Dissipative System Quantum decoherence Decoupling Optics
Maser ~
Fusor
Fissor
Mirror
Prot Neut Elect Ion - AC4
Mirror Particle
Emissions, Moderators, Generators, etc.
Angular Momentum
Vaccumes, Engines, Vortex, Spiral, etc.
Spont comb, sublim,
plasma,
arc, beam, etc.
(Genetics Keywords; Bioeng; Eng Keywords; Maths Keywords)
Ternary fission may happen during neutron-induced fission
absorption of the neutron, possibly because of the extra energy present in the nuclear reaction system of thermal neutron-induced fission.
Although particles as large as argon nuclei may be produced as the smaller (third) charged product in the usual ternary fission, the most common small fragments from ternary fission are helium-4 nuclei, which make up about 90% of the small fragment products. This high incidence is related to the stability (high binding energy) of the alpha particle, which makes more energy available to the reaction. The second-most common particles produced in ternary fission are Tritons (the nuclei of tritium), which make up 7% of the total small fragments, and the third-most are helium-6 nuclei (which decay in about 0.8 seconds to lithium-6). Protons and larger nuclei are in the small fraction (< 2%) which make up the remainder of the small charged products. The two larger charged particles from ternary fission, particularly when alphas are produced, are quite similar in size distribution to those produced in binary fission.
https://en.wikipedia.org/wiki/Ternary_fission
Tritium (/ˈtrɪtiəm/ or /ˈtrɪʃiəm/, from Ancient Greek τρίτος (trítos) 'third') or hydrogen-3 (symbol T or 3H) is a rare and radioactiveisotope of hydrogen. The nucleus of tritium (sometimes called a triton) contains one proton and two neutrons, whereas the nucleus of the common isotope hydrogen-1 (protium) contains just one proton, and that of hydrogen-2 (deuterium) contains one proton and one neutron.
Naturally occurring tritium is extremely rare on Earth. The atmosphere has only trace amounts, formed by the interaction of its gases with cosmic rays. It can be artificially produced by irradiating lithium metal or lithium-bearing ceramic pebbles in a nuclear reactor, and is a low-abundance byproduct in normal operations of nuclear reactors.
Tritium is used as the energy source in radioluminescent lights for watches, gun sights, numerous instruments and tools, and even novelty items such as self-illuminating key chains. It is used in a medical and scientific setting as a radioactive tracer. Tritium is also used as a nuclear fusion fuel, along with more abundant deuterium, in tokamak reactors and in hydrogen bombs.
https://en.wikipedia.org/wiki/Tritium
Spontaneous fission (SF) is a form of radioactive decay that is found only in very heavy chemical elements. The nuclear binding energy of the elements reaches its maximum at an atomic mass number of about 56; spontaneous breakdown into smaller nuclei and a few isolated nuclear particles becomes possible at greater atomic mass numbers.
By 1908, the process of alpha decay was known to consist of the ejection of helium nuclei from the decaying atom;[1] however, as with cluster decay, alpha decay is not typically categorized as a process of fission.[2]
The first nuclear fission process discovered was fission induced by neutrons. Because cosmic rays produce some neutrons, it was difficult to distinguish between induced and spontaneous events. Cosmic rays can be reliably shielded by a thick layer of rock or water. Spontaneous fission was identified in 1940 by Soviet physicists Georgy Flyorov and Konstantin Petrzhak[3][4] by their observations of uranium in the Moscow Metro Dinamo station, 60 metres (200 ft) underground.[5]
https://en.wikipedia.org/wiki/Spontaneous_fission
Proton emission (also known as proton radioactivity) is a rare type of radioactive decay in which a proton is ejected from a nucleus. Proton emission can occur from high-lying excited states in a nucleus following a beta decay, in which case the process is known as beta-delayed proton emission, or can occur from the ground state (or a low-lying isomer) of very proton-rich nuclei, in which case the process is very similar to alpha decay. For a proton to escape a nucleus, the proton separation energy must be negative—the proton is therefore unbound, and tunnelsout of the nucleus in a finite time. Proton emission is not seen in naturally occurring isotopes; proton emitters can be produced via nuclear reactions, usually using linear particle accelerators.
Although prompt (i.e. not beta-delayed) proton emission was observed from an isomer in cobalt-53 as early as 1969, no other proton-emitting states were found until 1981, when the proton radioactive ground states of lutetium-151 and thulium-147 were observed at experiments at the GSI in West Germany.[1] Research in the field flourished after this breakthrough, and to date more than 25 isotopes have been found to exhibit proton emission. The study of proton emission has aided the understanding of nuclear deformation, masses, and structure, and it is a pure example of quantum tunneling.
| Nuclear physics |
|---|
 |
| Nucleus · Nucleons (p, n) · Nuclear matter ·Nuclear force · Nuclear structure ·Nuclear reaction |
See also[edit]
- Nuclear drip line
- Diproton (a particle possibly involved in double proton decay)
- Free neutron
- Neutron emission
- Photodisintegration
References[edit]
- ^ S. Hofmann (1996). "Chapter 3: Proton radioactivity". In Dorin N. Poseru (ed.). Nuclear Decay Modes. Bristol: Institute of Physics Publishing. pp. 143–203. ISBN 0-7503-0338-7.
External links[edit]
 Nuclear Structure and Decay Data - IAEA with query on Proton Separation Energy
Nuclear Structure and Decay Data - IAEA with query on Proton Separation Energy
https://en.wikipedia.org/wiki/Proton_emission
Neutron emission is a mode of radioactive decay in which one or more neutrons are ejected from a nucleus. It occurs in the most neutron-rich/proton-deficient nuclides, and also from excited states of other nuclides as in photoneutron emission and beta-delayed neutron emission. As only a neutron is lost by this process the number of protons remains unchanged, and an atom does not become an atom of a different element, but a different isotope of the same element.
Neutrons are also produced in the spontaneous and induced fission of certain heavy nuclides.
https://en.wikipedia.org/wiki/Neutron_emission
Photodisintegration (also called phototransmutation, or a photonuclear reaction) is a nuclear process in which an atomic nucleus absorbs a high-energy gamma ray, enters an excited state, and immediately decays by emitting a subatomic particle. The incoming gamma ray effectively knocks one or more neutrons, protons, or an alpha particle out of the nucleus.[1] The reactions are called (γ,n), (γ,p), and (γ,α).
Photodisintegration is endothermic (energy absorbing) for atomic nuclei lighter than iron and sometimes exothermic (energy releasing) for atomic nuclei heavier than iron. Photodisintegration is responsible for the nucleosynthesis of at least some heavy, proton-rich elements via the p-process in supernovae.[which?] This causes the iron to further fuse into the heavier elements.[citation needed]
| Low-energy phenomena: |
|---|
| Photoelectric effect |
| Mid-energy phenomena: |
| Thomson scattering |
| Compton scattering |
| High-energy phenomena: |
| Pair production |
| Photodisintegration |
| Photofission |
Photodisintegration of deuterium[edit]
A photon carrying 2.22 MeV or more energy can photodisintegrate an atom of deuterium:
James Chadwick and Maurice Goldhaber used this reaction to measure the proton-neutron mass difference.[2] This experiment proves that a neutron is not a bound state of a proton and an electron,[why?][3] as had been proposed by Ernest Rutherford.
Hypernovae[edit]
In explosions of very large stars (250 or more solar masses), photodisintegration is a major factor in the supernova event. As the star reaches the end of its life, it reaches temperatures and pressures where photodisintegration's energy-absorbing effects temporarily reduce pressure and temperature within the star's core. This causes the core to start to collapse as energy is taken away by photodisintegration, and the collapsing core leads to the formation of a black hole. A portion of mass escapes in the form of relativistic jets, which could have "sprayed" the first metals into the universe.[7][8]
Photodisintegration in lightning[edit]
Terrestrial lightnings produce high-speed electrons that create bursts of gamma-rays as bremsstrahlung. The energy of these rays is sometimes sufficient to start photonuclear reactions resulting in emitted neutrons. One such reaction, 14
7N
(γ,n)13
7N
, is the only natural process other than those induced by cosmic rays in which 13
7N
is produced on Earth. The unstable isotopes remaining from the reaction may subsequently emit positrons by β+ decay.[9]
Photofission[edit]
Photofission is a similar but distinct process, in which a nucleus, after absorbing a gamma ray, undergoes nuclear fission (splits into two fragments of nearly equal mass).
See also[edit]
https://en.wikipedia.org/wiki/Photodisintegration
Helium-2 (diproton)[edit]
Helium-2 or 2
He
is an extremely unstable isotope of helium. Its nucleus, a diproton, consists of two protons with no neutrons. According to theoretical calculations, it would have been much more stable (although still undergoing β+ decay to deuterium) if the strong force had been 2% greater.[9] Its instability is due to spin–spin interactions in the nuclear force, and the Pauli exclusion principle, which forces the two protons to have anti-aligned spins and gives the diproton a negative binding energy.[10]
More evidence of 2
He
was found in 2008 at the Istituto Nazionale di Fisica Nucleare, in Italy.[8][13] A beam of 20
Ne
ions was directed at a target of beryllium foil. This collision converted some of the heavier neon nuclei in the beam into 18
Ne
nuclei. These nuclei then collided with a foil of lead. The second collision had the effect of exciting the 18
Ne
nucleus into a highly unstable condition. As in the earlier experiment at Oak Ridge, the 18
Ne
nucleus decayed into an 16
O
nucleus, plus two protons detected exiting from the same direction. The new experiment showed that the two protons were initially ejected together, correlated in a quasibound 1S configuration, before decaying into separate protons much less than a nanosecond later.
