A coenogamete is a multi-nucleate gamete (a gamete with more than one nucleus).[1] Fusion of coengametes creates Coenocytes.
They exist amongst members of the phylum Porifera (sponges) under kingdom Animalia and division Zygomycota of kingdom Fungi
https://en.wikipedia.org/wiki/Coenogamete
Anisogamy is a form of sexual reproduction that involves the union or fusion of two gametes that differ in size and/or form. The smaller gamete is male, a sperm cell, whereas the larger gamete is female, typically an egg cell. Anisogamy is predominant among multicellular organisms.[1] In both plants and animals gamete size difference is the fundamental difference between females and males.[2]
Anisogamy most likely evolved from isogamy.[3] Since the biological definition of male and female is based on gamete size, the evolution of anisogamy is viewed as the evolutionary origin of male and female sexes.[4][5] Anisogamy is the prerequisite to sexual selection,[6] and led the sexes to different primary and secondary sex characteristics[7] including sex differences in behavior.[8]
Geoff Parker, Robin Baker, and Vic Smith were the first to provide a mathematical model for the evolution of anisogamy that was consistent with modern evolutionary theory.[4] Their theory was widely accepted but there are alternative hypotheses about the evolution of anisogamy.[9][1]
https://en.wikipedia.org/wiki/Anisogamy
Isogamy is a form of sexual reproduction that involves gametes of the same morphology (indistinguishable in shape and size), found in most unicellular eukaryotes.[1] Because both gametes look alike, they generally cannot be classified as male or female.[2] Instead, organisms undergoing isogamy are said to have different mating types, most commonly noted as "+" and "−" strains.[3]
https://en.wikipedia.org/wiki/Isogamy
Jump to navigation Jump to search
A coenocyte (/ˈsiːnəˌsaɪt/) is a multinucleate cell which can result from multiple nuclear divisions without their accompanying cytokinesis, in contrast to a syncytium, which results from cellular aggregation followed by dissolution of the cell membranes inside the mass.[1] The word syncytium in animal embryology is used to refer to the coenocytic blastoderm of invertebrates.[2] A coenocytic cell is referred to as a coenobium (plural coenobia), and most coenobia are composed of a distinct number of cells, often as a multiple of two (4, 8, etc.).[3]
Research suggests that coenobium formation may be a defense against grazing in some species.[4]
https://en.wikipedia.org/wiki/Coenocyte
Multinucleate cells (also known as multinucleated or polynuclear cells) are eukaryotic cells that have more than one nucleus per cell, i.e., multiple nuclei share one common cytoplasm. Mitosis in multinucleate cells can occur either in a coordinated, synchronous manner where all nuclei divide simultaneously or asynchronously where individual nuclei divide independently in time and space. Certain organisms may have a multinuclear stage of their life cycle. For example, slime molds have a vegetative, multinucleate life stage called a plasmodium.[1]
Although not normally viewed as a case of multinucleation, plant cells share a common cytoplasm by plasmodesmata, and most cells in animal tissues are in communication with their neighbors via gap junctions.[2]
Multinucleate cells, depending on the mechanism by which they are formed, can be divided into[3][4] "syncytia" (formed by cell fusion) or "coenocytes" (formed by nuclear division not being followed by cytokinesis).
A number of dinoflagellates are known to have two nuclei. Unlike other multinucleated cells these nuclei contain two distinct lineages of DNA: one from the dinoflagellate and the other from a symbiotic diatom.[5]
Some bacteria, such as Mycoplasma pneumoniae, a pathogen of the respiratory tract, may display multinuclear filaments as a result of a delay between genome replication and cellular division.[6]
https://en.wikipedia.org/wiki/Multinucleate
Gap junctions are specialized intercellular connections between a multitude of animal cell-types.[1][2][3] They directly connect the cytoplasm of two cells, which allows various molecules, ions and electrical impulses to directly pass through a regulated gate between cells.[4][5]
One gap junction channel is composed of two protein hexamers (or hemichannels) called connexons in vertebrates and innexons in invertebrates. The hemichannel pair connect across the intercellular space bridging the gap between two cells.[4][5][6] Gap junctions are analogous to the plasmodesmata that join plant cells.[7]
Gap junctions occur in virtually all tissues of the body, with the exception of adult fully developed skeletal muscle and mobile cell types such as sperm or erythrocytes. Gap junctions are not found in simpler organisms such as sponges and slime molds.
https://en.wikipedia.org/wiki/Gap_junction
A syncytium (/sɪnˈsɪʃiəm/; plural syncytia; from Greek: σύν syn "together" and κύτος kytos "box, i.e. cell") or symplasm is a multinucleate cell which can result from multiple cell fusions of uninuclear cells (i.e., cells with a single nucleus), in contrast to a coenocyte, which can result from multiple nuclear divisions without accompanying cytokinesis.[1] The muscle cell that makes up animal skeletal muscle is a classic example of a syncytium cell. The term may also refer to cells interconnected by specialized membranes with gap junctions, as seen in the heart muscle cells and certain smooth muscle cells, which are synchronized electrically in an action potential.
The field of embryogenesis uses the word syncytium to refer to the coenocytic blastoderm embryos of invertebrates, such as Drosophila melanogaster.[2]
Contents
https://en.wikipedia.org/wiki/Syncytium
A blastoderm (germinal disc, blastodisc) is a single layer of embryonic epithelial tissue that makes up the blastula.[1] It encloses the fluid filled blastocoel. Gastrulation follows blastoderm formation, where the tips of the blastoderm begins the formation of the ectoderm, mesoderm, and endoderm.[2]
https://en.wikipedia.org/wiki/Blastoderm
Jump to navigation Jump to search
The blastodisc, also called the germinal disc, is the embryo-forming part on the yolk of the egg of an animal that undergoes discoidal meroblastic cleavage.[1] Discoidal cleavage occurs in those animals with a large proportion of yolk in their eggs, and include insects, fish, reptiles and birds.[2] The blastodisc is a small disc of cytoplasm that sits on top of the yolk. In birds it is a small, circular, white spot (approximately 1.5-3 mm across) on the surface of the yellow yolk of an egg, at the animal pole.[3]
https://en.wikipedia.org/wiki/Blastodisc
An action potential occurs when the membrane potential of a specific cell location rapidly rises and falls.[1] This depolarization then causes adjacent locations to similarly depolarize. Action potentials occur in several types of animal cells, called excitable cells, which include neurons, muscle cells, and in some plant cells. Certain endocrine cells such as pancreatic beta cells, and certain cells of the anterior pituitary gland are also excitable cells.[2]
In neurons, action potentials play a central role in cell-cell communication by providing for—or with regard to saltatory conduction, assisting—the propagation of signals along the neuron's axon toward synaptic boutons situated at the ends of an axon; these signals can then connect with other neurons at synapses, or to motor cells or glands. In other types of cells, their main function is to activate intracellular processes. In muscle cells, for example, an action potential is the first step in the chain of events leading to contraction. In beta cells of the pancreas, they provoke release of insulin.[a] Action potentials in neurons are also known as "nerve impulses" or "spikes", and the temporal sequence of action potentials generated by a neuron is called its "spike train". A neuron that emits an action potential, or nerve impulse, is often said to "fire".
