Tuesday, August 10, 2021
08-10-2021-1406 - Conspiracy (Two Doms)
A monoclonal antibody (mAb or moAb) is an antibody made by cloning a unique white blood cell. All subsequent antibodies derived this way trace back to a unique parent cell.
Monoclonal antibodies can have monovalent affinity, binding only to the same epitope (the part of an antigen that is recognized by the antibody). In contrast, polyclonal antibodies bind to multiple epitopes and are usually made by several different antibody-secreting plasma cell lineages. Bispecific monoclonal antibodies can also be engineered, by increasing the therapeutic targets of one monoclonal antibody to two epitopes.
It is possible to produce monoclonal antibodies that specifically bind to virtually any suitable substance; they can then serve to detect or purify it. This capability has become an important tool in biochemistry, molecular biology, and medicine. Monoclonal antibodies are being used on a clinical level for both the diagnosis and therapy of several diseases.[3] The administration of monoclonal antibodies was authorized by several countries for the treatment of moderate COVID-19 symptoms.[4]
https://en.wikipedia.org/wiki/Monoclonal_antibody
A bispecific monoclonal antibody (BsMAb, BsAb) is an artificial protein that can simultaneously bind to two different types of antigen or two different epitopes on the same antigen.[1] Naturally occurring antibodies typically only target one antigen. Upon development, BsAbs can be manufactured in several structural formats. Through different mechanism of action, BsAbs can be designed to recruit and activate immune cells, to interfere with receptor signaling and inactivate signaling ligands, and to force association of protein complexes.[2] BsAbs have advantages compared to ordinary monoclonal antibodies, while BsAbs have problems and disadvantages. The major current applications of BsAbs have been explored for cancer immunotherapy and drug delivery, while BsAbs can also be applied to treat other diseases, including Alzeimer's disease and so on. [1][3]
https://en.wikipedia.org/wiki/Bispecific_monoclonal_antibody
Bivalent and trivalent scFvs[edit]
Divalent (or bivalent) single-chain variable fragments (di-scFvs, bi-scFvs) can be engineered by linking two scFvs. This can be done by producing a single peptide chain with two VH and two VL regions, yielding tandem scFvs.[5][6] Another possibility is the creation of scFvs with linker peptides that are too short for the two variable regions to fold together (about five amino acids), forcing scFvs to dimerize. This type is known as diabodies.[7] Diabodies have been shown to have dissociation constants up to 40-fold lower than corresponding scFvs, meaning that they have a much higher affinity to their target. Consequently, diabody drugs could be dosed much lower than other therapeutic antibodies and are capable of highly specific targeting of tumors in vivo.[8] Still shorter linkers (one or two amino acids) lead to the formation of trimers, so-called triabodies or tribodies. Tetrabodies have also been produced. They exhibit an even higher affinity to their targets than diabodies.[9]
All of these formats can be composed from variable fragments with specificity for two different antigens, in which case they are types of bispecific antibodies.[10][11] The furthest developed of these are bispecific tandem di-scFvs, known as bi-specific T-cell engagers (BiTE antibody constructs).
https://en.wikipedia.org/wiki/Single-chain_variable_fragment#Bivalent_and_trivalent_scFvs
Antibody mimetics are organic compounds that, like antibodies, can specifically bind antigens, but that are not structurally related to antibodies. They are usually artificial peptides or proteins with a molar mass of about 3 to 20 kDa. (Antibodies are ~150 kDa.)
Nucleic acids and small molecules are sometimes considered antibody mimetics as well, but not artificial antibodies, antibody fragments and fusion proteins composed from these.
Common advantages over antibodies are better solubility, tissue penetration, stability towards heat and enzymes, and comparatively low production costs. Antibody mimetics are being developed as therapeutic and diagnostic agents.[1]
https://en.wikipedia.org/wiki/Antibody_mimetic
A protein mimetic is a molecule such as a peptide, a modified peptide or any other molecule that biologically mimics the action or activity of some other protein. Protein mimetics are commonly used in drug design and discovery.
- Phosphomimetics - An amino acid substitution or modification which mimic the effect of protein phosphorylation.
The vertebral column, also known as the backbone or spine, is part of the axial skeleton. The vertebral column is the defining characteristic of a vertebrate in which the notochord (a flexible rod of uniform composition) found in all chordates has been replaced by a segmented series of bone: vertebrae separated by intervertebral discs.[1] Individual vertebrae are named according to their region and position, and can be used as anatomical landmarks in order to guide procedures such as lumbar punctures. The vertebral column houses the spinal canal, a cavity that encloses and protects the spinal cord.
There are about 50,000 species of animals that have a vertebral column.[2] The human vertebral column is one of the most-studied examples. Many different diseases in humans can affect the spine, with Spina bifida and Scoliosis being recognisable examples.
The general structure of human vertebrae is fairly typical of that found in mammals, reptiles, and birds. The shape of the vertebral body does, however, vary somewhat between different groups.
https://en.wikipedia.org/wiki/Vertebral_column
Optimer ligands are short synthetic oligonucleotide molecules composed of DNA or RNA that bind to a specific target molecule. They are engineered to bind their target molecules with affinity typically in the low nanomolar range.[1] Optimers can be used as antibody mimetics in a range of applications,[2][3][4] and have been optimized to increase their stability, reduce their molecular weight, and offer increased scalability and consistency in manufacture compared to standard aptamer molecules.[5]
https://en.wikipedia.org/wiki/Optimer_ligand
Aptamers (from the Latin aptus – fit, and Greek meros – part) are oligonucleotide or peptide molecules that bind to a specific target molecule. Aptamers are usually created by selecting them from a large random sequence pool, but natural aptamers also exist in riboswitches. Aptamers can be used for both basic research and clinical purposes as macromolecular drugs. Aptamers can be combined with ribozymes to self-cleave in the presence of their target molecule. These compound molecules have additional research, industrial and clinical applications.
More specifically, aptamers can be classified as
- DNA or RNA or XNA aptamers. They consist of (usually short) strands of oligonucleotides.
- Peptide aptamers. They consist of one (or more) short variable peptide domains, attached at both ends to a protein scaffold.
Nucleic acid analogues are compounds which are analogous (structurally similar) to naturally occurring RNA and DNA, used in medicine and in molecular biology research. Nucleic acids are chains of nucleotides, which are composed of three parts: a phosphate backbone, a pentose sugar, either ribose or deoxyribose, and one of four nucleobases. An analogue may have any of these altered.[1] Typically the analogue nucleobases confer, among other things, different base pairing and base stacking properties. Examples include universal bases, which can pair with all four canonical bases, and phosphate-sugar backbone analogues such as PNA, which affect the properties of the chain (PNA can even form a triple helix).[2] Nucleic acid analogues are also called Xeno Nucleic Acid and represent one of the main pillars of xenobiology, the design of new-to-nature forms of life based on alternative biochemistries.
Artificial nucleic acids include peptide nucleic acid (PNA), Morpholino and locked nucleic acid (LNA), as well as glycol nucleic acid (GNA), threose nucleic acid (TNA) and hexitol nucleic acids (HNA). Each of these is distinguished from naturally occurring DNA or RNA by changes to the backbone of the molecule.
In May 2014, researchers announced that they had successfully introduced two new artificial nucleotides into bacterial DNA, and by including individual artificial nucleotides in the culture media, were able to passage the bacteria 24 times; they did not create mRNA or proteins able to use the artificial nucleotides. The artificial nucleotides featured 2 fused aromatic rings.
https://en.wikipedia.org/wiki/Nucleic_acid_analogue
The nucleic acid notation currently in use was first formalized by the International Union of Pure and Applied Chemistry (IUPAC) in 1970.[1] This universally accepted notation uses the Roman characters G, C, A, and T, to represent the four nucleotides commonly found in deoxyribonucleic acids (DNA).
Given the rapidly expanding role for genetic sequencing, synthesis, and analysis in biology, some researchers have developed alternate notations to further support the analysis and manipulation of genetic data. These notations generally exploit size, shape, and symmetry to accomplish these objectives.
https://en.wikipedia.org/wiki/Nucleic_acid_notation
A locked nucleic acid (LNA), also known as bridged nucleic acid (BNA),[1] and often referred to as inaccessible RNA, is a modified RNA nucleotide in which the ribose moiety is modified with an extra bridge connecting the 2' oxygen and 4' carbon. The bridge "locks" the ribose in the 3'-endo (North) conformation, which is often found in the A-form duplexes. This structure can be attributed to the increased stability against enzymatic degradation;[2][3][4][5] moreover the structure of LNA has improved specificity and affinity as a monomer or a constituent of an oligonucleotide.[6] LNA nucleotides can be mixed with DNA or RNA residues in the oligonucleotide, in effect hybridizing with DNA or RNA according to Watson-Crick base-pairing rules.
https://en.wikipedia.org/wiki/Locked_nucleic_acid
A-DNA is one of the possible double helical structures which DNA can adopt. A-DNA is thought to be one of three biologically active double helical structures along with B-DNA and Z-DNA. It is a right-handed double helix fairly similar to the more common B-DNA form, but with a shorter, more compact helical structure whose base pairs are not perpendicular to the helix-axis as in B-DNA. It was discovered by Rosalind Franklin, who also named the A and B forms. She showed that DNA is driven into the A form when under dehydrating conditions. Such conditions are commonly used to form crystals, and many DNA crystal structures are in the A form.[1] The same helical conformation occurs in double-stranded RNAs, and in DNA-RNA hybrid double helices.
https://en.wikipedia.org/wiki/A-DNA
A glycosyl group is a univalent free radical or substituent structure obtained by removing the hemiacetal hydroxyl group from the cyclic form of a monosaccharide and, by extension, of a lower oligosaccharide. Glycosyl also reacts with inorganic acids, such as phosphoric acid, forming an ester such as glucose 1-phosphate.[1]
https://en.wikipedia.org/wiki/Glycosyl
Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a weak acid with the chemical formula H
3PO
4. The pure compound is a colorless solid.
All three hydrogens are acidic to varying degrees and can be lost from the molecule as H+ ions (protons). When all three H+ ions are removed, the result is an orthophosphate ion PO43−, commonly called "phosphate". Removal of one or two protons gives dihydrogen phosphate ion H
2PO−
4, and the hydrogen phosphate ion HPO2−
4, respectively. Orthophosphoric acid also forms esters, called organophosphates.[15]
Phosphoric acid is commonly encountered in chemical laboratories as an 85% aqueous solution, which is a colourless, odourless, and non-volatile syrupy liquid. Although phosphoric acid does not meet the strict definition of a strong acid, the 85% solution can still severely irritate the skin and damage the eyes.
The name "orthophosphoric acid" can be used to distinguish this specific acid from other "phosphoric acids", such as pyrophosphoric acid. Nevertheless, the term "phosphoric acid" often means this specific compound; and that is the current IUPAC nomenclature.
https://en.wikipedia.org/wiki/Phosphoric_acid
Pyrophosphoric acid, also known as diphosphoric acid, is the inorganic compound with the formula H4P2O7 or, more descriptively, [(HO)2P(O)]2O. Colorless and odorless, it is soluble in water, diethyl ether, and ethyl alcohol. The anhydrous acid crystallizes in two polymorphs, which melt at 54.3 °C and 71.5 °C. The compound is not particularly useful, except that it is a component of polyphosphoric acid and the conjugate acid of the pyrophosphate anion. Anions, salts, and esters of pyrophosphoric acid are called pyrophosphates.
https://en.wikipedia.org/wiki/Pyrophosphoric_acid
In chemistry, a dehydration reaction is a chemical reaction that involves the loss of water from the reacting molecule or ion. Dehydration reactions are common processes, the reverse of a hydration reaction.
https://en.wikipedia.org/wiki/Dehydration_reaction
Desiccation (from Latin de- "thoroughly" + siccare "to dry") is the state of extreme dryness, or the process of extreme drying. A desiccant is a hygroscopic (attracts and holds water) substance that induces or sustains such a state in its local vicinity in a moderately sealed container.
https://en.wikipedia.org/wiki/Desiccation
Decussation is used in biological contexts to describe a crossing (due to the shape of the Roman numeral for ten, an uppercase 'X', which in Latin is called decussis, from decem 'ten' and as 'as'). In Latin anatomical terms, the form decussatio is used, e.g. decussatio pyramidum.
Similarly, the anatomical term chiasma is named after the Greek uppercase 'Χ' (chi). Whereas a decussation refers to a crossing within the central nervous system, various kinds of crossings in the peripheral nervous system are called chiasma.
Examples include:
- In the brain, where nerve fibers obliquely cross from one lateral side of the brain to the other, that is to say they cross at a level other than their origin. See for examples Decussation of pyramids and sensory decussation. In neuroanatomy, the term chiasma is reserved for crossing of- or within nerves such as in the optic chiasm.
- In botanical leaf taxology, the word decussate describes an opposite pattern of leaves which has successive pairs at right angles to each other (i.e. rotated 90 degrees along the stem when viewed from above). In effect, successive pairs of leaves cross each other. Basil is a classic example of a decussate leaf pattern.
- In tooth enamel, where bundles of rods cross each other as they travel from the enamel-dentine junction to the outer enamel surface, or near to it.
- In taxonomic description where decussate markings or structures occur, names such as decussatus or decussata or otherwise in part containing "decuss..." are common, especially in the specific epithet.[1]
| Chiasm | |
|---|---|
 Schema of the optic chiasm and the trochlear chiasm in vertebrates. | |
| Details | |
| Function | Anatomical feature where two structures cross |
| Identifiers | |
| Latin | chiasma |
| Anatomical terms of neuroanatomy | |
In anatomy a chiasm is the spot where two structures cross, forming an X-shape (from Greek letter χ, Chi). This can be:
- A tendinous chiasm is the spot where two tendons cross. For example, the tendon of the flexor digitorum superficialis muscle, and the tendon of the flexor digitorum longus muscle which even forms two chiasms.
- In neuroanatomy, a chiasm is the crossing of fibres of a nerve or the crossing of two nerves.[1]
Very different types of crossings of nerves are referred to as chiasm:
- Type I : Two nerves can cross one over the other (sagittal plane) without fusing, e.g., the trochlear nerve (see figure).
- Type II :Two nerves can merge while at least part of the fibres cross the midline (see figure 2).
- Type III : The fibres within a single nerve cross, such that the order of the functional map is reversed, e.g., the optic chiasms of various invertebrates such as insects[2] and cephalopods.[3]
- Type IV : A torsion or loop by 180 degrees of a nerve can also reverse the order of the functional map. This type is usually not referred to as chiasm.
Note that in the third type there is no crossing of the mid sagittal plane. Only in the first type, the crossing is complete.
There are other kinds of crossings of nerve fibres. The chiasm is distinguished from a decussation, which is a crossing of nerve fibres inside the central nervous system. A chiasm also differs from a ganglion in that axons run through it without making any synapses. A chiasm is thus not a nervous processing centre.
https://en.wikipedia.org/wiki/Chiasm_(anatomy)
Canthus (pl. canthi, palpebral commissures) is either corner of the eye where the upper and lower eyelids meet.[1] More specifically, the inner and outer canthi are, respectively, the medial and lateral ends/angles of the palpebral fissure.
The bicanthal plane is the transversal plane linking both canthi and defines the upper boundary of the midface.
https://en.wikipedia.org/wiki/Canthus
Nerve fibre crossings[edit]
Specific terms are also used to describe the route of a nerve or nerve fibre:
A chiasm (from Greek 'Chi') is used to describe different types of crossings of or within peripheral nerve fibres between the cerebral hemispheres. The major example in the human brain is the Optic chiasm.
A decussation (from Latin 'from 'deca', 10, which is written as a capital X') refers to nerve fibers that cross the sagittal plane from one side of the central nervous system to the other, and connect different brain regions. There are two kinds:
- Type 1 crosses the sagittal plane in the same brain region or spinal segment where the cell body is located. Examples are Mauthner cells in fish and amphibians.
- Type 2 crosses the sagittal plane in a different brain region. Example: pyramidal decussations.
The first type is known also for invertebrates, whereas the second type only occurs in vertebrates. The second type is thought to be due to an axial twist.
A commissure is a bilateral connection of axons connecting the left and right side of the same brain region. For example, nerve fibre tracts that cross between the two cerebral hemispheres, are the anterior commissure, posterior commissure, corpus callosum, hippocampal commissure, and habenular commissure. The spinal cord contains a commissure as well: the anterior white commissure.
A ganglion can also have the form of crossing nerves, but a ganglion always contains synapses between neurons as well as their cell bodies. The other kinds of nerve crossings never contain synapses of cell bodies of neurons.
The difference between a chiasm and a decussation is that the first refers to peripheral nerves whereas the latter refers to crossings inside central nervous system. A commissure connects the same brain region of each side whereas a decussation connects different brain regions.
Brain[edit]
Specific terms are used to represent the gross anatomy of the brain:
A gyrus is an outward folding of the brain, for example the precentral gyrus. A sulcus is an inward fold, or valley in the brain's surface - for example the central sulcus. Additional terms used to describe these may include:
- Annectent gyrus, for a small gyrus hidden in the depth of a sulcus
- sulcal fundus, for the bottom of a sulcus, an inward fold
A fissure is used to describe:
- A deep groove produced by opercularisation. An example is the Sylvian Fissure.
- A deep groove produced by the differentiation of the telencephalic vesicles. An example is the longitudinal fissure, also known as the interhemispheric fissure.
https://en.wikipedia.org/wiki/Anatomical_terms_of_neuroanatomy#Nerve_fibre_crossings
Virtual Fly Brain, or VFB, is an interactive, web-based tool that allows neurobiologists to explore the detailed neuroanatomy, transgene expression and associated phenotypes of the Drosophila melanogaster brain.[1] Users can browse painted 3D image stacks of the Drosophila brain, choosing any plane of section they want and clicking on painted regions to find names' definitions, references and synonyms for the chosen region. For each region, they can run queries to find neurons, transgene expression and phenotypes. For each neuron found, users can browse definitions, references and synonyms.
https://en.wikipedia.org/wiki/Virtual_Fly_Brain
| Look up supraesophageal in Wiktionary, the free dictionary. |
The supraesophageal ganglion (also "supraoesophageal ganglion", "arthropod brain" or "microbrain"[1]) is the first part of the arthropod, especially insect, central nervous system. It receives and processes information from the first, second, and third metameres. The supraesophageal ganglion lies dorsal to the esophagus and consists of three parts, each a pair of ganglia that may be more or less pronounced, reduced, or fused depending on the genus:
- The protocerebrum, associated with the eyes (compound eyes and ocelli).[2] Directly associated with the eyes is the optic lobe, as the visual center of the brain.