2
He
is an intermediate in the first step of the proton–proton chain reaction. The first step of the proton–proton chain reaction is a two-stage process; first, two protons fuse to form a diproton:
followed by the immediate beta-plus decay of the diproton to deuterium:
with the overall formula
https://en.wikipedia.org/wiki/Isotopes_of_helium#Helium-2_(diproton)
Proton–proton chain
The proton–proton chain, also commonly referred to as the p-p chain, is one of two known sets of nuclear fusionreactions by which stars convert hydrogen to helium. It dominates in stars with masses less than or equal to that of the Sun,[2] whereas the CNO cycle, the other known reaction, is suggested by theoretical models to dominate in stars with masses greater than about 1.3 times that of the Sun.[3]
In general, proton–proton fusion can occur only if the kinetic energy (i.e. temperature) of the protons is high enough to overcome their mutual electrostatic repulsion.[4]
In the Sun, deuterium-producing events are rare. Diprotons are the much more common result of proton–proton reactions within the star, and diprotons almost immediately decay back into two protons. Since the conversion of hydrogen to helium is slow, the complete conversion of the hydrogen initially in the core of the Sun is calculated to take more than ten billion years.[5]
Although sometimes called the "proton–proton chain reaction", it is not a chain reaction in the normal sense. In most nuclear reactions, a chain reaction designates a reaction that produces a product, such as neutrons given off during fission, that quickly induces another such reaction. The proton-proton chain is, like a decay chain, a series of reactions. The product of one reaction is the starting material of the next reaction. There are two main chains leading from Hydrogen to Helium in the Sun. One chain has five reactions, the other chain has six.
The theory that proton–proton reactions are the basic principle by which the Sun and other stars burn was advocated by Arthur Eddington in the 1920s. At the time, the temperature of the Sun was considered to be too low to overcome the Coulomb barrier. After the development of quantum mechanics, it was discovered that tunneling of the wavefunctions of the protons through the repulsive barrier allows for fusion at a lower temperature than the classical prediction.
In 1939, Hans Bethe attempted to calculate the rates of various reactions in stars. Starting with two protons combining to give deuterium and a positron he found what we now call Branch II of the proton-proton chain. But he did not consider the reaction of two 3
He nuclei (Branch I) which we now know to be important.[6] This was part of the body of work in stellar nucleosynthesis for which Bethe won the Nobel Prize in Physics in 1967.
https://en.wikipedia.org/wiki/Proton–proton_chain
The positron or antielectron is the antiparticle or the antimatter counterpart of the electron. The positron has an electric chargeof +1 e, a spin of 1/2 (the same as the electron), and has the same mass as an electron. When a positron collides with an electron, annihilation occurs. If this collision occurs at low energies, it results in the production of two or more photons.
Positrons can be created by positron emission radioactive decay (through weak interactions), or by pair production from a sufficiently energetic photon which is interacting with an atom in a material.
Cloud chamber photograph by C. D. Anderson of the first positron ever identified. A 6 mm lead plate separates the chamber. The deflection and direction of the particle's ion trail indicate that the particle is a positron. | |
| Composition | Elementary particle |
|---|---|
| Statistics | Fermionic |
| Family | Antilepton |
| Generation | First |
| Interactions | Gravity, Electromagnetic, Weak |
| Symbol | e+ , β+ |
| Antiparticle | Electron |
| Theorized | Paul Dirac (1928) |
| Discovered | Carl D. Anderson (1932) |
| Mass | me 9.10938356(11)×10−31 kg[1] |
| Mean lifetime | stable (same as electron) |
| Electric charge | +1 e +1.602176565(35)×10−19 C[1] |
| Spin | 12 (same as electron) |
| Weak isospin | LH: 0, RH: 12 |
https://en.wikipedia.org/wiki/Positron
The Dirac sea is a theoretical model of the vacuum as an infinite sea of particles with negative energy. It was first postulated by the British physicist Paul Dirac in 1930[1] to explain the anomalous negative-energy quantum states predicted by the Dirac equation for relativistic electrons (electrons traveling near the speed of light).[2] The positron, the antimatter counterpart of the electron, was originally conceived of as a hole in the Dirac sea, before its experimental discovery in 1932.[nb 1]
In hole theory, the solutions with negative time evolution factors[clarification needed] are reinterpreted as representing the positron, discovered by Carl Anderson. The interpretation of this result requires a Dirac sea, showing that the Dirac equation is not merely a combination of special relativity and quantum mechanics, but it also implies that the number of particles cannot be conserved.[3]
Dirac sea theory has been displaced by quantum field theory, though they are mathematically compatib
https://en.wikipedia.org/wiki/Dirac_sea
The anthropic principle is the principle that there is a restrictive lower bound on how statistically probable our observations of the universe are, given that we could only exist in the particular type of universe capable of developing and sustaining sentient life.[1] Proponents of the anthropic principle argue that it explains why this universe has the age and the fundamental physical constants necessary to accommodate conscious life, since if either had been different, we would not have been around to make observations. Anthropic reasoning is often used to deal with the notion that the universe seems to be fine tuned.[2]
There are many different formulations of the anthropic principle. Philosopher Nick Bostrom counts them at thirty, but the underlying principles can be divided into "weak" and "strong" forms, depending on the types of cosmological claims they entail. The weak anthropic principle (WAP), such as the one defined by Brandon Carter, states that the universe's ostensible fine tuning is the result of selection bias (specifically survivorship bias). Most often such arguments draw upon some notion of the multiverse for there to be a statistical population of universes to select from. However, a single vast universe is sufficient for most forms of the WAP that do not specifically deal with fine tuning. The strong anthropic principle (SAP), as proposed by John D. Barrow and Frank Tipler, states that the universe is in some sense compelled to eventually have conscious and sapient life emerge within it.
https://en.wikipedia.org/wiki/Anthropic_principle
A fusor is a device that uses an electric field to heat ions to nuclear fusion conditions. The machine induces a voltagebetween two metal cages, inside a vacuum. Positive ions fall down this voltage drop, building up speed. If they collide in the center, they can fuse. This is one kind of an inertial electrostatic confinement device – a branch of fusion research.
A Farnsworth–Hirsch fusor is the most common type of fusor.[1] This design came from work by Philo T. Farnsworth in 1964 and Robert L. Hirsch in 1967.[2][3] A variant type of fusor had been proposed previously by William Elmore, James L. Tuck, and Ken Watson at the Los Alamos National Laboratory[4] though they never built the machine.
https://en.wikipedia.org/wiki/Fusor
Cluster decay, also named heavy particle radioactivity or heavy ion radioactivity, is a rare type of nuclear decay in which an atomic nucleus emits a small "cluster" of neutrons and protons, more than in an alpha particle, but less than a typical binary fission fragment. Ternary fission into three fragments also produces products in the cluster size. The loss of protons from the parent nucleus changes it to the nucleus of a different element, the daughter, with a mass number Ad = A − Ae and atomic number Zd = Z− Ze, where Ae = Ne + Ze.[1] For example:
- 223
88Ra
→ 14
6C
+ 209
82Pb
This type of rare decay mode was observed in radioisotopes that decay predominantly by alpha emission, and it occurs only in a small percentage of the decays for all such isotopes.[2]
The branching ratio with respect to alpha decay is rather small (see the Table below).
Ta and Tc are the half-lives of the parent nucleus relative to alpha decay and cluster radioactivity, respectively.
https://en.wikipedia.org/wiki/Cluster_decay
Quantum tunnelling or tunneling (US) is the quantum mechanical phenomenon where a wavefunction can propagate through a potential barrier.
The transmission through the barrier can be finite and depends exponentially on the barrier height and barrier width. The wavefunction may disappear on one side and reappear on the other side. The wavefunction and its first derivative are continuous. In steady-state, the probability flux in the forward direction is spatially uniform. No particle or wave is lost. Tunneling occurs with barriers of thickness around 1–3 nm and smaller.[1]
Some authors also identify the mere penetration of the wavefunction into the barrier, without transmission on the other side as a tunneling effect. Quantum tunneling is not predicted by the laws of classical mechanics where surmounting a potential barrier requires potential energy.
Quantum tunneling plays an essential role in physical phenomena, such as nuclear fusion.[2] It has applications in the tunnel diode,[3]quantum computing, and in the scanning tunneling microscope.
The effect was predicted in the early 20th century. Its acceptance as a general physical phenomenon came mid-century.[4]
Quantum tunneling is projected to create physical limits to the size of the transistors used in microelectronics, due to electrons being able to tunnel past transistors that are too small.[5][6]
Tunneling may be explained in terms of the Heisenberg uncertainty principle in that a quantum object can be known as a wave or as a particle in general. In other words, the uncertainty in the exact location of light particles allows these particles to break rules of classical mechanics and move in space without passing over the potential energy barrier.