Action potentials are generated by special types of voltage-gated ion channels embedded in a cell's plasma membrane.[b] These channels are shut when the membrane potential is near the (negative) resting potential of the cell, but they rapidly begin to open if the membrane potential increases to a precisely defined threshold voltage, depolarising the transmembrane potential.[b] When the channels open, they allow an inward flow of sodium ions, which changes the electrochemical gradient, which in turn produces a further rise in the membrane potential towards zero. This then causes more channels to open, producing a greater electric current across the cell membrane and so on. The process proceeds explosively until all of the available ion channels are open, resulting in a large upswing in the membrane potential. The rapid influx of sodium ions causes the polarity of the plasma membrane to reverse, and the ion channels then rapidly inactivate. As the sodium channels close, sodium ions can no longer enter the neuron, and they are then actively transported back out of the plasma membrane. Potassium channels are then activated, and there is an outward current of potassium ions, returning the electrochemical gradient to the resting state. After an action potential has occurred, there is a transient negative shift, called the afterhyperpolarization.
In animal cells, there are two primary types of action potentials. One type is generated by voltage-gated sodium channels, the other by voltage-gated calcium channels. Sodium-based action potentials usually last for under one millisecond, but calcium-based action potentials may last for 100 milliseconds or longer.[citation needed] In some types of neurons, slow calcium spikes provide the driving force for a long burst of rapidly emitted sodium spikes. In cardiac muscle cells, on the other hand, an initial fast sodium spike provides a "primer" to provoke the rapid onset of a calcium spike, which then produces muscle contraction.[3]
https://en.wikipedia.org/wiki/Action_potential
ART techniques are also used to improve the profitability of agricultural animal species such as cows and pigs by enabling selective breeding for desired traits and/or to increase numbers of offspring.[44] For example, when allowed to breed naturally, cows typically produce one calf per year, whereas IVF increases offspring yield to 9-12 calves per year.[45] IVF and other ART techniques, including cloning via interspecies somatic cell nuclear transfer (iSCNT),[46] are also used in attempts to increase the numbers of endangered or vulnerable species, such as Northern white rhinos,[47] cheetahs,[48] and sturgeons.[49]
Fossilized animal embryos are known from the Precambrian, and are found in great numbers during the Cambrian period. Even fossilized dinosaur embryos have been discovered.[57]
https://en.wikipedia.org/wiki/Embryo#Development
Cytokinesis (/ˌsaɪtoʊkɪˈniːsɪs/) is the part of the cell division process during which the cytoplasm of a single eukaryotic cell divides into two daughter cells. Cytoplasmic division begins during or after the late stages of nuclear division in mitosis and meiosis. During cytokinesis the spindle apparatus partitions and transports duplicated chromatids into the cytoplasm of the separating daughter cells. It thereby ensures that chromosome number and complement are maintained from one generation to the next and that, except in special cases, the daughter cells will be functional copies of the parent cell. After the completion of the telophase and cytokinesis, each daughter cell enters the interphase of the cell cycle.
https://en.wikipedia.org/wiki/Cytokinesis
Oogamy is an extreme form of anisogamy where the gametes differ in both size and form. In oogamy the large female gamete (also known as ovum) is immobile, while the small male gamete (also known as sperm) is mobile.[1] Oogamy is a common form of anisogamy, with almost all animals and land plants being oogamous.
https://en.wikipedia.org/wiki/Oogamy
Glass sponges
Much of the body of Hexactinellid sponges is composed of syncitial tissue. This allows them to form their large siliceous spicules exclusively inside their cells.[8]
https://en.wikipedia.org/wiki/Syncytium#Glass_sponges
Hexactinellid sponges are sponges with a skeleton made of four- and/or six-pointed siliceous spicules, often referred to as glass sponges. They are usually classified along with other sponges in the phylum Porifera, but some researchers consider them sufficiently distinct to deserve their own phylum, Symplasma. Some experts believe glass sponges are the longest-lived animals on earth; these scientists tentatively estimate a maximum age of up to 15,000 years.
https://en.wikipedia.org/wiki/Hexactinellid
Jump to navigation Jump to search
A syncytium (/sɪnˈsɪʃiəm/; plural syncytia; from Greek: σύν syn "together" and κύτος kytos "box, i.e. cell") or symplasm is a multinucleate cell which can result from multiple cell fusions of uninuclear cells (i.e., cells with a single nucleus), in contrast to a coenocyte, which can result from multiple nuclear divisions without accompanying cytokinesis.[1] The muscle cell that makes up animal skeletal muscle is a classic example of a syncytium cell. The term may also refer to cells interconnected by specialized membranes with gap junctions, as seen in the heart muscle cells and certain smooth muscle cells, which are synchronized electrically in an action potential.
The field of embryogenesis uses the word syncytium to refer to the coenocytic blastoderm embryos of invertebrates, such as Drosophila melanogaster.[2]
https://en.wikipedia.org/wiki/Syncytium
Hexasterophora are a subclass of sponges, in the class Hexactinellida.[4] The Hexasterophora first appeared in the Ordovician and is separated into five recent orders, including the Lyssacinosa, the Hexactinosa, and the Lychniscosa, all of which have living representatives in the seas today.