- The deutocerebrum processes sensory information from the antennae.[2][3] It consists of two parts, the antennal lobe and the dorsal lobe.[3][4][5] The dorsal lobe also contains motor neurons which control the antennal muscles.[6]
- The tritocerebrum integrates sensory inputs from the previous two pairs of ganglia.[2] The lobes of the tritocerebrum split to circumvent the esophagus and begin the subesophageal ganglion.
The subesophageal ganglion continues the nervous system and lies ventral to the esophagus. Finally, the segmental ganglia of the ventral nerve cord are found in each body segment as a fused ganglion; they provide the segments with some autonomous control.
A locust brain dissection to expose the central brain and carry out electro-physiology recordings can be seen here. [7]
https://en.wikipedia.org/wiki/Supraesophageal_ganglion
Pigment dispersing factor (pdf) is a gene that encodes the protein PDF, which is part of a large family of neuropeptides.[1] Its hormonal product, pigment dispersing hormone (PDH), was named for the diurnal pigment movement effect it has in crustacean retinal cells upon its initial discovery in the central nervous system of arthropods.[1] The movement and aggregation of pigments in retina cells and extra-retinal cells is hypothesized to be under a split hormonal control mechanism.[1] One hormonal set is responsible for concentrating chromatophoral pigment by responding to changes in the organism's exposure time to darkness. Another hormonal set is responsible for dispersion and responds to the light cycle.[1] However, insect pdf genes do not function in such pigment migration since they lack the chromatophore.[2]
The gene was first isolated and studied in Drosophila by Jeffrey C. Hall's laboratory at Brandeis University in 1998, and has been found to function as a neuromodulator and coupling factor in controlling circadian rhythms.[3][4] A neuromodulator is a neuroregulator that can act on other neurons in close proximity or far away, altering the effect of neurotransmitters without itself initiating depolarization.[5]
https://en.wikipedia.org/wiki/Pigment_dispersing_factor
A spinal interneuron, found in the spinal cord, relays signals between (afferent) sensory neurons, and (efferent) motor neurons. Different classes of spinal interneurons are involved in the process of sensory-motor integration.[1] Most interneurons are found in the grey column, a region of grey matter in the spinal cord.
https://en.wikipedia.org/wiki/Spinal_interneuron
Accessory olfactory cortical areas are portions of the human amygdala that are homologous to those areas in other species that receive afferents from the accessory olfactory bulb. They include the caudal part of the medial amygdalar nucleus, and the cortical amygdalar nucleus.[1]
The trigeminal tubercle, or tuberculum cinereum[1] is a raised area between the rootlets of the accessory nerve and posterolateral sulcus. It overlies the spinal tract of the trigeminal nerve. It is an elevation in the lower part of medulla, lateral to the cuneate fasciculus, produced by a mass of grey matter called the spinal trigeminal nucleus.
The Protomap is a primordial molecular map of the functional areas of the mammalian cerebral cortex during early embryonic development, at a stage when neural stem cells are still the dominant cell type.[1] The protomap is a feature of the ventricular zone, which contains the principal cortical progenitor cells, known as radial glial cells.[2][3] Through a process called 'cortical patterning', the protomap is patterned by a system of signaling centers in the embryo, which provide positional information and cell fate instructions.[4][5][6] These early genetic instructions set in motion a development and maturation process that gives rise to the mature functional areas of the cortex, for example the visual, somatosensory, and motor areas. The term protomap was coined by Pasko Rakic.[1] The protomap hypothesis was opposed by the protocortex hypothesis, which proposes that cortical proto-areas initially have the same potential,[7][8] and that regionalization in large part is controlled by external influences, such as axonal inputs from the thalamus to the cortex.[9] However, a series of papers in the year 2000 and in 2001 provided strong evidence against the protocortex hypothesis, and the protomap hypothesis has been well accepted since then.[5][10][11] The protomap hypothesis, together with the related radial unit hypothesis, forms our core understanding of the embryonic development of the cerebral cortex. Once the basic structure is present and cortical neurons have migrated to their final destinations, many other processes contribute to the maturation of functional cortical circuits.[12]
See also[edit]
- Radial unit hypothesis
- Neural stem cell
- Stem cell
- Neurogenesis
- Cellular differentiation
- Cortical patterning
- Gyrification
Cortical patterning is a field of developmental neuroscience which aims to determine how the various functional areas of the cerebral cortex are generated, what size and shape they will be, and how their spatial pattern across the surface of the cortex is specified. Early brain lesion studies indicated that different parts of the cortex served different cognitive functions, such as visual, somatosensory, and motor functions, beautifully assimilated by Brodmann in 1909.[1] Today the field supports the idea of a 'protomap', which is a molecular pre-pattern of the cortical areas during early embryonic stages.[2] The protomap is a feature of the cortical ventricular zone, which contains the primary stem cells of the cortex known as radial glial cells. A system of signaling centers, positioned strategically at the midline and edges of the cortex, produce secreted signaling proteins that establish concentration gradients in the cortical primordium.[3][4][5] This provides positional information for each stem cell, and regulates proliferation, neurogenesis, and areal identity. After the initial establishment of areal identity, axons from the developing thalamus arrive at their correct cortical areal destination through the process of axon guidance and begin to form synapses. Many activity-dependent processes are then thought to play important roles in the maturation of each area.[6]
See also[edit]
- Protomap
- Radial unit hypothesis
- Neural stem cell
- Stem cell
- Neurogenesis
- Cellular differentiation
- Gyrification
Molecular diffusion, often simply called diffusion, is the thermal motion of all (liquid or gas) particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size (mass) of the particles. Diffusion explains the net flux of molecules from a region of higher concentration to one of lower concentration. Once the concentrations are equal the molecules continue to move, but since there is no concentration gradient the process of molecular diffusion has ceased and is instead governed by the process of self-diffusion, originating from the random motion of the molecules. The result of diffusion is a gradual mixing of material such that the distribution of molecules is uniform. Since the molecules are still in motion, but an equilibrium has been established, the result of molecular diffusion is called a "dynamic equilibrium". In a phase with uniform temperature, absent external net forces acting on the particles, the diffusion process will eventually result in complete mixing.
Consider two systems; S1 and S2 at the same temperature and capable of exchanging particles. If there is a change in the potential energy of a system; for example μ1>μ2 (μ is Chemical potential) an energy flow will occur from S1 to S2, because nature always prefers low energy and maximum entropy.
Molecular diffusion is typically described mathematically using Fick's laws of diffusion.
https://en.wikipedia.org/wiki/Molecular_diffusion
Gyrification is the process of forming the characteristic folds of the cerebral cortex.[1]
The peak of such a fold is called a gyrus (plural: gyri), and its trough is called a sulcus (plural: sulci). The neurons of the cerebral cortex reside in a thin layer of gray matter, only 2–4 mm thick, at the surface of the brain.[2] Much of the interior volume is occupied by white matter, which consists of long axonal projections to and from the cortical neurons residing near the surface. Gyrification allows a larger cortical surface area and hence greater cognitive functionality to fit inside a smaller cranium. In most mammals, gyrification begins during fetal development. Primates, cetaceans, and ungulates have extensive cortical gyri, with a few species exceptions, while rodents generally have none. Gyrification in some animals, for example the ferret, continues well into postnatal life.[3]
https://en.wikipedia.org/wiki/Gyrification
In vertebrates, the ventricular zone (VZ) is a transient embryonic layer of tissue containing neural stem cells, principally radial glial cells, of the central nervous system (CNS).[1][2] The VZ is so named because it lines the ventricular system, which contains cerebrospinal fluid (CSF). The embryonic ventricular system contains growth factors and other nutrients needed for the proper function of neural stem cells.[3] Neurogenesis, or the generation of neurons, occurs in the VZ during embryonic and fetal development as a function of the Notch pathway,[4][5] and the newborn neurons must migrate substantial distances to their final destination in the developing brain or spinal cord where they will establish neural circuits.[6][7] A secondary proliferative zone, the subventricular zone (SVZ), lies adjacent to the VZ. In the embryonic cerebral cortex, the SVZ contains intermediate neuronal progenitors that continue to divide into post-mitotic neurons.[8][9] Through the process of neurogenesis, the parent neural stem cell pool is depleted and the VZ disappears.[10] The balance between the rates of stem cell proliferation and neurogenesis changes during development,[11] and species from mouse to human show large differences in the number of cell cycles, cell cycle length, and other parameters, which is thought to give rise to the large diversity in brain size and structure.
Epigenetic DNA modifications appear to have a central role in regulating gene expression during differentiation of neural stem cells. One type of epigenetic modification occurring in the VZ is the formation of DNA 5-Methylcytosine from cytosine by DNA methyltransferases.[12] Another important type of epigenetic modification is the demethylation of 5mC catalyzed in several steps by TET enzymes and enzymes of the base excision repair pathway.[12]
See also[edit]
Cerebrospinal fluid (CSF) is a clear, colorless body fluid found within the tissue that surrounds the brain and spinal cord of all vertebrates.
CSF is produced by specialised ependymal cells in the choroid plexus of the ventricles of the brain, and absorbed in the arachnoid granulations. There is about 125 mL of CSF at any one time, and about 500 mL is generated every day. CSF acts as a cushion or buffer, providing basic mechanical and immunological protection to the brain inside the skull. CSF also serves a vital function in the cerebral autoregulation of cerebral blood flow.
The CSF occupies the subarachnoid space (between the arachnoid mater and the pia mater) and the ventricular system around and inside the brain and spinal cord. It fills the ventricles of the brain, cisterns, and sulci, as well as the central canal of the spinal cord. There is also a connection from the subarachnoid space to the bony labyrinth of the inner ear via the perilymphatic duct where the perilymph is continuous with the cerebrospinal fluid. The ependymal cells of the choroid plexus have multiple motile cilia on their apical surfaces that beat to move the CSF through the ventricles.
A sample of CSF can be taken from around the spinal cord via lumbar puncture. This can used to test the intracranial pressure, as well as indicate diseases including infections of the brain or the surrounding meninges.
Although noted by Hippocrates, it was forgotten for centuries, though later was described in the 18th century by Emanuel Swedenborg. In 1914, Harvey Cushing demonstrated that the CSF was secreted by the choroid plexus.
https://en.wikipedia.org/wiki/Cerebrospinal_fluid
Intracranial pressure (ICP) is the pressure exerted by fluids such as cerebrospinal fluid (CSF) inside the skull and on the brain tissue. ICP is measured in millimeters of mercury (mmHg) and at rest, is normally 7–15 mmHg for a supine adult.[1] The body has various mechanisms by which it keeps the ICP stable, with CSF pressures varying by about 1 mmHg in normal adults through shifts in production and absorption of CSF.
Changes in ICP are attributed to volume changes in one or more of the constituents contained in the cranium. CSF pressure has been shown to be influenced by abrupt changes in intrathoracic pressure during coughing (which is induced by contraction of the diaphragm and abdominal wall muscles, the latter of which also increases intra-abdominal pressure), the valsalva maneuver, and communication with the vasculature (venous and arterial systems).
Intracranial hypertension (IH), also called increased ICP (IICP) or raised intracranial pressure (RICP), is elevation of the pressure in the cranium. ICP is normally 7–15 mm Hg; at 20–25 mm Hg, the upper limit of normal, treatment to reduce ICP may be needed.[2]
https://en.wikipedia.org/wiki/Intracranial_pressure
Papilledema or papilloedema is optic disc swelling that is caused by increased intracranial pressure due to any cause. The swelling is usually bilateral and can occur over a period of hours to weeks.[1] Unilateral presentation is extremely rare.
In intracranial hypertension, the optic disc swelling most commonly occurs bilaterally. When papilledema is found on fundoscopy, further evaluation is warranted because vision loss can result if the underlying condition is not treated. Further evaluation with a CT or MRI of the brain and/or spine is usually performed. Recent research has shown that point-of-care ultrasound can be used to measure optic nerve sheath diameter for detection of increased intracranial pressure and shows good diagnostic test accuracy compared to CT.[2] Thus, if there is a question of papilledema on fundoscopic examination or if the optic disc cannot be adequately visualized, ultrasound can be used to rapidly assess for increased intracranial pressure and help direct further evaluation and intervention. Unilateral papilledema can suggest a disease in the eye itself, such as an optic nerve glioma.
https://en.wikipedia.org/wiki/Papilledema
The optic disc or optic nerve head is the point of exit for ganglion cell axons leaving the eye. Because there are no rods or cones overlying the optic disc, it corresponds to a small blind spot in each eye.
The ganglion cell axons form the optic nerve after they leave the eye. The optic disc represents the beginning of the optic nerve and is the point where the axons of retinal ganglion cells come together. The optic disc is also the entry point for the major blood vessels that supply the retina.[1] The optic disc in a normal human eye carries 1–1.2 million afferent nerve fibers from the eye towards the brain.
https://en.wikipedia.org/wiki/Optic_disc
Afferent nerve fibers are the axons (nerve fibers) carried by a sensory nerve that relay sensory information from sensory receptors to regions of the brain. Afferent projections arrive at a particular brain region. Efferent nerve fibers are carried by efferent nerves and exit a region to act on muscles and glands.
In the peripheral nervous system afferent and efferent nerve fibers are part of the somatic nervous system and arise from outside of the spinal cord. Sensory nerves carry the afferent fibers to enter into the spinal cord, and motor nerves carry the efferent fibers out of the spinal cord to act on skeletal muscles.
In the central nervous system non-motor efferents are carried in efferent nerves to act on glands.[1][2][3]
https://en.wikipedia.org/wiki/Afferent_nerve_fiber
Efferent nerve fibers refer to axonal projections that exit a particular region; as opposed to afferent projections that arrive at the region. These terms have a slightly different meaning in the context of the peripheral nervous system (PNS) and central nervous system (CNS). The efferent fiber is a long process projecting far from the neuron's body that carries nerve impulses away from the central nervous system toward the peripheral effector organs (mainly muscles and glands). A bundle of these fibers is called an efferent nerve (if it connects to muscles, then it is a motor nerve[1]). The opposite direction of neural activity is afferent conduction,[2][3][4] which carries impulses by way of the afferent nerve fibers of sensory neurons.
In the nervous system there is a "closed loop" system of sensation, decision, and reactions. This process is carried out through the activity of sensory neurons, interneurons, and motor neurons.
In the CNS, afferent and efferent projections can be from the perspective of any given brain region. That is, each brain region has its own unique set of afferent and efferent projections. In the context of a given brain region, afferents are arriving fibers while efferents are exiting fibers.
https://en.wikipedia.org/wiki/Efferent_nerve_fiber
| General visceral efferent fibers | |
|---|---|
 Scheme showing structure of a typical spinal nerve. 1. Somatic efferent. 2. Somatic afferent. 3,4,5. Sympathetic efferent. 6,7. Sympathetic afferent. | |
| Anatomical terminology |
General visceral efferent fibers (GVE) or visceral efferents or autonomic efferents, are the efferent nerve fibers of the autonomic nervous system (also known as the visceral efferent nervous system that provide motor innervation to smooth muscle, cardiac muscle, and glands (contrast with special visceral efferent (SVE) fibers) through postganglionic varicosities.[1][2]
GVE fibers may be either sympathetic or parasympathetic.[3]
The cranial nerves containing GVE fibers include the oculomotor nerve (CN III), the facial nerve (CN VII), the glossopharyngeal nerve (CN IX) and the vagus nerve (CN X).[4]
Additional images[edit]
See also[edit]
https://en.wikipedia.org/wiki/General_visceral_efferent_fibers
Intracellular signaling[edit]
During axonal development, the activity of PI3K is increased at the tip of destined axon. Disrupting the activity of PI3K inhibits axonal development. Activation of PI3K results in the production of phosphatidylinositol (3,4,5)-trisphosphate (PtdIns) which can cause significant elongation of a neurite, converting it into an axon. As such, the overexpression of phosphatases that dephosphorylate PtdIns leads into the failure of polarization.[28]
Cytoskeletal dynamics[edit]
The neurite with the lowest actin filament content will become the axon. PGMS concentration and f-actin content are inversely correlated; when PGMS becomes enriched at the tip of a neurite, its f-actin content is substantially decreased.[34] In addition, exposure to actin-depolimerizing drugs and toxin B (which inactivates Rho-signaling) causes the formation of multiple axons. Consequently, the interruption of the actin network in a growth cone will promote its neurite to become the axon.[35]
Growth[edit]
Growing axons move through their environment via the growth cone, which is at the tip of the axon. The growth cone has a broad sheet-like extension called a lamellipodium which contain protrusions called filopodia. The filopodia are the mechanism by which the entire process adheres to surfaces and explores the surrounding environment. Actin plays a major role in the mobility of this system. Environments with high levels of cell adhesion molecules (CAMs) create an ideal environment for axonal growth. This seems to provide a "sticky" surface for axons to grow along. Examples of CAM's specific to neural systems include N-CAM, TAG-1—an axonal glycoprotein—[36]—and MAG, all of which are part of the immunoglobulin superfamily. Another set of molecules called extracellular matrix-adhesion molecules also provide a sticky substrate for axons to grow along. Examples of these molecules include laminin, fibronectin, tenascin, and perlecan. Some of these are surface bound to cells and thus act as short range attractants or repellents. Others are difusible ligands and thus can have long range effects.
Cells called guidepost cells assist in the guidance of neuronal axon growth. These cells that help axon guidance, are typically other neurons that are sometimes immature. When the axon has completed its growth at its connection to the target, the diameter of the axon can increase by up to five times, depending on the speed of conduction required.[37]
It has also been discovered through research that if the axons of a neuron were damaged, as long as the soma (the cell body of a neuron) is not damaged, the axons would regenerate and remake the synaptic connections with neurons with the help of guidepost cells. This is also referred to as neuroregeneration.[38]
Nogo-A is a type of neurite outgrowth inhibitory component that is present in the central nervous system myelin membranes (found in an axon). It has a crucial role in restricting axonal regeneration in adult mammalian central nervous system. In recent studies, if Nogo-A is blocked and neutralized, it is possible to induce long-distance axonal regeneration which leads to enhancement of functional recovery in rats and mouse spinal cord. This has yet to be done on humans.[39] A recent study has also found that macrophages activated through a specific inflammatory pathway activated by the Dectin-1 receptor are capable of promoting axon recovery, also however causing neurotoxicity in the neuron.[40]
Length regulation[edit]
Axons vary largely in length from a few micrometers up to meters in some animals. This emphasizes that there must be a cellular length regulation mechanism allowing the neurons both to sense the length of their axons and to control their growth accordingly. It was discovered that motor proteins play an important role in regulating the length of axons.[41] Based on this observation, researchers developed an explicit model for axonal growth describing how motor proteins could affect the axon length on the molecular level.[42][43][44][45] These studies suggest that motor proteins carry signaling molecules from the soma to the growth cone and vice versa whose concentration oscillates in time with a length-dependent frequency.
