costs, labor, etc. Energy changes can come in the form of heating or cooling, pumping to increase pressure, frictional pressure loss or agitation.
| Part of a series on |
| Chemical engineering |
|---|
| Fundamentals |
| Unit processes |
| Aspects |
| Glossaries |
|
Chemical reaction engineering is the branch of chemical engineering which deals with chemical reactors and their design, especially by application of chemical kinetics to industrial systems.
Overview[edit]
The most common basic types of chemical reactors are tanks (where the reactants mix in the whole volume) and pipes or tubes (for laminar flow reactors and plug flow reactors)
Both types can be used as continuous reactors or batch reactors, and either may accommodate one or more solids (reagents, catalysts, or inert materials), but the reagents and products are typically fluids (liquids or gases). Reactors in continuous processes are typically run at steady-state, whereas reactors in batch processes are necessarily operated in a transient state. When a reactor is brought into operation, either for the first time or after a shutdown, it is in a transient state, and key process variables change with time.
There are three idealised models used to estimate the most important process variables of different chemical reactors:
- Batch reactor model,
- Continuous stirred-tank reactor model (CSTR), and
- Plug flow reactor model (PFR).
Many real-world reactors can be modeled as a combination of these basic types.
Key process variables include:
- Residence time (τ, lower case Greek tau)
- Volume (V)
- Temperature (T)
- Pressure (P)
- Concentrations of chemical species (C1, C2, C3, ... Cn)
- Heat transfer coefficients (h, U)
A tubular reactor can often be a packed bed. In this case, the tube or channel contains particles or pellets, usually a solid catalyst.[6] The reactants, in liquid or gas phase, are pumped through the catalyst bed.[7] A chemical reactor may also be a fluidized bed; see Fluidized bed reactor.
Chemical reactions occurring in a reactor may be exothermic, meaning giving off heat, or endothermic, meaning absorbing heat. A tank reactor may have a cooling or heating jacket or cooling or heating coils (tubes) wrapped around the outside of its vessel wall to cool down or heat up the contents, while tubular reactors can be designed like heat exchangers if the reaction is strongly exothermic, or like furnaces if the reaction is strongly endothermic.[8]
Types[edit]
Batch reactor[edit]
The simplest type of reactor is a batch reactor. Materials are loaded into a batch reactor, and the reaction proceeds with time. A batch reactor does not reach a steady state, and control of temperature, pressure and volume is often necessary. Many batch reactors therefore have ports for sensors and material input and output. Batch reactors are typically used in small-scale production and reactions with biological materials, such as in brewing, pulping, and production of enzymes. One example of a batch reactor is a pressure reactor.
CSTR (continuous stirred-tank reactor)[edit]
In a CSTR, one or more fluid reagents are introduced into a tank reactor which is typically stirred with an impeller to ensure proper mixing of the reagents while the reactor effluent is removed. Dividing the volume of the tank by the average volumetric flow rate through the tank gives the space time, or the time required to process one reactor volume of fluid. Using chemical kinetics, the reaction's expected percentcompletion can be calculated. Some important aspects of the CSTR:
- At steady-state, the mass flow rate in must equal the mass flow rate out, otherwise the tank will overflow or go empty (transient state). While the reactor is in a transient state the model equation must be derived from the differential mass and energy balances.
- The reaction proceeds at the reaction rate associated with the final (output) concentration, since the concentration is assumed to be homogenous throughout the reactor.
- Often, it is economically beneficial to operate several CSTRs in series. This allows, for example, the first CSTR to operate at a higher reagent concentration and therefore a higher reaction rate. In these cases, the sizes of the reactors may be varied in order to minimize the total capital investment required to implement the process.
- It can be demonstrated that an infinite number of infinitely small CSTRs operating in series would be equivalent to a PFR.[9]
The behavior of a CSTR is often approximated or modeled by that of a Continuous Ideally Stirred-Tank Reactor (CISTR). All calculations performed with CISTRs assume perfect mixing. If the residence time is 5-10 times the mixing time, this approximation is considered valid for engineering purposes. The CISTR model is often used to simplify engineering calculations and can be used to describe research reactors. In practice it can only be approached, particularly in industrial size reactors in which the mixing time may be very large.
A loop reactor is a hybrid type of catalytic reactor that physically resembles a tubular reactor, but operates like a CSTR. The reaction mixture is circulated in a loop of tube, surrounded by a jacket for cooling or heating, and there is a continuous flow of starting material in and product out.
PFR (plug flow reactor)[edit]
In a PFR, sometimes called continuous tubular reactor (CTR),[10] one or more fluid reagents are pumpedthrough a pipe or tube. The chemical reaction proceeds as the reagents travel through the PFR. In this type of reactor, the changing reaction rate creates a gradient with respect to distance traversed; at the inlet to the PFR the rate is very high, but as the concentrations of the reagents decrease and the concentration of the product(s) increases the reaction rate slows. Some important aspects of the PFR:
- The idealized PFR model assumes no axial mixing: any element of fluid traveling through the reactor doesn't mix with fluid upstream or downstream from it, as implied by the term "plug flow".
- Reagents may be introduced into the PFR at locations in the reactor other than the inlet. In this way, a higher efficiency may be obtained, or the size and cost of the PFR may be reduced.
- A PFR has a higher theoretical efficiency than a CSTR of the same volume. That is, given the same space-time (or residence time), a reaction will proceed to a higher percentage completion in a PFR than in a CSTR. This is not always true for reversible reactions.
For most chemical reactions of industrial interest, it is impossible for the reaction to proceed to 100% completion. The rate of reaction decreases as the reactants are consumed until the point where the system reaches dynamic equilibrium (no net reaction, or change in chemical species occurs). The equilibrium point for most systems is less than 100% complete. For this reason a separation process, such as distillation, often follows a chemical reactor in order to separate any remaining reagents or byproducts from the desired product. These reagents may sometimes be reused at the beginning of the process, such as in the Haber process. In some cases, very large reactors would be necessary to approach equilibrium, and chemical engineers may choose to separate the partially reacted mixture and recycle the leftover reactants.
Under laminar flow conditions, the assumption of plug flow is highly inaccurate, as the fluid traveling through the center of the tube moves much faster than the fluid at the wall. The continuous oscillatory baffled reactor (COBR) achieves thorough mixing by the combination of fluid oscillation and orifice baffles, allowing plug flow to be approximated under laminar flow conditions.
Semibatch reactor[edit]
A semibatch reactor is operated with both continuous and batch inputs and outputs. A fermenter, for example, is loaded with a batch of medium and microbes which constantly produces carbon dioxide that must be removed continuously. Similarly, reacting a gas with a liquid is usually difficult, because a large volume of gas is required to react with an equal mass of liquid. To overcome this problem, a continuous feed of gas can be bubbled through a batch of a liquid. In general, in semibatch operation, one chemical reactant is loaded into the reactor and a second chemical is added slowly (for instance, to prevent side reactions), or a product which results from a phase change is continuously removed, for example a gas formed by the reaction, a solid that precipitates out, or a hydrophobic product that forms in an aqueous solution.
Catalytic reactor[edit]
Although catalytic reactors are often implemented as plug flow reactors, their analysis requires more complicated treatment. The rate of a catalytic reaction is proportional to the amount of catalyst the reagents contact, as well as the concentration of the reactants. With a solid phase catalyst and fluid phase reagents, this is proportional to the exposed area, efficiency of diffusion of reagents in and products out, and efficacy of mixing. Perfect mixing usually cannot be assumed. Furthermore, a catalytic reaction pathway often occurs in multiple steps with intermediates that are chemically bound to the catalyst; and as the chemical binding to the catalyst is also a chemical reaction, it may affect the kinetics. Catalytic reactions often display so-called falsified kinetics, when the apparent kinetics differ from the actual chemical kinetics due to physical transport effects.
The behavior of the catalyst is also a consideration. Particularly in high-temperature petrochemical processes, catalysts are deactivated by processes such as sintering, coking, and poisoning.
A common example of a catalytic reactor is the catalytic converter that processes toxic components of automobile exhausts. However, most petrochemical reactors are catalytic, and are responsible for most industrial chemical production, with extremely high-volume examples including sulfuric acid, ammonia, reformate/BTEX(benzene, toluene, ethylbenzene and xylene), and fluid catalytic cracking. Various configurations are possible, see Heterogeneous catalytic reactor.
https://en.wikipedia.org/wiki/Chemical_reactor
Heterogenous catalytic reactors put emphasis on catalyst effectiveness factors and the heat and mass transfer implications. Heterogenous catalytic reactors are among the most commonly utilized chemical reactors in the chemical engineering industry.
https://en.wikipedia.org/wiki/Heterogeneous_catalytic_reactor
In chemical processing, a packed bed is a hollow tube, pipe, or other vessel that is filled with a packing material. The packing can be randomly filled with small objects like Raschig rings or else it can be a specifically designed structured packing. Packed beds may also contain catalyst particles or adsorbents such as zeolite pellets, granular activated carbon, etc.
The purpose of a packed bed is typically to improve contact between two phases in a chemical or similar process. Packed beds can be used in a chemical reactor, a distillation process, or a scrubber, but packed beds have also been used to store heat in chemical plants. In this case, hot gases are allowed to escape through a vessel that is packed with a refractory material until the packing is hot. Air or other cool gas is then fed back to the plant through the hot bed, thereby pre-heating the air or gas feed.
https://en.wikipedia.org/wiki/Packed_bed
Hydrodesulfurization (HDS) is a catalytic chemical process widely used to remove sulfur (S) from natural gas and from refined petroleum products, such as gasoline or petrol, jet fuel, kerosene, diesel fuel, and fuel oils.[1][2][3] The purpose of removing the sulfur, and creating products such as ultra-low-sulfur diesel, is to reduce the sulfur dioxide (SO
2) emissions that result from using those fuels in automotive vehicles, aircraft, railroad locomotives, ships, gas or oil burning power plants, residential and industrial furnaces, and other forms of fuel combustion.
Another important reason for removing sulfur from the naphtha streams within a petroleum refinery is that sulfur, even in extremely low concentrations, poisons the noble metal catalysts (platinum and rhenium) in the catalytic reforming units that are subsequently used to upgrade the octane rating of the naphtha streams.
The industrial hydrodesulfurization processes include facilities for the capture and removal of the resulting hydrogen sulfide (H
2S) gas. In petroleum refineries, the hydrogen sulfide gas is then subsequently converted into byproduct elemental sulfur or sulfuric acid (H
2SO
4). In fact, the vast majority of the 64,000,000 metric tons of sulfur produced worldwide in 2005 was byproduct sulfur from refineries and other hydrocarbon processing plants.[4][5]
An HDS unit in the petroleum refining industry is also often referred to as a hydrotreater.
https://en.wikipedia.org/wiki/Hydrodesulfurization
Reactors with insignificant motion of catalyst particles[edit]
Fixed bed reactors[edit]
A fixed bed reactor is a cylindrical tube filled with catalyst pellets with reactants flowing through the bed and being converted into products. The catalyst may have multiple configuration including: one large bed, several horizontal beds, several parallel packed tubes, multiple beds in their own shells. The various configurations may be adapted depending on the need to maintain temperature control within the system. Serial connection of two reactors with option to dose oxidant between the stages enable under optimal conditions to increase the product yield in oxidation catalysis.[1] By dosing intermediates or products between the stages, valuable information could be found concerning the reaction pathways.[2]
The catalyst pellets may be spherical, cylindrical, or randomly shaped pellets. They range from 0.25 cm to 1.0 cm in diameter. The flow of a fixed bed reactor is typically downward. Packed bed reactor.