https://en.wikipedia.org/wiki/Hexasterophora
Occurrence
Oogamy is found in most species that reproduce sexually, all higher species being oogamous.[2]
Oogamy is found in all land plants,[3] and in some red algae, brown algae and green algae.[4] Oogamy is favored in plants because only one gamete has to travel through harsh environments outside the plant.[5] Oogamy is also present in oomycetes.[6]
Almost all animals are oogamous.[7] There are exceptions, such as the opiliones that have immobile sperm.[8]
Etymology
The term oogamy was first used in the year 1888.[9]
Evolution
It is generally accepted that isogamy is the ancestral state[10] and that oogamy evolves from isogamy through anisogamy.[11][12] However, transitions do exist between anisogamy and oogamy.[13]
When oogamy has evolved, males and females typically differ in many aspects. According to David B. Dusenbery internal fertilization probably originated from oogamy.[7] But one study in 2014 on Colemanosphaera said that oogamy in Volvox may have evolved before the transition from external to internal fertilization.[14][non-primary source needed]
In streptophytes, oogamy likely first occurred before the split between algae and land plants.[15]
https://en.wikipedia.org/wiki/Oogamy
Jump to navigation Jump to search
| Unicellular organism | |
|---|---|
 Valonia ventricosa, a species of alga with a diameter that ranges typically from 1 to 4 centimetres (0.4 to 1.6 in) is among the largest unicellular species |
A unicellular organism, also known as a single-celled organism, is an organism that consists of a single cell, unlike a multicellular organism that consists of multiple cells. Organisms fall into two general categories: prokaryotic organisms and eukaryotic organisms. All prokaryotes are unicellular and are classified into bacteria and archaea. Many eukaryotes are multicellular, but some are unicellular such as protozoa, unicellular algae, and unicellular fungi. Unicellular organisms are thought to be the oldest form of life, with early protocells possibly emerging 3.8–4.0 billion years ago.[1][2]
https://en.wikipedia.org/wiki/Unicellular_organism
Volvox is a polyphyletic genus of chlorophyte green algae in the family Volvocaceae. It forms spherical colonies of up to 50,000 cells. They live in a variety of freshwater habitats, and were first reported by Antonie van Leeuwenhoek in 1700. Volvox diverged from unicellular ancestors approximately 200 million years ago.[1]
https://en.wikipedia.org/wiki/Volvox
Dictyostelium discoideum is a species of soil-dwelling amoeba belonging to the phylum Amoebozoa, infraphylum Mycetozoa. Commonly referred to as slime mold, D. discoideum is a eukaryote that transitions from a collection of unicellular amoebae into a multicellular slug and then into a fruiting body within its lifetime. Its unique asexual lifecycle consists of four stages: vegetative, aggregation, migration, and culmination. The lifecycle of D. discoideum is relatively short, which allows for timely viewing of all stages. The cells involved in the lifecycle undergo movement, chemical signaling, and development, which are applicable to human cancer research. The simplicity of its lifecycle makes D. discoideum a valuable model organism to study genetic, cellular, and biochemical processes in other organisms.[2]
| Dictyostelium discoideum | |
|---|---|

| |
| Fruiting bodies of D. discoideum | |
| 0:23 | |
| A migrating D. discoideum whose boundary is colored by curvature, scale bar: 5 µm, duration: 22 seconds | |
| Scientific classification | |
| Domain: | Eukaryota |
| Phylum: | Amoebozoa |
| Class: | Dictyostelia |
| Order: | Dictyosteliida |
| Family: | Dictyosteliidae |
| Genus: | Dictyostelium |
| Species: | D. discoideum
|
| Binomial name | |
| Dictyostelium discoideum | |
Natural habitat and diet
In the wild, D. discoideum can be found in soil and moist leaf litter. Its primary diet consists of bacteria, such as Escherichia coli, found in the soil and decaying organic matter. Uninucleate amoebae of D. discoideum consume bacteria found in their natural habitat, which includes deciduous forest soil and decaying leaves.[3]
Life cycle and reproduction
The life cycle of D. discoideum begins when spores are released from a mature sorocarp (fruiting body). Myxamoebae hatch from the spores under warm and moist conditions. During their vegetative stage, the myxamoebae divide by mitosis as they feed on bacteria. The bacteria secrete folic acid, which attracts the myxamoebae. When the supply of bacteria is depleted, the myxamoebae enter the aggregation stage.
During aggregation, starvation initiates the production of protein compounds such as glycoproteins and adenylyl cyclase.[4] The glycoproteins allow for cell-cell adhesion, and adenylyl cyclase creates cyclic AMP. Cyclic AMP is secreted by the amoebae to attract neighboring cells to a central location. As they move toward the signal, they bump into each other and stick together by the use of glycoprotein adhesion molecules.
The migration stage begins once the amoebae have formed a tight aggregate and the elongated mound of cells tips over to lie flat on the ground. The amoebae work together as a motile pseudoplasmodium, also known as a slug. The slug is about 2–4 mm long, composed of up to 100,000 cells,[5] and is capable of movement by producing a cellulose sheath in its anterior cells through which the slug moves.[6] Part of this sheath is left behind as a slimy trail as it moves toward attractants such as light, heat, and humidity in a forward-only direction.[6] Cyclic AMP and a substance called differentiation-inducing factor, help to form different cell types.[6] The slug becomes differentiated into prestalk and prespore cells that move to the anterior and posterior ends, respectively. Once the slug has found a suitable environment, the anterior end of the slug forms the stalk of the fruiting body and the posterior end forms the spores of the fruiting body.[6] Anterior-like cells, which have only been recently discovered, are also dispersed throughout the posterior region of the slug. These anterior-like cells form the very bottom of the fruiting body and the caps of the spores.[6] After the slug settles into one spot, the posterior end spreads out with the anterior end raised in the air, forming what is called the "Mexican hat", and the culmination stage begins.
The prestalk cells and prespore cells switch positions in the culmination stage to form the mature fruiting body.[6] The anterior end of the Mexican hat forms a cellulose tube, which allows the more posterior cells to move up the outside of the tube to the top, and the prestalk cells move down.[6] This rearrangement forms the stalk of the fruiting body made up of the cells from the anterior end of the slug, and the cells from the posterior end of the slug are on the top and now form the spores of the fruiting body. At the end of this 8– to 10-hour process, the mature fruiting body is fully formed.[6] This fruiting body is 1–2 mm tall and is now able to start the entire cycle over again by releasing the mature spores that become myxamoebae.
Sexual reproduction
In general, although D. discoideum generally reproduces asexually, D. discoideum is still capable of sexual reproduction if certain conditions are met. D. discoideum has three different mating types and studies have identified the sex locus that specifies these three mating types. Type I strains are specified by the gene called MatA, Type II strains have three different genes: MatB (homologous to Mat A), Mat C, and Mat D, and Type III strains have Mat S and Mat T genes (which are homologous to Mat C and Mat D).[7] These sexes can only mate with the two different sexes and not with its own.[7]
When incubated with their bacterial food supply, heterothallic or homothallic sexual development can occur, resulting in the formation of a diploid zygote.[8][9] Heterothallic mating occurs when two amoebae of different mating types are present in a dark and wet environment, where they can fuse during aggregation to form a giant zygote cell. The giant cell then releases cAMP to attract other cells, then engulfs the other cells cannibalistically in the aggregate. The consumed cells serve to encase the whole aggregate in a thick, cellulose wall to protect it. This is known as a macrocyst. Inside the macrocyst, the giant cell divides first through meiosis, then through mitosis to produce many haploid amoebae that will be released to feed as normal amoebae would. Homothallic D. discoideum strains AC4 and ZA3A are also able to produce macrocysts.[10] Each of these strains, unlike heterothallic strains, likely express both mating type alleles (matA and mata). While sexual reproduction is possible, it is very rare to see successful germination of a D. discoideum macrocyst under laboratory conditions. Nevertheless, recombination is widespread within D. discoideum natural populations, indicating that sex is likely an important aspect of their lifecycle.[9]
Use as a model organism
Because many of its genes are homologous to human genes, yet its lifecycle is simple, D. discoideum is commonly used as a model organism. It can be observed at organismic, cellular, and molecular levels primarily because of their restricted number of cell types and behaviors, and their rapid growth.[6] It is used to study cell differentiation, chemotaxis, and apoptosis, which are all normal cellular processes. It is also used to study other aspects of development, including cell sorting, pattern formation, phagocytosis, motility, and signal transduction.[11] These processes and aspects of development are either absent or too difficult to view in other model organisms. D. discoideum is closely related to higher metazoans. It carries similar genes and pathways, making it a good candidate for gene knockout.[12]
The cell differentiation process occurs when a cell becomes more specialized to develop into a multicellular organism. Changes in size, shape, metabolic activities, and responsiveness can occur as a result of adjustments in gene expression. Cell diversity and differentiation, in this species, involves decisions made from cell-cell interactions in pathways to either stalk cells or spore cells.[13] These cell fates depend on their environment and pattern formation. Therefore, the organism is an excellent model for studying cell differentiation.