Classification[edit]
The axons of neurons in the human peripheral nervous system can be classified based on their physical features and signal conduction properties. Axons were known to have different thicknesses (from 0.1 to 20 µm)[3] and these differences were thought to relate to the speed at which an action potential could travel along the axon – its conductance velocity. Erlanger and Gasser proved this hypothesis, and identified several types of nerve fiber, establishing a relationship between the diameter of an axon and its nerve conduction velocity. They published their findings in 1941 giving the first classification of axons.
Axons are classified in two systems. The first one introduced by Erlanger and Gasser, grouped the fibers into three main groups using the letters A, B, and C. These groups, group A, group B, and group C include both the sensory fibers (afferents) and the motor fibres (efferents). The first group A, was subdivided into alpha, beta, gamma, and delta fibers — Aα, Aβ, Aγ, and Aδ. The motor neurons of the different motor fibers, were the lower motor neurons – alpha motor neuron, beta motor neuron, and gamma motor neuron having the Aα, Aβ, and Aγ nerve fibers respectively.
Later findings by other researchers identified two groups of Aa fibers that were sensory fibers. These were then introduced into a system that only included sensory fibers (though some of these were mixed nerves and were also motor fibers). This system refers to the sensory groups as Types and uses Roman numerals: Type Ia, Type Ib, Type II, Type III, and Type IV.
https://en.wikipedia.org/wiki/Axon
Guidepost cells are cells which assist in the subcellular organization of both neural axon growth and migration.[1] They act as intermediate targets for long and complex axonal growths by creating short and easy pathways, leading axon growth cones towards their target area.[2][3]
https://en.wikipedia.org/wiki/Guidepost_cells
A growth cone is a large actin-supported extension of a developing or regenerating neurite seeking its synaptic target. It is the growth cone that drives axon growth.[1] Their existence was originally proposed by Spanish histologist Santiago Ramón y Cajal based upon stationary images he observed under the microscope. He first described the growth cone based on fixed cells as "a concentration of protoplasm of conical form, endowed with amoeboid movements" (Cajal, 1890).[2] Growth cones are situated on the tips of neurites, either dendrites or axons, of the nerve cell. The sensory, motor, integrative, and adaptive functions of growing axons and dendrites are all contained within this specialized structure.
Structure[edit]
The morphology of the growth cone can be easily described by using the hand as an analogy. The fine extensions of the growth cone are pointed filopodia known as microspikes.[3] The filopodia are like the "fingers" of the growth cone; they contain bundles of actin filaments (F-actin) that give them shape and support. Filopodia are the dominant structures in growth cones, and they appear as narrow cylindrical extensions which can extend several micrometres beyond the edge of the growth cone. The filopodia are bound by a membrane which contains receptors, and cell adhesion molecules that are important for axon growth and guidance.
In between filopodia—much like the webbing of the hands—are the "lamellipodia". These are flat regions of dense actin meshwork instead of bundled F-actin as in filopodia. They often appear adjacent to the leading edge of the growth cone and are positioned between two filopodia, giving them a "veil-like" appearance. In growth cones, new filopodia usually emerge from these inter-filopodial veils.
The growth cone is described in terms of three regions: the peripheral (P) domain, the transitional (T) domain, and the central (C) domain. The peripheral domain is the thin region surrounding the outer edge of the growth cone. It is composed primarily of an actin-based cytoskeleton, and contains the lamellipodia and filopodia which are highly dynamic. Microtubules, however, are known to transiently enter the peripheral region via a process called dynamic instability. The central domain is located in the center of the growth cone nearest to the axon. This region is composed primarily of a microtubule-based cytoskeleton, is generally thicker, and contains many organelles and vesicles of various sizes. The transitional domain is the region located in the thin band between the central and peripheral domains.
Growth cones are molecularly specialized, with transcriptomes and proteomes that are distinct from those of their parent cell bodies.[4] There are many cytoskeletal-associated proteins, which perform a variety of duties within the growth cone, such as anchoring actin and microtubules to each other, to the membrane, and to other cytoskeletal components. Some of these components include molecular motors that generate force within the growth cone and membrane-bound vesicles which are transported in and out of the growth cone via microtubules. Some examples of cytoskeletal-associated proteins are fascin and filamins (actin bundling), talin (actin anchoring), myosin (vesicle transport), and mDia (microtubule-actin linking).
Axon branching and outgrowth[edit]
The highly dynamic nature of growth cones allows them to respond to the surrounding environment by rapidly changing direction and branching in response to various stimuli. There are three stages of axon outgrowth, which are termed: protrusion, engorgement, and consolidation. During protrusion, there is a rapid extension of filopodia and lamellar extensions along the leading edge of the growth cone. Engorgement follows when the filopodia move to the lateral edges of the growth cone, and microtubules invade further into the growth cone, bringing vesicles and organelles such as mitochondria and endoplasmic reticulum. Finally, consolidation occurs when the F-actin at the neck of the growth cone depolymerizes and the filopodia retract. The membrane then shrinks to form a cylindrical axon shaft around the bundle of microtubules. One form of axon branching also occurs via the same process, except that the growth cone “splits” during the engorgement phase. This results in the bifurcation of the main axon. An additional form of axon branching is termed collateral (or interstitial) branching;.[5][6] Collateral branching, unlike axon bifurcations, involves the formation of a new branch from the established axon shaft and is independent of the growth cone at the tip of the growing axon. In this mechanism, the axon initially generates a filopodium or lamellipodium which following invasion by axonal microtubules can then develop further into a branch extending perpendicular from the axon shaft. Established collateral branches, like the main axon, exhibit a growth cone and develop independently of the main axon tip.
Overall, axon elongation is the product of a process known as tip growth. In this process, new material is added at the growth cone while the remainder of the axonal cytoskeleton remains stationary. This occurs via two processes: cytoskeletal-based dynamics and mechanical tension. With cytoskeletal dynamics, microtubules polymerize into the growth cone and deliver vital components. Mechanical tension occurs when the membrane is stretched due to force generation by molecular motors in the growth cone and strong adhesions to the substrate along the axon. In general, rapidly growing growth cones are small and have a large degree of stretching, while slow moving or paused growth cones are very large and have a low degree of stretching.
The growth cones are continually being built up through construction of the actin microfilaments and extension of the plasma membrane via vesicle fusion. The actin filaments depolymerize and disassemble on the proximal end to allow free monomers to migrate to the leading edge (distal end) of the actin filament where it can polymerize and thus reattach. Actin filaments are also constantly being transported away from the leading edge by a myosin-motor driven process known as retrograde F-actin flow. The actin filaments are polymerized in the peripheral region and then transported backward to the transitional region, where the filaments are depolymerized; thus freeing the monomers to repeat the cycle. This is different from actin treadmilling since the entire protein moves. If the protein were to simply treadmill, the monomers would depolymerize from one end and polymerize onto the other while the protein itself does not move.
The growth capacity of the axons lies in the microtubules which are located just beyond the actin filaments. Microtubules can rapidly polymerize into and thus “probe” the actin-rich peripheral region of the growth cone. When this happens, the polymerizing ends of microtubules come into contact with F-actin adhesion sites, where microtubule tip-associated proteins act as "ligands". Laminins of the basal membrane interact with the integrins of the growth cone to promote the forward movement of the growth cone. Additionally, axon outgrowth is also supported by the stabilization of the proximal ends of microtubules, which provide the structural support for the axon.
Axon guidance[edit]
Movement of the axons is controlled by an integration of its sensory and motor function (described above) which is established through second messengers such as calcium and cyclic nucleotides. The sensory function of axons is dependent on cues from the extracellular matrix which can be either attractive or repulsive, thus helping to guide the axon away from certain paths and attracting them to their proper target destinations. Attractive cues inhibit retrograde flow of the actin filaments and promote their assembly whereas repulsive cues have the exact opposite effect. Actin stabilizing proteins are also involved and are essential for continued protrusion of filopodia and lamellipodia in the presence of attractive cues, while actin destabilizing proteins are involved in the presence of a repulsive cue.
A similar process is involved with microtubules. In the presence of an attractive cue on one side of the growth cone, specific microtubules are targeted on that side by microtubule stabilizing proteins, resulting in growth cone turning in the direction of the positive stimulus. With repulsive cues, the opposite is true: microtubule stabilization is favored on the opposite side of the growth cone as the negative stimulus resulting in the growth cone turning away from the repellent. This process coupled with actin-associated processes result in the overall directed growth of an axon.
Growth cone receptors detect the presence of axon guidance molecules such as Netrin, Slit, Ephrins, and Semaphorins. It has more recently been shown that cell fate determinants such as Wnt or Shh can also act as guidance cues. The same guidance cue can act as an attractant or a repellent, depending on context. A prime example of this is Netrin-1, which signals attraction through the DCC receptor and repulsion through the Unc-5 receptor. Furthermore, it has been discovered that these same molecules are involved in guiding vessel growth. Axon guidance directs the initial wiring of the nervous system and is also important in axonal regeneration following an injury.[7]
https://en.wikipedia.org/wiki/Growth_cone
The lamellipodium (plural lamellipodia) (from Latin lamina, "thin sheet"; pod, "foot") is a cytoskeletal protein actin projection on the leading edge of the cell. It contains a quasi-two-dimensional actin mesh; the whole structure propels the cell across a substrate.[1] Within the lamellipodia are ribs of actin called microspikes, which, when they spread beyond the lamellipodium frontier, are called filopodia.[2] The lamellipodium is born of actin nucleation in the plasma membrane of the cell[1] and is the primary area of actin incorporation or microfilament formation of the cell.
https://en.wikipedia.org/wiki/Lamellipodium
https://en.wikipedia.org/wiki/Human_embryonic_development
https://en.wikipedia.org/wiki/Magnetic_cartridge
https://en.wikipedia.org/wiki/V-block
https://en.wikipedia.org/wiki/Magnetohydrodynamic_generator
https://en.wikipedia.org/wiki/Magnetic_circuit
https://en.wikipedia.org/wiki/Compass#Magnetic_compass
https://en.wikipedia.org/wiki/Magnetic_tape
https://en.wikipedia.org/wiki/Soundstream
https://en.wikipedia.org/wiki/Stereophonic_sound
https://en.wikipedia.org/wiki/Magnetogenetics
https://en.wikipedia.org/wiki/Cataclysmic_variable_star
https://en.wikipedia.org/wiki/Magnetic-core_memory
https://en.wikipedia.org/wiki/Tape_recorder
https://en.wikipedia.org/wiki/Chemical_shift
https://en.wikipedia.org/wiki/Gyrochronology
https://en.wikipedia.org/wiki/Centrifugal_pump#Magnetically_coupled_pumps
https://en.wikipedia.org/wiki/Hard_disk_drive
magnetic channel string mag channel, dot connect, etc.
magnetic draw line
https://en.wikipedia.org/wiki/Spider_silk
https://en.wikipedia.org/wiki/Inertial_confinement_fusion
https://en.wikipedia.org/wiki/Cold_fusion
https://en.wikipedia.org/wiki/Magnetic_confinement_fusion
https://en.wikipedia.org/wiki/Magnetic_pressure
https://en.wikipedia.org/wiki/Pressure_gradient
https://en.wikipedia.org/wiki/True_vertical_depth
https://en.wikipedia.org/wiki/Hydrostatics
https://en.wikipedia.org/wiki/Fluid_mechanics
https://en.wikipedia.org/wiki/Particle_image_velocimetry
https://en.wikipedia.org/wiki/Optics
https://en.wikipedia.org/wiki/Nuclear_fusion
https://en.wikipedia.org/wiki/Muon-catalyzed_fusion
https://en.wikipedia.org/wiki/Reduced_mass
https://en.wikipedia.org/wiki/Subatomic_particle
https://en.wikipedia.org/wiki/List_of_particles#Composite_particles
https://en.wikipedia.org/wiki/Table_of_nuclides
https://en.wikipedia.org/wiki/Exotic_atom
https://en.wikipedia.org/wiki/Wave_function_collapse
https://en.wikipedia.org/wiki/Reversible_process_(thermodynamics)
https://en.wikipedia.org/wiki/Quantum_decoherence
decoupling optics dissipative system
https://en.wikipedia.org/wiki/Cathode-ray_tube
https://en.wikipedia.org/wiki/Electrostatic_deflection
https://en.wikipedia.org/wiki/Analog_delay_line
abso reverb refract difract reflect mirror channel spinodal spinor tunnel
soft absorb hard mirror (optics perception of material solidity coherence congruency consistency etc.)
https://en.wikipedia.org/wiki/Electromagnetic_radiation
https://en.wikipedia.org/wiki/Dereverberation
https://en.wikipedia.org/wiki/Linear_prediction
https://en.wikipedia.org/wiki/Blind_deconvolution
https://en.wikipedia.org/wiki/Deconvolution
https://en.wikipedia.org/wiki/Acoustic
https://en.wikipedia.org/wiki/Analog
https://en.wikipedia.org/wiki/Eigenstrain
https://en.wikipedia.org/wiki/Deformation_(physics)
https://en.wikipedia.org/wiki/Yield_(engineering)
https://en.wikipedia.org/wiki/Viscoelasticity
https://en.wikipedia.org/wiki/Miller_index#Crystallographic_planes_and_directions
https://en.wikipedia.org/wiki/Rayleigh_scattering
https://en.wikipedia.org/wiki/Elastic_scattering
https://en.wikipedia.org/wiki/Dispersion_relation
https://en.wikipedia.org/wiki/Attenuation
https://en.wikipedia.org/wiki/Resonance
https://en.wikipedia.org/wiki/Sound_energy
https://en.wikipedia.org/wiki/Oscillation
https://en.wikipedia.org/wiki/energy
https://en.wikipedia.org/wiki/nuclear_transmutation
https://en.wikipedia.org/wiki/Harmonic_oscillator#Spring%E2%80%93mass_system
Resonance phenomena occur with all types of vibrations or waves: there is mechanical resonance, acoustic resonance, electromagnetic resonance, nuclear magnetic resonance (NMR), electron spin resonance (ESR) and resonance of quantum wave functions. Resonant systems can be used to generate vibrations of a specific frequency (e.g., musical instruments), or pick out specific frequencies from a complex vibration containing many frequencies (e.g., filters).
https://en.wikipedia.org/wiki/Resonance
https://en.wikipedia.org/wiki/Steady_state
https://en.wikipedia.org/wiki/Asymptote
https://en.wikipedia.org/wiki/Discrete_time_and_continuous_time
https://en.wikipedia.org/wiki/Analog
https://en.wikipedia.org/wiki/Linear_recurrence_with_constant_coefficients#Conversion_to_homogeneous_form
https://en.wikipedia.org/wiki/Characteristic_polynomial
https://en.wikipedia.org/wiki/Invariants_of_tensors
https://en.wikipedia.org/wiki/Secular_variation
https://en.wikipedia.org/wiki/Decomposition_of_time_series
https://en.wikipedia.org/wiki/Wold%27s_decomposition
Etymology[edit]
The word secular, from the Latin root saecularis ("of an age, occurring once in an age"),[1] has two basic meanings: I. Of or pertaining to the world (from which secularity is derived), and II. Of or belonging to an age or long period. The latter use appeared in the 18th century in the sense of "living or lasting for an age or ages". In the 19th century terms like secular acceleration and secular variation appeared in astronomy, and similar language was used in economics by 1895.[2]
Astronomy[edit]
In astronomy, secular variations are contrasted with periodic phenomena. In particular, astronomical ephemerides use secular to label the longest-lasting or non-oscillatory perturbations in the motion of planets, as opposed to periodic perturbations which exhibit repetition over the course of a time frame of interest. In this context it is referred to as secular motion. Solar System ephemerides are essential for the navigation of spacecraft and for all kinds of space observations of the planets, their natural satellites, stars and galaxies.
Most of the known perturbations to motion in stable, regular, and well-determined dynamical systems tend to be periodic at some level, but in many-body systems, chaotic dynamics result in some effects which are one-way (for example, planetary migration).
https://en.wikipedia.org/wiki/Secular_variation
In mathematics, an endomorphism is a morphism from a mathematical object to itself. An endomorphism that is also an isomorphism is an automorphism. For example, an endomorphism of a vector space V is a linear map f: V → V, and an endomorphism of a group G is a group homomorphism f: G → G. In general, we can talk about endomorphisms in any category. In the category of sets, endomorphisms are functions from a set S to itself.
In any category, the composition of any two endomorphisms of X is again an endomorphism of X. It follows that the set of all endomorphisms of X forms a monoid, the full transformation monoid, and denoted End(X) (or EndC(X) to emphasize the category C).
https://en.wikipedia.org/wiki/Endomorphism
Secular function and secular equation[edit]
Secular function[edit]
The term secular function has been used for what is now called characteristic polynomial (in some literature the term secular function is still used). The term comes from the fact that the characteristic polynomial was used to calculate secular perturbations (on a time scale of a century, that is, slow compared to annual motion) of planetary orbits, according to Lagrange's theory of oscillations.
Secular equation[edit]
Secular equation may have several meanings.
- In linear algebra it is sometimes used in place of characteristic equation.
- In astronomy it is the algebraic or numerical expression of the magnitude of the inequalities in a planet's motion that remain after the inequalities of a short period have been allowed for.[10]
- In molecular orbital calculations relating to the energy of the electron and its wave function it is also used instead of the characteristic equation.
In mathematics, a system of linear equations (or linear system) is a collection of one or more linear equations involving the same variables.[1][2][3][4][5] For example,
is a system of three equations in the three variables x, y, z. A solution to a linear system is an assignment of values to the variables such that all the equations are simultaneously satisfied. A solution to the system above is given by
since it makes all three equations valid. The word "system" indicates that the equations are to be considered collectively, rather than individually.
In mathematics, the theory of linear systems is the basis and a fundamental part of linear algebra, a subject which is used in most parts of modern mathematics. Computational algorithms for finding the solutions are an important part of numerical linear algebra, and play a prominent role in engineering, physics, chemistry, computer science, and economics. A system of non-linear equations can often be approximated by a linear system (see linearization), a helpful technique when making a mathematical model or computer simulation of a relatively complex system.
Very often, the coefficients of the equations are real or complex numbers and the solutions are searched in the same set of numbers, but the theory and the algorithms apply for coefficients and solutions in any field. For solutions in an integral domain like the ring of the integers, or in other algebraic structures, other theories have been developed, see Linear equation over a ring. Integer linear programming is a collection of methods for finding the "best" integer solution (when there are many). Gröbner basis theory provides algorithms when coefficients and unknowns are polynomials. Also tropical geometry is an example of linear algebra in a more exotic structure.
A system is said to be transient or in a transient state when a process variable or variables have been changed and the system has not yet reached a steady state. The time taken for the circuit to change from one steady state to another steady state is called the transient time.
https://en.wikipedia.org/wiki/Transient_state
Examples[edit]
Chemical Engineering[edit]
When a chemical reactor is being brought into operation, the concentrations, temperatures, species compositions, and reaction rates are changing with time until operation reaches its nominal process variables.
