Extrachromosomal DNA (abbreviated ecDNA) is any DNA that is found off the chromosomes, either inside or outside the nucleus of a cell. Most DNA in an individual genome is found in chromosomes contained in the nucleus. Multiple forms of extrachromosomal DNA exist and serve important biological functions,[1] e.g. they can play a role in disease, such as ecDNA in cancer.[2]
https://en.wikipedia.org/wiki/Extrachromosomal_DNA
Monday, September 6, 2021
---------------------------------------------------------------------------------------------------------
In chemistry, chemical synthesis (or combination) is the artificial execution of useful chemical reactions to obtain one or several products.[1]This occurs by physical and chemical manipulations usually involving one or more reactions. In modern laboratory uses, the process is reproducible and reliable.
A chemical synthesis involves one or more compounds (known as reagents or reactants) that will undergo a transformation when subjected to certain conditions. Various reaction types can be applied to formulate a desired product. This requires mixing the compounds in a reaction vessel, such as a chemical reactor or a simple round-bottom flask. Many reactions require some form of work-up or purification procedure to isolate the final product.[1]
The amount produced in chemical synthesis is known as the reaction yield. Typically, yields are expressed as a mass in grams (in a laboratory setting) or as a percentage of the total theoretical quantity that could be produced based on the limiting reagent. A side reaction is an unwanted chemical reaction taking place which reduces the desired yield. The word synthesis was first used by the chemist Hermann Kolbe.[2]
Strategies[edit]
Many strategies exist in chemical synthesis that go beyond converting reactant A to reaction product B in a single step. In multistep synthesis, a chemical compound is synthesized through a series of individual chemical reactions, each with its own work-up.[3] For example, a laboratory synthesis of paracetamol can consist of three individual synthetic steps. In cascade reactions multiple chemical transformations take place within a single reactant, in multi-component reactions up to 11 different reactants form a single reaction product and in a telescopic synthesis one reactant goes through multiple transformations without isolation of intermediates.
Organic synthesis[edit]
Organic synthesis is a special branch of chemical synthesis dealing with the synthesis of organic compounds. In the total synthesis of a complex product it may take multiple steps to synthesize the product of interest and an inordinate amount of time. Skill in organic synthesis is prized among chemists and the synthesis of exceptionally valuable or difficult compounds has won chemists such as Robert Burns Woodwardthe Nobel Prize for Chemistry. If a chemical synthesis starts from basic laboratory compounds, it is considered a purely synthetic process. If it starts from a product isolated from plants or animals and then proceeds to new compounds, the synthesis is described as a semisyntheticprocess.
Inorganic synthesis[edit]
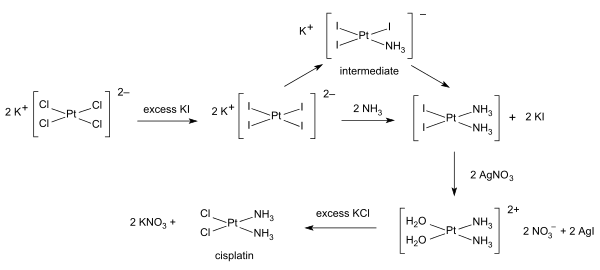
Inorganic synthesis and organometallic synthesis are applied to the preparation of compounds with significant non-organic content. An illustrative example is the preparation of the anti-cancer drug cisplatin from potassium tetrachloroplatinate.[4]
See also[edit]
Chemical synthesis
Branches of chemistry
https://en.wikipedia.org/wiki/Chemical_synthesis
No comments:
Post a Comment