Quantum tunnelling may be one of the mechanisms of proton decay.[7][8][9]
https://en.wikipedia.org/wiki/Quantum_tunnelling
Deuterium (or hydrogen-2, symbol 2
H
or
D
, also known as heavy hydrogen) is one of two stable isotopes of hydrogen (the other being protium, or hydrogen-1). The nucleus of a deuterium atom, called a deuteron, contains one proton and one neutron, whereas the far more common protium has no neutrons in the nucleus. Deuterium has a natural abundance in Earth's oceans of about one atom in 6420 of hydrogen. Thus deuterium accounts for approximately 0.0156% (0.0312% by mass) of all the naturally occurring hydrogen in the oceans, while protium accounts for more than 99.98%. The abundance of deuterium changes slightly from one kind of natural water to another (see Vienna Standard Mean Ocean Water).
https://en.wikipedia.org/wiki/Deuterium
Several sources have claimed that Dmitri Skobeltsyn first observed the positron long before 1930,[10] or even as early as 1923.[11] They state that while using a Wilson cloud chamber[12] in order to study the Compton effect, Skobeltsyn detected particles that acted like electrons but curved in the opposite direction in an applied magnetic field, and that he presented photographs with this phenomenon in a conference in Cambridge, on 23-27 July 1928. In his book[13] on the history of the positron discovery from 1963, Norwood Russell Hanson has given a detailed account of the reasons for this assertion, and this may have been the origin of the myth. But he also presented Skobeltsyn's objection to it in an appendix.[14] Later, Skobeltsyn has rejected this claim even more strongly, calling it "nothing but sheer nonsense".[15]
Skobeltsyn did pave the way for the eventual discovery of the positron by two important contributions: adding a magnetic field to his cloud chamber (in 1925[16]) , and by discovering charged particle cosmic rays,[17] for which he is credited in Carl Anderson's Nobel lecture.[18] Skobeltzyn did observe likely positron tracks on images taken in 1931,[19] but did not identify them as such at the time.
| Antimatter |
|---|
 |
β+
decay can be considered both artificial and natural production, as the generation of the radioisotope can be natural or artificial. Perhaps the best known naturally-occurring radioisotope which produces positrons is potassium-40, a long-lived isotope of potassium which occurs as a primordial isotope of potassium. Even though a small percent of potassium (0.0117%) it is the single most abundant radioisotope in the human body. In a human body of 70 kg mass, about 4,400 nuclei of 40K decay per second.[30] The activity of natural potassium is 31 Bq/g.[31] About 0.001% of these 40K decays produce about 4000 natural positrons per day in the human body.[32]These positrons soon find an electron, undergo annihilation, and produce pairs of 511 keV photons, in a process similar (but much lower intensity) to that which happens during a PET scan nuclear medicine procedure.[citation needed]
https://en.wikipedia.org/wiki/Positron
Positron emission, beta plus decay, or β+ decay is a subtype of radioactive decay called beta decay, in which a proton inside a radionuclide nucleus is converted into a neutron while releasing a positron and an electron neutrino (νe).[1] Positron emission is mediated by the weak force. The positron is a type of beta particle (β+), the other beta particle being the electron (β−) emitted from the β− decay of a nucleus.
An example of positron emission (β+ decay) is shown with magnesium-23 decaying into sodium-23:
- 23
12Mg
→ 23
11Na
+
e+
+
ν
e
Because positron emission decreases proton number relative to neutron number, positron decay happens typically in large "proton-rich" radionuclides. Positron decay results in nuclear transmutation, changing an atom of one chemical element into an atom of an element with an atomic number that is less by one unit.
Positron emission occurs only very rarely naturally on earth, when induced by a cosmic ray or from one in a hundred thousand decays of potassium-40, a rare isotope, 0.012% of that element on earth.
Positron emission should not be confused with electron emission or beta minus decay (β− decay), which occurs when a neutron turns into a proton and the nucleus emits an electron and an antineutrino.
Positron emission is different from proton decay, the hypothetical decay of protons, not necessarily those bound with neutrons, not necessarily through the emission of a positron, and not as part of nuclear physics, but rather of particle physics.
https://en.wikipedia.org/wiki/Positron_emission
Beta decay
| Nuclear physics |
|---|
 |
| Nucleus · Nucleons (p, n) · Nuclear matter ·Nuclear force · Nuclear structure ·Nuclear reaction |
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For example, beta decay of a neutrontransforms it into a proton by the emission of an electron accompanied by an antineutrino; or, conversely a proton is converted into a neutron by the emission of a positron with a neutrino in so-called positron emission. Neither the beta particle nor its associated (anti-)neutrino exist within the nucleus prior to beta decay, but are created in the decay process. By this process, unstable atoms obtain a more stable ratio of protons to neutrons. The probability of a nuclide decaying due to beta and other forms of decay is determined by its nuclear binding energy. The binding energies of all existing nuclides form what is called the nuclear band or valley of stability.[1]For either electron or positron emission to be energetically possible, the energy release (see below) or Q value must be positive.
Beta decay is a consequence of the weak force, which is characterized by relatively lengthy decay times. Nucleons are composed of up quarks and down quarks,[2] and the weak force allows a quark to change its flavour by emission of a W boson leading to creation of an electron/antineutrino or positron/neutrino pair. For example, a neutron, composed of two down quarks and an up quark, decays to a proton composed of a down quark and two up quarks.
Electron capture is sometimes included as a type of beta decay,[3] because the basic nuclear process, mediated by the weak force, is the same. In electron capture, an inner atomic electron is captured by a proton in the nucleus, transforming it into a neutron, and an electron neutrino is released.
Description[edit]
The two types of beta decay are known as beta minus and beta plus. In beta minus (β−) decay, a neutron is converted to a proton, and the process creates an electron and an electron antineutrino; while in beta plus (β+) decay, a proton is converted to a neutron and the process creates a positron and an electron neutrino. β+ decay is also known as positron emission.[4]
Beta decay conserves a quantum number known as the lepton number, or the number of electrons and their associated neutrinos (other leptons are the muon and tau particles). These particles have lepton number +1, while their antiparticles have lepton number −1. Since a proton or neutron has lepton number zero, β+ decay (a positron, or antielectron) must be accompanied with an electron neutrino, while β− decay (an electron) must be accompanied by an electron antineutrino.
An example of electron emission (β− decay) is the decay of carbon-14 into nitrogen-14 with a half-life of about 5,730 years:
- 14
6C
→ 14
7N
+
e−
+
ν
e
In this form of decay, the original element becomes a new chemical element in a process known as nuclear transmutation. This new element has an unchanged mass numberA, but an atomic number Z that is increased by one. As in all nuclear decays, the decaying element (in this case 14
6C
) is known as the parent nuclide while the resulting element (in this case 14
7N
) is known as the daughter nuclide.
Another example is the decay of hydrogen-3 (tritium) into helium-3 with a half-life of about 12.3 years:
- 3
1H
→ 3
2He
+
e−
+
ν
e
An example of positron emission (β+ decay) is the decay of magnesium-23 into sodium-23 with a half-life of about 11.3 s:
- 23
12Mg
→ 23
11Na
+
e+
+
ν
e
β+ decay also results in nuclear transmutation, with the resulting element having an atomic number that is decreased by one.
The beta spectrum, or distribution of energy values for the beta particles, is continuous. The total energy of the decay process is divided between the electron, the antineutrino, and the recoiling nuclide. In the figure to the right, an example of an electron with 0.40 MeV energy from the beta decay of 210Bi is shown. In this example, the total decay energy is 1.16 MeV, so the antineutrino has the remaining energy: 1.16 MeV − 0.40 MeV = 0.76 MeV. An electron at the far right of the curve would have the maximum possible kinetic energy, leaving the energy of the neutrino to be only its small rest mass.
History[edit]
Discovery and initial characterization[edit]
Radioactivity was discovered in 1896 by Henri Becquerel in uranium, and subsequently observed by Marie and Pierre Curie in thoriumand in the new elements polonium and radium. In 1899, Ernest Rutherford separated radioactive emissions into two types: alpha and beta (now beta minus), based on penetration of objects and ability to cause ionization. Alpha rays could be stopped by thin sheets of paper or aluminium, whereas beta rays could penetrate several millimetres of aluminium. In 1900, Paul Villard identified a still more penetrating type of radiation, which Rutherford identified as a fundamentally new type in 1903 and termed gamma rays. Alpha, beta, and gamma are the first three letters of the Greek alphabet.
In 1900, Becquerel measured the mass-to-charge ratio (m/e) for beta particles by the method of J.J. Thomson used to study cathode rays and identify the electron. He found that m/e for a beta particle is the same as for Thomson's electron, and therefore suggested that the beta particle is in fact an electron.[5]
In 1901, Rutherford and Frederick Soddy showed that alpha and beta radioactivity involves the transmutation of atoms into atoms of other chemical elements. In 1913, after the products of more radioactive decays were known, Soddy and Kazimierz Fajans independently proposed their radioactive displacement law, which states that beta (i.e.,
β−
) emission from one element produces another element one place to the right in the periodic table, while alpha emission produces an element two places to the left.
Neutrinos[edit]
The study of beta decay provided the first physical evidence for the existence of the neutrino. In both alpha and gamma decay, the resulting alpha or gamma particle has a narrow energy distribution, since the particle carries the energy from the difference between the initial and final nuclear states. However, the kinetic energy distribution, or spectrum, of beta particles measured by Lise Meitner and Otto Hahn in 1911 and by Jean Danysz in 1913 showed multiple lines on a diffuse background. These measurements offered the first hint that beta particles have a continuous spectrum.[6] In 1914, James Chadwick used a magnetic spectrometer with one of Hans Geiger's new counters to make more accurate measurements which showed that the spectrum was continuous.[6][7] The distribution of beta particle energies was in apparent contradiction to the law of conservation of energy. If beta decay were simply electron emission as assumed at the time, then the energy of the emitted electron should have a particular, well-defined value.[8] For beta decay, however, the observed broad distribution of energies suggested that energy is lost in the beta decay process. This spectrum was puzzling for many years.
A second problem is related to the conservation of angular momentum. Molecular band spectra showed that the nuclear spin of nitrogen-14 is 1 (i.e., equal to the reduced Planck constant) and more generally that the spin is integral for nuclei of even mass number and half-integral for nuclei of odd mass number. This was later explained by the proton-neutron model of the nucleus.[8] Beta decay leaves the mass number unchanged, so the change of nuclear spin must be an integer. However, the electron spin is 1/2, hence angular momentum would not be conserved if beta decay were simply electron emission.
From 1920 to 1927, Charles Drummond Ellis (along with Chadwick and colleagues) further established that the beta decay spectrum is continuous. In 1933, Ellis and Nevill Mottobtained strong evidence that the beta spectrum has an effective upper bound in energy. Niels Bohr had suggested that the beta spectrum could be explained if conservation of energy was true only in a statistical sense, thus this principle might be violated in any given decay.[8]:27 However, the upper bound in beta energies determined by Ellis and Mott ruled out that notion. Now, the problem of how to account for the variability of energy in known beta decay products, as well as for conservation of momentum and angular momentum in the process, became acute.
