Directly ionizing radiation[edit]
Ionizing radiation may be grouped as directly or indirectly ionizing.
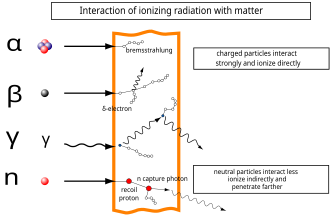
Any charged particle with mass can ionize atoms directly by fundamental interaction through the Coulomb force if it carries sufficient kinetic energy. Such particles include atomic nuclei, electrons, muons, charged pions, protons, and energetic charged nuclei stripped of their electrons. When moving at relativistic speeds (near the speed of light, c) these particles have enough kinetic energy to be ionizing, but there is considerable speed variation. For example, a typical alpha particle moves at about 5% of c, but an electron with 33 eV (just enough to ionize) moves at about 1% of c.
Two of the first types of directly ionizing radiation to be discovered are alpha particles which are helium nuclei ejected from the nucleus of an atom during radioactive decay, and energetic electrons, which are called beta particles.
Natural cosmic rays are made up primarily of relativistic protons but also include heavier atomic nuclei like heliumions and HZE ions. In the atmosphere such particles are often stopped by air molecules, and this produces short-lived charged pions, which soon decay to muons, a primary type of cosmic ray radiation that reaches the surface of the earth. Pions can also be produced in large amounts in particle accelerators.
Alpha particles[edit]
Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. Alpha particle emissions are generally produced in the process of alpha decay.
Alpha particles are a strongly ionizing form of radiation, but when emitted by radioactive decay they have low penetration power and can be absorbed by a few centimeters of air, or by the top layer of human skin. More powerful alpha particles from ternary fission are three times as energetic, and penetrate proportionately farther in air. The helium nuclei that form 10–12% of cosmic rays, are also usually of much higher energy than those produced by radioactive decay and pose shielding problems in space. However, this type of radiation is significantly absorbed by the Earth's atmosphere, which is a radiation shield equivalent to about 10 meters of water.[5]
Alpha particles are named after the first letter in the Greek alphabet, α. The symbol for the alpha particle is α or α2+. Because they are identical to helium nuclei, they are also sometimes written as He2+
or 4
2He2+
indicating a Helium ion with a +2 charge (missing its two electrons). If the ion gains electrons from its environment, the alpha particle can be written as a normal (electrically neutral) helium atom 4
2He.
Beta particles[edit]
Beta particles are high-energy, high-speed electrons or positrons emitted by certain types of radioactive nuclei, such as potassium-40. The production of beta particles is termed beta decay. They are designated by the Greek letterbeta (β). There are two forms of beta decay, β− and β+, which respectively give rise to the electron and the positron.[6] Beta particles are less penetrating than gamma radiation, but more penetrating than alpha particles.
High-energy beta particles may produce X-rays known as bremsstrahlung ("braking radiation") or secondary electrons (delta ray) as they pass through matter. Both of these can cause an indirect ionization effect. Bremsstrahlung is of concern when shielding beta emitters, as the interaction of beta particles with the shielding material produces Bremsstrahlung. This effect is greater with material of high atomic numbers, so material with low atomic numbers is used for beta source shielding.
Positrons and other types of antimatter[edit]
The positron or antielectron is the antiparticle or the antimatter counterpart of the electron. When a low-energy positron collides with a low-energy electron, annihilation occurs, resulting in their conversion into the energy of two or more gamma ray photons (see electron–positron annihilation). As positrons are positively charged particles they can directly ionize an atom through Coulomb interactions.
Positrons can be generated by positron emission nuclear decay (through weak interactions), or by pair production from a sufficiently energetic photon. Positrons are common artificial sources of ionizing radiation used in medical positron emission tomography (PET) scans.
Charged nuclei[edit]
Charged nuclei are characteristic of galactic cosmic rays and solar particle events and except for alpha particles (charged helium nuclei) have no natural sources on the earth. In space, however, very high energy protons, helium nuclei, and HZE ions can be initially stopped by relatively thin layers of shielding, clothes, or skin. However, the resulting interaction will generate secondary radiation and cause cascading biological effects. If just one atom of tissue is displaced by an energetic proton, for example, the collision will cause further interactions in the body. This is called "linear energy transfer" (LET), which utilizes elastic scattering.