Electrical engineering[edit]
When a switch is flipped in an appropriate electrical circuit containing a capacitor or inductor, the component draws out the resulting change in voltage or current (respectively), causing the system to take a substantial amount of time to reach a new steady state.
We can define a transient by saying that when a quantity is at rest or in uniform motion and a change in time takes place , changing the existing state , a transient has taken place.
When a SCR (four-layer PNPN Device) is switched on we have the problem of transients occurring as a result of high values of current and voltage oscillating around the point before normal levels are obtained again. Filtering can prevent damage to SCR by means of LC filters, zener diodes, trans-zorps, and varistors.[1]
https://en.wikipedia.org/wiki/Transient_state
A chemical reactor is an enclosed volume in which a chemical reaction takes place.[1][2][3][4] In chemical engineering, it is generally understood to be a process vessel used to carry out a chemical reaction,[5] which is one of the classic unit operations in chemical process analysis. The design of a chemical reactor deals with multiple aspects of chemical engineering. Chemical engineers design reactors to maximize net present value for the given reaction. Designers ensure that the reaction proceeds with the highest efficiency towards the desired output product, producing the highest yield of product while requiring the least amount of money to purchase and operate. Normal operating expenses include energy input, energy removal, raw material costs, labor, etc. Energy changes can come in the form of heating or cooling, pumping to increase pressure, frictional pressure loss or agitation.
| Part of a series on |
| Chemical engineering |
|---|
| Fundamentals |
| Unit processes |
| Aspects |
| Glossaries |
|
Chemical reaction engineering is the branch of chemical engineering which deals with chemical reactors and their design, especially by application of chemical kinetics to industrial systems.
Catalytic reactor[edit]
Although catalytic reactors are often implemented as plug flow reactors, their analysis requires more complicated treatment. The rate of a catalytic reaction is proportional to the amount of catalyst the reagents contact, as well as the concentration of the reactants. With a solid phase catalyst and fluid phase reagents, this is proportional to the exposed area, efficiency of diffusion of reagents in and products out, and efficacy of mixing. Perfect mixing usually cannot be assumed. Furthermore, a catalytic reaction pathway often occurs in multiple steps with intermediates that are chemically bound to the catalyst; and as the chemical binding to the catalyst is also a chemical reaction, it may affect the kinetics. Catalytic reactions often display so-called falsified kinetics, when the apparent kinetics differ from the actual chemical kinetics due to physical transport effects.
The behavior of the catalyst is also a consideration. Particularly in high-temperature petrochemical processes, catalysts are deactivated by processes such as sintering, coking, and poisoning.
A common example of a catalytic reactor is the catalytic converter that processes toxic components of automobile exhausts. However, most petrochemical reactors are catalytic, and are responsible for most industrial chemical production, with extremely high-volume examples including sulfuric acid, ammonia, reformate/BTEX (benzene, toluene, ethylbenzene and xylene), and fluid catalytic cracking. Various configurations are possible, see Heterogeneous catalytic reactor.
https://en.wikipedia.org/wiki/Chemical_reactor
Fluid Catalytic Cracking (FCC) is the conversion process used in petroleum refineries to convert the high-boiling point, high-molecular weight hydrocarbon fractions of petroleum (crude oils) into gasoline, olefinic gases, and other petroleum products.[1][2][3] The cracking of petroleum hydrocarbons was originally done by thermal cracking, now almost replaced by catalytic cracking, which yields greater volumes of high octane rating gasoline; and produces by-product gases, with more carbon-carbon double bonds (i.e. olefins), that are of greater economic value than the gases produced by thermal cracking.
The feedstock to the FCC conversion process usually is heavy gas oil (HGO), which is that portion of the petroleum (crude oil) that has an initial boiling-point temperature of 340 °C (644 °F) or higher, at atmospheric pressure, and that has an average molecular weight that ranges from about 200 to 600 or higher; heavy gas oil also is known as “heavy vacuum gas oil” (HVGO). In the fluid catalytic cracking process, the HGO feedstock is heated to a high temperature and to a moderate pressure, and then is placed in contact with a hot, powdered catalyst, which breaks the long-chain molecules of the high-boiling-point hydrocarbon liquids into short-chain molecules, which then are collected as a vapor.
https://en.wikipedia.org/wiki/Fluid_catalytic_cracking
Reactor and regenerator[edit]
The reactor and regenerator are considered to be the heart of the fluid catalytic cracking unit. The schematic flow diagram of a typical modern FCC unit in Figure 1 below is based upon the "side-by-side" configuration. The preheated high-boiling petroleum feedstock (at about 315 to 430 °C) consisting of long-chain hydrocarbon molecules is combined with recycle slurry oil from the bottom of the distillation column and injected into the catalyst riser where it is vaporised and cracked into smaller molecules of vapour by contact and mixing with the very hot powdered catalyst from the regenerator. All of the cracking reactions take place in the catalyst riser within a period of 2–4 seconds. The hydrocarbon vapours "fluidize" the powdered catalyst and the mixture of hydrocarbon vapors and catalyst flows upward to enter the reactor at a temperature of about 535 °C and a pressure of about 1.72 bar.
The reactor is a vessel in which the cracked product vapors are: (a) separated from the spent catalyst by flowing through a set of two-stage cyclones within the reactor and (b) the spent catalyst flows downward through a steam stripping section to remove any hydrocarbon vapors before the spent catalyst returns to the catalyst regenerator. The flow of spent catalyst to the regenerator is regulated by a slide valve in the spent catalyst line.
Since the cracking reactions produce some carbonaceous material (referred to as catalyst coke) that deposits on the catalyst and very quickly reduces the catalyst reactivity, the catalyst is regenerated by burning off the deposited coke with air blown into the regenerator. The regenerator operates at a temperature of about 715 °C and a pressure of about 2.41 bar, hence the regenerator operates at about 0.7 bar higher pressure than the reactor. The combustion of the coke is exothermic and it produces a large amount of heat that is partially absorbed by the regenerated catalyst and provides the heat required for the vaporization of the feedstock and the endothermic cracking reactions that take place in the catalyst riser. For that reason, FCC units are often referred to as being 'heat balanced'.
The hot catalyst (at about 715 °C) leaving the regenerator flows into a catalyst withdrawal well where any entrained combustion flue gases are allowed to escape and flow back into the upper part to the regenerator. The flow of regenerated catalyst to the feedstock injection point below the catalyst riser is regulated by a slide valve in the regenerated catalyst line. The hot flue gas exits the regenerator after passing through multiple sets of two-stage cyclones that remove entrained catalyst from the flue gas.
The amount of catalyst circulating between the regenerator and the reactor amounts to about 5 kg per kg of feedstock, which is equivalent to about 4.66 kg per litre of feedstock.[1][7] Thus, an FCC unit processing 75,000 barrels per day (11,900 m3/d) will circulate about 55,900 tonnes per day of catalyst.
Regenerator flue gas[edit]
Depending on the choice of FCC design, the combustion in the regenerator of the coke on the spent catalyst may or may not be complete combustion to carbon dioxide CO2. The combustion air flow is controlled so as to provide the desired ratio of carbon monoxide (CO) to carbon dioxide for each specific FCC design.[1][4]
In the design shown in Figure 1, the coke has only been partially combusted to CO2. The combustion flue gas (containing CO and CO2) at 715 °C and at a pressure of 2.41 bar is routed through a secondary catalyst separator containing swirl tubes designed to remove 70 to 90 percent of the particulates in the flue gas leaving the regenerator.[8] This is required to prevent erosion damage to the blades in the turbo-expander that the flue gas is next routed through.
The expansion of flue gas through a turbo-expander provides sufficient power to drive the regenerator's combustion air compressor. The electrical motor–generator can consume or produce electrical power. If the expansion of the flue gas does not provide enough power to drive the air compressor, the electric motor–generator provides the needed additional power. If the flue gas expansion provides more power than needed to drive the air compressor, then the electric motor–generator converts the excess power into electric power and exports it to the refinery's electrical system.[3]
The expanded flue gas is then routed through a steam-generating boiler (referred to as a CO boiler) where the carbon monoxide in the flue gas is burned as fuel to provide steam for use in the refinery as well as to comply with any applicable environmental regulatory limits on carbon monoxide emissions.[3]
The flue gas is finally processed through an electrostatic precipitator (ESP) to remove residual particulate matter to comply with any applicable environmental regulations regarding particulate emissions. The ESP removes particulates in the size range of 2 to 20 µm from the flue gas.[3] Particulate filter systems, known as Fourth Stage Separators (FSS) are sometimes required to meet particulate emission limits. These can replace the ESP when particulate emissions are the only concern.
The steam turbine in the flue gas processing system (shown in the above diagram) is used to drive the regenerator's combustion air compressor during start-ups of the FCC unit until there is sufficient combustion flue gas to take over that task.
https://en.wikipedia.org/wiki/Fluid_catalytic_cracking#Reactor_and_regenerator
Hermetically sealed, open, or semi-hermetic[edit]
Compressors used in refrigeration systems must exhibit near-zero leakage to avoid the loss of the refrigerant if they are to function for years without service. This necessitates the use of very effective seals, or even the elimination of all seals and openings to form a hermetic system. These compressors are often described as being either hermetic, open, or semi-hermetic, to describe how the compressor is enclosed and how the motor drive is situated in relation to the gas or vapor being compressed. Some compressors outside of refrigeration service may also be hermetically sealed to some extent, typically when handling toxic, polluting, or expensive gasses, with most non-refrigeration applications being in the petrochemical industry.
In hermetic and most semi-hermetic compressors, the compressor and motor driving the compressor are integrated, and operate within the pressurized gas envelope of the system. The motor is designed to operate in, and be cooled by, the refrigerant gas being compressed. Open compressors have an external motor driving a shaft that passes through the body of the compressor and rely on rotary seals around the shaft to retain the internal pressure.
The difference between the hermetic and semi-hermetic, is that the hermetic uses a one-piece welded steel casing that cannot be opened for repair; if the hermetic fails it is simply replaced with an entire new unit. A semi-hermetic uses a large cast metal shell with gasketed covers with screws that can be opened to replace motor and compressor components. The primary advantage of a hermetic and semi-hermetic is that there is no route for the gas to leak out of the system. The main advantages of open compressors is that they can be driven by any motive power source, allowing the most appropriate motor to be selected for the application, or even non-electric power sources such as an internal combustion engine or steam turbine, and secondly the motor of an open compressor can be serviced without opening any part of the refrigerant system.
An open pressurized system such as an automobile air conditioner can be more susceptible to leak its operating gases. Open systems rely on lubricant in the system to splash on pump components and seals. If it is not operated frequently enough, the lubricant on the seals slowly evaporates, and then the seals begin to leak until the system is no longer functional and must be recharged. By comparison, a hermetic or semi-hermetic system can sit unused for years, and can usually be started up again at any time without requiring maintenance or experiencing any loss of system pressure. Even well lubricated seals will leak a small amount of gas over time, particularly if the refrigeration gasses are soluble in the lubricating oil, but if the seals are well manufactured and maintained this loss is very low.
The disadvantage of hermetic compressors is that the motor drive cannot be repaired or maintained, and the entire compressor must be replaced if a motor fails. A further disadvantage is that burnt-out windings can contaminate the whole systems, thereby requiring the system to be entirely pumped down and the gas replaced (This can also happen in semi hermetic compressors where the motor operates in the refrigerant). Typically, hermetic compressors are used in low-cost factory-assembled consumer goods where the cost of repair and labor is high compared to the value of the device, and it would be more economical to just purchase a new device or compressor. Semi-hermetic compressors are used in mid-sized to large refrigeration and air conditioning systems, where it is cheaper to repair and/or refurbish the compressor compared to the price of a new one. A hermetic compressor is simpler and cheaper to build than a semi-hermetic or open compressor.
Aluminium chloride (AlCl3), also known as aluminium trichloride, describe compounds with the formula AlCl3(H2O)n (n = 0 or 6). They consist of aluminium and chlorine atoms in a 1:3 ratio, and one form also contains six waters of hydration. Both are white solids, but samples are often contaminated with iron(III) chloride, giving a yellow color.
The anhydrous material is important commercially. It has a low melting and boiling point. It is mainly produced and consumed in the production of aluminium metal, but large amounts are also used in other areas of the chemical industry.[7] The compound is often cited as a Lewis acid. It is an example of an inorganic compound that reversibly changes from a polymer to a monomer at mild temperature.
https://en.wikipedia.org/wiki/Aluminium_chloride
Carbon black (subtypes are acetylene black, channel black, furnace black, lamp black and thermal black) is a material produced by the incomplete combustion of heavy petroleum products such as fluid catalytic cracking tar, coal tar, ethylene cracking tar, or vegetable matter. Carbon black is a form of paracrystalline carbon that has a high surface-area-to-volume ratio, albeit lower than that of activated carbon. It is dissimilar to soot in its much higher surface-area-to-volume ratio and significantly lower (negligible and non-bioavailable) polycyclic aromatic hydrocarbon (PAH) content. However, carbon black is widely used as a model compound for diesel soot for diesel oxidation experiments.[2][better source needed] Carbon black is mainly used as a reinforcing filler in tires and other rubber products. In plastics, paints, and inks, carbon black is used as a color pigment.[3] It is used in some places, such as the EU, as a food colorant if produced from vegetable matter (E153).
The current International Agency for Research on Cancer (IARC) evaluation is that, "Carbon black is possibly carcinogenic to humans (Group 2B)".[4] Short-term exposure to high concentrations of carbon black dust may produce discomfort to the upper respiratory tract, through mechanical irritation.
https://en.wikipedia.org/wiki/Carbon_black
Black phosphorus[edit]
Black phosphorus is the thermodynamically stable form of phosphorus at room temperature and pressure, with a heat of formation of -39.3 kJ/mol (relative to white phosphorus which is defined as the standard state).[1] It was first synthesized by heating white phosphorus under high pressures (12,000 atmospheres) in 1914. As a 2D material, in appearance, properties, and structure, black phosphorus is very much like graphite with both being black and flaky, a conductor of electricity, and having puckered sheets of linked atoms.[18]
Black phosphorus has an orthorhombic pleated honeycomb structure and is the least reactive allotrope, a result of its lattice of interlinked six-membered rings where each atom is bonded to three other atoms.[19][20] In this structure, each phosphorous atom has 5 outer shell electrons.[21] Black and red phosphorus can also take a cubic crystal lattice structure.[22] The first high-pressure synthesis of black phosphorus crystals was made by the nobel-prize winner Percy Williams Bridgman in 1914.[23] Metal salts catalyze the synthesis of black phosphorus.[24]
Phosphorene[edit]
The similarities to graphite also include the possibility of scotch-tape delamination (exfoliation), resulting in phosphorene, a graphene-like 2D material with excellent charge transport properties, thermal transport properties and optical properties. Distinguishing features of scientific interest include a thickness dependent band-gap, which is not found in graphene.[25] This, combined with a high on/off ratio of ~105 makes phosphorene a promising candidate for field-effect transistors (FETs).[26] The tunable bandgap also suggests promising applications in mid-infrared photodetectors and LEDs.[27][28] Exfoliated black phosphorus sublimes at 400 °C in vacuum.[29] It gradually oxidizes when exposed to water in the presence of oxygen, which is a concern when contemplating it as a material for the manufacture of transistors, for example.[30][31] Exfoliated black phosphorus is an emerging anode material in the battery community, showing high stability and lithium storage.[32]
Reactions of violet phosphorus[edit]
It does not ignite in air until heated to 300 °C and is insoluble in all solvents. It is not attacked by alkali and only slowly reacts with halogens. It can be oxidised by nitric acid to phosphoric acid.
If it is heated in an atmosphere of inert gas, for example nitrogen or carbon dioxide, it sublimes and the vapour condenses as white phosphorus. If it is heated in a vacuum and the vapour condensed rapidly, violet phosphorus is obtained. It would appear that violet phosphorus is a polymer of high relative molecular mass, which on heating breaks down into P2 molecules. On cooling, these would normally dimerize to give P4 molecules (i.e. white phosphorus) but, in a vacuum, they link up again to form the polymeric violet allotrope.
https://en.wikipedia.org/wiki/Allotropes_of_phosphorus#Black_phosphorus
Biochar is charcoal added to compost; it is used as a soil conditioner[1][2] usually directly but occasionally via being fed to animals. Biochar is defined by the International Biochar Initiative as "the solid material obtained from the thermochemical conversion of biomass in an oxygen-limited environment".[3] Biochar is a stable solid that is rich in carbon and can endure in soil for thousands of years.[4]
The stability of biochar leads to the concept of pyrogenic carbon capture and storage (PyCCS),[5] i.e. carbon sequestration in the form of biochar.[4] It may be a means to mitigate climate change.[6][7][8] Biochar may increase the soil fertility of acidic soils and increase agricultural productivity.[9]
The pyrolysis (or devolatilization) process is the thermal decomposition of materials at elevated temperatures in an inert atmosphere.[1] It involves a change of chemical composition. The word is coined from the Greek-derived elements pyro "fire", "heat", "fever" and lysis "separating".
Pyrolysis is most commonly used in the treatment of organic materials. It is one of the processes involved in charring wood.[2] In general, pyrolysis of organic substances produces volatile products and leaves char, a carbon-rich solid residue. Extreme pyrolysis, which leaves mostly carbon as the residue, is called carbonization. Pyrolysis is considered the first step in the processes of gasification or combustion.[3][4]
The process is used heavily in the chemical industry, for example, to produce ethylene, many forms of carbon, and other chemicals from petroleum, coal, and even wood, to produce coke from coal. It is used also in the conversion of natural gas (primarily methane) into non-polluting hydrogen gas and non-polluting solid carbon char, recently on an industrial scale.[5] Aspirational applications of pyrolysis would convert biomass into syngas and biochar, waste plastics back into usable oil, or waste into safely disposable substances.
Ethylene[edit]
Pyrolysis is used to produce ethylene, the chemical compound produced on the largest scale industrially (>110 million tons/year in 2005). In this process, hydrocarbons from petroleum are heated to around 600 °C (1,112 °F) in the presence of steam; this is called steam cracking. The resulting ethylene is used to make antifreeze (ethylene glycol), PVC (via vinyl chloride), and many other polymers, such as polyethylene and polystyrene.[51]
https://en.wikipedia.org/wiki/Pyrolysis#Liquid_and_gaseous_biofuels
Microwave is a form of electromagnetic radiation with wavelengths ranging from about one meter to one millimeter corresponding to frequencies between 300 MHz and 300 GHz respectively.[1][2][3][4][5] Different sources define different frequency ranges as microwaves; the above broad definition includes both UHF and EHF (millimeter wave) bands. A more common definition in radio-frequency engineering is the range between 1 and 100 GHz (wavelengths between 0.3 m and 3 mm).[2] In all cases, microwaves include the entire SHF band (3 to 30 GHz, or 10 to 1 cm) at minimum. Frequencies in the microwave range are often referred to by their IEEE radar band designations: S, C, X, Ku, K, or Ka band, or by similar NATO or EU designations.