Chemotaxis is defined as a passage of an organism toward or away from a chemical stimulus along a chemical concentration gradient. Certain organisms demonstrate chemotaxis when they move toward a supply of nutrients. In D. discoideum, the amoeba secretes the signal, cAMP, out of the cell, attracting other amoebae to migrate toward the source. Every amoeba moves toward a central amoeba, the one dispensing the greatest amount of cAMP secretions. The secretion of the cAMP is then exhibited by all amoebae and is a call for them to begin aggregation. These chemical emissions and amoeba movement occur every six minutes. The amoebae move toward the concentration gradient for 60 seconds and stop until the next secretion is sent out. This behavior of individual cells tends to cause oscillations in a group of cells, and chemical waves of varying cAMP concentration propagate through the group in spirals.[14]: 174–175
An elegant set of mathematical equations that reproduces the spirals and the streaming patterns of D. discoideum was discovered by mathematical biologists Thomas Höfer and Martin Boerlijst. Mathematical biologist Cornelis J. Weijer has proven that similar equations can model its movement. The equations of these patterns are mainly influenced by the density of the amoeba population, the rate of the production of cyclic AMP and the sensitivity of individual amoebas to cyclic AMP. The spiraling pattern is formed by amoebas at the centre of a colony who rotate as they send out waves of cyclic AMP.[15][16]
The use of cAMP as a chemotactic agent is not established in any other organism. In developmental biology, this is one of the comprehensible examples of chemotaxis, which is important for an understanding of human inflammation, arthritis, asthma, lymphocyte trafficking, and axon guidance. Phagocytosis is used in immune surveillance and antigen presentation, while cell-type determination, cell sorting, and pattern formation are basic features of embryogenesis that may be studied with these organisms.[6]
Note, however, that cAMP oscillations may not be necessary for the collective cell migration at multicellular stages. A study has found that cAMP-mediated signaling changes from propagating waves to a steady state at a multicellular stage of D. discoideum.[17]
Thermotaxis is movement along a gradient of temperature. The slugs have been shown to migrate along extremely shallow gradients of only 0.05 °C/cm, but the direction chosen is complicated; it seems to be away from a temperature about 2 °C below the temperature to which they had been acclimated. This complicated behavior has been analyzed by computer modeling of the behavior and the periodic pattern of temperature changes in soil caused by daily changes in air temperature. The conclusion is that the behavior moves slugs a few centimeters below the soil surface up to the surface. This is an amazingly sophisticated behavior by a primitive organism with no apparent sense of gravity.[14]: 108–109
Apoptosis (programmed cell death) is a normal part of species development.[4] Apoptosis is necessary for the proper spacing and sculpting of complex organs. Around 20% of cells in D. discoideum altruistically sacrifice themselves in the formation of the mature fruiting body. During the pseudoplasmodium (slug or grex) stage of its lifecycle, the organism has formed three main types of cells: prestalk, prespore, and anterior-like cells. During culmination, the prestalk cells secrete a cellulose coat and extend as a tube through the grex.[4] As they differentiate, they form vacuoles and enlarge, lifting up the prespore cells. The stalk cells undergo apoptosis and die as the prespore cells are lifted high above the substrate. The prespore cells then become spore cells, each one becoming a new myxamoeba upon dispersal.[6] This is an example of how apoptosis is used in the formation of a reproductive organ, the mature fruiting body.
A recent major contribution from Dictyostelium research has come from new techniques allowing the activity of individual genes to be visualised in living cells.[18] This has shown that transcription occurs in "bursts" or "pulses" (transcriptional bursting) rather than following simple probabilistic or continuous behaviour. Bursting transcription now appears to be conserved between bacteria and humans. Another remarkable feature of the organism is that it has sets of DNA repair enzymes found in human cells, which are lacking from many other popular metazoan model systems.[19] Defects in DNA repair lead to devastating human cancers, so the ability to study human repair proteins in a simple tractable model will prove invaluable.
Lab cultivation
This organism's ability to be easily cultivated in the laboratory[6] adds to its appeal as a model organism. While D. discoideum can be grown in liquid culture, it is usually grown in Petri dishes containing nutrient agar and the surfaces are kept moist. The cultures grow best at 22–24 °C (room temperature). D. discoideum feed primarily on E. coli, which is adequate for all stages of the lifecycle. When the food supply is diminished, the myxamoebae aggregate to form pseudoplasmodia. Soon, the dish is covered with various stages of the lifecycle. Checking the dish often allows for detailed observations of development. The cells can be harvested at any stage of development and grown quickly.
While cultivating D. discoideum in a laboratory, it is important to take into account its behavioral responses. For example, it has an affinity toward light, higher temperatures, high humidity, low ionic concentrations, and the acidic side of the pH gradient. Experiments are often done to see how manipulations of these parameters hinder, stop, or accelerate development. Variations of these parameters can alter the rate and viability of culture growth. Also, the fruiting bodies, being that this is the tallest stage of development, are very responsive to air currents and physical stimuli. It is unknown if there is a stimulus involved with spore release.
Protein expression studies
Detailed analysis of protein expression in Dictyostelium has been hampered by large shifts in the protein expression profile between different developmental stages and a general lack of commercially available antibodies for Dictyostelium antigens.[20] In 2013, a group at the Beatson West of Scotland Cancer Centre reported an antibody-free protein visualization standard for immunoblotting based on detection of MCCC1 using streptavidin conjugates.[21]
Legionnaire's disease
The bacterial genus Legionella includes the species that causes legionnaire's disease in humans. D. discoideum is also a host for Legionella and is a suitable model for studying the infection process.[22] Specifically, D. discoideum shares with mammalian host cells a similar cytoskeleton and cellular processes relevant to Legionella infection, including phagocytosis, membrane trafficking, endocytosis, vesicle sorting, and chemotaxis.
"Farming"
A 2011 report in Nature published findings that demonstrated a "primitive farming behaviour" in D. discoideum colonies.[23][24] Described as a "symbiosis" between D. discoideum and bacterial prey, about one-third of wild-collected D. discoideum colonies engaged in the "husbandry" of the bacteria when the bacteria were included within the slime mold fruiting bodies.[24] The incorporation of the bacteria into the fruiting bodies allows the "seeding" of the food source at the location of the spore dispersal, which is particularly valuable if the new location is low in food resources.[24] Colonies produced from the "farming" spores typically also show the same behavior when sporulating. This incorporation has a cost associated with it: Those colonies that do not consume all of the prey bacteria produce smaller spores that cannot disperse as widely. In addition, much less benefit exists for bacteria-containing spores that land in a food-rich region. This balance of the costs and benefits of the behavior may contribute to the fact that a minority of D. discoideum colonies engage in this practice.[23][24]
D. discoideum is known for eating Gram-positive, as well as Gram-negative bacteria, but some of the phagocytized bacteria, including some human pathogens,[25] are able to live in the amoebae and exit without killing the cell. When they enter the cell, where they reside, and when they leave the cell are not known. The research is not yet conclusive but it is possible to draw a general lifecycle of D. discoideum adapted for farmer clones to better understand this symbiotic process.