LET can be visualized as a billiard ball hitting another in the manner of the conservation of momentum, sending both away with the energy of the first ball divided between the two unequally. When a charged nucleus strikes a relatively slow-moving nucleus of an object in space, LET occurs and neutrons, alpha particles, low-energy protons, and other nuclei will be released by the collisions and contribute to the total absorbed dose of tissue.[7]
Indirectly ionizing radiation[edit]
Indirectly ionizing radiation is electrically neutral and does not interact strongly with matter, therefore the bulk of the ionization effects are due to secondary ionization.
Photon radiation[edit]
Even though photons are electrically neutral, they can ionize atoms indirectly through the photoelectric effect and the Compton effect. Either of those interactions will cause the ejection of an electron from an atom at relativistic speeds, turning that electron into a beta particle (secondary beta particle) that will ionize other atoms. Since most of the ionized atoms are due to the secondary beta particles, photons are indirectly ionizing radiation.[8]
Radiated photons are called gamma rays if they are produced by a nuclear reaction, subatomic particle decay, or radioactive decay within the nucleus. They are called x-rays if produced outside the nucleus. The generic term "photon" is used to describe both.[9][10][11]
X-rays normally have a lower energy than gamma rays, and an older convention was to define the boundary as a wavelength of 10−11 m (or a photon energy of 100 keV).[12] That threshold was driven by historic limitations of older X-ray tubes and low awareness of isomeric transitions. Modern technologies and discoveries have shown an overlap between X-ray and gamma energies. In many fields they are functionally identical, differing for terrestrial studies only in origin of the radiation. In astronomy, however, where radiation origin often cannot be reliably determined, the old energy division has been preserved, with X-rays defined as being between about 120 eV and 120 keV, and gamma rays as being of any energy above 100 to 120 keV, regardless of source. Most astronomical "gamma-ray astronomy" are known not to originate in nuclear radioactive processes but, rather, result from processes like those that produce astronomical X-rays, except driven by much more energetic electrons.
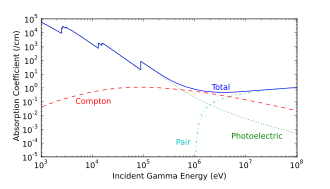
Photoelectric absorption is the dominant mechanism in organic materials for photon energies below 100 keV, typical of classical X-ray tube originated X-rays. At energies beyond 100 keV, photons ionize matter increasingly through the Compton effect, and then indirectly through pair production at energies beyond 5 MeV. The accompanying interaction diagram shows two Compton scatterings happening sequentially. In every scattering event, the gamma ray transfers energy to an electron, and it continues on its path in a different direction and with reduced energy.
Definition boundary for lower-energy photons[edit]
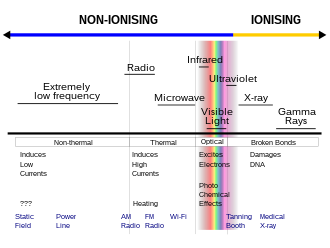
The lowest ionization energy of any element is 3.89 eV, for caesium. However, US Federal Communications Commission material defines ionizing radiation as that with a photon energy greater than 10 eV (equivalent to a far ultraviolet wavelength of 124 nanometers).[13] Roughly, this corresponds to both the first ionization energy of oxygen, and the ionization energy of hydrogen, both about 14 eV.[14] In some Environmental Protection Agency references, the ionization of a typical water molecule at an energy of 33 eV is referenced[15] as the appropriate biological threshold for ionizing radiation: this value represents the so-called W-value, the colloquial name for the ICRU's mean energy expended in a gas per ion pair formed,[16] which combines ionization energy plus the energy lost to other processes such as excitation.[17] At 38 nanometers wavelength for electromagnetic radiation, 33 eV is close to the energy at the conventional 10 nm wavelength transition between extreme ultraviolet and X-ray radiation, which occurs at about 125 eV. Thus, X-ray radiation is always ionizing, but only extreme-ultraviolet radiation can be considered ionizing under all definitions.