The prefix micro- in microwave is not meant to suggest a wavelength in the micrometer range. Rather, it indicates that microwaves are "small" (having shorter wavelengths), compared to the radio waves used prior to microwave technology. The boundaries between far infrared, terahertz radiation, microwaves, and ultra-high-frequency radio waves are fairly arbitrary and are used variously between different fields of study.
Microwaves travel by line-of-sight; unlike lower frequency radio waves they do not diffract around hills, follow the earth's surface as ground waves, or reflect from the ionosphere, so terrestrial microwave communication links are limited by the visual horizon to about 40 miles (64 km). At the high end of the band, they are absorbed by gases in the atmosphere, limiting practical communication distances to around a kilometer. Microwaves are widely used in modern technology, for example in point-to-point communication links, wireless networks, microwave radio relay networks, radar, satellite and spacecraft communication, medical diathermy and cancer treatment, remote sensing, radio astronomy, particle accelerators, spectroscopy, industrial heating, collision avoidance systems, garage door openers and keyless entry systems, and for cooking food in microwave ovens.
The ΛCDM (Lambda cold dark matter) or Lambda-CDM model is a parameterization of the Big Bang cosmological model in which the universe contains three major components: first, a cosmological constant denoted by Lambda (Greek Λ) associated with dark energy; second, the postulated cold dark matter (abbreviated CDM); and third, ordinary matter. It is frequently referred to as the standard model of Big Bang cosmology because it is the simplest model that provides a reasonably good account of the following properties of the cosmos:
- the existence and structure of the cosmic microwave background
- the large-scale structure in the distribution of galaxies
- the observed abundances of hydrogen (including deuterium), helium, and lithium
- the accelerating expansion of the universe observed in the light from distant galaxies and supernovae
The model assumes that general relativity is the correct theory of gravity on cosmological scales. It emerged in the late 1990s as a concordance cosmology, after a period of time when disparate observed properties of the universe appeared mutually inconsistent, and there was no consensus on the makeup of the energy density of the universe.
The ΛCDM model can be extended by adding cosmological inflation, quintessence and other elements that are current areas of speculation and research in cosmology.
Some alternative models challenge the assumptions of the ΛCDM model. Examples of these are modified Newtonian dynamics, entropic gravity, modified gravity, theories of large-scale variations in the matter density of the universe, bimetric gravity, scale invariance of empty space, and decaying dark matter (DDM).[1][2][3][4][5]
https://en.wikipedia.org/wiki/Lambda-CDM_model
The cosmic microwave background (CMB, CMBR), in Big Bang cosmology, is electromagnetic radiation which is a remnant from an early stage of the universe, also known as "relic radiation".[1] The CMB is faint cosmic background radiation filling all space. It is an important source of data on the early universe because it is the oldest electromagnetic radiation in the universe, dating to the epoch of recombination. With a traditional optical telescope, the space between stars and galaxies (the background) is completely dark. However, a sufficiently sensitive radio telescope shows a faint background noise, or glow, almost isotropic, that is not associated with any star, galaxy, or other object. This glow is strongest in the microwave region of the radio spectrum. The accidental discovery of the CMB in 1965 by American radio astronomers Arno Penzias and Robert Wilson[2][3] was the culmination of work initiated in the 1940s, and earned the discoverers the 1978 Nobel Prize in Physics.
CMB is landmark evidence of the Big Bang origin of the universe. When the universe was young, before the formation of stars and planets, it was denser, much hotter, and filled with an opaque fog of hydrogen plasma. As the universe expanded the plasma grew cooler and the radiation filling it expanded to longer wavelengths. When the temperature had dropped enough, protons and electrons combined to form neutral hydrogen atoms. Unlike the plasma, these newly conceived atoms could not scatter the thermal radiation by Thomson scattering, and so the universe became transparent.[4] Cosmologists refer to the time period when neutral atoms first formed as the recombination epoch, and the event shortly afterwards when photons started to travel freely through space is referred to as photon decoupling. The photons that existed at the time of photon decoupling have been propagating ever since, though growing less energetic, since the expansion of space causes their wavelength to increase over time (and wavelength is inversely proportional to energy according to Planck's relation). This is the source of the alternative term relic radiation. The surface of last scattering refers to the set of points in space at the right distance from us so that we are now receiving photons originally emitted from those points at the time of photon decoupling.
https://en.wikipedia.org/wiki/Cosmic_microwave_background
https://en.wikipedia.org/wiki/Grigory_Mairanovsky
https://en.wikipedia.org/wiki/Lambda-CDM_model
Pages in category "Voids (astronomy)"
The following 13 pages are in this category, out of 13 total. This list may not reflect recent changes (learn more).
The CMB Cold Spot or WMAP Cold Spot is a region of the sky seen in microwaves that has been found to be unusually large and cold relative to the expected properties of the cosmic microwave background radiation (CMBR). The "Cold Spot" is approximately 70 µK (0.00007 K) colder than the average CMB temperature (approximately 2.7 K), whereas the root mean square of typical temperature variations is only 18 µK.[1][note 1] At some points, the "cold spot" is 140 µK colder than the average CMB temperature.[2]
The radius of the "cold spot" subtends about 5°; it is centered at the galactic coordinate lII = 207.8°, bII = −56.3° (equatorial: α = 03h 15m 05s, δ = −19° 35′ 02″). It is, therefore, in the Southern Celestial Hemisphere, in the direction of the constellation Eridanus.
Typically, the largest fluctuations of the primordial CMB temperature occur on angular scales of about 1°. Thus a cold region as large as the "cold spot" appears very unlikely, given generally accepted theoretical models. Various alternative explanations exist, including a so-called Eridanus Supervoid or Great Void that may exist between us and the primordial CMB (foreground voids can cause cold spots against the CMB background). Such a void would affect the observed CMB via the integrated Sachs–Wolfe effect, and would be one of the largest structures in the observable universe. This would be an extremely large region of the universe, roughly 150 to 300 Mpc or 500 million to one billion light-years across and 6 to 10 billion light years away,[3] at redshift , containing a density of matter much smaller than the average density at that redshift.[citation needed]
Anisotropy (/ˌæn.ə-, ˌæn.aɪˈsɒtr.əp.i/) is the property of a material which allows it to change or assume different properties in different directions, as opposed to isotropy. It can be defined as a difference, when measured along different axes, in a material's physical or mechanical properties (absorbance, refractive index, conductivity, tensile strength, etc.).
An example of anisotropy is light coming through a polarizer. Another is wood, which is easier to split along its grain than across it.
Mathematics[edit]
Within mathematics, isotropy has a few different meanings:
- Isotropic manifolds
- A manifold is isotropic if the geometry on the manifold is the same regardless of direction. A similar concept is homogeneity.
- Isotropic quadratic form
- A quadratic form q is said to be isotropic if there is a non-zero vector v such that q(v) = 0; such a v is an isotropic vector or null vector. In complex geometry, a line through the origin in the direction of an isotropic vector is an isotropic line.
- Isotropic coordinates
- Isotropic coordinates are coordinates on an isotropic chart for Lorentzian manifolds.
- Isotropy group
- An isotropy group is the group of isomorphisms from any object to itself in a groupoid.[dubious ][1] An isotropy representation is a representation of an isotropy group.
- Isotropic position
- A probability distribution over a vector space is in isotropic position if its covariance matrix is the identity.
- Isotropic vector field
- The vector field generated by a point source is said to be isotropic if, for any spherical neighborhood centered at the point source, the magnitude of the vector determined by any point on the sphere is invariant under a change in direction. For an example, starlight appears to be isotropic.
Physics[edit]
- Quantum mechanics or particle physics
- When a spinless particle (or even an unpolarized particle with spin) decays, the resulting decay distribution must be isotropic in the rest frame of the decaying particle regardless of the detailed physics of the decay. This follows from rotational invariance of the Hamiltonian, which in turn is guaranteed for a spherically symmetric potential.
- Kinetic theory of gases is also an example of isotropy. It is assumed that the molecules move in random directions and as a consequence, there is an equal probability of a molecule moving in any direction. Thus when there are many molecules in the gas, with high probability there will be very similar numbers moving in one direction as any other, demonstrating approximate isotropy.
- Fluid dynamics
- Fluid flow is isotropic if there is no directional preference (e.g. in fully developed 3D turbulence). An example of anisotropy is in flows with a background density as gravity works in only one direction. The apparent surface separating two differing isotropic fluids would be referred to as an isotrope.
- Thermal expansion
- A solid is said to be isotropic if the expansion of solid is equal in all directions when thermal energy is provided to the solid.
- Electromagnetics
- An isotropic medium is one such that the permittivity, ε, and permeability, μ, of the medium are uniform in all directions of the medium, the simplest instance being free space.
- Optics
- Optical isotropy means having the same optical properties in all directions. The individual reflectance or transmittance of the domains is averaged for micro-heterogeneous samples if the macroscopic reflectance or transmittance is to be calculated. This can be verified simply by investigating, e.g., a polycrystalline material under a polarizing microscope having the polarizers crossed: If the crystallites are larger than the resolution limit, they will be visible.
- Cosmology
- The Big Bang theory of the evolution of the observable universe assumes that space is isotropic.[2] It also assumes that space is homogeneous.[2] These two assumptions together are known as the cosmological principle. As of 2006, the observations suggest that, on distance scales much larger than galaxies, galaxy clusters are "Great" features, but small compared to so-called multiverse scenarios. Here homogeneous means that the universe is the same everywhere (no preferred location) and isotropic implies that there is no preferred direction.
Materials science[edit]
In the study of mechanical properties of materials, "isotropic" means having identical values of a property in all directions. This definition is also used in geology and mineralogy. Glass and metals are examples of isotropic materials.[3] Common anisotropic materials include wood, because its material properties are different parallel and perpendicular to the grain, and layered rocks such as slate.
Isotropic materials are useful since they are easier to shape, and their behavior is easier to predict. Anisotropic materials can be tailored to the forces an object is expected to experience. For example, the fibers in carbon fiber materials and rebars in reinforced concrete are oriented to withstand tension.
Microfabrication[edit]
In industrial processes, such as etching steps, isotropic means that the process proceeds at the same rate, regardless of direction. Simple chemical reaction and removal of a substrate by an acid, a solvent or a reactive gas is often very close to isotropic. Conversely, anisotropic means that the attack rate of the substrate is higher in a certain direction. Anisotropic etch processes, where vertical etch-rate is high, but lateral etch-rate is very small are essential processes in microfabrication of integrated circuits and MEMS devices.
Antenna (radio)[edit]
An isotropic antenna is an idealized "radiating element" used as a reference; an antenna that broadcasts power equally (calculated by the Poynting vector) in all directions. The gain of an arbitrary antenna is usually reported in decibels relative to an isotropic antenna, and is expressed as dBi or dB(i).
In cells (a.k.a. muscle fibers), the term "isotropic" refers to the light bands (I bands) that contribute to the striated pattern of the cells.
- Pharmacology
- While it is well established that the skin provides an ideal site for the administration of local and systemic drugs, it presents a formidable barrier to the permeation of most substances.[4] Most recently, isotropic formulations have been used extensively in dermatology for drug delivery.[5]
Power (intensity) reflection and transmission coefficients[edit]
In the diagram on the right, an incident plane wave in the direction of the ray IO strikes the interface between two media of refractive indices n1 and n2 at point O. Part of the wave is reflected in the direction OR, and part refracted in the direction OT. The angles that the incident, reflected and refracted rays make to the normal of the interface are given as θi, θr and θt, respectively.
The relationship between these angles is given by the law of reflection:
and Snell's law:
The behavior of light striking the interface is solved by considering the electric and magnetic fields that constitute an electromagnetic wave, and the laws of electromagnetism, as shown below. The ratio of waves' electric field (or magnetic field) amplitudes are obtained, but in practice one is more often interested in formulae which determine power coefficients, since power (or irradiance) is what can be directly measured at optical frequencies. The power of a wave is generally proportional to the square of the electric (or magnetic) field amplitude.
We call the fraction of the incident power that is reflected from the interface the reflectance (or reflectivity, or power reflection coefficient) R, and the fraction that is refracted into the second medium is called the transmittance (or transmissivity, or power transmission coefficient) T . Note that these are what would be measured right at each side of an interface and do not account for attenuation of a wave in an absorbing medium following transmission or reflection.[2]
The reflectance for s-polarized light is
while the reflectance for p-polarized light is
where Z1 and Z2 are the wave impedances of media 1 and 2, respectively.
We assume that the media are non-magnetic (i.e., μ1 = μ2 = μ0), which is typically a good approximation at optical frequencies (and for transparent media at other frequencies).[3] Then the wave impedances are determined solely by the refractive indices n1 and n2:
The second form of each equation is derived from the first by eliminating θt using Snell's law and trigonometric identities.
As a consequence of conservation of energy, one can find the transmitted power (or more correctly, irradiance: power per unit area) simply as the portion of the incident power that isn't reflected: [4]
and
Note that all such intensities are measured in terms of a wave's irradiance in the direction normal to the interface; this is also what is measured in typical experiments. That number could be obtained from irradiances in the direction of an incident or reflected wave (given by the magnitude of a wave's Poynting vector) multiplied by cos θ for a wave at an angle θ to the normal direction (or equivalently, taking the dot product of the Poynting vector with the unit vector normal to the interface). This complication can be ignored in the case of the reflection coefficient, since cos θi = cos θr, so that the ratio of reflected to incident irradiance in the wave's direction is the same as in the direction normal to the interface.
Although these relationships describe the basic physics, in many practical applications one is concerned with "natural light" that can be described as unpolarized. That means that there is an equal amount of power in the s and p polarizations, so that the effective reflectivity of the material is just the average of the two reflectivities:
For low-precision applications involving unpolarized light, such as computer graphics, rather than rigorously computing the effective reflection coefficient for each angle, Schlick's approximation is often used.
Special cases[edit]
Normal incidence[edit]
For the case of normal incidence, , and there is no distinction between s and p polarization. Thus, the reflectance simplifies to
For common glass (n2 ≈ 1.5) surrounded by air (n1 = 1), the power reflectance at normal incidence can be seen to be about 4%, or 8% accounting for both sides of a glass pane.
Brewster's angle[edit]
At a dielectric interface from n1 to n2, there is a particular angle of incidence at which Rp goes to zero and a p-polarised incident wave is purely refracted, thus all reflected light is s-polarised. This angle is known as Brewster's angle, and is around 56° for n1 = 1 and n2 = 1.5 (typical glass).
Total internal reflection[edit]
When light travelling in a denser medium strikes the surface of a less dense medium (i.e., n1 > n2), beyond a particular incidence angle known as the critical angle, all light is reflected and Rs = Rp = 1. This phenomenon, known as total internal reflection, occurs at incidence angles for which Snell's law predicts that the sine of the angle of refraction would exceed unity (whereas in fact sin θ ≤ 1 for all real θ). For glass with n = 1.5 surrounded by air, the critical angle is approximately 41°.
Complex amplitude reflection and transmission coefficients[edit]
The above equations relating powers (which could be measured with a photometer for instance) are derived from the Fresnel equations which solve the physical problem in terms of electromagnetic field complex amplitudes, i.e., considering phase shifts in addition to their amplitudes. Those underlying equations supply generally complex-valued ratios of those EM fields and may take several different forms, depending on the formalism used. The complex amplitude coefficients for reflection and transmission are usually represented by lower case r and t (whereas the power coefficients are capitalized). As before, we are assuming the magnetic permeability, µ of both media to be equal to the permeability of free space µo as is essentially true of all dielectrics at optical frequencies.
In the following equations and graphs, we adopt the following conventions. For s polarization, the reflection coefficient r is defined as the ratio of the reflected wave's complex electric field amplitude to that of the incident wave, whereas for p polarization r is the ratio of the waves complex magnetic field amplitudes (or equivalently, the negative of the ratio of their electric field amplitudes). The transmission coefficient t is the ratio of the transmitted wave's complex electric field amplitude to that of the incident wave, for either polarization. The coefficients r and t are generally different between the s and p polarizations, and even at normal incidence (where the designations s and p do not even apply!) the sign of r is reversed depending on whether the wave is considered to be s or p polarized, an artifact of the adopted sign convention (see graph for an air-glass interface at 0° incidence).
The equations consider a plane wave incident on a plane interface at angle of incidence , a wave reflected at angle , and a wave transmitted at angle . In the case of an interface into an absorbing material (where n is complex) or total internal reflection, the angle of transmission does not generally evaluate to a real number. In that case, however, meaningful results can be obtained using formulations of these relationships in which trigonometric functions and geometric angles are avoided; the inhomogeneous waves launched into the second medium cannot be described using a single propagation angle.
One can see that ts = rs + 1[7] and n2n1tp=rp+1. One can write very similar equations applying to the ratio of the waves' magnetic fields, but comparison of the electric fields is more conventional.
Because the reflected and incident waves propagate in the same medium and make the same angle with the normal to the surface, the power reflection coefficient R is just the squared magnitude of r: [8]
On the other hand, calculation of the power transmission coefficient T is less straightforward, since the light travels in different directions in the two media. What's more, the wave impedances in the two media differ; power (irradiance) is given by the square of the electric field amplitude divided by the characteristic impedance of the medium (or by the square of the magnetic field multiplied by the characteristic impedance). This results in:[9]
using the above definition of t. The introduced factor of n2/n1 is the reciprocal of the ratio of the media's wave impedances. The cos(θ) factors adjust the waves' powers so they are reckoned in the direction normal to the interface, for both the incident and transmitted waves, so that full power transmission corresponds to T=1.
In the case of total internal reflection where the power transmission T is zero, t nevertheless describes the electric field (including its phase) just beyond the interface. This is an evanescent field which does not propagate as a wave (thus T = 0) but has nonzero values very close to the interface. The phase shift of the reflected wave on total internal reflection can similarly be obtained from the phase angles of rp and rs (whose magnitudes are unity in this case). These phase shifts are different for s and p waves, which is the well-known principle by which total internal reflection is used to effect polarization transformations.
Alternative forms[edit]
In the above formula for rs, if we put (Snell's law) and multiply the numerator and denominator by 1n1 sin θt, we obtain [10][11]
If we do likewise with the formula for rp, the result is easily shown to be equivalent to [12][13]
These formulas [14][15][16] are known respectively as Fresnel's sine law and Fresnel's tangent law.[17] Although at normal incidence these expressions reduce to 0/0, one can see that they yield the correct results in the limit as θi → 0.
