The magnate term, from the late Latin magnas, a great man, itself from Latin magnus, "great", means a man from the higher nobility, a man who belongs to the high office-holders, or a man in a high social position, by birth, wealth or other qualities in Western Christian countries since the medieval period. It also includes the members of the higher clergy, such as bishops, archbishops and cardinals. In reference to the medieval, the term is often used to distinguish higher territorial landowners and warlords, such as counts, earls, dukes, and territorial-princes from the baronage, and in Poland for the richest szlachta.
https://en.wikipedia.org/wiki/Magnate
 | |||||||||||||||||||||||||||||||||
| Copper | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Appearance | Red-orange metallic luster | ||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(Cu) | |||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||
| Copper in the periodic table | |||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||
| Atomic number (Z) | 29 | ||||||||||||||||||||||||||||||||
| Group | group 11 | ||||||||||||||||||||||||||||||||
| Period | period 4 | ||||||||||||||||||||||||||||||||
| Block | d-block | ||||||||||||||||||||||||||||||||
| Electron configuration | [Ar] 3d10 4s1 | ||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 1 | ||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||
| Phase at STP | solid | ||||||||||||||||||||||||||||||||
| Melting point | 1357.77 K (1084.62 °C, 1984.32 °F) | ||||||||||||||||||||||||||||||||
| Boiling point | 2835 K (2562 °C, 4643 °F) | ||||||||||||||||||||||||||||||||
| Density (near r.t.) | 8.96 g/cm3 | ||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 8.02 g/cm3 | ||||||||||||||||||||||||||||||||
| Heat of fusion | 13.26 kJ/mol | ||||||||||||||||||||||||||||||||
| Heat of vaporization | 300.4 kJ/mol | ||||||||||||||||||||||||||||||||
| Molar heat capacity | 24.440 J/(mol·K) | ||||||||||||||||||||||||||||||||
Vapor pressure
| |||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||
| Oxidation states | −2, 0,[2] +1, +2, +3, +4 (a mildly basic oxide) | ||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.90 | ||||||||||||||||||||||||||||||||
| Ionization energies |
| ||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 128 pm | ||||||||||||||||||||||||||||||||
| Covalent radius | 132±4 pm | ||||||||||||||||||||||||||||||||
| Van der Waals radius | 140 pm | ||||||||||||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||||||||||||||
| Crystal structure | face-centered cubic (fcc) | ||||||||||||||||||||||||||||||||
| Speed of sound thin rod | (annealed) 3810 m/s (at r.t.) | ||||||||||||||||||||||||||||||||
| Thermal expansion | 16.5 µm/(m⋅K) (at 25 °C) | ||||||||||||||||||||||||||||||||
| Thermal conductivity | 401 W/(m⋅K) | ||||||||||||||||||||||||||||||||
| Electrical resistivity | 16.78 nΩ⋅m (at 20 °C) | ||||||||||||||||||||||||||||||||
| Magnetic ordering | diamagnetic[3] | ||||||||||||||||||||||||||||||||
| Molar magnetic susceptibility | −5.46×10−6 cm3/mol[4] | ||||||||||||||||||||||||||||||||
| Young's modulus | 110–128 GPa | ||||||||||||||||||||||||||||||||
| Shear modulus | 48 GPa | ||||||||||||||||||||||||||||||||
| Bulk modulus | 140 GPa | ||||||||||||||||||||||||||||||||
| Poisson ratio | 0.34 | ||||||||||||||||||||||||||||||||
| Mohs hardness | 3.0 | ||||||||||||||||||||||||||||||||
| Vickers hardness | 343–369 MPa | ||||||||||||||||||||||||||||||||
| Brinell hardness | 235–878 MPa | ||||||||||||||||||||||||||||||||
| CAS Number | 7440-50-8 | ||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||
| Naming | after Cyprus, principal mining place in Roman era (Cyprium) | ||||||||||||||||||||||||||||||||
| Discovery | Middle East (9000 BC) | ||||||||||||||||||||||||||||||||
| Symbol | "Cu": from Latin cuprum | ||||||||||||||||||||||||||||||||
| Isotopes of copper | |||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||
Copper is a chemical element with the symbol Cu (from Latin: cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orange color. Copper is used as a conductor of heat and electricity, as a building material, and as a constituent of various metal alloys, such as sterling silver used in jewelry, cupronickel used to make marine hardware and coins, and constantan used in strain gauges and thermocouples for temperature measurement.
https://en.wikipedia.org/wiki/Copper
The Magnate conspiracy, also known as the Zrinski-Frankopan Conspiracy (Croatian: Zrinsko-frankopanska urota) in Croatia, and Wesselényi conspiracy (Hungarian: Wesselényi-összeesküvés) in Hungary, was a 17th-century attempt to throw off Habsburg and other foreign influences over Hungary and Croatia.[1] The attempted coup was caused by the unpopular Peace of Vasvár, struck in 1664 between Holy Roman Emperor Leopold I and the Ottoman Empire. The poorly organized attempt at revolt gave the Habsburgs reason to clamp down on their opponents. It was named after Hungarian Count Ferenc Wesselényi, and by Croatian counts, brothers Nikola Zrinski and Petar Zrinski and Petar's brother-in-law Fran Krsto Frankopan.