In a famous letter written in 1930, Wolfgang Pauli attempted to resolve the beta-particle energy conundrum by suggesting that, in addition to electrons and protons, atomic nuclei also contained an extremely light neutral particle, which he called the neutron. He suggested that this "neutron" was also emitted during beta decay (thus accounting for the known missing energy, momentum, and angular momentum), but it had simply not yet been observed. In 1931, Enrico Fermi renamed Pauli's "neutron" the "neutrino" ('little neutral one' in Italian). In 1933, Fermi published his landmark theory for beta decay, where he applied the principles of quantum mechanics to matter particles, supposing that they can be created and annihilated, just as the light quanta in atomic transitions. Thus, according to Fermi, neutrinos are created in the beta-decay process, rather than contained in the nucleus; the same happens to electrons. The neutrino interaction with matter was so weak that detecting it proved a severe experimental challenge. Further indirect evidence of the existence of the neutrino was obtained by observing the recoil of nuclei that emitted such a particle after absorbing an electron. Neutrinos were finally detected directly in 1956 by Clyde Cowan and Frederick Reines in the Cowan–Reines neutrino experiment.[9] The properties of neutrinos were (with a few minor modifications) as predicted by Pauli and Fermi.
β+
decay and electron capture[edit]
In 1934, Frédéric and Irène Joliot-Curie bombarded aluminium with alpha particles to effect the nuclear reaction 4
2He
+ 27
13Al
→ 30
15P
+ 1
0n
, and observed that the product isotope 30
15P
emits a positron identical to those found in cosmic rays (discovered by Carl David Anderson in 1932). This was the first example of
β+
decay (positron emission), which they termed artificial radioactivity since 30
15P
is a short-lived nuclide which does not exist in nature. In recognition of their discovery the couple were awarded the Nobel Prize in Chemistry in 1935.[10]
The theory of electron capture was first discussed by Gian-Carlo Wick in a 1934 paper, and then developed by Hideki Yukawa and others. K-electron capture was first observed in 1937 by Luis Alvarez, in the nuclide 48V.[11][12][13] Alvarez went on to study electron capture in 67Ga and other nuclides.[11][14][15]
Non-conservation of parity[edit]
In 1956, Tsung-Dao Lee and Chen Ning Yang noticed that there was no evidence that parity was conserved in weak interactions, and so they postulated that this symmetry may not be preserved by the weak force. They sketched the design for an experiment for testing conservation of parity in the laboratory.[16] Later that year, Chien-Shiung Wu and coworkers conducted the Wu experiment showing an asymmetrical beta decay of cobalt-60 at cold temperatures that proved that parity is not conserved in beta decay.[17][18]This surprising result overturned long-held assumptions about parity and the weak force. In recognition of their theoretical work, Lee and Yang were awarded the Nobel Prize for Physics in 1957. However Wu, who was female, was not awarded the Nobel prize.[19]
β− decay[edit]
In
β−
decay, the weak interaction converts an atomic nucleus into a nucleus with atomic number increased by one, while emitting an electron (
e−
) and an electron antineutrino (
ν
e).
β−
decay generally occurs in neutron-rich nuclei.[22] The generic equation is:
- A
ZX
→ A
Z+1X′
+
e−
+
ν
e[1]
where A and Z are the mass number and atomic number of the decaying nucleus, and X and X′ are the initial and final elements, respectively.
Another example is when the free neutron (1
0n
) decays by
β−
decay into a proton (
p
):
n
→
p
+
e−
+
ν
e.
At the fundamental level (as depicted in the Feynman diagram on the right), this is caused by the conversion of the negatively charged (−13 e) down quark to the positively charged (+23 e) up quark by emission of a
W−
boson; the
W−
boson subsequently decays into an electron and an electron antineutrino:
d
→
u
+
e−
+
ν
e.
β+ decay[edit]
In
β+
decay, or positron emission, the weak interaction converts an atomic nucleus into a nucleus with atomic number decreased by one, while emitting a positron (
e+
) and an electron neutrino (
ν
e).
β+
decay generally occurs in proton-rich nuclei. The generic equation is:
- A
ZX
→ A
Z−1X′
+
e+
+
ν
e[1]
This may be considered as the decay of a proton inside the nucleus to a neutron:
- p → n +
e+
+
ν
e[1]
However,
β+
decay cannot occur in an isolated proton because it requires energy, due to the mass of the neutron being greater than the mass of the proton.
β+
decay can only happen inside nuclei when the daughter nucleus has a greater binding energy (and therefore a lower total energy) than the mother nucleus. The difference between these energies goes into the reaction of converting a proton into a neutron, a positron and a neutrino and into the kinetic energy of these particles. This process is opposite to negative beta decay, in that the weak interaction converts a proton into a neutron by converting an up quark into a down quark resulting in the emission of a
W+
or the absorption of a
W−
. When a
W+
boson is emitted, it decays into a positron and an electron neutrino:
u
→
d
+
e+
+
ν
e.
Electron capture (K-capture)[edit]
In all cases where
β+
decay (positron emission) of a nucleus is allowed energetically, so too is electron capture allowed. This is a process during which a nucleus captures one of its atomic electrons, resulting in the emission of a neutrino:
- A
ZX
+
e−
→ A
Z−1X′
+
ν
e
An example of electron capture is one of the decay modes of krypton-81 into bromine-81:
- 81
36Kr
+
e−
→ 81
35Br
+
ν
e
All emitted neutrinos are of the same energy. In proton-rich nuclei where the energy difference between the initial and final states is less than 2mec2,
β+
decay is not energetically possible, and electron capture is the sole decay mode.[23]
If the captured electron comes from the innermost shell of the atom, the K-shell, which has the highest probability to interact with the nucleus, the process is called K-capture.[24] If it comes from the L-shell, the process is called L-capture, etc.
Electron capture is a competing (simultaneous) decay process for all nuclei that can undergo β+ decay. The converse, however, is not true: electron capture is the only type of decay that is allowed in proton-rich nuclides that do not have sufficient energy to emit a positron and neutrino.[23]
Nuclear transmutation[edit]
If the proton and neutron are part of an atomic nucleus, the above described decay processes transmute one chemical element into another. For example:
137
55Cs
→ 137
56Ba
+
e−
+
ν
e(beta minus decay) 22
11Na
→ 22
10Ne
+
e+
+
ν
e(beta plus decay) 22
11Na
+
e−
→ 22
10Ne
+
ν
e(electron capture)
Beta decay does not change the number (A) of nucleons in the nucleus, but changes only its charge Z. Thus the set of all nuclides with the same A can be introduced; these isobaric nuclides may turn into each other via beta decay. For a given A there is one that is most stable. It is said to be beta stable, because it presents a local minimum of the mass excess: if such a nucleus has (A, Z) numbers, the neighbour nuclei (A, Z−1) and (A, Z+1) have higher mass excess and can beta decay into (A, Z), but not vice versa. For all odd mass numbers A, there is only one known beta-stable isobar. For even A, there are up to three different beta-stable isobars experimentally known; for example, 124
50Sn
, 124
52Te
, and 124
54Xe
are all beta-stable. There are about 350 known beta-decay stable nuclides.[25]
Competition of beta decay types[edit]
Usually unstable nuclides are clearly either "neutron rich" or "proton rich", with the former undergoing beta decay and the latter undergoing electron capture (or more rarely, due to the higher energy requirements, positron decay). However, in a few cases of odd-proton, odd-neutron radionuclides, it may be energetically favorable for the radionuclide to decay to an even-proton, even-neutron isobar either by undergoing beta-positive or beta-negative decay. An often-cited example is the single isotope 64
29Cu
(29 protons, 35 neutrons), which illustrates three types of beta decay in competition. Copper-64 has a half-life of about 12.7 hours. This isotope has one unpaired proton and one unpaired neutron, so either the proton or the neutron can decay. This particular nuclide (though not all nuclides in this situation) is almost equally likely to decay through proton decay by positron emission (18%) or electron capture (43%) to 64
28Ni
, as it is through neutron decay by electron emission (39%) to 64
30Zn
.[26]
Stability of naturally occurring nuclides[edit]
Most naturally occurring nuclides on earth are beta stable. Those that are not have half-lives ranging from under a second to periods of time significantly greater than the age of the universe. One common example of a long-lived isotope is the odd-proton odd-neutron nuclide 40
19K
, which undergoes all three types of beta decay (
β−
,
β+
and electron capture) with a half-life of 1.277×109 years.[27]
Conservation rules for beta decay[edit]
Baryon number is conserved[edit]
where
- is the number of constituent quarks, and
- is the number of constituent antiquarks.
Beta decay just changes neutron to proton or, in the case of positive beta decay (electron capture) proton to neutron so the number of individual quarks doesn't change. It is only the baryon flavor that changes, here labelled as the isospin.
Up and down quarks have total isospin and isospin projections
All other quarks have I = 0.
In general
Lepton number is conserved[edit]
so all leptons have assigned a value of +1, antileptons −1, and non-leptonic particles 0.
Angular momentum[edit]
For allowed decays, the net orbital angular momentum is zero, hence only spin quantum numbers are considered.
The electron and antineutrino are fermions, spin-1/2 objects, therefore they may couple to total (parallel) or (anti-parallel).
For forbidden decays, orbital angular momentum must also be taken into consideration.
Energy release[edit]
The Q value is defined as the total energy released in a given nuclear decay. In beta decay, Q is therefore also the sum of the kinetic energies of the emitted beta particle, neutrino, and recoiling nucleus. (Because of the large mass of the nucleus compared to that of the beta particle and neutrino, the kinetic energy of the recoiling nucleus can generally be neglected.) Beta particles can therefore be emitted with any kinetic energy ranging from 0 to Q.[1] A typical Q is around 1 MeV, but can range from a few keV to a few tens of MeV.
Since the rest mass of the electron is 511 keV, the most energetic beta particles are ultrarelativistic, with speeds very close to the speed of light. In the case of 187Re, the maximum speed of the beta particle is only 9.8% of the speed of light.