Trickle-bed reactors[edit]
A trickle-bed reactor is a fixed bed where liquid flows without filling the spaces between particles. Like with the fixed bed reactors, the liquid typically flows downward. At the same time, gas is flowing upward. The primary use for trickle-bed reactors is hydrotreatment reactions (hydrodesulfurization and hydrodemetalation of heavy crude oil,[3] hydrodeasphaltenization of coal tar[4]). This reactor is often utilized in order to handle feeds with extremely high boiling points..
Moving bed reactors[edit]
A moving bed reactor has a fluid phase that passes up through a packed bed. Solid is fed into the top of the reactor and moves down. It is removed at the bottom. Moving bed reactors require special control valves to maintain close control of the solids. For this reason, moving bed reactors are less frequently used than the above two reactors. Moving bed reactors are most suitable for solid content below 10% and is generally used where the solids (primarily catalyst) have high surface area due to its size in microns.
Rotating bed reactors[edit]
A rotating bed reactor (RBR) holds a packed bed fixed within a basket with a central hole. When the basket is spinning immersed in a fluid phase, the inertia forces created by the spinning motion forces the fluid outwards, thereby creating a circulating flow through the rotating packed bed. The rotating bed reactor is a rather new invention that shows high rates of mass transfer and good fluid mixing. RBR type reactors have mostly been applied in biocatalysis reactions or decoloration applications. RBR is majorly used in high value products where Capex is justified with smaller reactor capacity. It is not widely popular due to difficulty in manufacturing.
Reactors with significant motion of catalyst particles[edit]
Fluidized bed reactors[edit]
A fluidized bed reactor suspends small particles of catalyst by the upward motion of the fluid to be reacted. The fluid is typically a gas with a flow rate high enough to mix the particles without carrying them out of the reactor. The particles are much smaller than those for the above reactors. Typically on the scale of 10-300 microns. One key advantage of using a fluidized bed reactor is the ability to achieve a highly uniform temperature in the reactor. The fluidized bed reactors are best for bio-catalysts or enzymes doped on solids since the solid are fluidized by the working fluid and there is no mechanical impact on the solids.
Slurry reactors[edit]
A slurry reactor contains the catalyst in a powdered or granular form.[5] This reactor is typically used when one reactant is a gas and the other a liquid while the catalyst is a solid. The reactant gas is put through the liquid and dissolved. It then diffuses onto the catalyst surface. Slurry reactors can use very fine particles and this can lead to problems of separation of catalyst from the liquid. Trickle-bed reactors don't have this problem and this is a big advantage of trickle-bed reactor. Unfortunately these large particles in trickle bed means much lower reaction rate. Overall, the trickle bed is simpler, the slurry reactors usually has a high reaction rate and the fluidized bed is somewhat in-between.
https://en.wikipedia.org/wiki/Heterogeneous_catalytic_reactor
Sintering or frittage is the process of compacting and forming a solid mass of material by heat[1] or pressure[2] without melting it to the point of liquefaction.
Sintering happens as part of a manufacturing process used with metals, ceramics, plastics, and other materials. The atoms in the materials diffuse across the boundaries of the particles, fusing the particles together and creating one solid piece. Because the sintering temperature does not have to reach the melting point of the material, sintering is often chosen as the shaping process for materials with extremely high melting points such as tungsten and molybdenum. The study of sintering in metallurgy powder-related processes is known as powder metallurgy. An example of sintering can be observed when ice cubes in a glass of water adhere to each other, which is driven by the temperature difference between the water and the ice. Examples of pressure-driven sintering are the compacting of snowfall to a glacier, or the forming of a hard snowball by pressing loose snow together.
The material produced by sintering is called sinter. The word sinter comes from the Middle High German sinter, a cognate of English cinder.
https://en.wikipedia.org/wiki/Sintering
Catalyst poisoning refers to the partial or total deactivation of a catalyst by a chemical compound. Poisoning refers specifically to chemical deactivation, rather than other mechanisms of catalyst degradation such as thermal decomposition or physical damage.[1][2] Although usually undesirable, poisoning may be helpful when it results in improved catalyst selectivity (e.g. Lindlar's catalyst). An important historic example was the poisoning of catalytic converters by leaded fuel.
Poisoning of Pd catalysts[edit]
Organic functional groups and inorganic anions often have the ability to strongly adsorb to metal surfaces. Common catalyst poisons include carbon monoxide, halides, cyanides, sulfides, sulfites, phosphates, phosphites and organic molecules such as nitriles, nitro compounds, oximes, and nitrogen-containing heterocycles. Agents vary their catalytic properties because of the nature of the transition metal. Lindlar catalysts are prepared by the reduction of palladium chloride in a slurry of calcium carbonate (CaCO3) followed by poisoning with lead acetate.[3] In a related case, the Rosenmund reduction of acyl halides to aldehydes, the palladium catalyst (over barium sulfate or calcium carbonate) is intentionally poisoned by the addition of sulfur or quinoline in order to lower the catalyst activity and thereby prevent over-reduction of the aldehyde product to the primary alcohol.
Poisoning process[edit]
Poisoning often involves compounds that chemically bond to a catalyst's active sites. Poisoning decreases the number of active sites, and the average distance that a reactant molecule must diffuse through the pore structure before undergoing reaction increases as a result.[4] As a result, poisoned sites can no longer accelerate the reaction with which the catalyst was supposed to catalyze.[5] Large scale production of substances such as ammonia in the Haber–Bosch process include steps to remove potential poisons from the product stream. When the poisoning reaction rate is slow relative to the rate of diffusion, the poison will be evenly distributed throughout the catalyst and will result in homogeneous poisoning of the catalyst. Conversely, if the reaction rate is fast compared to the rate of diffusion, a poisoned shell will form on the exterior layers of the catalyst, a situation known as "pore-mouth" poisoning, and the rate of catalytic reaction may become limited by the rate of diffusion through the inactive shell.[4]
Selective poisoning[edit]
If the catalyst and reaction conditions are indicative of low effectiveness, selective poisoning may be observed, where poisoning of only a small fraction of the catalyst's surface gives a disproportionately large drop in activity.[4]
If η is the effectiveness factor of the poisoned surface and hp is the Thiele modulus for the poisoned case:
When the ratio of the reaction rates of the poisoned pore to the unpoisoned pore is considered:
where F is the ratio of poisoned to unpoisoned pores, hT is the Thiele modulus for the unpoisoned case, and α is the fraction of the surface that is poisoned.
The above equation simplifies depending on the value of hT. When the surface is available, hT is negligible:
This represents the "classical case" of nonselective poisoning where the fraction of the activity remaining is equal to the fraction of the unpoisoned surface remaining.
When hT is very large, it becomes:
In this case, the catalyst effectiveness factors are considerably less than unity, and the effects of the portion of the poison adsorbed near the closed end of the pore are not as apparent as when hT is small.
The rate of diffusion of the reactant through the poisoned region is equal to the rate of reaction and is given by:
And the rate of reaction within a pore is given by:
The fraction of the catalyst surface available for reaction can be obtained from the ratio of the poisoned reaction rate to the unpoisoned reaction rate:
or
- [4]: 465
Benefits of selective poisoning[edit]
Usually, catalyst poisoning is undesirable as it leads to the wasting of expensive metals or their complexes. However, poisoning of catalysts can be used to improve selectivity of reactions. Poisoning can allow for selective intermediates to be isolated and desirable final products to be produced.
Hydrodesulfurization catalysts[edit]
In the purification of petroleum products, the process of hydrodesulfurization is utilized.[6] Thiols, such as thiophene, are reduced using H2 to produce H2S and hydrocarbons of varying chain length. Common catalysts used are tungsten and molybdenum sulfide. Adding cobalt and nickel [7] to either edges or partially incorporating them into the crystal lattice structure can improve the catalyst's efficiency. The synthesis of the catalyst creates a supported hybrid that prevents poisoning of the cobalt nuclei.
Other examples[edit]
- In catalytic converters used in automobiles, tetraethyllead is converted into elemental lead which alloys with the metals present in the catalyst, reducing the converter's ability to reduce NOx emissions.
- In fuel cells using platinum catalysts, the fuels must be free of sulfur and carbon monoxide, unless a desulfurization system is used.
- Ziegler-Natta catalysts for the production of polyolefins (e.g. polyethylene, polypropylene, etc) are poisoned by water and oxygen. This poisoning applies to both homogeneous catalysts and heterogeneous catalysts for olefin polymerization. This requires the monomers (ethylene, propylene, etc.) to be purified.
See also[edit]
https://en.wikipedia.org/wiki/Catalyst_poisoning
For both chemical and biological engineering, Semibatch (semiflow) reactors operate much like batch reactors in that they take place in a single stirred tank with similar equipment.[1] However, they are modified to allow reactant addition and/or product removal in time.
A normal batch reactor is filled with reactants in a single stirred tank at time and the reaction proceeds. A semibatch reactor, however, allows partial filling of reactants with the flexibility of adding more as time progresses. Stirring in both types is very efficient, which allows batch and semibatch reactors to assume a uniform composition and temperature throughout.
https://en.wikipedia.org/wiki/Semibatch_reactor
A Continuous Oscillatory Baffled Reactor (COBR) is a specially designed chemical reactor to achieve plug flow under laminar flow conditions. Achieving plug flow has previously been limited to either a large number of continuous stir tank reactors (CSTR) in series or conditions with high turbulent flow. The technology incorporates annular baffles to a tubular reactor framework to create eddies when liquid is pushed up through the tube. Likewise, when liquid is on a downstroke through the tube, eddies are created on the other side of the baffles. Eddy generation on both sides of the baffles creates very effective mixing while still maintaining plug flow. By using COBR, potentially higher yields of product can be made with greater control and reduced waste.[1]
Design[edit]
A standard COBR consists of a 10-150mm ID tube with equally spaced baffles throughout. There are typically two pumps in a COBR; one pump is reciprocating to generate continuous oscillatory flow and a second pump creates net flow through the tube. This design offers a control over mixing intensity that conventional tubular reactors cannot achieve.[2] Each baffled cell acts as a CSTR and because a secondary pump is creating a net laminar flow, much longer residence times can be achieved relative to turbulent flow systems.[3]
With conventional tubular reactors, mixing is accomplished through stirring mechanisms or turbulent flow conditions, which is difficult to control. By changing variable values such as baffle spacing or thickness, COBRs can operate with much better mixing control. For instance, it has been found that a spacing of 1.5 times tube diameter size is the most effective mixing condition; furthermore, vortex deformation increases with increase in baffle thickness greater than 3mm.[2]
Biological applications[edit]
The low shear rate and enhanced mass transfer provided by the COBR makes it an ideal reactor for various biological processes. For shear rate, it has been found that COBRs have an evenly distributed, five-fold reduction in shear rate relative to conventional tubular reactors; this is especially important for biological process given that high shear rates can damage microorganisms.