In the picture, one can see the different stages. First, in the starvation stage, bacteria are enclosed within D. discoideum,[25] after entry into amoebae, in a phagosome the fusion with lysosomes is blocked and these unmatured phagosomes are surrounded by host cell organelles such as mitochondria, vesicles, and a multilayer membrane derived from the rough endoplasmic reticulum (RER) of amoebae. The role of the RER in the intracellular infection is not known, but the RER is not required as a source of proteins for the bacteria.[26] The bacteria reside within these phagosomes during the aggregation and the multicellular development stages. The amoebae preserve their individuality and each amoeba has its own bacterium. During the culmination stage, when the spores are produced, the bacteria pass from the cell to the sorus with the help of a cytoskeletal structure that prevents host cell destruction.[27] Some results suggest the bacteria exploit the exocytosis without killing the cell.[27] Free-living amoebae seem to play a crucial role for persistence and dispersal of some pathogens in the environment. Transient association with amoebae has been reported for a number of different bacteria, including Legionella pneumophila, many Mycobacterium species, Francisella tularensis, and Escherichia coli, among others.[26] Agriculture seems to play a crucial role for pathogens' survival, as they can live and replicate inside D. discoideum, making husbandry. Nature’s report has made an important advance in the knowledge of amoebic behavior, and the famous Spanish phrase translated as “you are more stupid than an amoeba” is losing the sense because amoebae are an excellent example of social behavior with an amazing coordination and sense of sacrifice for the benefit of the species.
Sentinel cells
Sentinel cells in Dictyostelium discoideum are phagocytic cells responsible for removing toxic material from the slug stage of the social cycle. Generally round in shape, these cells are present within the slug sheath where they are found to be circulating freely. The detoxification process occurs when these cells engulf toxins and pathogens within the slug through phagocytosis. Then, the cells clump into groups of five to ten cells, which then attach to the inner sheath of the slug. The sheath is sloughed off as the slug migrates to a new site in search of food bacteria.
Sentinel cells make up approximately 1% of the total number of slug cells, and the number of sentinel cells remains constant even as they are being released. This indicates a constant regeneration of sentinel cells within the slugs as they are being removed along with toxins and pathogens. Sentinel cells are present in the slug even when there are no toxins or pathogens to be removed. Sentinel cells have been located in five other species of Dictyostelia, which suggests that sentinel cells can be described as a general characteristic of the innate immune system in social amoebae.[28]
Effects of farming status on sentinel cells
The number of sentinel cells varies depending on the farming status of wild D. discoideum. When exposed to a toxic environment created by the use of ethidium bromide, it was shown that the number of sentinel cells per millimeter was lower for farmers than non-farmers. This was concluded by observing the trails left behind as the slugs migrated and counting the number of sentinel cells present in a millimeter. However, the number of sentinel cells does not affect the spore production and viability in farmers. Farmers exposed to a toxic environment produce the same number of spores as farmers in a non-toxic environment, and the spore viability was the same between the farmers and non-farmers. When Clade 2 Burkholderia, or farmer-associated bacteria, are removed from the farmers, spore production and viability were similar to that of the non-farmers. Thus, it is suggested that bacteria carried by the farmers provide an additional role of protection for the farmers against potential harm due to toxins or pathogens.[29]
Classification and phylogeny
In older classifications, Dictyostelium was placed in the defunct polyphyletic class Acrasiomycetes. This was a class of cellular slime molds, which was characterized by the aggregation of individual amoebae into a multicellular fruiting body, making it an important factor that related the acrasids to the dictyostelids.[30]
More recent genomic studies have shown that Dictyostelium has maintained more of its ancestral genome diversity than plants and animals, although proteome-based phylogeny confirms that amoebozoa diverged from the animal–fungal lineage after the plant–animal split.[31] Subclass Dictyosteliidae, order Dictyosteliales is a monophyletic assemblage within the Mycetozoa, a group that includes the protostelid, dictyostelid, and myxogastrid slime molds. Elongation factor-1α (EF-1α) data analyses support Mycetozoa as a monophyletic group, though rRNA trees place it as a polyphyletic group. Further, these data support the idea that the dictyostelid and myxogastrid are more closely related to each other than they are the protostelids. EF-1α analysis also placed the Mycetozoa as the immediate outgroup for the animal-fungal clade.[32] Latest phylogenetic data place dictyostelids firmly within supergroup Amoebozoa, along with myxomycetes. Meanwhile, protostelids have turned out to be polyphyletic, their stalked fruiting bodies a convergent feature of multiple unrelated lineages.[33]
Genome
The D. discoideum genome sequencing project was completed and published in 2005 by an international collaboration of institutes. This was the first free-living protozoan genome to be fully sequenced. D. discoideum consists of a 34-Mb haploid genome with a base composition of 77% [A+T] and contains six chromosomes that encode around 12,500 proteins.[3] Sequencing of the D. discoideum genome provides a more intricate study of its cellular and developmental biology.
Tandem repeats of trinucleotides are very abundant in this genome; one class of the genome is clustered, leading researchers to believe it serves as centromeres. The repeats correspond to repeated sequences of amino acids and are thought to be expanded by nucleotide expansion.[3] Expansion of trinucleotide repeats also occurs in humans, in general leading to many diseases. Learning how D. discoideum cells endure these amino acid repeats may provide insight to allow humans to tolerate them.
Every genome sequenced plays an important role in identifying genes that have been gained or lost over time. Comparative genomic studies allow for comparison of eukaryotic genomes. A phylogeny based on the proteome showed that the amoebozoa deviated from the animal-fungal lineage after the plant-animal split.[3] The D. discoideum genome is noteworthy because its many encoded proteins are commonly found in fungi, plants, and animals.[3]
Databases
- DictyBase - general genomic database about Dictyostelium discoideum
- Membranome database provides information about single-pass transmembrane proteins from Dictyostelium and several other organisms
References
- Shadwick, LL; Spiegel, FW; Shadwick, JD; Brown, MW; Silberman, JD (2009). "Eumycetozoa = Amoebozoa?: SSUrDNA Phylogeny of Protosteloid Slime Molds and Its Significance for the Amoebozoan Supergroup". PLOS ONE. 4 (8): e6754. doi:10.1371/journal.pone.0006754. PMC 2727795. PMID 19707546.
Further reading
- Mary S. Tyler (2000). Developmental Biology: A Guide for Experimental Study.2nd ed. Sinauer Associates. pp. 31–34. ISBN 978-0-87893-843-8.
- Scott F. Gilbert (2006). Developmental Biology. 8th ed. Sinauer. pp. 36–39. ISBN 978-0-87893-250-4.