The following table gives some examples:
| Isotope | Energy (keV) | Decay mode | Comments |
|---|---|---|---|
| free Neutron | 782.33 | β− | |
| 3H (Tritium) | 18.59 | β− | Second lowest known β− energy, being used in the KATRIN experiment. |
| 11C | 960.4 1982.4 | β+ ε | |
| 14C | 156.475 | β− | |
| 20F | 5390.86 | β− | |
| 37K | 5125.48 6147.48 | β+ ε | |
| 163Ho | 2.555 | ε | |
| 187Re | 2.467 | β− | Lowest known β− energy, being used in the Microcalorimeter Arrays for a Rhenium Experiment experiment |
| 210Bi | 1162.2 | β− |
β− decay[edit]
Consider the generic equation for beta decay
- A
ZX
→ A
Z+1X′
+
e−
+
ν
e.
The Q value for this decay is
- ,
where is the mass of the nucleus of the A
ZX
atom, is the mass of the electron, and is the mass of the electron antineutrino. In other words, the total energy released is the mass energy of the initial nucleus, minus the mass energy of the final nucleus, electron, and antineutrino. The mass of the nucleus mN is related to the standard atomic mass m by
- .
That is, the total atomic mass is the mass of the nucleus, plus the mass of the electrons, minus the sum of all electron binding energies Bi for the atom. This equation is rearranged to find , and is found similarly. Substituting these nuclear masses into the Q-value equation, while neglecting the nearly-zero antineutrino mass and the difference in electron binding energies, which is very small for high-Z atoms, we have
This energy is carried away as kinetic energy by the electron and neutrino.
Because the reaction will proceed only when the Q value is positive, β− decay can occur when the mass of atom A
ZX
is greater than the mass of atom A
Z+1X′
.[28]
β+ decay[edit]
The equations for β+ decay are similar, with the generic equation
- A
ZX
→ A
Z−1X′
+
e+
+
ν
e
giving
- .
However, in this equation, the electron masses do not cancel, and we are left with
Because the reaction will proceed only when the Q value is positive, β+ decay can occur when the mass of atom A
ZX
exceeds that of A
Z-1X′
by at least twice the mass of the electron.[28]
Electron capture[edit]
The analogous calculation for electron capture must take into account the binding energy of the electrons. This is because the atom will be left in an excited state after capturing the electron, and the binding energy of the captured innermost electron is significant. Using the generic equation for electron capture
- A
ZX
+
e−
→ A
Z−1X′
+
ν
e
we have
- ,
which simplifies to
- ,
where Bn is the binding energy of the captured electron.
Because the binding energy of the electron is much less than the mass of the electron, nuclei that can undergo β+ decay can always also undergo electron capture, but the reverse is not true.[28]
Beta emission spectrum[edit]
Beta decay can be considered as a perturbation as described in quantum mechanics, and thus Fermi's Golden Rule can be applied. This leads to an expression for the kinetic energy spectrum N(T) of emitted betas as follows:[29]
where T is the kinetic energy, CL is a shape function that depends on the forbiddenness of the decay (it is constant for allowed decays), F(Z, T) is the Fermi Function (see below) with Z the charge of the final-state nucleus, E=T + mc2 is the total energy, p=√(E/c)2 − (mc)2 is the momentum, and Q is the Q value of the decay. The kinetic energy of the emitted neutrino is given approximately by Q minus the kinetic energy of the beta.
As an example, the beta decay spectrum of 210Bi (originally called RaE) is shown to the right.
Fermi function[edit]
The Fermi function that appears in the beta spectrum formula accounts for the Coulomb attraction / repulsion between the emitted beta and the final state nucleus. Approximating the associated wavefunctions to be spherically symmetric, the Fermi function can be analytically calculated to be:[30]
where p is the final momentum, Γ the Gamma function, and (if α is the fine-structure constant and rN the radius of the final state nucleus) S=√1 − α2 Z2, η=±Ze2c⁄ℏp (+ for electrons, − for positrons), and ρ=rN⁄ℏ.
For non-relativistic betas (Q ≪ mec2), this expression can be approximated by:[31]
Other approximations can be found in the literature.[32][33]
Kurie plot[edit]
A Kurie plot (also known as a Fermi–Kurie plot) is a graph used in studying beta decay developed by Franz N. D. Kurie, in which the square root of the number of beta particles whose momenta (or energy) lie within a certain narrow range, divided by the Fermi function, is plotted against beta-particle energy.[34][35] It is a straight line for allowed transitions and some forbidden transitions, in accord with the Fermi beta-decay theory. The energy-axis (x-axis) intercept of a Kurie plot corresponds to the maximum energy imparted to the electron/positron (the decay's Q value). With a Kurie plot one can find the limit on the effective mass of a neutrino.[36]
Helicity (polarization) of neutrinos, electrons and positrons emitted in beta decay[edit]
After the discovery of parity non-conservation (see History), it was found that, in beta decay, electrons are emitted mostly with negative helicity, i.e., they move, naively speaking, like left-handed screws driven into a material (they have negative longitudinal polarization).[37] Conversely, positrons have mostly positive helicity, i.e., they move like right-handed screws. Neutrinos (emitted in positron decay) have negative helicity, while antineutrinos (emitted in electron decay) have positive helicity.[38]
The higher the energy of the particles, the higher their polarization.
Types of beta decay transitions[edit]
Beta decays can be classified according to the angular momentum (L value) and total spin (S value) of the emitted radiation. Since total angular momentum must be conserved, including orbital and spin angular momentum, beta decay occurs by a variety of quantum state transitions to various nuclear angular momentum or spin states, known as "Fermi" or "Gamow–Teller" transitions. When beta decay particles carry no angular momentum (L = 0), the decay is referred to as "allowed", otherwise it is "forbidden".
Other decay modes, which are rare, are known as bound state decay and double beta decay.
Fermi transitions[edit]
A Fermi transition is a beta decay in which the spins of the emitted electron (positron) and anti-neutrino (neutrino) couple to total spin , leading to an angular momentum change between the initial and final states of the nucleus (assuming an allowed transition). In the non-relativistic limit, the nuclear part of the operator for a Fermi transition is given by
with the weak vector coupling constant, the isospin raising and lowering operators, and running over all protons and neutrons in the nucleus.
Gamow–Teller transitions[edit]
A Gamow–Teller transition is a beta decay in which the spins of the emitted electron (positron) and anti-neutrino (neutrino) couple to total spin , leading to an angular momentum change between the initial and final states of the nucleus (assuming an allowed transition). In this case, the nuclear part of the operator is given by
with the weak axial-vector coupling constant, and the spin Pauli matrices, which can produce a spin-flip in the decaying nucleon.
Forbidden transitions[edit]
When L > 0, the decay is referred to as "forbidden". Nuclear selection rules require high L values to be accompanied by changes in nuclear spin (J) and parity (π). The selection rules for the Lth forbidden transitions are:
where Δπ = 1 or −1 corresponds to no parity change or parity change, respectively. The special case of a transition between isobaric analogue states, where the structure of the final state is very similar to the structure of the initial state, is referred to as "superallowed" for beta decay, and proceeds very quickly. The following table lists the ΔJ and Δπ values for the first few values of L:
| Forbiddenness | ΔJ | Δπ |
|---|---|---|
| Superallowed | 0 | no |
| Allowed | 0, 1 | no |
| First forbidden | 0, 1, 2 | yes |
| Second forbidden | 1, 2, 3 | no |
| Third forbidden | 2, 3, 4 | yes |
Rare decay modes[edit]
Bound-state β− decay[edit]
A very small minority of free neutron decays (about four per million) are so-called "two-body decays", in which the proton, electron and antineutrino are produced, but the electron fails to gain the 13.6 eV energy necessary to escape the proton, and therefore simply remains bound to it, as a neutral hydrogen atom.[39] In this type of beta decay, in essence all of the neutron decay energy is carried off by the antineutrino.
For fully ionized atoms (bare nuclei), it is possible in likewise manner for electrons to fail to escape the atom, and to be emitted from the nucleus into low-lying atomic bound states (orbitals). This cannot occur for neutral atoms with low-lying bound states which are already filled by electrons.
Bound-state β decays were predicted by Daudel, Jean, and Lecoin in 1947,[40] and the phenomenon in fully ionized atoms was first observed for 163Dy66+ in 1992 by Jung et al. of the Darmstadt Heavy-Ion Research group. Although neutral 163Dy is a stable isotope, the fully ionized 163Dy66+ undergoes β decay into the K and L shells with a half-life of 47 days.[41]
Another possibility is that a fully ionized atom undergoes greatly accelerated β decay, as observed for 187Re by Bosch et al., also at Darmstadt. Neutral 187Re does undergo β decay with a half-life of 41.6 × 109 years,[42] but for fully ionized 187Re75+ this is shortened to only 32.9 years.[43] For comparison the variation of decay rates of other nuclear processes due to chemical environment is less than 1%.
Double beta decay[edit]
Some nuclei can undergo double beta decay (ββ decay) where the charge of the nucleus changes by two units. Double beta decay is difficult to study, as the process has an extremely long half-life. In nuclei for which both β decay and ββ decay are possible, the rarer ββ decay process is effectively impossible to observe. However, in nuclei where β decay is forbidden but ββ decay is allowed, the process can be seen and a half-life measured.[44] Thus, ββ decay is usually studied only for beta stable nuclei. Like single beta decay, double beta decay does not change A; thus, at least one of the nuclides with some given A has to be stable with regard to both single and double beta decay.
"Ordinary" double beta decay results in the emission of two electrons and two antineutrinos. If neutrinos are Majorana particles (i.e., they are their own antiparticles), then a decay known as neutrinoless double beta decay will occur. Most neutrino physicists believe that neutrinoless double beta decay has never been observed.[44]
See also[edit]
- Neutrino
- Betavoltaics
- Particle radiation
- Radionuclide
- Tritium illumination, a form of fluorescent lighting powered by beta decay
- Pandemonium effect
- Total absorption spectroscopy
References[edit]
- ^ a b c d e Konya, J.; Nagy, N. M. (2012). Nuclear and Radio-chemistry. Elsevier. pp. 74–75. ISBN 978-0-12-391487-3.