For the case of mass transfer, COBR fluid mechanics allows for an increase in oxygen gas residence time. Furthermore, the vortexes created in the COBRs causes a gas bubble break-up and thus an increase in surface area for gas transfer. For aerobic biological processes, therefore, COBRs again present an advantage. An especially promising aspect of the COBR technology is its ability to scale-up processes while still retaining the advantages in shear rate and mass transfer.
Limitations[edit]
Though the prospect for COBR applications in fields like bioprocessing are very promising, there are a number of necessary improvements to be made before more global use. Clearly, there is additional complexity in the COBR design relative to other bioreactors, which can introduce complications in operation. Furthermore, for bioprocessing it is possible that fouling of baffles and internal surfaces becomes an issue. Perhaps the most significant needed advancement moving forward is further comprehensive studies that COBR technology can indeed be useful in industry. There are currently no COBRs in use at industrial bioprocessing plants and the evidence of its effectiveness, though very promising and theoretically an improvement relative to current reactors in industry, is limited to smaller laboratory-scale experiments.[3]
https://en.wikipedia.org/wiki/Oscillatory_baffled_reactor
Distillation, or classical distillation, is the process of separating the components or substances from a liquid mixture by using selective boiling and condensation. Dry distillation is the heating of solid materials to produce gaseous products (which may condense into liquids or solids). Dry distillation may involve chemical changes such as destructive distillation or cracking and is not discussed under this article. Distillation may result in essentially complete separation (nearly pure components), or it may be a partial separation that increases the concentration of selected components in the mixture. In either case, the process exploits differences in the relative volatility of the mixture's components. In industrial applications, distillation is a unit operationof practically universal importance, but it is a physical separation process, not a chemical reaction.
Distillation has many applications. For example:
- The distillation of fermented products produces distilled beverages with a high alcohol content, or separates other fermentation products of commercial value.
- Distillation is an effective and traditional method of desalination.
- In the petroleum industry, oil stabilization is a form of partial distillation that reduces the vapor pressure of crude oil, thereby making it safe for storage and transport as well as reducing the atmospheric emissions of volatile hydrocarbons. In midstream operations at oil refineries, fractional distillation is a major class of operation for transforming crude oil into fuelsand chemical feed stocks.[2][3][4]
- Cryogenic distillation leads to the separation of air into its components – notably oxygen, nitrogen, and argon – for industrial use.
- In the chemical industry, large amounts of crude liquid products of chemical synthesis are distilled to separate them, either from other products, from impurities, or from unreacted starting materials.
An installation used for distillation, especially of distilled beverages, is a distillery. The distillation equipment itself is a still.
Laboratory procedures[edit]
Laboratory scale distillations are almost exclusively run as batch distillations. The device used in distillation, sometimes referred to as a still, consists at a minimum of a reboiler or pot in which the source material is heated, a condenser in which the heated vapor is cooled back to the liquid state, and a receiver in which the concentrated or purified liquid, called the distillate, is collected. Several laboratory scale techniques for distillation exist (see also distillation types).
A completely sealed distillation apparatus could experience extreme and rapidly varying internal pressure, which could cause it to burst open at the joints. Therefore, some path is usually left open (for instance, at the receiving flask) to allow the internal pressure to equalize with atmospheric pressure. Alternatively, a vacuum pumpmay be used to keep the apparatus at a lower than atmospheric pressure. If the substances involved are air- or moisture-sensitive, the connection to the atmosphere can be made through one or more drying tubes packed with materials that scavenge the undesired air components, or through bubblers that provide a movable liquid barrier. Finally, the entry of undesired air components can be prevented by pumping a low but steady flow of suitable inert gas, like nitrogen, into the apparatus.
Simple distillation[edit]
In simple distillation, the vapor is immediately channeled into a condenser. Consequently, the distillate is not pure but rather its composition is identical to the composition of the vapors at the given temperature and pressure. That concentration follows Raoult's law.
As a result, simple distillation is effective only when the liquid boiling points differ greatly (rule of thumb is 25 °C)[32]or when separating liquids from non-volatile solids or oils. For these cases, the vapor pressures of the components are usually different enough that the distillate may be sufficiently pure for its intended purpose.
A cutaway schematic of a simple distillation operation is shown at right. The starting liquid 15 in the boiling flask 2 is heated by a combined hotplate and magnetic stirrer 13 via a silicone oil bath (orange, 14). The vapor flows through a short Vigreux column 3, then through a Liebig condenser 5, is cooled by water (blue) that circulates through ports 6 and 7. The condensed liquid drips into the receiving flask 8, sitting in a cooling bath (blue, 16). The adapter 10 has a connection 9 that may be fitted to a vacuum pump. The components are connected by ground glass joints.
Fractional distillation[edit]
For many cases, the boiling points of the components in the mixture will be sufficiently close that Raoult's law must be taken into consideration. Therefore, fractional distillation must be used in order to separate the components by repeated vaporization-condensation cycles within a packed fractionating column. This separation, by successive distillations, is also referred to as rectification.[33]
As the solution to be purified is heated, its vapors rise to the fractionating column. As it rises, it cools, condensing on the condenser walls and the surfaces of the packing material. Here, the condensate continues to be heated by the rising hot vapors; it vaporizes once more. However, the composition of the fresh vapors are determined once again by Raoult's law. Each vaporization-condensation cycle (called a theoretical plate) will yield a purer solution of the more volatile component.[34] In reality, each cycle at a given temperature does not occur at exactly the same position in the fractionating column; theoretical plate is thus a concept rather than an accurate description.
More theoretical plates lead to better separations. A spinning band distillation system uses a spinning band of Teflon or metal to force the rising vapors into close contact with the descending condensate, increasing the number of theoretical plates.[35]
Steam distillation[edit]
Like vacuum distillation, steam distillation is a method for distilling compounds which are heat-sensitive.[1]: 151–153 The temperature of the steam is easier to control than the surface of a heating element, and allows a high rate of heat transfer without heating at a very high temperature. This process involves bubbling steam through a heated mixture of the raw material. By Raoult's law, some of the target compound will vaporize (in accordance with its partial pressure). The vapor mixture is cooled and condensed, usually yielding a layer of oil and a layer of water.
Steam distillation of various aromatic herbs and flowers can result in two products; an essential oil as well as a watery herbal distillate. The essential oils are often used in perfumery and aromatherapy while the watery distillates have many applications in aromatherapy, food processing and skin care.
Vacuum distillation[edit]
Some compounds have very high boiling points. To boil such compounds, it is often better to lower the pressure at which such compounds are boiled instead of increasing the temperature. Once the pressure is lowered to the vapor pressure of the compound (at the given temperature), boiling and the rest of the distillation process can commence. This technique is referred to as vacuum distillation and it is commonly found in the laboratory in the form of the rotary evaporator.
This technique is also very useful for compounds which boil beyond their decomposition temperature at atmospheric pressure and which would therefore be decomposed by any attempt to boil them under atmospheric pressure.
Short path and molecular distillation[edit]
Molecular distillation is vacuum distillation below the pressure of 0.01 torr. 0.01 torr is one order of magnitude above high vacuum, where fluids are in the free molecular flowregime, i.e. the mean free path of molecules is comparable to the size of the equipment. The gaseous phase no longer exerts significant pressure on the substance to be evaporated, and consequently, rate of evaporation no longer depends on pressure. That is, because the continuum assumptions of fluid dynamics no longer apply, mass transport is governed by molecular dynamics rather than fluid dynamics. Thus, a short path between the hot surface and the cold surface is necessary, typically by suspending a hot plate covered with a film of feed next to a cold plate with a line of sight in between. Molecular distillation is used industrially for purification of oils.
Short path distillation is a distillation technique that involves the distillate travelling a short distance, often only a few centimeters, and is normally done at reduced pressure.[1]: 150 A classic example would be a distillation involving the distillate travelling from one glass bulb to another, without the need for a condenser separating the two chambers. This technique is often used for compounds which are unstable at high temperatures or to purify small amounts of compound. The advantage is that the heating temperature can be considerably lower (at reduced pressure) than the boiling point of the liquid at standard pressure, and the distillate only has to travel a short distance before condensing. A short path ensures that little compound is lost on the sides of the apparatus. The Kugelrohr apparatus is a kind of short path distillation method which often contains multiple chambers to collect distillate fractions.
Air-sensitive vacuum distillation[edit]
Some compounds have high boiling points as well as being air sensitive. A simple vacuum distillation system as exemplified above can be used, whereby the vacuum is replaced with an inert gas after the distillation is complete. However, this is a less satisfactory system if one desires to collect fractions under a reduced pressure. To do this a "cow" or "pig" adaptor can be added to the end of the condenser, or for better results or for very air sensitive compounds a Perkin triangle apparatus can be used.
The Perkin triangle, has means via a series of glass or Teflon taps to allows fractions to be isolated from the rest of the still, without the main body of the distillation being removed from either the vacuum or heat source, and thus can remain in a state of reflux. To do this, the sample is first isolated from the vacuum by means of the taps, the vacuum over the sample is then replaced with an inert gas (such as nitrogen or argon) and can then be stoppered and removed. A fresh collection vessel can then be added to the system, evacuated and linked back into the distillation system via the taps to collect a second fraction, and so on, until all fractions have been collected.
Zone distillation[edit]
Zone distillation is a distillation process in a long container with partial melting of refined matter in moving liquid zone and condensation of vapor in the solid phase at condensate pulling in cold area. The process is worked in theory. When zone heater is moving from the top to the bottom of the container then solid condensate with irregular impurity distribution is forming. Then most pure part of the condensate may be extracted as product. The process may be iterated many times by moving (without turnover) the received condensate to the bottom part of the container on the place of refined matter. The irregular impurity distribution in the condensate (that is efficiency of purification) increases with the number of iterations. Zone distillation is the distillation analog of zone recrystallization. Impurity distribution in the condensate is described by known equations of zone recrystallization – with the replacement of the distribution co-efficient k of crystallization - for the separation factor α of distillation.[36][37][38]
Closed-system vacuum distillation (cryovap)[edit]
Non-condensable gas can be expelled from the apparatus by the vapor of relatively volatile co-solvent, which spontaneously evaporates during initial pumping, and this can be achieved with regular oil or diaphragm pump.[39][40]
Other types[edit]
- The process of reactive distillation involves using the reaction vessel as the still. In this process, the product is usually significantly lower-boiling than its reactants. As the product is formed from the reactants, it is vaporized and removed from the reaction mixture. This technique is an example of a continuous vs. a batch process; advantages include less downtime to charge the reaction vessel with starting material, and less workup. Distillation "over a reactant" could be classified as a reactive distillation. It is typically used to remove volatile impurity from the distallation feed. For example, a little lime may be added to remove carbon dioxide from water followed by a second distillation with a little sulfuric acid added to remove traces of ammonia.
- Catalytic distillation is the process by which the reactants are catalyzed while being distilled to continuously separate the products from the reactants. This method is used to assist equilibrium reactions in reaching completion.