External links
https://en.wikipedia.org/wiki/Dictyostelium_discoideum
A model organism is one that is extensively studied to understand particular biological phenomena, with the expectation that discoveries made in the model organism will provide insight into the workings of other organisms.
https://en.wikipedia.org/wiki/Category:Model_organisms
Spicules are structural elements found in most sponges. The meshing of many spicules serves as the sponge's skeleton and thus it provides structural support and potentially defense against predators.[1]
Sponge spicules are made of calcium carbonate or silica. Large spicules visible to the naked eye are referred to as megascleres, while smaller, microscopic ones are termed microscleres. The composition, size, and shape of spicules are major characters in sponge systematics and taxonomy.
https://en.wikipedia.org/wiki/Sponge_spicule
| Part of a series related to |
| Biomineralization |
|---|
 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
https://en.wikipedia.org/wiki/Sponge_spicule
- Hyalonema fruticosum Schulze, 1893
- Monorhaphis dives Schulze, 1904
- Monorhaphis intermedia Li Jinhe, 1987
| Monorhaphididae chuni | |
|---|---|

| |
| Two black and white photographs of M. chuni | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Porifera |
| Class: | Hexactinellida |
| Order: | Amphidiscosida |
| Family: | Monorhaphididae Iijima, 1927 |
| Genus: | Monorhaphis Schulze, 1904 |
| Species: | M. chuni
|
| Binomial name | |
| Monorhaphis chuni Schulze, 1904
| |
| Synonyms[1] | |
|
| |
https://en.wikipedia.org/wiki/Monorhaphis
Amphidiscosida is an order of hexactinellid sponges characterized by amphidisc spicules, that is, spicules having a stellate disk at each end.[2] They are in the class Hexactinellida and are the only order classified in the monotypic subclass Amphidiscophora.[3] Species of the order Amphidiscosida have existed since the Ordovician period, and still flourish today.[4]
Families
- Hyalonematidae Gray, 1857[5]
- Monorhaphididae Ijima, 1927[6]
- Pheronematidae Gray, 1870[7]
https://en.wikipedia.org/wiki/Amphidiscosida
picules are formed by a proteinaceous scaffold which mediates the formation of siliceous lamellae in which the proteins are encased. Up to eight hundred 5 to 10 μm thick lamellae can be concentrically arranged around an axial canal. The silica matrix is composed of almost pure silicon and oxygen, providing it with unusual optophysical properties superior to man-made waveguides.[19]
The spicules, the elements from which their skeletons are constructed, are built in a variety of distinct shapes, and are made from silica that is deposited in the form of amorphous opal (SiO2·nH2O).[19]
In evolution, after the Ediacaran period, a third class of Porifera appeared, the Calcarea, which has a calcium-carbonate skeleton.[22][19]
https://en.wikipedia.org/wiki/Sponge_spicule
A waveguide is a structure that guides waves, such as electromagnetic waves or sound, with minimal loss of energy by restricting the transmission of energy to one direction. Without the physical constraint of a waveguide, wave intensities decrease according to the inverse square law as they expand into three-dimensional space.
https://en.wikipedia.org/wiki/Waveguide
Spongin, a modified type of collagen protein, forms the fibrous skeleton of most organisms among the phylum Porifera, the sponges. It is secreted by sponge cells known as spongocytes.[1]
Spongin gives a sponge its flexibility. True spongin is found only in members of the class Demospongiae.[2]
https://en.wikipedia.org/wiki/Spongin
Monaxons form simple cylinders with pointed ends. The ends of diactinal monaxons are similar, whereas monactinal monaxons have different ends: one pointed, one rounded. Diactinal monaxons are classified by the nature of their ends: oxea have pointed ends, and strongyles are rounded. Spine-covered oxea and strongyles are termed acanthoxea and acanthostrongyles, respectively.[31]: 2 Monactical monaxons always have one pointed end; they are termed styles if the other end is blunt, tylostyles if their blunt end forms a knob; and acanthostyles if they are covered in spines.
Triaxons have three axes; in triods, each axis bears a similar ray; in pentacts, the triaxon has five rays, four of which lie in a single plane; and pinnules are pentacts with large spines on the non-planar ray.[31]
Tetraxons have four axes, and polyaxons more (description of types to be incorporated from [31]). Sigma-C spicules have the shape of a C.[31]
Dendroclones might be unique to extinct sponges[32] and are branching spicules that may take irregular forms, or may form structures with an I, Y or X shape.[33][34]
- Megascleres are large spicules measuring from 60-2000 μm and often function as the main support elements in the skeleton.[35]
- Acanthostyles are spiny styles.
- Anatriaenes, orthotriaenes and protriaenes are triaenes[36] - megascleres with one long and three short rays.
- Strongyles are megascleres with both ends blunt or rounded.
- Styles are megascleres with one end pointed and the other end rounded.
- Tornotes are megascleres with spear shaped ends.
- Tylotes are megascleres with knobs on both ends.
- Microscleres are small spicules measuring from 10-60 μm and are
scattered throughout the tissue and are not part of the main support
element.[35]
- Chelae are microscleres with shovel-like structures on the ends. Anisochelas are microscleres with dissimilar ends. Isochelas are microscleres with two similar ends.
- Euasters are star-shaped microscleres with multiple rays radiating from a common centre. Exemples are oxyasters (euasters with pointed rays) or sterrasters (ball-shaped euasters).
- Forceps are microscleres bent back on themselves.
- Microstrongyles are small rods with both ends blunt or rounded.
- Microxeas are small rods with both ends pointed.
- Sigmas are "C" or "S" shaped microscleres.
Calcareous spicules
Animal biomineralization is a controlled process and leads to the production of mineral–organic composite materials that considerably differ in shape and material properties from their purely inorganic counterparts. The ability to form functional biominerals, such as endoskeletons and exoskeletons, protective shells, or teeth, had been a significant step in animal evolution. Calcium carbonate biomineralization, the most widespread type among animal phyla,[37] evolved several times independently, resulting in multiple recruitments of the same genes for biomineralization in different lineages.[38]
Among these genes, members of the alpha carbonic anhydrase gene family (CAs) are essential for biomineralization.[39] CAs are zinc-binding enzymes that catalyze the reversible conversion of carbon dioxide and water to bicarbonate and one proton.[40] The zinc-binding is mediated by three histidine residues essential for the protein's catalytic function.[41][42] CAs are involved in many physiological processes requiring ion regulation or carbon transport,[43] both of which are crucial for the controlled precipitation of carbonate biominerals. In mammals, where they are best studied, 16 different CAs are expressed in specific tissues and active in defined subcellular compartments.[44] Cytosolic, mitochondrial, membrane-bound, and secreted CA forms can be distinguished, and these groups got expanded and reduced in different animal groups.[39][45] Specific CAs are involved in the carbonate biomineralization in distinct animal lineages,[39] including sponges.[46][45][47][48]
Among extant sponges, only the calcareous sponges can produce calcite spicules, whereas other classes' spicules are siliceous. Some lineages among demosponges and a few calcareans have massive calcium carbonate basal skeletons, the so-called coralline sponges or sclerosponges. The biomineralizing CAs used by carbonate-producing demosponges are not orthologous to the CAs involved in the spicule formation of calcareous sponges,[45] suggesting that the two biomineralization types evolved independently. This observation agrees with the idea that the formation of calcitic spicules is an evolutionary innovation of calcareous sponges.[49][48]
Spicules are formed by sclerocytes, which are derived from archaeocytes. The sclerocyte begins with an organic filament, and adds silica to it. Spicules are generally elongated at a rate of 1-10 μm per hour. Once the spicule reaches a certain length it protrudes from the sclerocyte cell body, but remains within the cell's membrane. On occasion, sclerocytes may begin a second spicule while the first is still in progress.[51]
The shapes of calcareous sponge spicules are simple compared with the sometimes very elaborate siliceous spicules found in the other sponge classes. With only a few exceptions, calcareous sponge spicules can be of three basic types: monaxonic, two-tipped diactines, triactines with three spicules rays, and four-rayed tetractines. Specialized cells, the sclerocytes, produce these spicules, and only a few sclerocytes interact in the formation of one specific spicule: Two sclerocytes produce a diactine, six sclerocytes form a triactine, and seven a tetractines.[52][53][54] A pair of sclerocytes is involved in the growth of each actine of these spicules. After an initial phase, the so-called founder cell promotes actine elongation, the second, so-called thickener cell in some, but not all species deposit additional calcium carbonate on the actine, as it migrates back toward the founder cell.[54][55] Calcareous sponges can possess only one or any combination of the three spicule types in their body, and in many cases, certain spicule types are restricted to specific body parts. This indicates that spicule formation is under strict genetic control in calcareous sponges, and specific CAs play an essential role in this genetic control[50][48]
Siliceous spicules
Shown left: The largest biosilica structure on Earth is the giant basal spicule from the deep-sea glass sponge Monorhaphis chuni.[19]
The largest biosilica structure on Earth is the giant basal spicule from the deep-sea glass sponge Monorhaphis chuni.[19] The diagram on the right shows:
- (a) Young specimens of M. chuni anchored to the muddy substratum by one single giant basal spicule (gbs). The body (bo) surrounds the spicule as a continuous, round cylinder.