- ^ Bijker, R.; Santopinto, E. (2015). "Valence and sea quarks in the nucleon". Journal of Physics: Conference Series. 578 (1): 012015. arXiv:1412.5559. Bibcode:2015JPhCS.578a2015B. doi:10.1088/1742-6596/578/1/012015. S2CID 118499855.
- ^ Cottingham, W. N.; Greenwood, D. A. (1986). An introduction to nuclear physics. Cambridge University Press. p. 40. ISBN 978-0-521-31960-7.
- ^ Basdevant, J.-L.; Rich, J.; Spiro, M. (2005). Fundamentals in Nuclear Physics: From Nuclear Structure to Cosmology. Springer. ISBN 978-0387016726.
- ^ L'Annunziata, Michael (2012). Handbook of Radioactivity Analysis (Third ed.). Elsevier Inc. p. 3. ISBN 9780123848741. Retrieved 4 October 2017.
- ^ a b Jensen, C. (2000). Controversy and Consensus: Nuclear Beta Decay 1911-1934. Birkhäuser Verlag. ISBN 978-3-7643-5313-1.
- ^ Chadwick, J. (1914). "Intensitätsverteilung im magnetischen Spektren der β-Strahlen von Radium B + C". Verhandlungen der Deutschen Physikalischen Gesellschaft (in German). 16: 383–391.
- ^ a b c Brown, L. M. (1978). "The idea of the neutrino". Physics Today. 31 (9): 23–8. Bibcode:1978PhT....31i..23B. doi:10.1063/1.2995181.
- ^ Cowan, C. L., Jr.; Reines, F.; Harrison, F. B.; Kruse, H. W.; McGuire, A. D. (1956). "Detection of the Free Neutrino: a Confirmation". Science. 124 (3212): 103–104. Bibcode:1956Sci...124..103C. doi:10.1126/science.124.3212.103. PMID 17796274.
- ^ "The Nobel Prize in Chemistry 1935". www.nobelprize.org. Retrieved 2018-04-25.
- ^ a b Segré, E. (1987). "K-Electron Capture by Nuclei". In Trower, P. W. (ed.). Discovering Alvarez: Selected Works of Luis W. Alvarez. University of Chicago Press. pp. 11–12. ISBN 978-0-226-81304-2.
- ^ "The Nobel Prize in Physics 1968: Luis Alvarez". The Nobel Foundation. Retrieved 2009-10-07.
- ^ Alvarez, L. W. (1937). "Nuclear K Electron Capture". Physical Review. 52 (2): 134–135. Bibcode:1937PhRv...52..134A. doi:10.1103/PhysRev.52.134.
- ^ Alvarez, L. W. (1938). "Electron Capture and Internal Conversion in Gallium 67". Physical Review. 53 (7): 606. Bibcode:1938PhRv...53..606A. doi:10.1103/PhysRev.53.606.
- ^ Alvarez, L. W. (1938). "The Capture of Orbital Electrons by Nuclei". Physical Review. 54 (7): 486–497. Bibcode:1938PhRv...54..486A. doi:10.1103/PhysRev.54.486.
- ^ Lee, T. D.; Yang, C. N. (1956). "Question of Parity Conservation in Weak Interactions". Physical Review. 104 (1): 254–258. Bibcode:1956PhRv..104..254L. doi:10.1103/PhysRev.104.254.
- ^ Wu, C.-S.; Ambler, E.; Hayward, R. W.; Hoppes, D. D.; Hudson, R. P. (1957). "Experimental Test of Parity Conservation in Beta Decay". Physical Review. 105 (4): 1413–1415. Bibcode:1957PhRv..105.1413W. doi:10.1103/PhysRev.105.1413.
- ^ Weinstock, Maia. "Channeling Ada Lovelace: Chien-Shiung Wu, Courageous Hero of Physics". scientificamerican.com.
- ^ "The Nobel Prize in Physics 1957". The Nobel Foundation. Retrieved March 24,2015.
- ^ Ivanov, A. N.; Höllwieser, R.; Troitskaya, N. I.; Wellenzohn, M.; Berdnikov, Ya. A. (2017-06-26). "Precision theoretical analysis of neutron radiative beta decay to order O ( α 2 / π 2 )". Physical Review D. 95 (11): 113006. arXiv:1706.08687. Bibcode:2017PhRvD..95k3006I. doi:10.1103/PhysRevD.95.113006. ISSN 2470-0010. S2CID 119103283.
- ^ Ivanov, A. N.; Höllwieser, R.; Troitskaya, N. I.; Wellenzohn, M.; Berdnikov, Ya. A. (2018-11-30). "Gauge properties of hadronic structure of nucleon in neutron radiative beta decay to order O(α/π) in standard V − A effective theory with QED and linear sigma model of strong low-energy interactions". International Journal of Modern Physics A. 33 (33): 1850199. arXiv:1805.09702. doi:10.1142/S0217751X18501993. ISSN 0217-751X. S2CID 119088802.
- ^ Loveland, W. D. (2005). Modern Nuclear Chemistry. Wiley. p. 232. ISBN 978-0471115328.
- ^ a b Zuber, K. (2011). Neutrino Physics (2nd ed.). CRC Press. p. 466. ISBN 978-1420064711.
- ^ Jevremovic, T. (2009). Nuclear Principles in Engineering. Springer Science + Business Media. p. 201. ISBN 978-0-387-85608-7.
- ^ "Interactive Chart of Nuclides". National Nuclear Data Center, Brookhaven National Laboratory. Retrieved 2014-09-18.
- ^ "WWW Table of Radioactive Isotopes, Copper 64". LBNL Isotopes Project. Lawrence Berkeley National Laboratory. Archived from the original on 2013-12-14. Retrieved 2014-09-18.
- ^ "WWW Table of Radioactive Isotopes, Potassium 40". LBNL Isotopes Project. Lawrence Berkeley National Laboratory. Archived from the original on 2013-10-09. Retrieved 2014-09-18.
- ^ a b c Kenneth S. Krane (5 November 1987). Introductory Nuclear Physics. Wiley. ISBN 978-0-471-80553-3.
- ^ Nave, C. R. "Energy and Momentum Spectra for Beta Decay". HyperPhysics. Retrieved 2013-03-09.
- ^ Fermi, E. (1934). "Versuch einer Theorie der β-Strahlen. I". Zeitschrift für Physik. 88(3–4): 161–177. Bibcode:1934ZPhy...88..161F. doi:10.1007/BF01351864. S2CID 125763380.
- ^ Mott, N. F.; Massey, H. S. W. (1933). The Theory of Atomic Collisions. Clarendon Press. LCCN 34001940.
- ^ Venkataramaiah, P.; Gopala, K.; Basavaraju, A.; Suryanarayana, S. S.; Sanjeeviah, H. (1985). "A simple relation for the Fermi function". Journal of Physics G. 11 (3): 359–364. Bibcode:1985JPhG...11..359V. doi:10.1088/0305-4616/11/3/014.
- ^ Schenter, G. K.; Vogel, P. (1983). "A simple approximation of the fermi function in nuclear beta decay". Nuclear Science and Engineering. 83 (3): 393–396. doi:10.13182/NSE83-A17574. OSTI 5307377.
- ^ Kurie, F. N. D.; Richardson, J. R.; Paxton, H. C. (1936). "The Radiations Emitted from Artificially Produced Radioactive Substances. I. The Upper Limits and Shapes of the β-Ray Spectra from Several Elements". Physical Review. 49 (5): 368–381. Bibcode:1936PhRv...49..368K. doi:10.1103/PhysRev.49.368.
- ^ Kurie, F. N. D. (1948). "On the Use of the Kurie Plot". Physical Review. 73 (10): 1207. Bibcode:1948PhRv...73.1207K. doi:10.1103/PhysRev.73.1207.
- ^ Rodejohann, W. (2012). "Neutrinoless double beta decay and neutrino physics". Journal of Physics G: Nuclear and Particle Physics. 39 (12): 124008. arXiv:1206.2560. Bibcode:2012JPhG...39l4008R. doi:10.1088/0954-3899/39/12/124008. S2CID 119158221.
- ^ Frauenfelder, H.; et al. (1957). "Parity and the Polarization of Electrons fromCo60". Physical Review. 106 (2): 386–387. Bibcode:1957PhRv..106..386F. doi:10.1103/physrev.106.386.
- ^ Konopinski, E. J.; Rose, M. E. (1966). "The Theory of nuclear Beta Decay". In Siegbhan, K. (ed.). Alpha-, Beta- and Gamma-Ray Spectroscopy. 2. North-Holland Publishing Company.
- ^ An Overview Of Neutron Decay J. Byrne in Quark-Mixing, CKM Unitarity (H. Abele and D. Mund, 2002), see p.XV
- ^ Daudel, Raymond; Jean, Maurice; Lecoin, Marcel (1947). "Sur la possibilité d'existence d'un type particulier de radioactivité phénomène de création e". J. Phys. Radium. 8 (8): 238–243. doi:10.1051/jphysrad:0194700808023800.
- ^ Jung, M.; et al. (1992). "First observation of bound-state β− decay". Physical Review Letters. 69 (15): 2164–2167. Bibcode:1992PhRvL..69.2164J. doi:10.1103/PhysRevLett.69.2164. PMID 10046415.
- ^ Smoliar, M.I.; Walker, R.J.; Morgan, J.W. (1996). "Re-Os ages of group IIA, IIIA, IVA, and IVB iron meteorites". Science. 271 (5252): 1099–1102. Bibcode:1996Sci...271.1099S. doi:10.1126/science.271.5252.1099. S2CID 96376008.
- ^ Bosch, F.; et al. (1996). "Observation of bound-state beta minus decay of fully ionized 187Re: 187Re–187Os Cosmochronometry". Physical Review Letters. 77 (26): 5190–5193. Bibcode:1996PhRvL..77.5190B. doi:10.1103/PhysRevLett.77.5190. PMID 10062738.