- Pervaporation is a method for the separation of mixtures of liquids by partial vaporization through a non-porous membrane.
- Extractive distillation is defined as distillation in the presence of a miscible, high boiling, relatively non-volatile component, the solvent, that forms no azeotrope with the other components in the mixture.
- Flash evaporation (or partial evaporation) is the partial vaporization that occurs when a saturated liquid stream undergoes a reduction in pressure by passing through a throttling valve or other throttling device. This process is one of the simplest unit operations, being equivalent to a distillation with only one equilibrium stage.
- Codistillation is distillation which is performed on mixtures in which the two compounds are not miscible. In the laboratory, the Dean-Stark apparatus is used for this purpose to remove water from synthesis products. The Bleidner apparatus is another example with two refluxing solvents.
- Membrane distillation is a type of distillation in which vapors of a mixture to be separated are passed through a membrane, which selectively permeates one component of mixture. Vapor pressure difference is the driving force. It has potential applications in seawater desalination and in removal of organic and inorganic components.
The unit process of evaporation may also be called "distillation":
- In rotary evaporation a vacuum distillation apparatus is used to remove bulk solvents from a sample. Typically the vacuum is generated by a water aspirator or a membrane pump.
- In a Kugelrohr apparatus a short path distillation apparatus is typically used (generally in combination with a (high) vacuum) to distill high boiling (> 300 °C) compounds. The apparatus consists of an oven in which the compound to be distilled is placed, a receiving portion which is outside of the oven, and a means of rotating the sample. The vacuum is normally generated by using a high vacuum pump.
Other uses:
- Dry distillation or destructive distillation, despite the name, is not truly distillation, but rather a chemical reaction known as pyrolysis in which solid substances are heated in an inert or reducing atmosphere and any volatile fractions, containing high-boiling liquids and products of pyrolysis, are collected. The destructive distillation of wood to give methanol is the root of its common name – wood alcohol.
- Freeze distillation is an analogous method of purification using freezing instead of evaporation. It is not truly distillation, but a recrystallization where the product is the mother liquor, and does not produce products equivalent to distillation. This process is used in the production of ice beer and ice wine to increase ethanol and sugar content, respectively. It is also used to produce applejack. Unlike distillation, freeze distillation concentrates poisonous congeners rather than removing them; As a result, many countries prohibit such applejack as a health measure. Also, distillation by evaporation can separate these since they have different boiling points.
- Distillation by filtration: In early alchemy and chemistry, otherwise known as natural philosophy, a form of "distillation" by capillary filtration was known as a form of distillation at the time. In this, a series of cups or bowls were set upon a stepped support with a "wick" of cotton or felt-like material, which had been wetted with water or a clear liquid with each step dripping down through the wetted cloth through capillary action in succeeding steps, creating a "purification" of the liquid, leaving solid materials behind in the upper bowls and purifying the succeeding product through capillary action through the moistened cloth. This was called "distillatio" by filtration by those using the method.
Azeotropic process[edit]
Interactions between the components of the solution create properties unique to the solution, as most processes entail non-ideal mixtures, where Raoult's law does not hold. Such interactions can result in a constant-boiling azeotrope which behaves as if it were a pure compound (i.e., boils at a single temperature instead of a range). At an azeotrope, the solution contains the given component in the same proportion as the vapor, so that evaporation does not change the purity, and distillation does not effect separation. For example, ethyl alcohol and water form an azeotrope of 95.6% at 78.1 °C.
If the azeotrope is not considered sufficiently pure for use, there exist some techniques to break the azeotrope to give a pure distillate. This set of techniques are known as azeotropic distillation. Some techniques achieve this by "jumping" over the azeotropic composition (by adding another component to create a new azeotrope, or by varying the pressure). Others work by chemically or physically removing or sequestering the impurity. For example, to purify ethanol beyond 95%, a drying agent (or desiccant, such as potassium carbonate) can be added to convert the soluble water into insoluble water of crystallization. Molecular sieves are often used for this purpose as well.
Immiscible liquids, such as water and toluene, easily form azeotropes. Commonly, these azeotropes are referred to as a low boiling azeotrope because the boiling point of the azeotrope is lower than the boiling point of either pure component. The temperature and composition of the azeotrope is easily predicted from the vapor pressure of the pure components, without use of Raoult's law. The azeotrope is easily broken in a distillation set-up by using a liquid–liquid separator (a decanter) to separate the two liquid layers that are condensed overhead. Only one of the two liquid layers is refluxed to the distillation set-up.
High boiling azeotropes, such as a 20 percent by weight mixture of hydrochloric acid in water, also exist. As implied by the name, the boiling point of the azeotrope is greater than the boiling point of either pure component.
To break azeotropic distillations and cross distillation boundaries, such as in the DeRosier Problem, it is necessary to increase the composition of the light key in the distillate.
Breaking an azeotrope with unidirectional pressure manipulation[edit]
The boiling points of components in an azeotrope overlap to form a band. By exposing an azeotrope to a vacuum or positive pressure, it's possible to bias the boiling point of one component away from the other by exploiting the differing vapor pressure curves of each; the curves may overlap at the azeotropic point, but are unlikely to remain identical further along the pressure axis to either side of the azeotropic point. When the bias is great enough, the two boiling points no longer overlap and so the azeotropic band disappears.
This method can remove the need to add other chemicals to a distillation, but it has two potential drawbacks.
Under negative pressure, power for a vacuum source is needed and the reduced boiling points of the distillates requires that the condenser be run cooler to prevent distillate vapors being lost to the vacuum source. Increased cooling demands will often require additional energy and possibly new equipment or a change of coolant.
Alternatively, if positive pressures are required, standard glassware can not be used, energy must be used for pressurization and there is a higher chance of side reactions occurring in the distillation, such as decomposition, due to the higher temperatures required to effect boiling.
A unidirectional distillation will rely on a pressure change in one direction, either positive or negative.
Pressure-swing distillation[edit]
Pressure-swing distillation is essentially the same as the unidirectional distillation used to break azeotropic mixtures, but here both positive and negative pressures may be employed.
This improves the selectivity of the distillation and allows a chemist to optimize distillation by avoiding extremes of pressure and temperature that waste energy. This is particularly important in commercial applications.
One example of the application of pressure-swing distillation is during the industrial purification of ethyl acetate after its catalytic synthesis from ethanol.
Industrial process[edit]
Large scale industrial distillation applications include both batch and continuous fractional, vacuum, azeotropic, extractive, and steam distillation. The most widely used industrial applications of continuous, steady-state fractional distillation are in petroleum refineries, petrochemical and chemical plants and natural gas processing plants.
To control and optimize such industrial distillation, a standardized laboratory method, ASTM D86, is established. This test method extends to the atmospheric distillation of petroleum products using a laboratory batch distillation unit to quantitatively determine the boiling range characteristics of petroleum products.
Industrial distillation[33][41] is typically performed in large, vertical cylindrical columns known as distillation towers or distillation columns with diameters ranging from about 0.65 to 16 metres (2 ft 2 in to 52 ft 6 in) and heights ranging from about 6 to 90 metres (20 to 295 ft) or more. When the process feed has a diverse composition, as in distilling crude oil, liquid outlets at intervals up the column allow for the withdrawal of different fractions or products having different boiling points or boiling ranges. The "lightest" products (those with the lowest boiling point) exit from the top of the columns and the "heaviest" products (those with the highest boiling point) exit from the bottom of the column and are often called the bottoms.
Industrial towers use reflux to achieve a more complete separation of products. Reflux refers to the portion of the condensed overhead liquid product from a distillation or fractionation tower that is returned to the upper part of the tower as shown in the schematic diagram of a typical, large-scale industrial distillation tower. Inside the tower, the downflowing reflux liquid provides cooling and condensation of the upflowing vapors thereby increasing the efficiency of the distillation tower. The more reflux that is provided for a given number of theoretical plates, the better the tower's separation of lower boiling materials from higher boiling materials. Alternatively, the more reflux that is provided for a given desired separation, the fewer the number of theoretical plates required. Chemical engineers must choose what combination of reflux rate and number of plates is both economically and physically feasible for the products purified in the distillation column.
Such industrial fractionating towers are also used in cryogenic air separation, producing liquid oxygen, liquid nitrogen, and high purity argon. Distillation of chlorosilanes also enables the production of high-purity silicon for use as a semiconductor.
Design and operation of a distillation tower depends on the feed and desired products. Given a simple, binary component feed, analytical methods such as the McCabe–Thiele method[33][42] or the Fenske equation[33] can be used. For a multi-component feed, simulationmodels are used both for design and operation. Moreover, the efficiencies of the vapor–liquid contact devices (referred to as "plates" or "trays") used in distillation towers are typically lower than that of a theoretical 100% efficient equilibrium stage. Hence, a distillation tower needs more trays than the number of theoretical vapor–liquid equilibrium stages. A variety of models have been postulated to estimate tray efficiencies.
In modern industrial uses, a packing material is used in the column instead of trays when low pressure drops across the column are required. Other factors that favor packing are: vacuum systems, smaller diameter columns, corrosive systems, systems prone to foaming, systems requiring low liquid holdup, and batch distillation. Conversely, factors that favor plate columns are: presence of solids in feed, high liquid rates, large column diameters, complex columns, columns with wide feed composition variation, columns with a chemical reaction, absorption columns, columns limited by foundation weight tolerance, low liquid rate, large turn-down ratio and those processes subject to process surges.
This packing material can either be random dumped packing (25–76 millimetres (1–3 in) wide) such as Raschig rings or structured sheet metal. Liquids tend to wet the surface of the packing and the vapors pass across this wetted surface, where mass transfertakes place. Unlike conventional tray distillation in which every tray represents a separate point of vapor–liquid equilibrium, the vapor–liquid equilibrium curve in a packed column is continuous. However, when modeling packed columns, it is useful to compute a number of "theoretical stages" to denote the separation efficiency of the packed column with respect to more traditional trays. Differently shaped packings have different surface areas and void space between packings. Both of these factors affect packing performance.
Another factor in addition to the packing shape and surface area that affects the performance of random or structured packing is the liquid and vapor distribution entering the packed bed. The number of theoretical stages required to make a given separation is calculated using a specific vapor to liquid ratio. If the liquid and vapor are not evenly distributed across the superficial tower area as it enters the packed bed, the liquid to vapor ratio will not be correct in the packed bed and the required separation will not be achieved. The packing will appear to not be working properly. The height equivalent to a theoretical plate (HETP) will be greater than expected. The problem is not the packing itself but the mal-distribution of the fluids entering the packed bed. Liquid mal-distribution is more frequently the problem than vapor. The design of the liquid distributors used to introduce the feed and reflux to a packed bed is critical to making the packing perform to it maximum efficiency. Methods of evaluating the effectiveness of a liquid distributor to evenly distribute the liquid entering a packed bed can be found in references.[44][45] Considerable work has been done on this topic by Fractionation Research, Inc. (commonly known as FRI).[46]
Multi-effect distillation[edit]
The goal of multi-effect distillation is to increase the energy efficiency of the process, for use in desalination, or in some cases one stage in the production of ultrapure water. The number of effects is inversely proportional to the kW·h/m3 of water recovered figure, and refers to the volume of water recovered per unit of energy compared with single-effect distillation. One effect is roughly 636 kW·h/m3.