- (b) The growth phases of the sessile animal with its GBS (gbs) which anchors it to the substratum and holds the surrounding soft body (bo). The characteristic habitus displays linearly arranged large atrial openings (at) of approximately 2 cm in diameter. With growth, the soft body dies off in the basal region and exposes the bare GBS (a to c).
- (c) Part of the body (bo) with its atrial openings (at). The body surface is interspersed with ingestion openings allowing a continuous water flow though canals in the interior which open into oscules that are centralized in atrial openings, the sieve-plates.
- (d) M. chuni in its natural soft bottom habitat of bathyal slopes off New Caledonia. The specimens live at a depth of 800–1,000 m [23]. In this region, the sponge occurs at a population density of 1-2 individuals per m2. The animals reach sizes of around 1 m in length.
- (e) Drawings of different glass sponges (hexactinellids).[19]
Not to scale — sizes vary between 0.01 and 1 mm
Geodia atlantica; (h) is the hilum; the arrow points to a smooth rosette made up of rays. Left scale 20 μm, right scale 2 μm.
Geodia macandrewii, left scale 50 μm, right scale 2 μm
Siliceous spicules in demosponges exist in a variety of shapes, some of which look like minute spheres of glass. They are called sterrasters when they belong to the Geodiidae family and selenasters when they belong to the Placospongiidae family.[58]
Siliceous spicules were first described and illustrated in 1753 by Vitaliano Donati,[59] who found them in the species Geodia cydonium from the Adriatic Sea: he called these spicules "little balls". They are later called globular crystalloids, globate spicules, or globostellates by sponge taxonomists, until 1888 when William Sollas [60] finally coins the term "sterraster" from the Greek sterros meaning "solid" or "firm" – see diagram on the right. Meanwhile, similar ball-shaped spicules are observed in another genus, Placospongia, and these are at first considered as "sterrasters" [60] before Richard Hanitsch coins the term "selenaster" in 1895 [61] for these different spicules (coming from the Greek selene for "moon", referring to the "half-moon" shape). Finally, an additional term "aspidaster" is created by von Lendenfeld in 1910,[62] convinced that the flattened sterrasters in the genus Erylus are significantly different from those in Geodia.[58]
Today, the Geodiidae represent a highly diverse sponge family with more than 340 species, occurring in shallow to deep waters worldwide apart from the Antarctic. Sterrasters/aspidaster spicules are currently the main synapomorphy of the Geodiidae. The family currently includes five genera with sterrasters and several others that have secondarily lost their sterrasters.[63][64] The Geodia can be massive animals more than a meter across.[65][66][67][58]
Selenasters are the main synapomorphy of Placospongia (family Placospongiidae, order Clionaida), a well-supported monophyletic genus [68] from shallow temperate/tropical waters worldwide. It is not a very diverse genus with only 10 species currently described (WPD) and a handful of undescribed species.[69][68] Placospongia species are usually small, encrusting, and never occur in high densities.[58]
Sterrasters/selenasters are big enough to examine in some detail their surfaces with an optical microscope. However, the use of the scanning electron microscope (SEM) enabled a significantly better understanding of the surface microornamentations. A few descriptive terms have also appeared to describe and compare in greater detail the microornamentations of these ball-shaped spicules. polyaxial spicules such as the sterrasters and aspidasters, are the result of fused "actines" (= branches of asters, from the Greek for "star"), later covered with "rosettes" made of different "rays". The "hilum" (Latin for a "little thing" or "trifle" or the "eye of a bean") is a small area without rosettes or any kind of surface pattern. There are no particular terms to describe the surface of selenasters, except for the "hilum", also present. Although there appears to be no significant variation in the size of the rosettes and hilum between species,[70][67] noticed that rosettes could be smooth or warty and hypothesized that this character could be of phylogenetic value if studied more broadly. Furthermore, the rosette morphology also seemed to be variable between Geodia, Pachymatisma, and Caminella [71][72] which suggests that a more detailed study of the sterraster/aspidaster surface would potentially bring new characters for Geodiidae genera identification.[58]
Spicule "life cycle"
From formation to deposition
The formation of spicules is controlled genetically.[73] In most cases, the first growth phase is intracellular; it starts in sclerocytes (amoeboid cells responsible for spicule formation) in mesohyl [74][75] and is mediated by silicatein, a special enzyme that initiates formation of the axial filament (harboured by the axial canal) which provides the vertical axis of the spicule.[76] The axial canal is filled with organic proteinaceous material which usually extends to the tip of the newly-formed spicule.[77] The cross-section of the axial canal differs across major sponge clades that produce siliceous spicules (it is triangular in demosponges,[78] irregular in homoscleromorphs [14] and quadrangular in hexactinellids.[79] In calcareans (producing calcareous spicules) the axial canal is not developed.[79] The geometry and the length of the axial filament determines the shape of the spicule.[80] In desmoid spicules of 'lithistids' (an informal group of demosponges with articulated skeletons), however, the axial filament is shorter than the spicule arms and it is possible that only organic molecules are involved in the spicule-forming process.[80][16]
During formation of the siliceous spicules (Calcarea displays different mechanisms of spicule biomineralization), sponges obtain silicon in the form of soluble silicic acid and deposit it around the axial filament, [78][14] within a special membrane called silicalemma.[81][82] Silica is first laid out as small 2 μm granules [78][80] that are fused to bigger spheres (or fused together within process of biosintering in Hexactinellida.[83] After some time, amorphous silica is added, forming evenly-deposited concentric layers,[14] separated from each other by ultrathin organic interlayers.[84] At this stage, immature spicules are secreted from the sclerocyte and covered by pseudopodia of one to several cells, and the process of silica deposition and spicule growth continues.[78][16]
After completing the deposition of silica (or during this phase), the spicule is transported to the right place in the sponge body by crawling mesohyl cells, where spongocytes secrete spongin fibrils around them and connect them with adjacent spicules.[14] In some hexactinellids, that are characterized by rigid skeleton, the fusion of spicules appears to occur parallel to spicule secretion.[85][16]
When sponges are alive, their spicules provide a structural "framework". Following their death, the body and the skeleton structure, especially that of demosponges in which the spicules are connected to each other only by perishable collagen fibres, rapidly disintegrate leaving the spicules "free". Because of this, sponges are rarely wholly preserved in the fossil record. Their spicules, however, are incorporated into sediments, often becoming one of the main components of sedimentary rocks.[86][87] Sometimes spicules accumulate into enormous agglomerations called spicule mats or beds.[88] These accumulations are characteristic for polar waters.[89][90] Spicules can fossilize to form special type of rocks called the spiculites ("spongillites" for freshwater sponge spicules); these types of rocks are known globally,[91][92][93][94] and have been formed through the whole Phanerozoic.[92] Biosiliceous sedimentation occasionally results in the formation of spiculitic cherts (in so called glass ramps) which are recorded from the Permian to Eocene of many parts of the world.[95][96][16]