- ^ a b Bilenky, S. M. (2010). "Neutrinoless double beta-decay". Physics of Particles and Nuclei. 41 (5): 690–715. arXiv:1001.1946. Bibcode:2010PPN....41..690B. doi:10.1134/S1063779610050035. hdl:10486/663891. S2CID 55217197.
Bibliography[edit]
- Tomonaga, S.-I. (1997). The Story of Spin. University of Chicago Press.
- Tuli, J. K. (2011). Nuclear Wallet Cards (PDF) (8th ed.). Brookhaven National Laboratory.
External links[edit]
 The Live Chart of Nuclides - IAEA with filter on decay type
The Live Chart of Nuclides - IAEA with filter on decay type
In particle physics, proton decay is a hypothetical form of particle decay in which the proton decays into lighter subatomic particles, such as a neutral pion and a positron.[1] The proton decay hypothesis was first formulated by Andrei Sakharov in 1967. Despite significant experimental effort, proton decay has never been observed. If it does decay via a positron, the proton's half-life is constrained to be at least 1.67×1034 years.[2]
According to the Standard Model, the proton, a type of baryon, is stable because baryon number (quark number) is conserved (under normal circumstances; see chiral anomaly for exception). Therefore, protons will not decay into other particles on their own, because they are the lightest (and therefore least energetic) baryon. Positron emission – a form of radioactive decay which sees a proton become a neutron – is not proton decay, since the proton interacts with other particles within the atom.
Some beyond-the-Standard Model grand unified theories (GUTs) explicitly break the baryon number symmetry, allowing protons to decay via the Higgs particle, magnetic monopoles, or new X bosons with a half-life of 1031 to 1036 years. For comparison, the universe is roughly 1010 years old.[3] To date, all attempts to observe new phenomena predicted by GUTs (like proton decay or the existence of magnetic monopoles) have failed.
Quantum tunnelling may be one of the mechanisms of proton decay.[4][5][6]
Quantum gravity[7] (via virtual black holes and Hawking radiation) may also provide a venue of proton decay at magnitudes or lifetimes well beyond the GUT scale decay range above, as well as extra dimensions in supersymmetry.[8][9][10][11]
There are theoretical methods of baryon violation other than proton decay including interactions with changes of baryon and/or lepton number other than 1 (as required in proton decay). These included B and/or L violations of 2, 3, or other numbers, or B − L violation. Such examples include neutron oscillations and the electroweak sphaleron anomaly at high energies and temperatures that can result between the collision of protons into antileptons[12] or vice versa (a key factor in leptogenesis and non-GUT baryogenesis
https://en.wikipedia.org/wiki/Proton_decay
Potassium-40 (40K) is a radioactive isotope of potassium which has a long half-life of 1.251×109 years. It makes up 0.012% (120 ppm) of the total amount of potassium found in nature.
Potassium-40 is a rare example of an isotope that undergoes both types of beta decay. In about 89.28% of events, it decays to calcium-40 (40Ca) with emission of a beta particle (β−, an electron) with a maximum energy of 1.31 MeV and an antineutrino. In about 10.72% of events, it decays to argon-40 (40Ar) by electron capture (EC), with the emission of a neutrino and then a 1.460 MeV gamma ray.[1] The radioactive decay of this particular isotope explains the large abundance of argon (nearly 1%) in the Earth's atmosphere, as well as prevalence of 40Ar over other isotopes. Very rarely (0.001% of events), it decays to 40Ar by emitting a positron (β+) and a neutrino.[2]
https://en.wikipedia.org/wiki/Potassium-40
Artificial production[edit]
Physicists at the Lawrence Livermore National Laboratory in California have used a short, ultra-intense laser to irradiate a millimeter-thick gold target and produce more than 100 billion positrons.[42] Presently significant lab production of 5 MeV positron-electron beams allows investigation of multiple characteristics such as how different elements react to 5 MeV positron interactions or impacts, how energy is transferred to particles, and the shock effect of gamma-ray bursts (GRBs).[43]
Applications[edit]
Certain kinds of particle accelerator experiments involve colliding positrons and electrons at relativistic speeds. The high impact energy and the mutual annihilation of these matter/antimatter opposites create a fountain of diverse subatomic particles. Physicists study the results of these collisions to test theoretical predictions and to search for new kinds of particles.[citation needed]
The ALPHA experiment combines positrons with antiprotons to study properties of antihydrogen.[citation needed]
Gamma rays, emitted indirectly by a positron-emitting radionuclide (tracer), are detected in positron emission tomography (PET) scanners used in hospitals. PET scanners create detailed three-dimensional images of metabolic activity within the human body.[44]
An experimental tool called positron annihilation spectroscopy (PAS) is used in materials research to detect variations in density, defects, displacements, or even voids, within a solid material.[45]
See also[edit]
The PEP reaction[edit]
Deuterium can also be produced by the rare pep (proton–electron–proton) reaction (electron capture):
In the Sun, the frequency ratio of the pep reaction versus the p–p reaction is 1:400. However, the neutrinos released by the pep reaction are far more energetic: while neutrinos produced in the first step of the p–p reaction range in energy up to 0.42 MeV, the pep reaction produces sharp-energy-line neutrinos of 1.44 MeV. Detection of solar neutrinos from this reaction were reported by the Borexino collaboration in 2012.[14]
Both the pep and p–p reactions can be seen as two different Feynman representations of the same basic interaction, where the electron passes to the right side of the reaction as a positron. This is represented in the figure of proton–proton and electron-capture reactions in a star, available at the NDM'06 web site.[15]
https://en.wikipedia.org/wiki/Proton–proton_chain
Antihydrogen (
H
) is the antimatter counterpart of hydrogen. Whereas the common hydrogen atom is composed of an electron and proton, the antihydrogen atom is made up of a positron and antiproton. Scientists hope studying antihydrogen may shed light on the question of why there is more matter than antimatter in the observable universe, known as the baryon asymmetry problem.[1]Antihydrogen is produced artificially in particle accelerators.
https://en.wikipedia.org/wiki/Antihydrogen
The buffer-gas trap (BGT) is a device used to accumulate positrons (the antiparticles of electrons) efficiently while minimizing positron loss due to annihilation, which occurs when an electron and positron collide and the energy is converted to gamma rays. The BGT is used for a variety of research applications, particularly those that benefit from specially tailored positron gases, plasmas and/or pulsed beams. Examples include use of the BGT to create antihydrogen and the positronium molecule.
https://en.wikipedia.org/wiki/Buffer-gas_trap
In particle physics, proton decay is a hypothetical form of particle decay in which the proton decays into lighter subatomic particles, such as a neutral pion and a positron.[1] The proton decay hypothesis was first formulated by Andrei Sakharov in 1967. Despite significant experimental effort, proton decay has never been observed. If it does decay via a positron, the proton's half-life is constrained to be at least 1.67×1034 years.[2]
https://en.wikipedia.org/wiki/Proton_decay
Positronium (Ps) is a system consisting of an electron and its anti-particle, a positron, bound together into an exotic atom, specifically an onium. The system is unstable: the two particles annihilate each other to predominantly produce two or three gamma-rays, depending on the relative spin states. The energy levels of the two particles are similar to that of the hydrogen atom (which is a bound state of a protonand an electron). However, because of the reduced mass, the frequencies of the spectral lines are less than half of those for the corresponding hydrogen lines.
https://en.wikipedia.org/wiki/Positronium
The triple-alpha process is a set of nuclear fusion reactions by which three helium-4 nuclei (alpha particles) are transformed into carbon.[1][2]
https://en.wikipedia.org/wiki/Triple-alpha_process
An example of positron emission (β+ decay) is the decay of magnesium-23 into sodium-23 with a half-life of about 11.3 s:
- 23
12Mg
→ 23
11Na
+
e+
+
ν
e
β+ decay also results in nuclear transmutation, with the resulting element having an atomic number that is decreased by one.
https://en.wikipedia.org/wiki/Beta_decay#β%E2%88%92_decay
For helium-3 to form a superfluid, it must be cooled to a temperature of 0.0025 K, or almost a thousand times lower than helium-4 (2.17 K). This difference is explained by quantum statistics, since helium-3 atoms are fermions, while helium-4 atoms are bosons, which condense to a superfluid more easily.
https://en.wikipedia.org/wiki/Isotopes_of_helium#Helium-2_(diproton)
The CNO cycle (for carbon–nitrogen–oxygen; sometimes called Bethe–Weizsäcker cycle after Hans Albrecht Bethe and Carl Friedrich von Weizsäcker) is one of the two known sets of fusion reactions by which stars convert hydrogen to helium, the other being the proton–proton chain reaction (p-p cycle), which is more efficient at the Sun's core temperature. The CNO cycle is hypothesized to be dominant in stars that are more than 1.3 times as massive as the Sun.[1]
https://en.wikipedia.org/wiki/CNO_cycle
Although the ternary fission process is less common than the binary process, it still produces significant helium-4 and tritium gas buildup in the fuel rods of modern nuclear reactors.[2] This phenomenon was initially detected in 1957, within the environs of the Savannah River National Laboratory.[3]
https://en.wikipedia.org/wiki/Ternary_fission
Nuclear transmutation is the conversion of one chemical element or an isotope into another chemical element.[1] Because any element (or isotope of one) is defined by its number of protons (and neutrons) in its atoms, i.e. in the atomic nucleus, nuclear transmutation occurs in any process where the number of protons or neutrons in the nucleus is changed.
A transmutation can be achieved either by nuclear reactions (in which an outside particle reacts with a nucleus) or by radioactive decay, where no outside cause is needed.
https://en.wikipedia.org/wiki/Nuclear_transmutation
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radioactive decay.