- Multi-stage flash distillation can achieve more than 20 effects with thermal energy input, as mentioned in the article.
- Vapor compression evaporation – Commercial large-scale units can achieve around 72 effects with electrical energy input, according to manufacturers.
There are many other types of multi-effect distillation processes, including one referred to as simply multi-effect distillation (MED), in which multiple chambers, with intervening heat exchangers, are employed.
In food processing[edit]
Beverages[edit]
Carbohydrate-containing plant materials are allowed to ferment, producing a dilute solution of ethanol in the process. Spirits such as whiskey and rum are prepared by distilling these dilute solutions of ethanol. Components other than ethanol, including water, esters, and other alcohols, are collected in the condensate, which account for the flavor of the beverage. Some of these beverages are then stored in barrels or other containers to acquire more flavor compounds and characteristic flavors.
Gallery[edit]
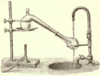 | Chemistry in its beginnings used retorts as laboratory equipment exclusively for distillation processes. |
 | A simple set-up to distill dry and oxygen-free toluene. |
 | Diagram of an industrial-scale vacuum distillation column as commonly used in oil refineries |
 | A rotary evaporator is able to distill solvents more quickly at lower temperatures through the use of a vacuum. |
 | Distillation using semi-microscale apparatus. The jointless design eliminates the need to fit pieces together. The pear-shaped flask allows the last drop of residue to be removed, compared with a similarly-sized round-bottom flask. The small holdup volume prevents losses. A "pig" is used to channel the various distillates into three receiving flasks. If necessary the distillation can be carried out under vacuum using the vacuum adapter at the pig. |
See also[edit]
- Atmospheric distillation of crude oil
- Clyssus
- Fragrance extraction
- Low-temperature distillation
- Microdistillery
- Sublimation
- Dixon rings
- Random column packing
https://en.wikipedia.org/wiki/Distillation
A separation process is a method that converts a mixture or solution of chemical substances into two or more distinct product mixtures.[1] In other words, it's a scientific process of distinguishing to two or more substance in order to obtain purity. At least one product mixture of the separation is enriched in one or more of the source mixture's constituents. In some cases, a separation may fully divide the mixture into pure constituents. Separations exploit differences in chemical properties or physical properties (such as size, shape, mass, density, or chemical affinity) between the constituents of a mixture.
Processes are often classified according to the particular differences they use to achieve separation. If no single difference can be used to accomplish the desired separation, multiple operations can often be combined to achieve the desired end.
With a few exceptions, elements or compounds exist in nature in an impure state. Often these raw materials must go through a separation before they can be put to productive use, making separation techniques essential for the modern industrial economy.
The purpose of separation may be analytical, i.e. to identify the size of each fraction of a mixture is attributable to each component without attempting to harvest the fractions. The purpose of a separation may maybe preparative, i.e. to "prepare" fractions for input into processes that benefit when components are separated.
Separations may be performed on a small scale, as in a laboratory for analytical purposes. Separations may also be performed on a large scale, as in a chemical plant.
Complete and incomplete separation[edit]
Some types of separation require complete purification of a certain component. An example is the production of aluminum metal from bauxite ore through electrolysis refining. In contrast, an incomplete separation process may specify an output to consist of a mixture instead of a single pure component. A good example of an incomplete separation technique is oil refining. Crude oil occurs naturally as a mixture of various hydrocarbons and impurities. The refining process splits this mixture into other, more valuable mixtures such as natural gas, gasoline and chemical feedstocks, none of which are pure substances, but each of which must be separated from the raw crude.
In both cases of complete and incomplete separation, a series of separations may be necessary to obtain the desired end products. In the case of oil refining, crude is subjected to a long series of individual distillation steps, each of which produces a different product or intermediate.
List of separation techniques[edit]
- Sponge, adhesion of atoms, ions or molecules of gas, liquid, or dissolved solids to a surface
- Centrifugation and cyclonic separation, separates based on density differences
- Chelation
- Filtration
- Centrifugation
Chromatography[edit]
Chromatography separates dissolved substances by different interaction with (i.e., travel through) a material.
- High-performance liquid chromatography (HPLC)
- Thin-layer chromatography (TLC)
- Countercurrent chromatography (CCC)
- Droplet countercurrent chromatography (DCC)
- Paper chromatography
- Ion chromatography
- Size-exclusion chromatography
- Affinity chromatography
- Centrifugal partition chromatography
- Gas chromatography and Inverse gas chromatography
- Crystallization
- Decantation
- Demister (vapor), removes liquid droplets from gas streams
- Distillation, used for mixtures of liquids with different boiling points
- Drying, removes liquid from a solid by vaporization or evaporation
Electrophoresis[edit]
Electrophoresis, separates organic molecules based on their different interaction with a gel under an electric potential (i.e., different travel)
- Electrostatic separation, works on the principle of corona discharge, where two plates are placed close together and high voltage is applied. This high voltage is used to separate the ionized particles.
- Elutriation
- Evaporation
Extraction[edit]
Flotation[edit]
- Flotation
- Dissolved air flotation, removes suspended solids non-selectively from slurry by bubbles that are generated by air coming out of solution
- Froth flotation, recovers valuable, hydrophobic solids by attachment to air bubbles generated by mechanical agitation of an air-slurry mixture, which floats, and are recovered
- Deinking, separating hydrophobic ink particles from the hydrophilic paper pulp in paper recycling
- Flocculation, separates a solid from a liquid in a colloid, by use of a flocculant, which promotes the solid clumping into flocs
- Filtration – Mesh, bag and paper filters are used to remove large particulates suspended in fluids (e.g., fly ash) while membrane processes including microfiltration, ultrafiltration, nanofiltration, reverse osmosis, dialysis (biochemistry) utilising synthetic membranes, separates micrometre-sized or smaller species
- Fractional distillation
- Fractional freezing
- Oil-water separation, gravimetrically separates suspended oil droplets from waste water in oil refineries, petrochemical and chemical plants, natural gas processingplants and similar industries
- Magnetic separation
- Precipitation
- Recrystallization
- Scrubbing, separation of particulates (solids) or gases from a gas stream using liquid.
- Sedimentation, separates using vocal density pressure differences
- Sieving
- Stripping
- Sublimation
- Vapor-liquid separation, separates by gravity, based on the Souders-Brown equation
- Winnowing
- Zone refining
See also[edit]
- Analytical chemistry – Study of the separation, identification, and quantification of the chemical components of materials
- High-performance liquid chromatography – Technique used in analytical chemistry
- Unit operation
- Filtration – Process that separates solids from fluids
https://en.wikipedia.org/wiki/Separation_process
Category:Unit operations
Unit operations is the terminology that is used to describe the individual pieces/sub systems of Chemical Engineering.
Subcategories
This category has the following 2 subcategories, out of 2 total.
P
S
Pages in category "Unit operations"
The following 19 pages are in this category, out of 19 total. This list may not reflect recent changes (learn more).
https://en.wikipedia.org/wiki/Category:Unit_operations
Fins are extensions on exterior surfaces of objects that increase the rate of heat transfer to or from the object by increasing convection. This is achieved by increasing the surface area of the body, which in turn increases the heat transfer rate by a sufficient degree. This is an efficient way of increasing the rate, since the alternative way of doing so is by increasing either the heat transfer coefficient (which depends on the nature of materials being used and the conditions of use) or the temperature gradient (which depends on the conditions of use). Clearly, changing the shape of the bodies is more convenient. Fins are therefore a very popular solution to increase the heat transfer from surfaces and are widely used in a number of objects. The fin material should preferably have high thermal conductivity. In most applications the fin is surrounded by a fluid in motion,[1] which heats or cools it quickly due to the large surface area, and subsequently the heat gets transferred to or from the body quickly due to the high thermal conductivity of the fin. For optimal Heat transfer performance with minimal cost, the dimensions and shape of the fin have to be calculated for specific applications, and this is called design of a fin. A common way of doing so is by creating a model of the fin and then simulating it under required service conditions.
https://en.wikipedia.org/wiki/Heat_transfer_through_fins
In centrifugation the clearing factor or k factor represents the relative pelleting efficiency of a given centrifuge rotor at maximum rotation speed. It can be used to estimate the time (in hours) required for sedimentation of a fraction with a known sedimentation coefficient (in svedbergs):
The value of the clearing factor depends on the maximum angular velocity of a centrifuge (in rad/s) and the minimum and maximum radius of the rotor:
As the rotational speed of a centrifuge is usually specified in RPM, the following formula is often used for convenience:[1]
Centrifuge manufacturers usually specify the minimum, maximum and average radius of a rotor, as well as the factor of a centrifuge-rotor combination.
For runs with a rotational speed lower than the maximum rotor-speed, the factor has to be adjusted:
- 2
The K-factor is related to the sedimentation coefficient by the formula:
Where is the time to pellet a certain particle in hours. Since is a constant for a certain particle, this relationship can be used to interconvert between different rotors.
Where is the time to pellet in one rotor, and is the K-factor of that rotor. is the K-factor of the other rotor, and , the time to pellet in the other rotor, can be calculated. In this manner, one does not need access to the exact rotor cited in a protocol, as long as the K-factor can be calculated. Many online calculators are available to perform the calculations for common rotors.
References[edit]
https://en.wikipedia.org/wiki/Clearing_factor
Pultrusion is a continuous process for manufacture of fibre-reinforced plastics with constant cross-section. The term is a portmanteau word, combining "pull" and "extrusion". As opposed to extrusion, which pushes the material, pultrusion pulls the material.
A very early pultrusions type patent was filed by J.H. Watson in 1944. This was followed by M.J. Meek's filing of 1950. The first commercial pultrusions were provided by Glastic Company of Cleveland, Ohio under the patent filed in 1952 by Rodger B. White. The patent issued to W. B. Goldsworthy in 1959 helped initiate the promotion and knowledge spread within the industry. W. Brandt Goldsworthy is widely regarded as the inventor of pultrusion.[1]
Parallel to the work of Goldsworthy, who concentrated his work on unsaturated polyester resins, Ernst Kühne in Germany developed a quite similar process in 1954 based on epoxy resin.
Invention, development and the issuance of patents continue in the pultrusion field through today. A later innovation in this field has been developed and patented by Thomas GmbH + Co. Technik + Innovation KG in Germany 2008 and is described below.
https://en.wikipedia.org/wiki/Pultrusion
Aqueous biphasic systems (ABS) or aqueous two-phase systems (ATPS) are clean alternatives for traditional organic-water solvent extraction systems.