Multiple surfaces[edit]
When light makes multiple reflections between two or more parallel surfaces, the multiple beams of light generally interfere with one another, resulting in net transmission and reflection amplitudes that depend on the light's wavelength. The interference, however, is seen only when the surfaces are at distances comparable to or smaller than the light's coherence length, which for ordinary white light is few micrometers; it can be much larger for light from a laser.
An example of interference between reflections is the iridescent colours seen in a soap bubble or in thin oil films on water. Applications include Fabry–Pérot interferometers, antireflection coatings, and optical filters. A quantitative analysis of these effects is based on the Fresnel equations, but with additional calculations to account for interference.
The transfer-matrix method, or the recursive Rouard method [18] can be used to solve multiple-surface problems.
A Fresnel rhomb is an optical prism that introduces a 90° phase difference between two perpendicular components of polarization, by means of two total internal reflections. If the incident beam is linearly polarized at 45° to the plane of incidence and reflection, the emerging beam is circularly polarized, and vice versa. If the incident beam is linearly polarized at some other inclination, the emerging beam is elliptically polarized with one principal axis in the plane of reflection, and vice versa.
The rhomb usually takes the form of a right parallelepiped — that is, a right parallelogram-based prism. If the incident ray is perpendicular to one of the smaller rectangular faces, the angle of incidence and reflection at both of the longer faces is equal to the acute angle of the parallelogram. This angle is chosen so that each reflection introduces a phase difference of 45° between the components polarized parallel and perpendicular to the plane of reflection. For a given, sufficiently high refractive index, there are two angles meeting this criterion; for example, an index of 1.5 requires an angle of 50.2° or 53.3°.
Conversely, if the angle of incidence and reflection is fixed, the phase difference introduced by the rhomb depends only on its refractive index, which typically varies only slightly over the visible spectrum. Thus the rhomb functions as if it were a wideband quarter-wave plate — in contrast to a conventional birefringent (doubly-refractive) quarter-wave plate, whose phase difference is more sensitive to the frequency (color) of the light. The material of which the rhomb is made — usually glass — is specifically not birefringent.
The Fresnel rhomb is named after its inventor, the French physicist Augustin-Jean Fresnel, who developed the device in stages between 1817 [1] and 1823.[2] During that time he deployed it in crucial experiments involving polarization, birefringence, and optical rotation,[3][4][5] all of which contributed to the eventual acceptance of his transverse-wave theory of light.
Photolithography[edit]
Antireflective coatings (ARC) are often used in microelectronic photolithography to help reduce image distortions associated with reflections off the surface of the substrate. Different types of antireflective coatings are applied either before (Bottom ARC, or BARC) or after the photoresist, and help reduce standing waves, thin-film interference, and specular reflections.[3][4]
Crown glass is a type of optical glass used in lenses and other optical components. It has relatively low refractive index (≈1.52) and low dispersion (with Abbe numbers around 60). Crown glass is produced from alkali-lime silicates containing approximately 10% potassium oxide and is one of the earliest low dispersion glasses.
As well as the specific material named crown glass, there are other optical glasses with similar properties that are also called crown glasses. Generally, this is any glass with Abbe numbers in the range 50 to 85. For example, the borosilicate glass Schott BK7[1] is an extremely common crown glass, used in precision lenses. Borosilicates contain about 10% boric oxide, have good optical and mechanical characteristics, and are resistant to chemical and environmental damage. Other additives used in crown glasses include zinc oxide, phosphorus pentoxide, barium oxide, fluorite and lanthanum oxide.
BAK-4 barium crown glass has a higher index of refraction than BK7, and is used for prisms in high-end binoculars. In that application, it gives better image quality and a round exit pupil.
A concave lens of flint glass is commonly combined with a convex lens of crown glass to produce an achromatic doublet. The dispersions of the glasses partially compensate for each other, producing reduced chromatic aberration compared to a singlet lens with the same focal length.
https://en.wikipedia.org/wiki/Crown_glass_(optics)
Sulfur trioxide (alternative spelling sulphur trioxide, also known as nisso sulfan) is the chemical compound with the formula SO3. It has been described as "unquestionably the most important economically" sulfur oxide.[1] It is prepared on an industrial scale as a precursor to sulfuric acid.
Sulfur trioxide exists in several forms - gaseous monomer, crystalline trimer, and solid polymer. Sulfur trioxide is a solid at just below room temperature with a relatively narrow liquid range. Gaseous SO3 is the primary precursor to acid rain.[6]
https://en.wikipedia.org/wiki/Sulfur_trioxide
Thionyl chloride is an inorganic compound with the chemical formula SOCl
2. It is a moderately volatile colourless liquid with an unpleasant acrid odour. Thionyl chloride is primarily used as a chlorinating reagent, with approximately 45,000 tonnes (50,000 short tons) per year being produced during the early 1990s,[5] but is occasionally also used as a solvent.[6][7][8] It is toxic, reacts with water, and is also listed under the Chemical Weapons Convention as it may be used for the production of chemical weapons.
Thionyl chloride is sometimes confused with sulfuryl chloride, SO2Cl2, but the properties of these compounds differ significantly. Sulfuryl chloride is a source of chlorine whereas thionyl chloride is a source of chloride ions.
https://en.wikipedia.org/wiki/Thionyl_chloride
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption or adsorption from the surrounding environment, which is usually at normal or room temperature. If water molecules become suspended among the substance's molecules, adsorbing substances can become physically changed, e.g., changing in volume, boiling point, viscosity or some other physical characteristic or property of the substance.
Deliquescent materials are sufficiently hygroscopic that they absorb so much water that they become liquid and form an aqueous solution.
https://en.wikipedia.org/wiki/Hygroscopy
Hygroscopic substances include cellulose fibers (such as cotton and paper), sugar, caramel, honey, glycerol, ethanol, wood, methanol, sulfuric acid, many fertilizer chemicals, many salts (like calcium chloride, bases like sodium hydroxide etc.), and a wide variety of other substances.[1]
A bimetallic strip is used to convert a temperature change into mechanical displacement. The strip consists of two strips of different metals which expand at different rates as they are heated. The different expansions force the flat strip to bend one way if heated, and in the opposite direction if cooled below its initial temperature. The metal with the higher coefficient of thermal expansion is on the outer side of the curve when the strip is heated and on the inner side when cooled.
The invention of the bimetallic strip is generally credited to John Harrison, an eighteenth-century clockmaker who made it for his third marine chronometer (H3) of 1759 to compensate for temperature-induced changes in the balance spring.[1] Harrison's invention is recognized in the memorial to him in Westminster Abbey, England.
This effect is used in a range of mechanical and electrical devices.
https://en.wikipedia.org/wiki/Bimetallic_strip
A humectant /hjuːˈmɛktənt/ is a hygroscopic substance used to keep things moist. They are used in many products, including food, cosmetics, medicines and pesticides. When used as a food additive, a humectant has the effect of keeping moisture in the food.[1] Humectants are sometimes used as a component of antistatic coatings for plastics.
A humectant attracts and retains the moisture in the air nearby via absorption, drawing the water vapor into or beneath the organism's or object's surface.[2][3] This is the opposite use of a hygroscopic material where it is used as a desiccant used to draw moisture away.
In pharmaceuticals and cosmetics, humectants can be used in topical dosage forms to increase the solubility of a chemical compound's active ingredients, increasing the active ingredients' ability to penetrate skin, or its activity time. This hydrating property can also be needed to counteract a dehydrating active ingredient (e.g., soaps, corticoids, and some alcohols), which is why humectants are common ingredients in a wide range of cosmetic and personal care products that make moisturization claims (e.g., hair conditioners, body lotions, face or body cleansers, lip balms, and eye creams).
https://en.wikipedia.org/wiki/Humectant
https://en.wikipedia.org/wiki/Hygroscopy
Potassium phosphate is a generic term for the salts of potassium and phosphate ions including:[1]
- Monopotassium phosphate (KH2PO4) (Molar mass approx: 136 g/mol)
- Dipotassium phosphate (K2HPO4) (Molar mass approx: 174 g/mol)
- Tripotassium phosphate (K3PO4) (Molar mass approx: 212.27 g/mol)
As food additives, potassium phosphates have the E number E340.
https://en.wikipedia.org/wiki/Potassium_phosphate
A desiccant is a hygroscopic substance that is used to induce or sustain a state of dryness (desiccation) in its vicinity; it is the opposite of a humectant. Commonly encountered pre-packaged desiccants are solids that absorb water. Desiccants for specialized purposes may be in forms other than solid, and may work through other principles, such as chemical bonding of water molecules. They are commonly encountered in foods to retain crispness. Industrially, desiccants are widely used to control the level of water in gas streams.
https://en.wikipedia.org/wiki/Desiccant
Air-free techniques refer to a range of manipulations in the chemistry laboratory for the handling of compounds that are air-sensitive. These techniques prevent the compounds from reacting with components of air, usually water and oxygen; less commonly carbon dioxide and nitrogen. A common theme among these techniques is the use of a fine (100–10−3 Torr) or high (10−3–10−6 Torr) vacuum to remove air, and the use of an inert gas: preferably argon, but often nitrogen.
https://en.wikipedia.org/wiki/Air-free_technique
Degassing, also known as degasification, is the removal of dissolved gases from liquids, especially water or aqueous solutions. There are numerous methods for removing gases from liquids.
Gases are removed for various reasons. Chemists remove gases from solvents when the compounds they are working on are possibly air- or oxygen-sensitive (air-free technique), or when bubble formation at solid-liquid interfaces becomes a problem. The formation of gas bubbles when a liquid is frozen can also be undesirable, necessitating degassing beforehand.
https://en.wikipedia.org/wiki/Degassing
A deaerator is a device that removes oxygen and other dissolved gases from liquids and pumpable compounds.
https://en.wikipedia.org/wiki/Deaerator
Vacuum deaerator[edit]
Deaerators are also used to remove dissolved gases from products such as food, personal care products, cosmetic products, chemicals, and pharmaceuticals to increase the dosing accuracy in the filling process, to increase product shelf stability, to prevent oxidative effects (e.g. discolouration, changes of smell or taste, rancidity), to alter pH, and to reduce packaging volume. Vacuum deaerators are also used in the petrochemical field. [20]
In 1921 a tank with vacuum pump for removing gases was used in Pittsburgh.[21] In 1934 and 1940 a tank with vacuum pump for removing gases were used in Indiana.[22] [23]
https://en.wikipedia.org/wiki/Deaerator#Vacuum_deaerator
A defoamer or an anti-foaming agent is a chemical additive that reduces and hinders the formation of foam in industrial process liquids. The terms anti-foam agent and defoamer are often used interchangeably. Strictly speaking, defoamers eliminate existing foam and anti-foamers prevent the formation of further foam. Commonly used agents are insoluble oils, polydimethylsiloxanes and other silicones, certain alcohols, stearates and glycols. The additive is used to prevent formation of foam or is added to break a foam already formed.
In industrial processes, foams pose serious problems. They cause defects on surface coatings and prevent the efficient filling of containers. A variety of chemical formulae are available to prevent formation of foams.[1]
https://en.wikipedia.org/wiki/Defoamer
https://en.wikipedia.org/wiki/Diol
https://en.wikipedia.org/wiki/Aliphatic_compound
https://en.wikipedia.org/wiki/Saturated_and_unsaturated_compounds
https://en.wikipedia.org/wiki/Surface_science
https://en.wikipedia.org/wiki/Interface_and_colloid_science
https://en.wikipedia.org/wiki/Colloid
https://en.wikipedia.org/wiki/Suspension_(chemistry)
https://en.wikipedia.org/wiki/Aerosol
https://en.wikipedia.org/wiki/Bioaerosol
https://en.wikipedia.org/wiki/Lipopolysaccharide
Bioaerosols (short for biological aerosols) are a subcategory of particles released from terrestrial and marine ecosystems into the atmosphere. They consist of both living and non-living components, such as fungi, pollen, bacteria and viruses.[1] Common sources of bioaerosols include soil, water, and sewage.
Bioaerosols are typically introduced into the air via wind turbulence over a surface. Once in the atmosphere, they can be transported locally or globally: common wind patterns/strengths are responsible for local dispersal, while tropical storms and dust plumes can move bioaerosols between continents.[2] Over ocean surfaces, bioaerosols are generated via sea spray and bubbles
Bioaerosols can transmit microbial pathogens, endotoxins, and allergens to which humans are sensitive. A well-known case was the meningococcal meningitis outbreak in sub-Saharan Africa, which was linked to dust storms during dry seasons. Other outbreaks linked to dust events including Mycoplasma pneumonia and tuberculosis.[2]
Another instance was an increase in human respiratory problems in the Caribbean that may have been caused by traces of heavy metals, microorganism bioaerosols, and pesticides transported via dust clouds passing over the Atlantic Ocean.
https://en.wikipedia.org/wiki/Bioaerosol
Mycoplasma pneumonia (also known as "walking pneumonia") is a form of bacterial pneumonia caused by the bacterial species Mycoplasma pneumoniae. It is also known as PPLO, which is an acronym for Pleuro Pneumonia Like Organism.
https://en.wikipedia.org/wiki/Mycoplasma_pneumonia
https://en.wikipedia.org/wiki/Respiratory_droplet
https://en.wikipedia.org/wiki/Binary_fission
Fission, in biology, is the division of a single entity into two or more parts and the regeneration of those parts to separate entities resembling the original. The object experiencing fission is usually a cell, but the term may also refer to how organisms, bodies, populations, or species split into discrete parts.[1][2][3] The fission may be binary fission, in which a single organism produces two parts, or multiple fission, in which a single entity produces multiple parts.
Binary fission[edit]
Organisms in the domains of Archaea and Bacteria reproduce with binary fission. This form of asexual reproduction and cell division is also used by some organelles within eukaryotic organisms (e.g., mitochondria). Binary fission results in the reproduction of a living prokaryotic cell (or organelle) by dividing the cell into two parts, each with the potential to grow to the size of the original.
Fission of prokaryotes[edit]
The single DNA molecule first replicates, then attaches each copy to a different part of the cell membrane. When the cell begins to pull apart, the replicated and original chromosomes are separated. The consequence of this asexual method of reproduction is that all the cells are genetically identical, meaning that they have the same genetic material (barring random mutations). Unlike the processes of mitosis and meiosis used by eukaryotic cells, binary fission takes place without the formation of a spindle apparatus on the cell. Like in mitosis (and unlike in meiosis), the parental identity is lost.
Process of FtsZ-dependent fission[edit]
FtsZ is homologous to β-tubulin, the building block of the microtubule cytoskeleton used during mitosis in eukaryotes.[4] FtsZ is thought to be the first protein to localize to the site of future division in bacteria, and it assembles into a Z ring, anchored by FtsZ-binding proteins and defines the division plane between the two daughter cells.[5][4] MinC and MinD function together as division inhibitors, blocking formation of the FtsZ ring. MinE stops the MinCD activity midcell, allowing FtsZ to take over for binary fission.[6]
More specifically, the following steps occur:
- The bacterium before binary fission is when the DNA is tightly coiled.
- The DNA of the bacterium has uncoiled and duplicated.
- The DNA is pulled to the separate poles of the bacterium as it increases the size to prepare for splitting.
- The growth of a new cell wall begins to separate the bacterium (triggered by FtsZ polymerization and "Z-ring" formation)[7]
- The new cell wall (septum) fully develops, resulting in the complete split of the bacterium.
- The new daughter cells have tightly coiled DNA rods, ribosomes, and plasmids; these are now brand-new organisms.
Studies of bacteria made to not produce a cell wall, called L-form bacteria, shows that FtsZ requires a cell wall to work. Little is known about how bacteria that naturally don't grow a cell wall divides, but it is thought to resemble the L-form's budding-like division process of extrusion and separation.[8][9]
Speed of FtsZ-dependent Fission[edit]
Binary fission is generally rapid though its speed varies between species. For E. coli, cells typically divide about every 20 minutes at 37 °C.[10] Because the new cells will, in turn, undergo binary fission on their own, the time binary fission requires is also the time the bacterial culture requires to double in the number of cells it contains. This time period can, therefore, be referred to as the doubling time. Some species other than E. coli may have faster or slower doubling times: some strains of Mycobacterium tuberculosis may have doubling times of nearly 100 hours.[11] Bacterial growth is limited by factors including nutrient availability and available space, so binary fission occurs at much lower rates in bacterial cultures once they enter the stationary phase of growth.
In archaea[edit]
Crenarchaeota possess neither a cell wall nor the FtsZ mechanism. They use a primitive version of the eukaryotic ESCRT-III system (also known as Cdv) to manipulate the membrane into separating, specifically by coming into the middle of the two soon-to-be daughter cells.[12][9] Euryarchaeota use FtsZ like bacteria do.[4][13]
Fission of organelles[edit]
Some organelles in eukaryotic cells reproduce using binary fission. Mitochondrial fission occurs frequently within the cell, even when the cell is not actively undergoing mitosis, and is necessary to regulate the cell's metabolism.[14] All chloroplasts and some mitochrondria (not in animals), both organelles derived from endosymbiosis of bacteria, also use FtsZ in a bacteria-like fashion.[4][15]
Types of binary[edit]
Binary fission in organisms can occur in four ways, irregular, longitudinal, transverse, oblique.i.e.left oblique & right oblique
- Irregular: In this fission, cytokinesis may take place along any plane but it is always perpendicular to the plane of karyokinesis (nuclear division). e.g. amoeba
- Longitudinal: Here cytokinesis takes place along the longitudinal axis. e.g. in flagellates like Euglena.
- Transverse: Here cytokinesis takes place along the transverse axis. e.g. in ciliate protozoans like Paramecium.
- Oblique: In this type of binary fission cytokinesis occurs obliquely. Example Ceratium
Binary fission means "division into two". It is the simplest and most common method of asexual reproduction.
Multiple fission[edit]
Fission of protists[edit]
Multiple fission at the cellular level occurs in many protistists, e.g. sporozoans and algae. The nucleus of the parent cell divides several times by amitosis, producing several nuclei. The cytoplasm then separates, creating multiple daughter cells.[16][17][18]
Some parasitic, single-celled organisms undergo a multiple fission-like process to produce numerous daughter cells from a single parent cell. Isolates of the human parasite Blastocystis hominis were observed to begin such a process within 4 to 6 days.[19] Cells of the fish parasite Trypanosoma borreli have also been observed participating in both binary and multiple fission.[20]
Fission of apicomplexans[edit]
In the apicomplexans, a phylum of parasitic protists, multiple fission, or schizogony, is manifested either as merogony, sporogony or gametogony. Merogony results in merozoites, which are multiple daughter cells, that originate within the same cell membrane,[21][22] sporogony results in sporozoites, and gametogony results in microgametes.