Nuclear fission of heavy elements was discovered on 17 December 1938, by German Otto Hahn and his assistant Fritz Strassmann in cooperation with Austrian-Swedish physicist Lise Meitner. Hahn understood that a "burst" of the atomic nuclei had occurred.[1][2] Meitner explained it theoretically in January 1939 along with her nephew Otto Robert Frisch. Frisch named the process by analogy with biological fission of living cells. For heavy nuclides, it is an exothermic reaction which can release large amounts of energy both as electromagnetic radiation and as kinetic energy of the fragments (heating the bulk material where fission takes place). Like nuclear fusion, in order for fission to produce energy, the total binding energy of the resulting elements must be greater than that of the starting element.
Fission is a form of nuclear transmutation because the resulting fragments (or daughter atoms) are not the same element as the original parent atom. The two (or more) nuclei produced are most often of comparable but slightly different sizes, typically with a mass ratio of products of about 3 to 2, for common fissile isotopes.[3][4] Most fissions are binary fissions (producing two charged fragments), but occasionally (2 to 4 times per 1000 events), three positively charged fragments are produced, in a ternary fission. The smallest of these fragments in ternary processes ranges in size from a proton to an argon nucleus.
https://en.wikipedia.org/wiki/Nuclear_fission
Photodisintegration (also called phototransmutation, or a photonuclear reaction) is a nuclear process in which an atomic nucleus absorbs a high-energy gamma ray, enters an excited state, and immediately decays by emitting a subatomic particle. The incoming gamma ray effectively knocks one or more neutrons, protons, or an alpha particle out of the nucleus.[1] The reactions are called (γ,n), (γ,p), and (γ,α).
https://en.wikipedia.org/wiki/Photodisintegration
Cluster decay, also named heavy particle radioactivity or heavy ion radioactivity, is a rare type of nuclear decay in which an atomic nucleus emits a small "cluster" of neutrons and protons, more than in an alpha particle, but less than a typical binary fission fragment. Ternary fission into three fragments also produces products in the cluster size.
https://en.wikipedia.org/wiki/Cluster_decay
A gamma ray, also known as gamma radiation (symbol γ or ), is a penetrating form of electromagnetic radiation arising from the radioactive decay of atomic nuclei. It consists of the shortest wavelength electromagnetic waves and so imparts the highest photon energy. Paul Villard, a French chemist and physicist, discovered gamma radiation in 1900 while studying radiation emitted by radium. In 1903, Ernest Rutherford named this radiation gamma rays based on their relatively strong penetration of matter; in 1900 he had already named two less penetrating types of decay radiation (discovered by Henri Becquerel) alpha rays and beta rays in ascending order of penetrating power.
https://en.wikipedia.org/wiki/Gamma_ray
Spontaneous fission (SF) is a form of radioactive decay that is found only in very heavy chemical elements. The nuclear binding energy of the elements reaches its maximum at an atomic mass number of about 56; spontaneous breakdown into smaller nuclei and a few isolated nuclear particles becomes possible at greater atomic mass numbers.
https://en.wikipedia.org/wiki/Spontaneous_fission
The slow neutron-capture process, or s-process, is a series of reactions in nuclear astrophysics that occur in stars, particularly AGB stars. The s-process is responsible for the creation (nucleosynthesis) of approximately half the atomic nuclei heavier than iron.
In the s-process, a seed nucleus undergoes neutron capture to form an isotope with one higher atomic mass. If the new isotope is stable, a series of increases in mass can occur, but if it is unstable, then beta decay will occur, producing an element of the next higher atomic number. The process is slow (hence the name) in the sense that there is sufficient time for this radioactive decay to occur before another neutron is captured. A series of these reactions produces stable isotopes by moving along the valley of beta-decay stable isobars in the table of nuclides.
A range of elements and isotopes can be produced by the s-process, because of the intervention of alpha decay steps along the reaction chain. The relative abundances of elements and isotopes produced depends on the source of the neutrons and how their flux changes over time. Each branch of the s-process reaction chain eventually terminates at a cycle involving lead, bismuth, and polonium.
The s-process contrasts with the r-process, in which successive neutron captures are rapid: they happen more quickly than the beta decay can occur. The r-process dominates in environments with higher fluxes of free neutrons; it produces heavier elements and more neutron-rich isotopes than the s-process. Together the two processes account for most of the relative abundance of chemical elements heavier than iron.
https://en.wikipedia.org/wiki/S-process
The oxygen-burning process is a set of nuclear fusion reactions that take place in massive stars that have used up the lighter elements in their cores. Oxygen-burning is preceded by the neon-burning process and succeeded by the silicon-burning process. As the neon-burning process ends, the core of the star contracts and heats until it reaches the ignition temperature for oxygen burning. Oxygen burning reactions are similar to those of carbon burning; however, they must occur at higher temperatures and densities due to the larger Coulomb barrier of oxygen.
https://en.wikipedia.org/wiki/Oxygen-burning_process
The alpha process, also known as the alpha ladder, is one of two classes of nuclear fusion reactions by which stars convert helium into heavier elements, the other being the triple-alpha process.[1] The triple-alpha process consumes only helium, and produces carbon. After enough carbon has accumulated, the reactions below take place, all consuming only helium and the product of the previous reaction.
https://en.wikipedia.org/wiki/Alpha_process
The carbon-burning process or carbon fusion is a set of nuclear fusion reactions that take place in the cores of massive stars (at least 8 at birth) that combines carbon into other elements. It requires high temperatures (> 5×108 K or 50 keV) and densities (> 3×109 kg/m3).[1]
These figures for temperature and density are only a guide. More massive stars burn their nuclear fuel more quickly, since they have to offset greater gravitational forces to stay in (approximate) hydrostatic equilibrium. That generally means higher temperatures, although lower densities, than for less massive stars.[2] To get the right figures for a particular mass, and a particular stage of evolution, it is necessary to use a numerical stellar model computed with computer algorithms.[3] Such models are continually being refined based on nuclear physics experiments (which measure nuclear reaction rates) and astronomical observations (which include direct observation of mass loss, detection of nuclear products from spectrum observations after convection zones develop from the surface to fusion-burning regions – known as dredge-up events – and so bring nuclear products to the surface, and many other observations relevant to models).[4]
Fusion reactions[edit]
The principal reactions are:[5]
12
6C
+ 12
6C
→ 20
10Ne
+ 4
2He
+ 4.617 MeV 12
6C
+ 12
6C
→ 23
11Na
+ 1
1H
+ 2.241 MeV 12
6C
+ 12
6C
→ 23
12Mg
+ 1n − 2.599 MeV Alternatively: 12
6C
+ 12
6C
→ 24
12Mg
+
γ
+ 13.933 MeV 12
6C
+ 12
6C
→ 16
8O
+ 2 4
2He
− 0.113 MeV
https://en.wikipedia.org/wiki/Carbon-burning_process
Carbon Silicide
A silicide is a compound that has silicon with (usually) more electropositive elements.
Silicon is more electropositive than carbon. Silicides are structurally closer to borides than to carbides.
Similar to borides and carbides, the composition of silicides cannot be easily specified as covalent molecules. The chemical bonds in silicides range from conductive metal-like structures to covalent or ionic. Silicides of all non-transition metals, with exception of beryllium, have been described.
https://en.wikipedia.org/wiki/Silicide


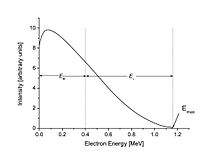


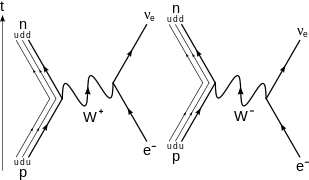
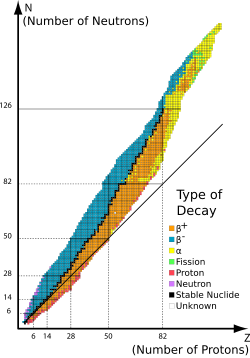










![{\displaystyle Q=\left[m_{N}\left({\ce {^{\mathit {A}}_{\mathit {Z}}X}}\right)-m_{N}\left({\ce {^{\mathit {A}}_{{\mathit {Z}}+1}X'}}\right)-m_{e}-m_{{\overline {\nu }}_{e}}\right]c^{2}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/c5e1eec434e575480cf49bcab3a7f465e7ebc685)





![{\displaystyle Q=\left[m\left({\ce {^{\mathit {A}}_{\mathit {Z}}X}}\right)-m\left({\ce {^{\mathit {A}}_{{\mathit {Z}}+1}X'}}\right)\right]c^{2}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/821caea8313714b516ee3aeb19c7ae54c61a4c20)
![{\displaystyle Q=\left[m_{N}\left({\ce {^{\mathit {A}}_{\mathit {Z}}X}}\right)-m_{N}\left({\ce {^{\mathit {A}}_{{\mathit {Z}}-1}X'}}\right)-m_{e}-m_{\nu _{e}}\right]c^{2}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/72f1a09e1e9730a9b58c78990998ac6b48823fd6)
![{\displaystyle Q=\left[m\left({\ce {^{\mathit {A}}_{\mathit {Z}}X}}\right)-m\left({\ce {^{\mathit {A}}_{{\mathit {Z}}-1}X'}}\right)-2m_{e}\right]c^{2}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/23c492148b9f6db6f0e9b5b410bf0dfdfad0cedc)
![{\displaystyle Q=\left[m_{N}\left({\ce {^{\mathit {A}}_{\mathit {Z}}X}}\right)+m_{e}-m_{N}\left({\ce {^{\mathit {A}}_{{\mathit {Z}}-1}X'}}\right)-m_{\nu _{e}}\right]c^{2}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/e4debc0451faba32fb46957aec92e8eef0872ac5)
![{\displaystyle Q=\left[m\left({\ce {^{\mathit {A}}_{\mathit {Z}}X}}\right)-m\left({\ce {^{\mathit {A}}_{{\mathit {Z}}-1}X'}}\right)\right]c^{2}-B_{n}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/2624d17cab17c7089236309097f02cfcec938dde)



















No comments:
Post a Comment