ABS are formed when either two polymers, one polymer and one kosmotropic salt, or two salts (one chaotropic salt and the other a kosmotropic salt) are mixed at appropriate concentrations or at a particular temperature. The two phases are mostly composed of water and non volatile components, thus eliminating volatile organic compounds. They have been used for many years in biotechnological applications as non-denaturing and benign separation media. Recently, it has been found that ATPS can be used for separations of metal ions like mercury and cobalt,[1] carbon nanotubes,[2][3][4] environmental remediation, metallurgical applications and as a reaction media.
https://en.wikipedia.org/wiki/Aqueous_two-phase_system
Homogenization or homogenisation is any of several processes used to make a mixture of two mutually non-soluble liquids the same throughout. This is achieved by turning one of the liquids into a state consisting of extremely small particles distributed uniformly throughout the other liquid. A typical example is the homogenization of milk, wherein the milk fat globules are reduced in size and dispersed uniformly through the rest of the milk.[1]
https://en.wikipedia.org/wiki/Homogenization_(chemistry)
Extrusion is a process used to create objects of a fixed cross-sectional profile by pushing material through a die of the desired cross-section. Its two main advantages over other manufacturing processes are its ability to create very complex cross-sections; and to work materials that are brittle, because the material encounters only compressive and shear stresses. It also creates excellent surface finish and gives considerable freedom of form in the design process.[1]
Drawing is a similar process, using the tensile strength of the material to pull it through the die. It limits the amount of change that can be performed in one step, so it is limited to simpler shapes, and multiple stages are usually needed. Drawing is the main way to produce wire. Metal bars and tubes are also often drawn.
Extrusion may be continuous (theoretically producing indefinitely long material) or semi-continuous (producing many pieces). It can be done with hot or cold material. Commonly extruded materials include metals, polymers, ceramics, concrete, modelling clay, and foodstuffs. Products of extrusion are generally called extrudates.
Also referred to as "hole flanging", hollow cavities within extruded material cannot be produced using a simple flat extrusion die, because there would be no way to support the centre barrier of the die. Instead, the die assumes the shape of a block with depth, beginning first with a shape profile that supports the center section. The die shape then internally changes along its length into the final shape, with the suspended center pieces supported from the back of the die. The material flows around the supports and fuses to create the desired closed shape.
The extrusion of metals can also increase their strength.
https://en.wikipedia.org/wiki/Extrusion
Pasteurization or pasteurisation is a process in which packaged and non-packaged foods (such as milk and fruit juices) are treated with mild heat, usually to less than 100 °C (212 °F), to eliminate pathogens and extend shelf life. The process is intended to destroy or deactivate organisms and enzymes that contribute to spoilage or risk of disease, including vegetative bacteria, but not bacterial spores.[1][2]
The process was named after the French microbiologist, Louis Pasteur, whose research in the 1860s demonstrated that thermal processing would deactivate unwanted microorganisms in wine.[2][3] Spoilage enzymes are also inactivated during pasteurization. Today, pasteurization is used widely in the dairy industry and other food processing industries to achieve food preservation and food safety.[3]
By the year 1999, most liquid products were heat treated in a continuous system where heat can be applied using a plate heat exchanger or the direct or indirect use of hot water and steam. Due to the mild heat, there are minor changes to the nutritional quality and sensory characteristics of the treated foods.[4] Pascalization or high pressure processing (HPP) and pulsed electric field (PEF) are non-thermal processes that are also used to pasteurize foods.[1]
https://en.wikipedia.org/wiki/Pasteurization
Vapor-compression evaporation is the evaporation method by which a blower, compressor or jet ejector is used to compress, and thus, increase the pressure of the vapor produced. Since the pressure increase of the vapor also generates an increase in the condensation temperature, the same vapor can serve as the heating medium for its "mother" liquid or solution being concentrated, from which the vapor was generated to begin with. If no compression was provided, the vapor would be at the same temperature as the boiling liquid/solution, and no heat transfer could take place.
It is also sometimes called vapor compression distillation (VCD). If compression is performed by a mechanically driven compressor or blower, this evaporation process is usually referred to as MVR (mechanical vapor recompression). In case of compression performed by high pressure motive steam ejectors, the process is usually called thermocompression, steam compression or ejectocompression.
https://en.wikipedia.org/wiki/Vapor-compression_evaporation
Micronization is the process of reducing the average diameter of a solid material's particles. Traditional techniques for micronization focus on mechanical means, such as milling and grinding. Modern techniques make use of the properties of supercritical fluids and manipulate the principles of solubility.
The term micronization usually refers to the reduction of average particle diameters to the micrometer range, but can also describe further reduction to the nanometerscale. Common applications include the production of active chemical ingredients, foodstuff ingredients, and pharmaceuticals. These chemicals need to be micronized to increase efficacy.
Traditional techniques[edit]
Traditional micronization techniques are based on friction to reduce particle size. Such methods include milling, bashing and grinding. A typical industrial mill is composed of a cylindrical metallic drum that usually contains steel spheres. As the drum rotates the spheres inside collide with the particles of the solid, thus crushing them towards smaller diameters. In the case of grinding, the solid particles are formed when the grinding units of the device rub against each other while particles of the solid are trapped in between.
Methods like crushing and cutting are also used for reducing particle diameter, but produce more rough particles compared to the two previous techniques (and are therefore the early stages of the micronization process). Crushing employs hammer-like tools to break the solid into smaller particles by means of impact. Cutting uses sharp blades to cut the rough solid pieces into smaller ones.
Modern techniques[edit]
Modern methods use supercritical fluids in the micronization process. These methods use supercritical fluids to induce a state of supersaturation, which leads to precipitation of individual particles. The most widely applied techniques of this category include the RESS process (Rapid Expansion of Supercritical Solutions), the SAS method (Supercritical Anti-Solvent) and the PGSS method (Particles from Gas Saturated Solutions). These modern techniques allow for greater tuneability of the process. Parameters like relative pressure and temperature, solute concentration, and antisolvent to solvent ratio are varied to adjust the output to the producer's needs. The supercritical fluid methods result in finer control over particle diameters, distribution of particle size and consistency of morphology.[1][2][3] Because of the relatively low pressure involved, many supercritical fluid methods can incorporate thermolabile materials. Modern techniques involve renewable, nonflammable and nontoxic chemicals.[4]
RESS[edit]
In the case of RESS (Rapid Expansion of Supercritical Solutions), the supercritical fluid is used to dissolve the solid material under high pressure and temperature, thus forming a homogeneous supercritical phase. Thereafter, the mixture is expanded through a nozzle to form the smaller particles. Immediately upon exiting the nozzle, rapid expansion occurs, lowering the pressure. The pressure will drop below supercritical pressure, causing the supercritical fluid - usually carbon dioxide - to return to the gas state. This phase change severely decreases the solubility of the mixture and results in precipitation of particles.[5] The less time it takes the solution to expand and the solute to precipitate, the narrower the particle size distribution will be. Faster precipitation times also tend to result in smaller particle diameters.[6]
SAS[edit]
In the SAS method (Supercritical Anti-Solvent), the solid material is dissolved in an organic solvent. The supercritical fluid is then added as an antisolvent, which decreases the solubility of the system. As a result, particles of small diameter are formed.[3] There are various submethods to SAS which differ in the method of introduction of the supercritical fluid into the organic solution.[7]
PGSS[edit]
In the PGSS method (Particles from Gas Saturated Solutions) the solid material is melted and the supercritical fluid is dissolved in it.[8] However, in this case the solution is forced to expand through a nozzle, and in this way nanoparticles are formed. The PGSS method has the advantage that because of the supercritical fluid, the melting point of the solid material is reduced. Therefore, the solid melts at a lower temperature than the normal melting temperature at ambient pressure.
Applications[edit]
Pharmaceuticals and foodstuff ingredients are the main industries in which micronization is utilized. Particles with reduced diameters have higher dissolution rates, which increases efficacy.[4] Progesterone, for example, can be micronized by making very tiny crystals of the progesterone.[9] Micronized progesterone is manufactured in a laboratory from plants. It is available for use as HRT, infertility treatment, treat progesterone deficiency treatment, including dysfunctional uterine bleeding in premenopausal women. Compounding pharmacies can supply micronized progesterone in sublingual tablets, oil caps, or transdermal creams.[10] Creatine is among the other drugs that are micronized.[6]
https://en.wikipedia.org/wiki/Micronization
Shear strength is a term used in soil mechanics to describe the magnitude of the shear stress that a soil can sustain. The shear resistance of soil is a result of friction and interlocking of particles, and possibly cementation or bonding at particle contacts. Due to interlocking, particulate material may expand or contract in volume as it is subject to shear strains. If soil expands its volume, the density of particles will decrease and the strength will decrease; in this case, the peak strength would be followed by a reduction of shear stress. The stress-strain relationship levels off when the material stops expanding or contracting, and when interparticle bonds are broken. The theoretical state at which the shear stress and density remain constant while the shear strain increases may be called the critical state, steady state, or residual strength.
The volume change behavior and interparticle friction depend on the density of the particles, the intergranular contact forces, and to a somewhat lesser extent, other factors such as the rate of shearing and the direction of the shear stress. The average normal intergranular contact force per unit area is called the effective stress.
If water is not allowed to flow in or out of the soil, the stress path is called an undrained stress path. During undrained shear, if the particles are surrounded by a nearly incompressible fluid such as water, then the density of the particles cannot change without drainage, but the water pressure and effective stress will change. On the other hand, if the fluids are allowed to freely drain out of the pores, then the pore pressures will remain constant and the test path is called a drained stress path. The soil is free to dilate or contract during shear if the soil is drained. In reality, soil is partially drained, somewhere between the perfectly undrained and drained idealized conditions.
The shear strength of soil depends on the effective stress, the drainage conditions, the density of the particles, the rate of strain, and the direction of the strain.
For undrained, constant volume shearing, the Tresca theory may be used to predict the shear strength, but for drained conditions, the Mohr–Coulomb theory may be used.
Two important theories of soil shear are the critical state theory and the steady state theory. There are key differences between the critical state condition and the steady state condition and the resulting theory corresponding to each of these conditions.
Factors controlling shear strength of soils[edit]
The stress-strain relationship of soils, and therefore the shearing strength, is affected (Poulos 1989) by:
- soil composition (basic soil material): mineralogy, grain size and grain size distribution, shape of particles, pore fluid type and content, ions on grain and in pore fluid.
- state (initial): Defined by the initial void ratio, effective normal stress and shear stress (stress history). State can be described by terms such as: loose, dense, overconsolidated, normally consolidated, stiff, soft, contractive, dilative, etc.
- structure: Refers to the arrangement of particles within the soil mass; the manner the particles are packed or distributed. Features such as layers, joints, fissures, slickensides, voids, pockets, cementation, etc., are part of the structure. Structure of soils is described by terms such as: undisturbed, disturbed, remolded, compacted, cemented; flocculent, honey-combed, single-grained; flocculated, deflocculated; stratified, layered, laminated; isotropic and anisotropic.
- Loading conditions: Effective stress path, i.e., drained, and undrained; and type of loading, i.e., magnitude, rate (static, dynamic), and time history (monotonic, cyclic).
https://en.wikipedia.org/wiki/Shear_strength_(soil)
Critical state soil mechanics is the area of soil mechanics that encompasses the conceptual models that represent the mechanical behavior of saturated remolded soils based on the Critical State concept.
https://en.wikipedia.org/wiki/Critical_state_soil_mechanics
Soil mechanics is a branch of soil physics and applied mechanics that describes the behavior of soils. It differs from fluid mechanics and solid mechanics in the sense that soils consist of a heterogeneous mixture of fluids (usually air and water) and particles (usually clay, silt, sand, and gravel) but soil may also contain organic solids and other matter.[1][2][3][4] Along with rock mechanics, soil mechanics provides the theoretical basis for analysis in geotechnical engineering,[5] a subdiscipline of civil engineering, and engineering geology, a subdiscipline of geology. Soil mechanics is used to analyze the deformations of and flow of fluids within natural and man-made structures that are supported on or made of soil, or structures that are buried in soils.[6] Example applications are building and bridge foundations, retaining walls, dams, and buried pipeline systems. Principles of soil mechanics are also used in related disciplines such as geophysical engineering, coastal engineering, agricultural engineering, hydrology and soil physics.