In the second half of the 17th century, Vienna was interested in centralising the administration of the state so that it could introduce a consistent economic policy of mercantilism and so lay the foundations for an absolute monarchy.[2] The main obstacle on that path was the independence of magnates. Nikola and Petar Zrinski and their associate Fran Krsto Frankapan resisted Vienna's policy and were angered by its leniency toward the Ottomans. The Habsburgs were paying more attention to their Western European goals and less to freeing Croatia and Hungary from Ottomans.[2]
https://en.wikipedia.org/wiki/Magnate_conspiracy
| Magnesite | |
|---|---|
 Magnesite crystals from Brazil (11.4 x 9.2 x 3.6 cm) | |
| General | |
| Category | Carbonate mineral |
| Formula (repeating unit) | MgCO3 |
| IMA symbol | Mgs[1] |
| Strunz classification | 5.AB.05 |
| Crystal system | Trigonal |
| Crystal class | Hexagonal scalenohedral (3m) H-M symbol: (3 2/m) |
| Space group | R3c |
| Identification | |
| Color | Colorless, white, pale yellow, pale brown, faintly pink, lilac-rose |
| Crystal habit | Usually massive, rarely as rhombohedrons or hexagonal prisms |
| Cleavage | [1011] perfect |
| Fracture | Conchoidal |
| Tenacity | Brittle |
| Mohs scale hardness | 3.5 – 4.5 |
| Luster | Vitreous |
| Streak | white |
| Diaphaneity | Transparent to translucent |
| Specific gravity | 3.0 – 3.2 |
| Optical properties | Uniaxial (-) |
| Refractive index | nω=1.508 – 1.510 nε=1.700 |
| Birefringence | 0.191 |
| Fusibility | infusible |
| Solubility | Effervesces in hot HCl |
| Other characteristics | May exhibit pale green to pale blue fluorescence and phosphorescence under UV; triboluminescent |
| References | [2][3][4][5] |
Magnesite is a mineral with the chemical formula MgCO
3 (magnesium carbonate). Iron, manganese, cobalt, and nickel may occur as admixtures, but only in small amounts.
https://en.wikipedia.org/wiki/Magnesite
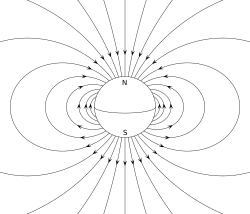
A magnet is a material or object that produces a magnetic field. This magnetic field is invisible but is responsible for the most notable property of a magnet: a force that pulls on other ferromagnetic materials, such as iron, steel, nickel, cobalt, etc. and attracts or repels other magnets.
A permanent magnet is an object made from a material that is magnetized and creates its own persistent magnetic field. An everyday example is a refrigerator magnet used to hold notes on a refrigerator door. Materials that can be magnetized, which are also the ones that are strongly attracted to a magnet, are called ferromagnetic (or ferrimagnetic). These include the elements iron, nickel and cobalt and their alloys, some alloys of rare-earth metals, and some naturally occurring minerals such as lodestone. Although ferromagnetic (and ferrimagnetic) materials are the only ones attracted to a magnet strongly enough to be commonly considered magnetic, all other substances respond weakly to a magnetic field, by one of several other types of magnetism.
https://en.wikipedia.org/wiki/Magnet

A magnetometer is a device that measures magnetic field or magnetic dipole moment. Different types of magnetometers measure the direction, strength, or relative change of a magnetic field at a particular location. A compass is one such device, one that measures the direction of an ambient magnetic field, in this case, the Earth's magnetic field. Other magnetometers measure the magnetic dipole moment of a magnetic material such as a ferromagnet, for example by recording the effect of this magnetic dipole on the induced current in a coil.
The first magnetometer capable of measuring the absolute magnetic intensity at a point in space was invented by Carl Friedrich Gauss in 1833 and notable developments in the 19th century included the Hall effect, which is still widely used.
Magnetometers are widely used for measuring the Earth's magnetic field, in geophysical surveys, to detect magnetic anomalies of various types, and to determine the dipole moment of magnetic materials. In an aircraft's attitude and heading reference system, they are commonly used as a heading reference. Magnetometers are also used by the military as a triggering mechanism in magnetic mines to detect submarines. Consequently, some countries, such as the United States, Canada and Australia, classify the more sensitive magnetometers as military technology, and control their distribution.