Fission of green algae[edit]
Green algae can divide into more than two daughter cells. The exact number of daughter cells depends on the species of algae and is an effect of temperature and light.[23]
Multiple fission of bacteria[edit]
Most species of bacteria primarily undergo binary reproduction. Some species and groups of bacteria may undergo multiple fission as well, sometimes beginning or ending with the production of spores.[24] The species Metabacterium polyspora, a symbiont of guinea pigs, has been found to produce multiple endospores in each division.[25] Some species of cyanobacteria have also been found to reproduce through multiple fission.[26]
Plasmotomy[edit]
Some protozoans reproduce by yet another mechanism of fission called as plasmotomy. In this type of fission, a multinucleate adult parent undergoes cytokinesis to form two multinucleate (or coenocytic) daughter cells. The daughter cells so produced undergo further mitosis.
Opalina and Pelomyxa reproduce in this way.
Clonal fragmentation[edit]
Fragmentation in multicellular or colonial organisms is a form of asexual reproduction or cloning, where an organism is split into fragments. Each of these fragments develop into mature, fully grown individuals that are clones of the original organism. In echinoderms, this method of reproduction is usually known as fissiparity.[27]
Population fission[edit]
Any splitting of a single population of individuals into discrete parts may be considered fission. A population may undergo fission process for a variety of reasons, including migration or geographic isolation. Since the fission leads to genetic variance in the newly isolated, smaller populations, population fission is a precursor to speciation.[28][29]
See also[edit]
- Cytokinesis, cell division in eukaryotes
- Divisome, protein complex that initiates cell division in bacteria
- Fission-fusion society, a type of social organization that is notable among primates
- Mitochondrial fusion, a reverse fission
- Mitosis
- Paratomy
- Speciation
- Cytoskeleton
https://en.wikipedia.org/wiki/Binary_fission
Mitochondria are dynamic organelles with the ability to fuse and divide (fission), forming constantly changing tubular networks in most eukaryotic cells. These mitochondrial dynamics, first observed over a hundred years ago[1] are important for the health of the cell, and defects in dynamics lead to genetic disorders. Through fusion, mitochondria can overcome the dangerous consequences of genetic malfunction.[2] The process of mitochondrial fusion involves a variety of proteins that assist the cell throughout the series of events that form this process.
In mammals[edit]
Both proteins, Mfn1 and Mfn2, can act either together or separately during mitochondrial fusion. Mfn1 and Mfn2 are 81% similar to each other and about 51% similar to the Drosophila protein Fzo. Results published for a study to determine the impact of fusion on mitochondrial structure revealed that Mfn-deficient cells demonstrated either elongated cells (majority) or small, spherical cells upon observation.
The Mfn protein has three different methods of action: Mfn1 homotypic oligomers, Mfn2 homotypic oligomers and Mfn1-Mfn2 heterotypic oligomers. It has been suggested that the type of cell determines the method of action but it has yet to be concluded whether or not Mfn1 and Mfn2 perform the same function in the process or if they are separate. Cells lacking this protein are subject to severe cellular defects such as poor cell growth, heterogeneity of mitochondrial membrane potential and decreased cellular respiration.[7]
Mitochondrial fusion plays an important role in the process of embryonic development, as shown through the Mfn1 and Mfn2 proteins. Using Mfn1 and Mfn2 knock-out mice, which die in utero at midgestation due to a placental deficiency, mitochondrial fusion was shown not to be essential for cell survival in vitro, but necessary for embryonic development and cell survival throughout later stages of development. Mfn1 Mfn2 double knock-out mice, which die even earlier in development, were distinguished from the "single" knock-out mice. Mouse embryo fibroblasts (MEFs) originated from the double knock-out mice, which do survive in culture even though there is a complete absence of fusion, but parts of their mitochondria show a reduced mitochondrial DNA (mtDNA) copy number and lose membrane potential. This series of events causes problems with adenosine triphosphate (ATP) synthesis.
The Mitochondrial Inner/Outer Membrane Fusion (MMF) Family[edit]
The Mitochondrial Inner/Outer Membrane Fusion (MMF) Family (TC# 9.B.25) is a family of proteins that play a role in mitochondrial fusion events. This family belongs to the larger Mitochondrial Carrier (MC) Superfamily. The dynamic nature of mitochondria is critical for function. Chen and Chan (2010) have discussed the molecular basis of mitochondrial fusion, its protective role in neurodegeneration, and its importance in cellular function.[8] The mammalian mitofusins Mfn1 and Mfn2, GTPases localized to the outer membrane, mediate outer-membrane fusion. OPA1, a GTPase associated with the inner membrane, mediates subsequent inner-membrane fusion. Mutations in Mfn2 or OPA1 cause neurodegenerative diseases. Mitochondrial fusion enables content mixing within a mitochondrial population, thereby preventing permanent loss of essential components. Cells with reduced mitochondrial fusion show a subpopulation of mitochondria that lack mtDNA nucleoids. Such mtDNA defects lead to respiration-deficient mitochondria, and their accumulation in neurons leads to impaired outgrowth of cellular processes and consequent neurodegeneration.
Family members[edit]
A representative list of the proteins belonging to the MMF family is available in the Transporter Classification Database.
- 9.B.25.1.1 - The mitochondrial inner/outer membrane fusion complex, Fzo/Mgm1/Ugo1. Only the Ugo1 protein is a member of the MC superfamily.
- 9.B.25.2.1 - The mammalian mitochondrial membrane fusion complex, Mitofusin 1 (Mfn1)/Mfn2/Optical Atrophy Protein 1 (OPA1) complex. This subfamily includes mitofusins 1 and 2.
Mitofusins: Mfn1 and Mfn2[edit]
Mfn1 and Mfn2 (TC# 9.B.25.2.1; Q8IWA4 and O95140, respectively), in mammalian cells are required for mitochondrial fusion, Mfn1 and Mfn2 possess functional distinctions. For instance, the formation of tethered structures in vitro occurs more readily when mitochondria are isolated from cells overexpressing Mfn1 than Mfn2.[9] In addition, Mfn2 specifically has been shown to associate with Bax and Bak (Bcl-2 family, TC#1.A.21), resulting in altered Mfn2 activity, indicating that the mitofusins possess unique functional characteristics. Lipidic holes may open on opposing bilayers as intermediates, and fusion in cardiac myocytes is coupled with outer mitochondrial membrane destabilization that is opportunistically employed during the mitochondrial permeability transition.[10]
Mutations in Mfn2 (but not Mfn1) result in the neurological disorder Charcot-Marie-Tooth syndrome. These mutations can be complemented by the formation of Mfn1–Mfn2CMT2A hetero-oligomers but not homo-oligomers of Mfn2+–Mfn2CMT2A.[11] This suggests that within the Mfn1–Mfn2 hetero-oligomeric complex, each molecule is functionally distinct. This suggests that control of the expression levels of each protein likely represents the most basic form of regulation to alter mitochondrial dynamics in mammalian tissues. Indeed, the expression levels of Mfn1 and Mfn2 vary according to cell or tissue type as does the mitochondrial morphology.[12]
Yeast mitochondrial fusion proteins[edit]
In yeast, three proteins are essential for mitochondrial fusion. Fzo1 (P38297) and Mgm1 (P32266) are conserved guanosine triphosphatases that reside in the outer and inner membranes, respectively. At each membrane, these conserved proteins are required for the distinct steps of membrane tethering and lipid mixing. The third essential component is Ugo1, an outer membrane protein with a region homologous to but distantly related to a region in the Mitochondrial Carrier (MC) family. Hoppins et al., 2009 showed that Ugo1 is a modified member of this family, containing three transmembrane domains and existing as a dimer, a structure that is critical for the fusion function of Ugo1.[13] Their analyses of Ugo1 indicate that it is required for both outer and inner membrane fusion after membrane tethering, indicating that it operates at the lipid-mixing step of fusion. This role is distinct from the fusion dynamin-related proteins and thus demonstrates that at each membrane, a single fusion protein is not sufficient to drive the lipid-mixing step. Instead, this step requires a more complex assembly of proteins. The formation of a fusion pore has not yet been demonstrated.[13][14] The Ugo1 protein is a member of the MC superfamily.
See also[edit]
https://en.wikipedia.org/wiki/Mitochondrial_fusion
Mitochondrial fission is the process where mitochondria divide or segregate into two separate mitochondrial organelles. Mitochondrial fission is counteracted by the process of mitochondrial fusion, whereby two separate mitochondria can fuse together to form a large one.[1] Mitochondrial fusion in turn can result in elongated mitochondrial networks. Both mitochondrial fission and fusion are balanced in the cell, and mutations interfering with either processes are associated with a variety of diseases. Mitochondria can divide by prokaryotic binary fission and since they require mitochondrial DNA for their function, fission is coordinated with DNA replication.[2] Some of the proteins that are involved in mitochondrial fission have been identified and some of them are associated with mitochondrial diseases.[3] Mitochondrial fission has significant implications in stress response and apoptosis.[4]
https://en.wikipedia.org/wiki/Mitochondrial_fission
A mitochondrion (/ˌmaɪtəˈkɒndriən/;[1] pl. mitochondria) is a double-membrane-bound organelle found in most eukaryotic organisms. Mitochondria generate most of the cell's supply of adenosine triphosphate (ATP), subsequently utilized as a source of chemical energy, using the energy of oxygen released in aerobic respiration at the inner mitochondrial membrane.[2][3] They were first discovered by Albert von Kölliker in 1857[4] in the voluntary muscles of insects. The term mitochondrion was coined by Carl Benda in 1898. The mitochondrion is popularly nicknamed the "powerhouse of the cell", a phrase coined by Philip Siekevitz in a 1957 article of the same name.[5]
Some cells in some multicellular organisms lack mitochondria (for example, mature mammalian red blood cells). A large number of unicellular organisms, such as microsporidia, parabasalids and diplomonads, have reduced or transformed their mitochondria into other structures.[6] One eukaryote, Monocercomonoides, is known to have completely lost its mitochondria,[7] and one multicellular organism, Henneguya salminicola, is known to have retained mitochondrion-related organelles in association with a complete loss of their mitochondrial genome.[7][8][9]
Mitochondria are commonly between 0.75 and 3 μm2 in area,[10] but vary considerably in size and structure. Unless specifically stained, they are not visible. In addition to supplying cellular energy, mitochondria are involved in other tasks, such as signaling, cellular differentiation, and cell death, as well as maintaining control of the cell cycle and cell growth.[11] Mitochondrial biogenesis is in turn temporally coordinated with these cellular processes.[12][13] Mitochondria have been implicated in several human disorders and conditions, such as mitochondrial diseases,[14] cardiac dysfunction,[15] heart failure[16] and autism.[17]
The number of mitochondria in a cell can vary widely by organism, tissue, and cell type. A mature red blood cell has no mitochondria,[18] whereas a liver cell can have more than 2000.[19][20] The mitochondrion is composed of compartments that carry out specialized functions. These compartments or regions include the outer membrane, intermembrane space, inner membrane, cristae and matrix.
Although most of a cell's DNA is contained in the cell nucleus, the mitochondrion has its own genome ("mitogenome") that is substantially similar to bacterial genomes.[21] Mitochondrial proteins (proteins transcribed from mitochondrial DNA) vary depending on the tissue and the species. In humans, 615 distinct types of proteins have been identified from cardiac mitochondria,[22] whereas in rats, 940 proteins have been reported.[23] The mitochondrial proteome is thought to be dynamically regulated.[24]
https://en.wikipedia.org/wiki/Mitochondrion
The proteome is the entire set of proteins that is, or can be, expressed by a genome, cell, tissue, or organism at a certain time. It is the set of expressed proteins in a given type of cell or organism, at a given time, under defined conditions. Proteomics is the study of the proteome.
https://en.wikipedia.org/wiki/Proteome
Silica gel was in existence as early as the 1640s as a scientific curiosity.[5] It was used in World War I for the adsorption of vapors and gases in gas mask canisters.[6] The synthetic route for producing silica gel was patented in 1918 by Walter A. Patrick, a chemistry professor at Johns Hopkins University.
White phosphorus[edit]
White phosphorus, yellow phosphorus or simply tetraphosphorus () exists as molecules made up of four atoms in a tetrahedral structure. The tetrahedral arrangement results in ring strain and instability. The molecule is described as consisting of six single P–P bonds. Two crystalline forms are known. The α form is defined as the standard state of the element, but is actually metastable under standard conditions.[1] It has a body-centered cubic crystal structure, and transforms reversibly into the β form at 195.2 K. The β form is believed to have a hexagonal crystal structure.[2]
White phosphorus is a translucent waxy solid that quickly becomes yellow when exposed to light. For this reason it is also called yellow phosphorus. It glows greenish in the dark (when exposed to oxygen) and is highly flammable and pyrophoric (self-igniting) upon contact with air. It is toxic, causing severe liver damage on ingestion and phossy jaw from chronic ingestion or inhalation. The odour of combustion of this form has a characteristic garlic smell, and samples are commonly coated with white "diphosphorus pentoxide", which consists of tetrahedral with oxygen inserted between the phosphorus atoms and at their vertices. White phosphorus is only slightly soluble in water and can be stored under water. Indeed, white phosphorus is safe from self-igniting only[citation needed] when it is submerged in water; due to this, unreacted white phosphorus can pose hazardous to beachcombers who may collect washed-up samples while unaware of their true nature.[3][4] is soluble in benzene, oils, carbon disulfide, and disulfur dichloride.
According to a U.S. Government Accountability Office (GAO) report published on 4 October 2007, a total of 1,356 CDC/USDA registered BSL-3 facilities were identified throughout the United States.[31] Approximately 36% of these laboratories are located in academia. 15 BSL-4 facilities were identified in the U.S. in 2007, including nine at federal labs.[31]
Embryonic development[edit]
The fertilized zygote undergoes rotational holoblastic cleavage.
Sperm entry into the oocyte commences formation of an anterior-posterior axis.[34] The sperm microtubule organizing center directs the movement of the sperm pronucleus to the future posterior pole of the embryo, while also inciting the movement of PAR proteins, a group of cytoplasmic determination factors, to their proper respective locations.[35] As a result of the difference in PAR protein distribution, the first cell division is highly asymmetric.[36] C. elegans embryogenesis is among the best understood examples of asymmetric cell division.[37]
All cells of the germline arise from a single primordial germ cell, called the P4 cell, established early in embryogenesis.[38][39] This primordial cell divides to generate two germline precursors that do not divide further until after hatching.[39]
Caenorhabditis elegans (/ˌsiːnoʊræbˈdaɪtəs ˈɛləɡæns/[6]) is a free-living transparent nematode about 1 mm in length[7] that lives in temperate soil environments. It is the type species of its genus.[8] The name is a blend of the Greek caeno- (recent), rhabditis (rod-like)[9] and Latin elegans (elegant). In 1900, Maupas initially named it Rhabditides elegans. Osche placed it in the subgenus Caenorhabditis in 1952, and in 1955, Dougherty raised Caenorhabditis to the status of genus.[10]
C. elegans is an unsegmented pseudocoelomate and lacks respiratory or circulatory systems.[11] Most of these nematodes are hermaphrodites and a few are males.[12] Males have specialised tails for mating that include spicules.
In 1963, Sydney Brenner proposed research into C. elegans, primarily in the area of neuronal development. In 1974, he began research into the molecular and developmental biology of C. elegans, which has since been extensively used as a model organism.[13] It was the first multicellular organism to have its whole genome sequenced, and as of 2019, is the only organism to have its connectome (neuronal "wiring diagram") completed.[14][15][16]
https://en.wikipedia.org/wiki/Caenorhabditis_elegans#Embryonic_development
Collagen (/ˈkɒlədʒən/) is the main structural protein in the extracellular matrix found in the body's various connective tissues. As the main component of connective tissue, it is the most abundant protein in mammals,[1] making up from 25% to 35% of the whole-body protein content. Collagen consists of amino acids bound together to form a triple helix of elongated fibril[2] known as a collagen helix. It is mostly found in connective tissue such as cartilage, bones, tendons, ligaments, and skin.