This article describes the genesis and composition of soil, the distinction between pore water pressure and inter-granular effective stress, capillary action of fluids in the soil pore spaces, soil classification, seepage and permeability, time dependent change of volume due to squeezing water out of tiny pore spaces, also known as consolidation, shear strength and stiffness of soils. The shear strength of soils is primarily derived from friction between the particles and interlocking, which are very sensitive to the effective stress.[7][6] The article concludes with some examples of applications of the principles of soil mechanics such as slope stability, lateral earth pressure on retaining walls, and bearing capacity of foundations.
https://en.wikipedia.org/wiki/Soil_mechanics
A triaxial shear test is a common method to measure the mechanical properties of many deformable solids, especially soil(e.g., sand, clay) and rock, and other granular materials or powders. There are several variations on the test.[1][2][3][4]
In a triaxial shear test, stress is applied to a sample of the material being tested in a way which results in stresses along one axis being different from the stresses in perpendicular directions. This is typically achieved by placing the sample between two parallel platens which apply stress in one (usually vertical) direction, and applying fluid pressure to the specimen to apply stress in the perpendicular directions. (Testing apparatus which allows application of different levels of stress in each of three orthogonal directions are discussed below, under "True Triaxial test".)
The application of different compressive stresses in the test apparatus causes shear stress to develop in the sample; the loads can be increased and deflections monitored until failure of the sample. During the test, the surrounding fluid is pressurized, and the stress on the platens is increased until the material in the cylinder fails and forms sliding regions within itself, known as shear bands. The geometry of the shearing in a triaxial test typically causes the sample to become shorter while bulging out along the sides. The stress on the platen is then reduced and the water pressure pushes the sides back in, causing the sample to grow taller again. This cycle is usually repeated several times while collecting stress and strain data about the sample. During the test the pore pressures of fluids (e.g., water, oil) or gasses in the sample may be measured using Bishop's pore pressure apparatus.
From the triaxial test data, it is possible to extract fundamental material parameters about the sample, including its angle of shearing resistance, apparent cohesion, and dilatancy angle. These parameters are then used in computer models to predict how the material will behave in a larger-scale engineering application. An example would be to predict the stability of the soil on a slope, whether the slope will collapse or whether the soil will support the shear stresses of the slope and remain in place. Triaxial tests are used along with other tests to make such engineering predictions.
During the shearing, a granular material will typically have a net gain or loss of volume. If it had originally been in a dense state, then it typically gains volume, a characteristic known as Reynolds' dilatancy. If it had originally been in a very loose state, then contraction may occur before the shearing begins or in conjunction with the shearing.
Sometimes, testing of cohesive samples is done with no confining pressure, in an unconfined compression test. This requires much simpler and less expensive apparatus and sample preparation, though the applicability is limited to samples that the sides won't crumble when exposed, and the confining stress being lower than the in-situ stress gives results which may be overly conservative. The compression test performed for concrete strength testing is essentially the same test, on apparatus designed for the larger samples and higher loads typical of concrete testing.
https://en.wikipedia.org/wiki/Triaxial_shear_test
The shear strength of a discontinuity in a soil or rock mass may have a strong impact on the mechanical behavior of a soil or rock mass.[1][2][3][4][5][6] The shear strength of a discontinuity is often considerably lower than the shear strength of the blocks of intact material in between the discontinuities, and therefore influences, for example, tunnel, foundation, or slope engineering, but also the stability of natural slopes. Many slopes, natural and man-made, fail due to a low shear strength of discontinuities in the soil or rock mass in the slope. The deformation characteristics of a soil or rock mass are also influenced by the shear strength of the discontinuities. For example, the modulus of deformation is reduced, and the deformation becomes plastic (i.e. non-reversible deformation on reduction of stress) rather than elastic (i.e. reversible deformation). This may cause, for example, larger settlement of foundations, which is also permanent even if the load is only temporary. Furthermore, the shear strength of discontinuities influences the stress distribution in a soil or rock mass.[7]
https://en.wikipedia.org/wiki/Shear_strength_(discontinuity)
Soil consolidation refers to the mechanical process by which soil changes volume gradually in response to a change in pressure. This happens because soil is a two-phase material, comprising soil grains and pore fluid, usually groundwater. When soil saturated with water is subjected to an increase in pressure, the high volumetric stiffness of water compared to the soil matrix means that the water initially absorbs all the change in pressure without changing volume, creating excess pore water pressure. As water diffuses away from regions of high pressure due to seepage, the soil matrix gradually takes up the pressure change and shrinks in volume. The theoretical framework of consolidation is therefore closely related to the diffusion equation, the concept of effective stress, and hydraulic conductivity.
In the narrow sense, "consolidation" refers strictly to this delayed volumetric response to pressure change due to gradual movement of water. Some publications also use "consolidation" in the broad sense, to refer to any process by which soil changes volume due to a change in applied pressure. This broader definition encompasses the overall concept of soil compaction, subsidence, and heave. Some types of soil, mainly those rich in organic matter, show significant creep, whereby the soil changes volume slowly at constant effective stress over a longer time-scale than consolidation due to the diffusion of water. To distinguish between the two mechanisms, "primary consolidation" refers to consolidation due to dissipation of excess water pressure, while "secondary consolidation" refers to the creep process.
The effects of consolidation are most conspicuous where a building sits over a layer of soil with low stiffness and low permeability, such as marine clay, leading to large settlement over many years. Types of construction project where consolidation often poses technical risk include land reclamation, the construction of embankments, and tunnel and basement excavation in clay.
Geotechnical engineers use oedometers to quantify the effects of consolidation. In an oedometer test, a series of known pressures are applied to a thin disc of soil sample, and the change of sample thickness with time is recorded. This allows the consolidation characteristics of the soil to be quantified in terms of the coefficient of consolidation () and hydraulic conductivity ().
Clays undergo consolidation settlement not only the action action of external loads (surcharge loads) but also under its own weight or weight of soils that exist above the clay.
Clays also undergo settlement when dewatered (groundwater pumping) because the effective stress on the clay increases.
Coarse-grained soils do not undergo consolidation settlement due to relativity high hydraulic conductivity compared to clays. Instead, Coarse-grained soils undergo the immediate settlement.
https://en.wikipedia.org/wiki/Soil_consolidation
Preconsolidation pressure is the maximum effective vertical overburden stress that a particular soil sample has sustained in the past.[1] This quantity is important in geotechnical engineering, particularly for finding the expected settlement of foundations and embankments. Alternative names for the preconsolidation pressure are preconsolidation stress, pre-compression stress, pre-compaction stress, and preload stress.[2] A soil is called overconsolidated if the current effective stress acting on the soil is less than the historical maximum.
The preconsolidation pressure can help determine the largest overburden pressure that can be exerted on a soil without irrecoverable volume change. This type of volume change is important for understanding shrinkage behavior, crack and structure formation and resistance to shearing stresses.[3] Previous stresses and other changes in a soil's history are preserved within the soil's structure.[4] If a soil is loaded beyond this point the soil is unable to sustain the increased load and the structure will break down.[4] This breakdown can cause a number of different things depending on the type of soil and its geologic history.
Preconsolidation pressure cannot be measured directly, but can be estimated using a number of different strategies. Samples taken from the field are subjected to a variety of tests, like the constant rate of strain test (CRS) or the incremental loading test (IL). These tests can be costly due to expensive equipment and the long period of time they require. Each sample must be undisturbed and can only undergo one test with satisfactory results.[5] It is important to execute these tests precisely to ensure an accurate resulting plot. There are various methods for determining the preconsolidation pressure from lab data. The data is usually arranged on a semilog plot of the effective stress (frequently represented as σ'vc) versus the void ratio. This graph is commonly called the e log p curve or the consolidation curve.
https://en.wikipedia.org/wiki/Preconsolidation_pressure
Pore water pressure (sometimes abbreviated to pwp) refers to the pressure of groundwater held within a soil or rock, in gaps between particles (pores). Pore water pressures below the phreatic level of the groundwater are measured with piezometers. The vertical pore water pressure distribution in aquifers can generally be assumed to be close to hydrostatic.
In the unsaturated ("vadose") zone, the pore pressure is determined by capillarity and is also referred to as tension, suction, or matric pressure. Pore water pressures under unsaturated conditions are measured with tensiometers, which operate by allowing the pore water to come into equilibrium with a reference pressure indicator through a permeable ceramic cup placed in contact with the soil.
Pore water pressure is vital in calculating the stress state in the ground soil mechanics, from Terzaghi's expression for the effective stress of a soil.
https://en.wikipedia.org/wiki/Pore_water_pressure
Hydraulic conductivity, symbolically represented as K (unit: m/s), is a property of porous materials, soils and rocks, that describes the ease with which a fluid (usually water) can move through the pore space, or fractures network. It depends on the intrinsic permeability (k, unit: m2) of the material, the degree of saturation, and on the density and viscosity of the fluid. Saturated hydraulic conductivity, Ksat, describes water movement through saturated media. By definition, hydraulic conductivity is the ratio of volume flux to hydraulic gradient yielding a quantitative measure of a saturated soil's ability to transmit water when subjected to a hydraulic gradient.
https://en.wikipedia.org/wiki/Hydraulic_conductivity
Drainage is the natural or artificial removal of a surface's water and sub-surface water from an area with excess of water. The internal drainage of most agricultural soils is good enough to prevent severe waterlogging (anaerobic conditions that harm root growth), but many soils need artificial drainage to improve production or to manage water supplies.
https://en.wikipedia.org/wiki/Drainage
Grain size (or particle size) is the diameter of individual grains of sediment, or the lithified particles in clastic rocks. The term may also be applied to other granular materials. This is different from the crystallite size, which refers to the size of a single crystal inside a particle or grain. A single grain can be composed of several crystals. Granular material can range from very small colloidal particles, through clay, silt, sand, gravel, and cobbles, to boulders.
https://en.wikipedia.org/wiki/Grain_size
The table lists various objects and units by the order of magnitude of their volume.