Magnetometers can be used as metal detectors: they can detect only magnetic (ferrous) metals, but can detect such metals at a much greater distance than conventional metal detectors, which rely on conductivity. Magnetometers are capable of detecting large objects, such as cars, at over 10 metres (33 ft), while a conventional metal detector's range is rarely more than 2 metres (6 ft 7 in).
In recent years, magnetometers have been miniaturized to the extent that they can be incorporated in integrated circuits at very low cost and are finding increasing use as miniaturized compasses (MEMS magnetic field sensor).
https://en.wikipedia.org/wiki/Magnetometer
|
|
|
|
|
|
|
|
|
|
|
|
In electromagnetism, the magnetic moment is the magnetic strength and orientation of a magnet or other object that produces a magnetic field. Examples of objects that have magnetic moments include loops of electric current (such as electromagnets), permanent magnets, elementary particles (such as electrons), various molecules, and many astronomical objects (such as many planets, some moons, stars, etc).
More precisely, the term magnetic moment normally refers to a system's magnetic dipole moment, the component of the magnetic moment that can be represented by an equivalent magnetic dipole: a magnetic north and south pole separated by a very small distance. The magnetic dipole component is sufficient for small enough magnets or for large enough distances. Higher-order terms (such as the magnetic quadrupole moment) may be needed in addition to the dipole moment for extended objects.
The magnetic dipole moment of an object is readily defined in terms of the torque that the object experiences in a given magnetic field. The same applied magnetic field creates larger torques on objects with larger magnetic moments. The strength (and direction) of this torque depends not only on the magnitude of the magnetic moment but also on its orientation relative to the direction of the magnetic field. The magnetic moment may be considered, therefore, to be a vector. The direction of the magnetic moment points from the south to north pole of the magnet (inside the magnet).
The magnetic field of a magnetic dipole is proportional to its magnetic dipole moment. The dipole component of an object's magnetic field is symmetric about the direction of its magnetic dipole moment, and decreases as the inverse cube of the distance from the object.
https://en.wikipedia.org/wiki/Magnetic_moment
A dipole glass is an analog of a glass where the dipoles are frozen below a given freezing temperature Tf introducing randomness thus resulting in a lack of long-range ferroelectric order.[1] A dipole glass is very similar to the concept of a spin glass where the atomic spins don't all align in the same direction (like in a ferromagnetic material) and thus result in a net-zero magnetization. The randomness of dipoles in a dipole glass creates local fields resulting in short-range order but no long-range order.
The dipole glass like state was first observed in Alkali halide crystal-type dielectrics containing dipole impurities.[2][3][4] The dipole impurities in these materials result in off-center ions which results in anomalies in certain properties like specific heat, thermal conductivity as well as some spectroscopic properties.[1] Other materials which show a dipolar glass phase include Rb(1-x)(NH4)xH2PO4 (RADP) and Rb(1-x)(ND4)xD2PO4 (DRADP).[5][6] In materials like DRADP the dipole moment is introduced due to the deuteron O-D--O bond. Dipole glass like behavior is also observed in materials like ceramics,[7] 3D water framework[8] and perovskites.[9][10]
https://en.wikipedia.org/wiki/Dipole_glass
In physics, a dipole (from Greek δίς (dis) 'twice', and πόλος (polos) 'axis'[1][2][3]) is an electromagnetic phenomenon which occurs in two ways:
- An electric dipole deals with the separation of the positive and negative electric charges found in any electromagnetic system. A simple example of this system is a pair of charges of equal magnitude but opposite sign separated by some typically small distance. (A permanent electric dipole is called an electret.)
- A magnetic dipole is the closed circulation of an electric current system. A simple example is a single loop of wire with constant current through it. A bar magnet is an example of a magnet with a permanent magnetic dipole moment.[4][5]
https://en.wikipedia.org/wiki/Dipole
The only known mechanisms for the creation of magnetic dipoles are by current loops or quantum-mechanical spin since the existence of magnetic monopoles has never been experimentally demonstrated.
https://en.wikipedia.org/wiki/Dipole
A dipole speaker enclosure in its simplest form is constructed by mounting a loudspeaker driver on a flat panel. The panel may be folded to conserve space.
The term dipole derives from the fact that the polar response consists of two lobes, with equal radiation forwards and backwards, and none perpendicular to the axis. (By comparison a monopole response consists of one lobe.) This can be useful in reducing the stimulation of resonant room modes at low frequencies. It also results in high frequencies being reflected from any rear wall, which can enhance the naturalness of the sound in typical listening rooms by creating more diffuse reverberation, though in theory it could detract from stereo localization. For this reason dipole speakers are often used as surround channel speakers, where a diffuse sound is desired to create ambience.
https://en.wikipedia.org/wiki/Dipole_speaker





No comments:
Post a Comment