Neutrons[edit]
Neutrons have a neutral electrical charge often misunderstood as zero electrical charge and thus often do not directly cause ionization in a single step or interaction with matter. However, fast neutrons will interact with the protons in hydrogen via LET, and this mechanism scatters the nuclei of the materials in the target area, causing direct ionization of the hydrogen atoms. When neutrons strike the hydrogen nuclei, proton radiation (fast protons) results. These protons are themselves ionizing because they are of high energy, are charged, and interact with the electrons in matter.
Neutrons that strike other nuclei besides hydrogen will transfer less energy to the other particle if LET does occur. But, for many nuclei struck by neutrons, inelastic scattering occurs. Whether elastic or inelastic scatter occurs is dependent on the speed of the neutron, whether fast or thermal or somewhere in between. It is also dependent on the nuclei it strikes and its neutron cross section.
In inelastic scattering, neutrons are readily absorbed in a type of nuclear reaction called neutron capture and attributes to the neutron activation of the nucleus. Neutron interactions with most types of matter in this manner usually produce radioactive nuclei. The abundant oxygen-16 nucleus, for example, undergoes neutron activation, rapidly decays by a proton emission forming nitrogen-16, which decays to oxygen-16. The short-lived nitrogen-16 decay emits a powerful beta ray. This process can be written as:
While not a favorable reaction, the 16O (n,p) 16N reaction is a major source of X-rays emitted from the cooling water of a pressurized water reactor and contributes enormously to the radiation generated by a water-cooled nuclear reactor while operating.
For the best shielding of neutrons, hydrocarbons that have an abundance of hydrogen are used.
In fissile materials, secondary neutrons may produce nuclear chain reactions, causing a larger amount of ionization from the daughter products of fission.
Outside the nucleus, free neutrons are unstable and have a mean lifetime of 14 minutes, 42 seconds. Free neutrons decay by emission of an electron and an electron antineutrino to become a proton, a process known as beta decay:[18]
In the adjacent diagram, a neutron collides with a proton of the target material, and then becomes a fast recoil proton that ionizes in turn. At the end of its path, the neutron is captured by a nucleus in an (n,γ)-reaction that leads to the emission of a neutron capture photon. Such photons always have enough energy to qualify as ionizing radiation.
Ionizing radiation (ionising radiation) consists of subatomic particles or electromagnetic waves that have sufficient energy to ionize atoms or moleculesby detaching electrons from them.[1] The particles generally travel at a speed that is greater than 1% of that of light, and the electromagnetic waves are on the high-energy portion of the electromagnetic spectrum.
Gamma rays, X-rays and the higher ultraviolet part of the electromagnetic spectrum are ionizing radiation, whereas the lower energy ultraviolet, visible light, nearly all types of laser light, infrared, microwaves, and radio waves are non-ionizing radiation. The boundary between ionizing and non-ionizing radiation in the ultraviolet area is not sharply defined, since different molecules and atoms ionize at different energies, but is between 10 electronvolts (eV) and 33 eV.
Typical ionizing subatomic particles include alpha particles, beta particles and neutrons. These are typically created due to radioactive decay, and almost all are energetic enough to be ionizing. Secondary cosmic particles produced after cosmic rays interact with Earth's atmosphere, and include muons, mesons, and positrons.[2][3] Cosmic rays may also produce radioisotopes on Earth (for example, carbon-14), which in turn decay and emit ionizing radiation. Cosmic rays and the decay of radioactive isotopes are the primary sources of natural ionizing radiation on Earth, contributing to background radiation. Ionizing radiation is also generated artificially by X-ray tubes, particle accelerators, and nuclear fission.
Ionizing radiation is not detectable by human senses, so instruments such as Geiger counters must be used to detect and measure it. However, very high intensities can produce visible light, such as in Cherenkov radiation.
https://en.wikipedia.org/wiki/Ionizing_radiation


No comments:
Post a Comment