https://en.wikipedia.org/wiki/Collagen
https://en.wikipedia.org/wiki/Biomineralization
biofilm plaque tartar calculus mineralization calcification liquefication
cation solvent
buoyancy
bone ligaments (carb or abio min basing; macrophage, ascaries, mitochondrite)
channulation neg mag vac vort spinor spinodal phosphorous
https://en.wikipedia.org/wiki/Pyrophoricity
https://en.wikipedia.org/wiki/Magnetotactic_bacteria
https://en.wikipedia.org/wiki/Magnetotaxis
https://en.wikipedia.org/wiki/Greigite
https://en.wikipedia.org/wiki/Arkose
https://en.wikipedia.org/wiki/Pyrite
https://en.wikipedia.org/wiki/Galena
https://en.wikipedia.org/wiki/Ferrimagnetism
https://en.wikipedia.org/wiki/Antiferromagnetism
https://en.wikipedia.org/wiki/Remanence
https://en.wikipedia.org/wiki/Degaussing
https://en.wikipedia.org/wiki/Cathode-ray_tube
https://en.wikipedia.org/wiki/Deflection_yoke
https://en.wikipedia.org/wiki/Deflection_(physics)
https://en.wikipedia.org/wiki/Thermodynamics
https://en.wikipedia.org/wiki/Magnetite
https://en.wikipedia.org/wiki/Abiogenesis
https://en.wikipedia.org/wiki/Shock_(mechanics)
https://en.wikipedia.org/wiki/Deflection_(physics)
https://en.wikipedia.org/wiki/mirror
https://en.wikipedia.org/wiki/spider
https://en.wikipedia.org/wiki/Explosion
https://en.wikipedia.org/wiki/mass_weight
https://en.wikipedia.org/wiki/dark_matter
spinor spinodal vortex vac void
phos
acid particle hard mat mirror
https://en.wikipedia.org/wiki/Pressure
https://en.wikipedia.org/wiki/Transonic
https://en.wikipedia.org/wiki/Prandtl%E2%80%93Meyer_expansion_fan
https://en.wikipedia.org/wiki/Stagnation_point
https://en.wikipedia.org/wiki/Compressible_flow
https://en.wikipedia.org/wiki/Friction
https://en.wikipedia.org/wiki/Stiction
https://en.wikipedia.org/wiki/Hydrogen_bond
https://en.wikipedia.org/wiki/Hydrogen_chalcogenide
https://en.wikipedia.org/wiki/Hydronium
https://en.wikipedia.org/wiki/Solvation_shell#Dehydrons
https://en.wikipedia.org/wiki/Metal_aquo_complex
https://en.wikipedia.org/wiki/Homoleptic
https://en.wikipedia.org/wiki/Tetrakis(triphenylphosphine)palladium(0)
https://en.wikipedia.org/wiki/One-pot_synthesis
https://en.wikipedia.org/wiki/Tetramethyllead
https://en.wikipedia.org/wiki/Dimethylmercury
https://en.wikipedia.org/wiki/Potassium_cyanide
https://en.wikipedia.org/wiki/Genetics
https://en.wikipedia.org/wiki/Sodium_cyanide
https://en.wikipedia.org/wiki/Hydrogen_cyanide
https://en.wikipedia.org/wiki/Adiponitrile
https://en.wikipedia.org/wiki/Linear_molecular_geometry
https://en.wikipedia.org/wiki/Xenon_difluoride
https://en.wikipedia.org/wiki/Chemical_decomposition
https://en.wikipedia.org/wiki/Dioxygen_difluoride
https://en.wikipedia.org/wiki/Iodine_pentafluoride
https://en.wikipedia.org/wiki/glass
https://en.wikipedia.org/wiki/pyrex
https://en.wikipedia.org/wiki/Phosphoric_acids_and_phosphates
https://en.wikipedia.org/wiki/Iodine
https://en.wikipedia.org/wiki/Iodine_monofluoride
https://en.wikipedia.org/wiki/Triphosphoric_acid
https://en.wikipedia.org/wiki/Silver(I)_fluoride
https://en.wikipedia.org/wiki/Anhydrous
https://en.wikipedia.org/wiki/Desiccator
https://en.wikipedia.org/wiki/Condensation_reaction
https://en.wikipedia.org/wiki/Condensation
https://en.wikipedia.org/wiki/Atom_cluster
https://en.wikipedia.org/wiki/Hydride
https://en.wikipedia.org/wiki/Ligand
https://en.wikipedia.org/wiki/Metal
https://en.wikipedia.org/wiki/Coordinate_covalent_bond
https://en.wikipedia.org/wiki/Isocyanide
https://en.wikipedia.org/wiki/Borane
https://en.wikipedia.org/wiki/Freezing
https://en.wikipedia.org/wiki/Nanoparticle
https://en.wikipedia.org/wiki/Brownian_motion
https://en.wikipedia.org/wiki/Nanocellulose
https://en.wikipedia.org/wiki/Thixotropy
https://en.wikipedia.org/wiki/Homogenization_(chemistry)
https://en.wikipedia.org/wiki/Pulp_(paper)
https://en.wikipedia.org/wiki/Liquid_crystal
https://en.wikipedia.org/wiki/Aerogel
https://en.wikipedia.org/wiki/Pulp_(paper)
https://en.wikipedia.org/wiki/Chemical_vapor_deposition
https://en.wikipedia.org/wiki/Silane
https://en.wikipedia.org/wiki/Emulsion
https://en.wikipedia.org/wiki/Coalescence_(chemistry)
https://en.wikipedia.org/wiki/repulsion
Bio-based electronics and energy storage[edit]
Nanocellulose can pave the way for a new type of "bio-based electronics" where interactive materials are mixed with nanocellulose to enable the creation of new interactive fibers, films, aerogels, hydrogels and papers.[90] E.g. nanocellulose mixed with conducting polymers such as PEDOT:PSS show synergetic effects resulting in extraordinary[91] mixed electronic and ionic conductivity, which is important for energy storage applications. Filaments spun from a mix of nanocellulose and carbon nanotubes show good conductivity and mechanical properties.[92] Nanocellulose aerogels decorated with carbon nanotubes can be constructed into robust compressible 3D supercapacitor devices.[93][94] Structures from nanocellulose can be turned into bio-based triboelectric generators[95] and sensors.
Bio-based sequins for fashion[edit]
Cellulose nanocrystals have shown the possibility to self organize into chiral nematic structures[96] with angle-dependent iridescent colours. It is thus possible to manufacture totally bio-based sequins having a metallic glare and a small footprint compared to fossil-based sequins.
Other potential applications[edit]
- As a highly scattering material for ultra-white coatings.[97]
- Activate the dissolution of cellulose in different solvents
- Regenerated cellulose products, such as fibers films, cellulose derivatives
- Tobacco filter additive
- Organometallic modified nanocellulose in battery separators
- Reinforcement of conductive materials
- Loud-speaker membranes
- High-flux membranes
- Computer components[31][98]
- Capacitors[94]
- Lightweight body armour and ballistic glass[31]
- Corrosion inhibitors[99]
- Radio lenses [100]
https://en.wikipedia.org/wiki/Nanocellulose
https://en.wikipedia.org/wiki/Hydrophobe
https://en.wikipedia.org/wiki/Lipophilicity
https://en.wikipedia.org/wiki/Hydrophile
https://en.wikipedia.org/wiki/Contact_angle
https://en.wikipedia.org/wiki/3D_cell_culture_in_wood-based_nanocellulose_hydrogel
https://en.wikipedia.org/wiki/Desiccator
https://en.wikipedia.org/wiki/Self-organization
https://en.wikipedia.org/wiki/Oxidative_dissolution_of_silver_nanoparticles
https://en.wikipedia.org/wiki/Titanium_dioxide_nanoparticle
https://en.wikipedia.org/wiki/Polyvalent_DNA_gold_nanoparticles
https://en.wikipedia.org/wiki/Solid_lipid_nanoparticle
https://en.wikipedia.org/wiki/Monoglyceride
https://en.wikipedia.org/wiki/Glycerolysis
https://en.wikipedia.org/wiki/Microemulsion
https://en.wikipedia.org/wiki/Endocytosis
https://en.wikipedia.org/wiki/Phagocytosis
https://en.wikipedia.org/wiki/Receptor-mediated_endocytosis
Endocytosis pathways[edit]
Endocytosis pathways can be subdivided into four categories: namely, receptor-mediated endocytosis (also known as clathrin-mediated endocytosis), caveolae, pinocytosis, and phagocytosis.[3]
- Clathrin-mediated endocytosis is mediated by the production of small (approx. 100 nm in diameter) vesicles that have a morphologically characteristic coat made up of the cytosolic protein clathrin.[4] Clathrin-coated vesicles (CCVs) are found in virtually all cells and form domains of the plasma membrane termed clathrin-coated pits. Coated pits can concentrate large extracellular molecules that have different receptors responsible for the receptor-mediated endocytosis of ligands, e.g. low density lipoprotein, transferrin, growth factors, antibodies and many others.[5]
https://en.wikipedia.org/wiki/Endocytosis
https://en.wikipedia.org/wiki/Eisosome
https://en.wikipedia.org/wiki/Phosphorylation
Vesicles selectively concentrate and exclude certain proteins during formation and are not representative of the membrane as a whole. AP2 adaptors are multisubunit complexes that perform this function at the plasma membrane. The best-understood receptors that are found concentrated in coated vesicles of mammalian cells are the LDL receptor (which removes LDL from circulating blood), the transferrin receptor (which brings ferric ions bound by transferrin into the cell) and certain hormone receptors (such as that for EGF).
At any one moment, about 25% of the plasma membrane of a fibroblast is made up of coated pits. As a coated pit has a life of about a minute before it buds into the cell, a fibroblast takes up its surface by this route about once every 16 minutes. Coated vesicles formed from the plasma membrane have a diameter of about 36 nm and a lifetime measured in a few seconds. Once the coat has been shed, the remaining vesicle fuses with endosomes and proceeds down the endocytic pathway. The actual budding-in process, whereby a pit is converted to a vesicle, is carried out by clathrin assisted by a set of cytoplasmic proteins, which includes dynamin and adaptors such as adaptin.
Coated pits and vesicles were first seen in thin sections of tissue in the electron microscope by Matt Lions and Parker George. The importance of them for the clearance of LDL from blood was discovered by Richard G. Anderson, Michael S. Brown and Joseph L. Goldstein in 1977.[26] Coated vesicles were first purified by Barbara Pearse, who discovered the clathrin coat molecule in 1976.[27]
Gallery[edit]
- Endocytosis. For example, coronavirus SARS-CoV-2 binds to the ACE2 receptor of the epithelial cell.
See also[edit]
- Active transport
- Emperipolesis
- RAP6 (Rab5-activating protein 6)
- Exocytosis
- Phagocytosis
- Pinocytosis
- Trans-endocytosis
https://en.wikipedia.org/wiki/Endocytosis
https://en.wikipedia.org/wiki/Emperipolesis
- Leukocyte migration from the blood stream to tissues through endothelial cells, in a process also known as transcellular migration and is akin to diapedesis (paracellular migration).
https://en.wikipedia.org/wiki/Emperipolesis
Symbiogenesis, endosymbiotic theory, or serial endosymbiotic theory,[1] is the leading evolutionary theory of the origin of eukaryotic cells from prokaryotic organisms.[2] The theory holds that mitochondria, plastids such as chloroplasts, and possibly other organelles of eukaryotic cells are descended from formerly free-living prokaryotes (more closely related to bacteria than to archaea) taken one inside the other in endosymbiosis. The idea that chloroplasts were originally independent organisms that merged into a symbiotic relationship with other one-celled organisms dates back to the 19th century, when it was espoused by researchers such as Andreas Schimper.
Mitochondria appear to be phylogenetically related to Rickettsiales proteobacteria, and chloroplasts to nitrogen-fixing filamentous cyanobacteria. The endosymbiotic theory was articulated in 1905 and 1910 by the Russian botanist Konstantin Mereschkowski, and advanced and substantiated with microbiological evidence by Lynn Margulis in 1967. Among the many lines of evidence supporting symbiogenesis are that new mitochondria and plastids are formed only through binary fission, and that cells cannot create new ones otherwise; that the transport proteins called porins are found in the outer membranes of mitochondria, chloroplasts, and bacterial cell membranes; that cardiolipin is found only in the inner mitochondrial membrane and bacterial cell membranes; and that some mitochondria and plastids contain single circular DNA molecules similar to the circular chromosomes of bacteria.
https://en.wikipedia.org/wiki/Symbiogenesis
Hemophagocytic lymphohistiocytosis (HLH), also known as haemophagocytic lymphohistiocytosis (British spelling), and hemophagocytic or haemophagocytic syndrome,[1] is an uncommon hematologic disorder seen more often in children than in adults. It is a life-threatening disease of severe hyperinflammation caused by uncontrolled proliferation of activated lymphocytes and macrophages, characterised by proliferation of morphologically benign lymphocytes and macrophages that secrete high amounts of inflammatory cytokines. It is classified as one of the cytokine storm syndromes. There are inherited and non-inherited (acquired) causes of hemophagocytic lymphohistiocytosis (HLH).
https://en.wikipedia.org/wiki/Hemophagocytic_lymphohistiocytosis
Chronic myelogenous leukemia (CML), also known as chronic myeloid leukemia, is a cancer of the white blood cells. It is a form of leukemia characterized by the increased and unregulated growth of myeloid cells in the bone marrow and the accumulation of these cells in the blood. CML is a clonal bone marrow stem cell disorder in which a proliferation of mature granulocytes (neutrophils, eosinophils and basophils) and their precursors is found. It is a type of myeloproliferative neoplasm associated with a characteristic chromosomal translocation called the Philadelphia chromosome.
CML is largely treated with targeted drugs called tyrosine-kinase inhibitors (TKIs) which have led to dramatically improved long-term survival rates since 2001. These drugs have revolutionized treatment of this disease and allow most patients to have a good quality of life when compared to the former chemotherapy drugs. In Western countries, CML accounts for 15–25% of all adult leukemias and 14% of leukemias overall (including the pediatric population, where CML is less common).[3]
https://en.wikipedia.org/wiki/Chronic_myelogenous_leukemia
Myeloproliferative neoplasms (MPNs) are a group of rare blood cancers in which excess red blood cells, white blood cells or platelets are produced in the bone marrow. Myelo refers to the bone marrow, proliferative describes the rapid growth of blood cells and neoplasm describes that growth as abnormal and uncontrolled.
The overproduction of blood cells is often associated with a somatic mutation, for example in the JAK2, CALR, TET2, and MPL gene markers.
In rare cases, some MPNs such as primary myelofibrosis may accelerate and turn into acute myeloid leukemia.[1]
https://en.wikipedia.org/wiki/Myeloproliferative_neoplasm
A myelodysplastic syndrome (MDS) is one of a group of cancers in which immature blood cells in the bone marrow do not mature, so do not become healthy blood cells.[3] Early on, no symptoms typically are seen.[3] Later, symptoms may include feeling tired, shortness of breath, bleeding disorders, anemia, or frequent infections.[3] Some types may develop into acute myeloid leukemia.[3]
https://en.wikipedia.org/wiki/Myelodysplastic_syndrome
https://en.wikipedia.org/wiki/Granulocyte
https://en.wikipedia.org/wiki/Karyorrhexis
https://en.wikipedia.org/wiki/Perls_Prussian_blue
https://en.wikipedia.org/wiki/Arsenic_poisoning
https://en.wikipedia.org/wiki/Chronic_myelomonocytic_leukemia
https://en.wikipedia.org/wiki/Myelodysplastic%E2%80%93myeloproliferative_diseases
https://en.wikipedia.org/wiki/Anti-thymocyte_globulin
https://en.wikipedia.org/wiki/Hematopoietic_stem_cell
https://en.wikipedia.org/wiki/Agranulocyte
https://en.wikipedia.org/wiki/Natural_killer_T_cell
https://en.wikipedia.org/wiki/Cytotoxic_T_cell
https://en.wikipedia.org/wiki/Cytokine
https://en.wikipedia.org/wiki/Tumor_necrosis_factor_superfamily
https://en.wikipedia.org/wiki/Protein_trimer
https://en.wikipedia.org/wiki/Escherichia_virus_T4
https://en.wikipedia.org/wiki/Bacteriophage
https://en.wikipedia.org/wiki/Myoviridae
https://en.wikipedia.org/wiki/Plasmid
https://en.wikipedia.org/wiki/Fungus
https://en.wikipedia.org/wiki/Holomycota
https://en.wikipedia.org/wiki/Autograph_(manuscript)
https://en.wikipedia.org/wiki/Macrophage
https://en.wikipedia.org/wiki/Holography
https://en.wikipedia.org/wiki/Ferris_wheel
https://en.wikipedia.org/wiki/Amusement_park
https://en.wikipedia.org/wiki/Ascaris_lumbricoides
https://en.wikipedia.org/wiki/Helminthiasis
https://en.wikipedia.org/wiki/Toxocaridae
https://en.wikipedia.org/wiki/Secernentea
https://en.wikipedia.org/wiki/Strongylida
https://en.wikipedia.org/wiki/Spiruria
https://en.wikipedia.org/wiki/Mansonella_perstans
https://en.wikipedia.org/wiki/Spirurida
https://en.wikipedia.org/wiki/Rhabditida
https://en.wikipedia.org/wiki/Onchocercidae
https://en.wikipedia.org/wiki/Anisakis
https://en.wikipedia.org/wiki/Cloacinidae
https://en.wikipedia.org/wiki/Oxyurida
https://en.wikipedia.org/wiki/Johann_August_Ephraim_Goeze
https://en.wikipedia.org/wiki/Edward_Tyson
https://en.wikipedia.org/wiki/Echinococcus_granulosus
https://en.wikipedia.org/wiki/Lymphatic_filariasis
Eggs are introduced into the body through ingestion. This can occur when eggs are deposited on the hands or face, after handling infected dogs or cats. In some cases, they can also get them from undercooked meat. In children without exposure to animals, eggs can be introduced by directly ingesting egg-contaminated soil while playing in a yard or on a playground. Usually, the scenario involves a young child with a new puppy. Unfortunately, many young children who have been infected with these larvae, causing ocular larva migrans in the eye, have been misdiagnosed to have retinoblastoma and have had their eyes erroneously removed.
https://en.wikipedia.org/wiki/Toxocaridae
https://en.wikipedia.org/wiki/Thelaziasis
https://en.wikipedia.org/wiki/Post-Soviet_states
Phosphorous acid (or phosphonic acid (singular)) is the compound described by the formula H3PO3. This acid is diprotic (readily ionizes two protons), not triprotic as might be suggested by this formula. Phosphorous acid is an intermediate in the preparation of other phosphorus compounds. Organic derivatives of phosphorous acid, compounds with the formula RPO3H2, are called phosphonic acids.
https://en.wikipedia.org/wiki/Phosphorous_acid
https://en.wikipedia.org/wiki/Category:Spiro_compounds
https://en.wikipedia.org/wiki/Helminthiasis
https://en.wikipedia.org/wiki/Ascaris_lumbricoides
https://en.wikipedia.org/wiki/Quinoline
https://en.wikipedia.org/wiki/Piperazine
https://en.wikipedia.org/wiki/Niclosamide
https://en.wikipedia.org/wiki/Antimony_potassium_tartrate
lead antimony arsenic phosphorous phos componds acid dessicant salt iodine silver
https://en.wikipedia.org/wiki/Quinoline
Subversion, Disease Dissemination/Procreation, Genetic Disease aid, sustenance occurrence, theft, Entreatment of aid with pursuant enhostagement and conspiracy cover, Theft of intellectual property to expand with public terrorism and subjugation/damages/etc., Mass Trafficking, use of cloak stolen/original design family defamation/reckless endangerment with penalty to endangered/etc., etc.

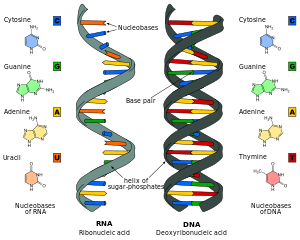





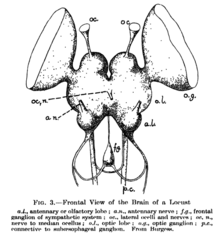




































![{\displaystyle {\begin{aligned}r_{\text{s}}&={\frac {n_{1}\cos \theta _{\text{i}}-n_{2}\cos \theta _{\text{t}}}{n_{1}\cos \theta _{\text{i}}+n_{2}\cos \theta _{\text{t}}}},\\[3pt]t_{\text{s}}&={\frac {2n_{1}\cos \theta _{\text{i}}}{n_{1}\cos \theta _{\text{i}}+n_{2}\cos \theta _{\text{t}}}},\\[3pt]r_{\text{p}}&={\frac {n_{2}\cos \theta _{\text{i}}-n_{1}\cos \theta _{\text{t}}}{n_{2}\cos \theta _{\text{i}}+n_{1}\cos \theta _{\text{t}}}},\\[3pt]t_{\text{p}}&={\frac {2n_{1}\cos \theta _{\text{i}}}{n_{2}\cos \theta _{\text{i}}+n_{1}\cos \theta _{\text{t}}}}.\end{aligned}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/175a7f341dbca168e50878dc6b1fdefca1d4bdf2)













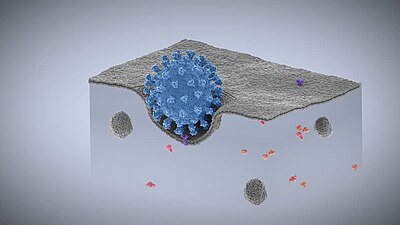




No comments:
Post a Comment