https://en.wikipedia.org/wiki/Orders_of_magnitude_(volume)
https://en.wikipedia.org/wiki/Unified_Soil_Classification_System
https://en.wikipedia.org/wiki/International_unit
https://en.wikipedia.org/wiki/International_System_of_Units
https://en.wikipedia.org/wiki/Scale_(analytical_tool)
https://en.wikipedia.org/wiki/Scale_(chemistry)
https://en.wikipedia.org/wiki/Scale_(anatomy)
https://en.wikipedia.org/wiki/Scale_(insect_anatomy)
https://en.wikipedia.org/wiki/Weighing_scale
https://en.wikipedia.org/wiki/Vernier_scale
https://en.wikipedia.org/wiki/Spatial_scale
https://en.wikipedia.org/wiki/Time_scale
https://en.wikipedia.org/wiki/Geologic_time_scale
https://en.wikipedia.org/wiki/Nuclear_timescale
https://en.wikipedia.org/wiki/Rhythm
https://en.wikipedia.org/wiki/Pattern
https://en.wikipedia.org/wiki/Façade
https://en.wikipedia.org/wiki/Loanword
https://en.wikipedia.org/wiki/Metaphor
https://en.wikipedia.org/wiki/Language
https://en.wikipedia.org/wiki/Cognate
https://en.wikipedia.org/wiki/Calque
https://en.wikipedia.org/wiki/Rhetoric
https://en.wikipedia.org/wiki/Literal_translation
https://en.wikipedia.org/wiki/Linguistics
https://en.wikipedia.org/wiki/Metaphrase
https://en.wikipedia.org/wiki/Semantics
https://en.wikipedia.org/wiki/Syntax
https://en.wikipedia.org/wiki/Diction
https://en.wikipedia.org/wiki/Connotation
https://en.wikipedia.org/wiki/Denotation
https://en.wikipedia.org/wiki/Pragmatics
https://en.wikipedia.org/wiki/Context_(language_use)
https://en.wikipedia.org/wiki/Content
https://en.wikipedia.org/wiki/Register_(sociolinguistics)
https://en.wikipedia.org/wiki/Indexicality
https://en.wikipedia.org/wiki/Deixis
https://en.wikipedia.org/wiki/Reference
https://en.wikipedia.org/wiki/Formal_language
https://en.wikipedia.org/wiki/Referent
https://en.wikipedia.org/wiki/Grammatical_modifier
https://en.wikipedia.org/wiki/qualifier
https://en.wikipedia.org/wiki/Descriptor
https://en.wikipedia.org/wiki/Nominal
https://en.wikipedia.org/wiki/ordinal
https://en.wikipedia.org/wiki/classification
https://en.wikipedia.org/wiki/category
https://en.wikipedia.org/wiki/system
https://en.wikipedia.org/wiki/method
https://en.wikipedia.org/wiki/systematized
https://en.wikipedia.org/wiki/systematology
https://en.wikipedia.org/wiki/study
https://en.wikipedia.org/wiki/structure
https://en.wikipedia.org/wiki/hierarchy
https://en.wikipedia.org/wiki/order
https://en.wikipedia.org/wiki/precedence
https://en.wikipedia.org/wiki/order_of_precedence
https://en.wikipedia.org/wiki/Nominalization
https://en.wikipedia.org/wiki/Natural_language
https://en.wikipedia.org/wiki/International_auxiliary_language
https://en.wikipedia.org/wiki/Controlled_natural_language
https://en.wikipedia.org/wiki/Conversion_(word_formation)
https://en.wikipedia.org/wiki/Autological_word
https://en.wikipedia.org/wiki/Self-reference
https://en.wikipedia.org/wiki/Ideas_and_delusions_of_reference
https://en.wikipedia.org/wiki/Wikipedia:Manual_of_Style/Self-references_to_avoid
https://en.wikipedia.org/wiki/Paradox
https://en.wikipedia.org/wiki/Recursion
https://en.wikipedia.org/wiki/Impredicativity
https://en.wikipedia.org/wiki/Halting_problem
https://en.wikipedia.org/wiki/Strange_loop
https://en.wikipedia.org/wiki/Use–mention_distinction
https://en.wikipedia.org/wiki/Woozle_effect
https://en.wikipedia.org/wiki/Factoid
https://en.wikipedia.org/wiki/List_of_common_misconceptions
https://en.wikipedia.org/wiki/Conventional_wisdom
https://en.wikipedia.org/wiki/Moral_panic
https://en.wikipedia.org/wiki/Pseudoscience
https://en.wikipedia.org/wiki/Humanoid
https://en.wikipedia.org/wiki/Truthiness
https://en.wikipedia.org/wiki/Category:Doubt
https://en.wikipedia.org/wiki/Disinformation
https://en.wikipedia.org/wiki/Propaganda
https://en.wikipedia.org/wiki/Great_Soviet_Encyclopedia
https://en.wikipedia.org/wiki/Black_propaganda
https://en.wikipedia.org/wiki/Misinformation
https://en.wikipedia.org/wiki/Information
device element grammar
The free-fall time is the characteristic time that would take a body to collapse under its own gravitational attraction, if no other forces existed to oppose the collapse. As such, it plays a fundamental role in setting the timescale for a wide variety of astrophysical processes—from star formation to helioseismology to supernovae—in which gravity plays a dominant role.
https://en.wikipedia.org/wiki/Free-fall_time
Terms Level of Measurement nominal ordinal interval ratio cardinal [data measurement scales]
https://www.studysmarter.us/explanations/psychology/data-handling-and-analysis/levels-of-measurement/
https://www.statisticshowto.com/probability-and-statistics/statistics-definitions/nominal-ordinal-interval-ratio/
https://www.displayr.com/what-are-data-measurement-scales/
Microwave ovens[edit]
- Microwave ovens are not tuned to any specific resonance frequency for water molecules in the food, but rather produce a broad spectrum of frequencies,[44][45][46]cooking food via dielectric heating of polar molecules, including water. Several absorption peaks for water lie within the microwave range, and while it is true that these peaks are caused by quantization of molecular energy levels corresponding to a single frequency,[47] water absorbs radiation across the entire microwave spectrum.
- Microwave ovens do not cook food from the inside out. 2.45 GHz microwaves can only penetrate approximately 1 centimeter (3⁄8 inch) into most foods. The inside portions of thicker foods are mainly heated by heat conducted from the outer portions.[48]
- Microwave ovens do not cause cancer, as microwave radiation is non-ionizing, and therefore does not have the cancer risks associated with ionizing radiation such as X-rays. No studies have found that microwave radiation causes cancer, even with exposure levels far greater than normal radiation leakage.[49]
- Microwaving food does not reduce its nutritive value, and may preserve it better than other cooking processes due to shorter cooking times.[50]
There is another opinion regarding the universal definition of information. It lies in the fact that the concept itself has changed along with the change of various historical epochs, and in order to find such a definition, it is necessary to find common features and patterns of this transformation. For example, researchers in the field of information Petrichenko E. A. and Semenova V. G., based on a retrospective analysis of changes in the concept of information, give the following universal definition: "Information is a form of transmission of human experience (knowledge)." In their opinion, the change in the essence of the concept of information occurs after various breakthrough technologies for the transfer of experience (knowledge), i.e. the appearance of writing, the printing press, the first encyclopedias, the telegraph, the development of cybernetics, the creation of a microprocessor, the Internet, smartphones, etc. Each new form of experience transfer is a synthesis of the previous ones. That is why we see such a variety of definitions of information, because, according to the law of dialectics "negation-negation", all previous ideas about information are contained in a "filmed" form and in its modern representation.[8]
Applications of fundamental topics of information theory include source coding/data compression (e.g. for ZIP files), and channel coding/error detection and correction(e.g. for DSL). Its impact has been crucial to the success of the Voyager missions to deep space, the invention of the compact disc, the feasibility of mobile phones and the development of the Internet. The theory has also found applications in other areas, including statistical inference,[9] cryptography, neurobiology,[10] perception,[11]linguistics, the evolution[12] and function[13] of molecular codes (bioinformatics), thermal physics,[14] quantum computing, black holes, information retrieval, intelligence gathering, plagiarism detection,[15] pattern recognition, anomaly detection[16] and even art creation.
If, however, the premise of "influence" implies that information has been perceived by a conscious mind and also interpreted by it, the specific context associated with this interpretation may cause the transformation of the information into knowledge. Complex definitions of both "information" and "knowledge" make such semantic and logical analysis difficult, but the condition of "transformation" is an important point in the study of information as it relates to knowledge, especially in the business discipline of knowledge management. In this practice, tools and processes are used to assist a knowledge worker in performing research and making decisions, including steps such as:
- Review information to effectively derive value and meaning
- Reference metadata if available
- Establish relevant context, often from many possible contexts
- Derive new knowledge from the information
- Make decisions or recommendations from the resulting knowledge
The application of information study[edit]
The information cycle (addressed as a whole or in its distinct components) is of great concern to information technology, information systems, as well as information science. These fields deal with those processes and techniques pertaining to information capture (through sensors) and generation (through computation, formulationor composition), processing (including encoding, encryption, compression, packaging), transmission (including all telecommunication methods), presentation (including visualization / display methods), storage (such as magnetic or optical, including holographic methods), etc.
Information visualization (shortened as InfoVis) depends on the computation and digital representation of data, and assists users in pattern recognition and anomaly detection.
Information security (shortened as InfoSec) is the ongoing process of exercising due diligence to protect information, and information systems, from unauthorized access, use, disclosure, destruction, modification, disruption or distribution, through algorithms and procedures focused on monitoring and detection, as well as incident response and repair.
Information analysis is the process of inspecting, transforming, and modelling information, by converting raw data into actionable knowledge, in support of the decision-making process.
Information quality (shortened as InfoQ) is the potential of a dataset to achieve a specific (scientific or practical) goal using a given empirical analysis method.
Information communication represents the convergence of informatics, telecommunication and audio-visual media & content.
See also[edit]
- Abstraction
- Accuracy and precision
- Classified information
- Complex adaptive system
- Complex system
- Cybernetics
- Data storage device#Recording media
- Engram
- Exformation
- Free Information Infrastructure
- Freedom of information
- Informatics
- Information and communication technologies
- Information architecture
- Information broker
- Information continuum
- Information ecology
- Information engineering
- Information geometry
- Information inequity
- Information infrastructure
- Information management
- Information metabolism
- Information overload
- Information processor
- Information quality(InfoQ)
- Information science
- Information sensitivity
- Information superhighway
- Information technology
- Information theory
- Information warfare
- Infosphere
- Internet forum
- Lexicographic information cost
- Library science
- Meme
- Philosophy of information
- Propaganda model
- Quantum information
- Receiver operating characteristic
- Satisficing












![{\displaystyle F={\frac {\tanh[(1-\alpha )h_{\rm {T}}]}{\tanh h_{\rm {T}}}}{\frac {1}{1+\alpha h_{\rm {T}}\tanh[(1-\alpha )h_{\rm {T}}]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/a5f90a34d050a51a338db061e9803d5e0efbcc0e)



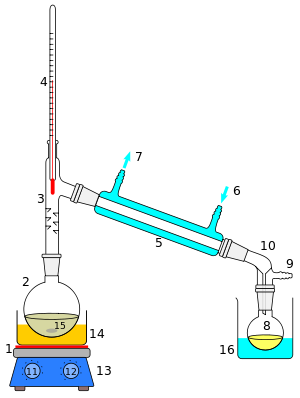


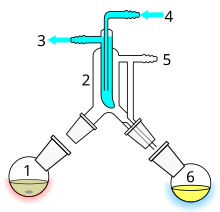


























No comments:
Post a Comment