| |||

| |||
| Names | |||
|---|---|---|---|
| Other names
Diphosphorus pentoxide
Phosphorus(V) oxide Phosphoric anhydride Tetraphosphorus decaoxide Tetraphosphorus decoxide | |||
| Identifiers | |||
| |||
3D model (JSmol)
|
| ||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.013.852 | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| Properties | |
|---|---|
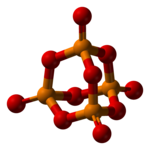
| P4O10 | |
| Molar mass | 283.9 g mol−1 |
| Appearance | White powder Very deliquescent |
| Odor | Odorless |
| Density | 2.39 g/cm3 |
| Melting point | 340 °C (644 °F; 613 K) |
| Boiling point | 360 °C (sublimes) |
| exothermic hydrolysis | |
| Vapor pressure | 1 mmHg @ 385 °C (stable form) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
reacts with water, strong dehydrating agent, corrosive |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Phosphorus pentoxide is a chemical compound with molecular formula P4O10 (with its common name derived from its empirical formula, P2O5). This white crystalline solid is the anhydride of phosphoric acid. It is a powerful desiccant and dehydrating agent.
https://en.wikipedia.org/wiki/Phosphorus_pentoxide



No comments:
Post a Comment