Myosin V
Myosin V is an unconventional myosin motor, which is processive as a dimer and has a step size of 36 nm.[23] It translocates (walks) along actin filaments traveling towards the barbed end (+ end) of the filaments. Myosin V is involved in the transport of cargo (e.g. RNA, vesicles, organelles, mitochondria) from the center of the cell to the periphery, but has been furthermore shown to act like a dynamic tether, retaining vesicles and organelles in the actin-rich periphery of cells.[24][25] A recent single molecule in vitro reconstitution study on assembling actin filaments suggests that Myosin V travels farther on newly assembling (ADP-Pi rich) F-actin, while processive runlengths are shorter on older (ADP-rich) F-actin.[26]
The Myosin V motor head can be subdivided into the following functional regions:[27]
- Nucleotide-binding site - These elements together coordinate di-valent metal cations (usually magnesium) and catalyze hydrolysis:
- Switch I - This contains a highly conserved SSR motif. Isomerizes in the presence of ATP.
- Switch II - This is the Kinase-GTPase version of the Walker B motif DxxG. Isomerizes in the presence of ATP.
- P-loop - This contains the Walker A motif GxxxxGK(S,T). This is the primary ATP binding site.
- Transducer - The seven β-strands that underpin the motor head's structure.[28]
- U50 and L50 - The Upper (U50) and Lower (L50) domains are each around 50kDa. Their spatial separation[29] forms a cleft critical for binding to actin and some regulatory compounds.
- SH1 helix and Relay - These elements together provide an essential mechanism for coupling the enzymatic state of the motor domain to the powerstroke-producing region (converter domain, lever arm, and light chains).[30][31]
- Converter - This converts a change of conformation in the motor head to an angular displacement of the lever arm (in most cases reinforced with light chains).[31]
Myosin VI
Myosin VI is an unconventional myosin motor, which is primarily processive as a dimer, but also acts as a nonprocessive monomer. It walks along actin filaments, travelling towards the pointed end (- end) of the filaments.[33] Myosin VI is thought to transport endocytic vesicles into the cell.[34]
Myosin VII
Myosin VII is an unconventional myosin with two FERM domains in the tail region. It has an extended lever arm consisting of five calmodulin binding IQ motifs followed by a single alpha helix (SAH)[35] Myosin VII is required for phagocytosis in Dictyostelium discoideum, spermatogenesis in C. elegans and stereocilia formation in mice and zebrafish.[36]
Myosin VIII
Myosin VIII is a plant-specific myosin linked to cell division;[37] specifically, it is involved in regulating the flow of cytoplasm between cells[38] and in the localization of vesicles to the phragmoplast.[39]
Myosin IX
Myosin IX is a group of single-headed motor proteins. It was first shown to be minus-end directed,[40] but a later study showed that it is plus-end directed.[41] The movement mechanism for this myosin is poorly understood.
Myosin X
Myosin X is an unconventional myosin motor, which is functional as a dimer. The dimerization of myosin X is thought to be antiparallel.[42] This behavior has not been observed in other myosins. In mammalian cells, the motor is found to localize to filopodia. Myosin X walks towards the barbed ends of filaments. Some research suggests it preferentially walks on bundles of actin, rather than single filaments.[43] It is the first myosin motor found to exhibit this behavior.
Myosin XI
Myosin XI directs the movement of organelles such as plastids and mitochondria in plant cells.[44] It is responsible for the light-directed movement of chloroplasts according to light intensity and the formation of stromules interconnecting different plastids. Myosin XI also plays a key role in polar root tip growth and is necessary for proper root hair elongation.[45] A specific Myosin XI found in Nicotiana tabacum was discovered to be the fastest known processive molecular motor, moving at 7μm/s in 35 nm steps along the actin filament.[46]
Myosin XII
Myosin XIII
Myosin XIV
This myosin group has been found in the Apicomplexa phylum.[47] The myosins localize to plasma membranes of the intracellular parasites and may then be involved in the cell invasion process.[48]
This myosin is also found in the ciliated protozoan Tetrahymena thermaphila. Known functions include: transporting phagosomes to the nucleus and perturbing the developmentally regulated elimination of the macronucleus during conjugation.
Myosin XV
Myosin XV is necessary for the development of the actin core structure of the non-motile stereocilia located in the inner ear. It is thought to be functional as a monomer.
Myosin XVI
Myosin XVII
Myosin XVIII
MYO18A A gene on chromosome 17q11.2 that encodes actin-based motor molecules with ATPase activity, which may be involved in maintaining stromal cell scaffolding required for maintaining intercellular contact.
Myosin XIX
Unconventional myosin XIX (Myo19) is a mitochondrial associated myosin motor.[49]
Genes in humans
Note that not all of these genes are active.
- Class I: MYO1A, MYO1B, MYO1C, MYO1D, MYO1E, MYO1F, MYO1G, MYO1H
- Class II: MYH1, MYH2, MYH3, MYH4, MYH6, MYH7, MYH7B, MYH8, MYH9, MYH10, MYH11, MYH13, MYH14, MYH15, MYH16
- Class III: MYO3A, MYO3B
- Class V: MYO5A, MYO5B, MYO5C
- Class VI: MYO6
- Class VII: MYO7A, MYO7B
- Class IX: MYO9A, MYO9B
- Class X: MYO10
- Class XV: MYO15A
- Class XVIII: MYO18A, MYO18B
Myosin light chains are distinct and have their own properties. They are not considered "myosins" but are components of the macromolecular complexes that make up the functional myosin enzymes.
Paramyosin
Paramyosin is a large, 93-115kDa muscle protein that has been described in a number of diverse invertebrate phyla.[50] Invertebrate thick filaments are thought to be composed of an inner paramyosin core surrounded by myosin. The myosin interacts with actin, resulting in fibre contraction.[51] Paramyosin is found in many different invertebrate species, for example, Brachiopoda, Sipunculidea, Nematoda, Annelida, Mollusca, Arachnida, and Insecta.[50] Paramyosin is responsible for the "catch" mechanism that enables sustained contraction of muscles with very little energy expenditure, such that a clam can remain closed for extended periods.
Paramyosins can be found in seafood. A recent computational study showed that following human intestinal digestion, paramyosins of common octopus, Humboldt squid, Japanese abalone, Japanese scallop, Mediterranean mussel, Pacific oyster, sea cucumber, and Whiteleg shrimp could release short peptides that inhibit the enzymatic activities of angiotensin converting enzyme and dipeptidyl peptidase.[52]
https://en.wikipedia.org/wiki/Myosin#Myosin_V
https://en.wikipedia.org/wiki/Amoeba
https://en.wikipedia.org/wiki/Protoplasm
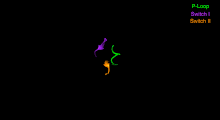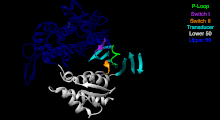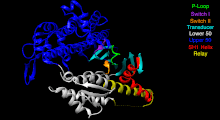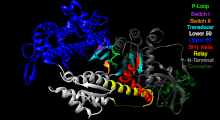

No comments:
Post a Comment