Locomotion
In 2016 a newly discovered demosponge community living under arctic ice were found to have moved across the sea floor by extending their spicules and then retracting their body in the direction of motion.[97]
Spiculites
When dead sponge bodies disintegrate, spicules become incorporated into marine sediments and sometimes accumulate into enormous agglomerations called spicule mats or beds, or fossilize to form special type of rocks called the spiculites.[16]
The record of fossil and subfossil sponge spicules is extraordinarily rich and often serves as a basis for far-reaching reconstructions of sponge communities, though spicules are also bearers of significant ecological and environmental information. Specific requirements and preferences of sponges can be used to interpret the environment in which they lived, and reconstruct oscillations in water depths, pH, temperatures, and other parameters, providing snapshots of past climate conditions. In turn, the silicon isotope compositions in spicules (δ30Si) are being increasingly often used to estimate the level of silicic acid in the marine settings throughout the geological history, which enables the reconstruction of past silica cycle and ocean circulation.[16]
Spicules provide structural support for maintaining the vertical body position, minimize the metabolic cost of water exchange, [98][14] and may even deter predators.[14] They often develop in different sizes [12] and a wide variety of three dimensional shapes, with many being unique to clade- or even species-level taxa. Demosponges are characterized by spicules of monaxonic or tetraxonic symmetry.[12] Hexactinellids produce spicules of hexactinic or triaxonic (cubic) symmetry or shapes that are clearly derived from such morpohologies.[21] The spicules of homoscleromorphs represent peculiar tetractines (calthrops) and their derivatives that originate through reduction or ramification of the clads.[99] Spicules of Calcarea are produced in three basic forms: diactines, triactines and tetractines.[100][16]
The mineral composition of sponge spicules makes these structures the most resistant parts of the sponge bodies [79] and ensures the ability of spicules to withstand various taphonomic processes,[86][101] resulting in that they often constitute the only evidence of the presence of some sponges in an ecosystem.[102] Even though sponges are often known from rich assemblages of bodily-preserved specimens,[103][104][105] a significant part of their fossil and subfossil record is also represented by their spicules. Having that in mind, spicules can be of crucial importance for reconstructions of extinct or cryptic (hiding in cervices and caves) sponge communities; and, indeed, they have been investigated especially with respect to their taxonomic significance.[106][12] The morphologies of spicules and their arrangement, together with other important sponge features, such as the shape, consistency, and color, are essential when identifying sponges.[107][16]
In contrast to whole-bodied sponge fossils, spicules are common in many depositional environments.[108] Their significance, however, is often underestimated, which is mostly due to the difficulties in assigning disassociated spicules to sponge taxa or due to the scarcity of the material.[16]
Interaction with light
Research on the Euplectella aspergillum (Venus' Flower Basket) demonstrated that the spicules of certain deep-sea sponges have similar traits to Optical fibre. In addition to being able to trap and transport light, these spicules have a number of advantages over commercial fibre optic wire. They are stronger, resist stress easier, and form their own support elements. Also, the low-temperature formation of the spicules, as compared to the high temperature stretching process of commercial fibre optics, allows for the addition of impurities which improve the refractive index. In addition, these spicules have built-in lenses in the ends which gather and focus light in dark conditions. It has been theorized that this ability may function as a light source for symbiotic algae (as with Rosella racovitzae) or as an attractor for shrimp which live inside the Venus' Flower Basket. However, a conclusive decision has not been reached; it may be that the light capabilities are simply a coincidental trait from a purely structural element. [51][109][110] Spicules funnel light deep inside sea sponges.[111][112]
Spicules of sponge (SEM)
See also
https://en.wikipedia.org/wiki/Sponge_spicule
Biogenic silica (bSi), also referred to as opal, biogenic opal, or amorphous opaline silica, forms one of the most widespread biogenic minerals. For example, microscopic particles of silica called phytoliths can be found in grasses and other plants.
https://en.wikipedia.org/wiki/Biogenic_silica
Research directions
Use in the removal of phenolic compounds from wastewater
Researchers have found spongin to be useful in the photocatalytic degradation and removal of bisphenols (such as BPA) in wastewater. A heterogeneous catalyst consisting of a spongin scaffold for iron phthalocyanine (SFe) in conjunction with peroxide and UV radiation has been shown to remove phenolic wastes more quickly and efficiently than conventional methods.[3] Other research using spongin scaffolds for the immobilization of Trametes versicolor Laccase has shown similar results in phenol degradation.[4]
https://en.wikipedia.org/wiki/Spongin
Silica is an amorphous metal oxide formed by complex inorganic polymerization processes. This is opposed to the other major biogenic minerals, comprising carbonate and phosphate, which occur in nature as crystalline iono-covalent solids (e.g. salts) whose precipitation is dictated by solubility equilibria.[1] Chemically, bSi is hydrated silica (SiO2·nH2O), which is essential to many plants and animals.
Diatoms in both fresh and salt water extract dissolved silica from the water to use as a component of their cell walls. Likewise, some holoplanktonic protozoa (Radiolaria), some sponges, and some plants (leaf phytoliths) use silicon as a structural material. Silicon is known to be required by chicks and rats for growth and skeletal development. Silicon is in human connective tissues, bones, teeth, skin, eyes, glands and organs.
Silica in marine environments
Silicate, or silicic acid (H4SiO4), is an important nutrient in the ocean. Unlike the other major nutrients such as phosphate, nitrate, or ammonium, which are needed by almost all marine plankton, silicate is an essential chemical requirement for very specific biota, including diatoms, radiolaria, silicoflagellates, and siliceous sponges. These organisms extract dissolved silicate from open ocean surface waters for the buildup of their particulate silica (SiO2), or opaline, skeletal structures (i.e. the biota's hard parts).[2][3] Some of the most common siliceous structures observed at the cell surface of silica-secreting organisms include: spicules, scales, solid plates, granules, frustules, and other elaborate geometric forms, depending on the species considered.[4]
Marine sources of silica
Five major sources of dissolved silica to the marine environment can be distinguished:[3]
- Riverine influx of dissolved silica to the oceans: 4.2 ± 0.8 × 1014 g SiO2 yr−1
- Submarine volcanism and associated hydrothermal emanations: 1.9 ± 1.0 × 1014 g SiO2 yr−1
- Glacial weathering: 2 × 1012 g SiO2 yr−1
- Low temperature submarine weathering of oceanic basalts
- Some silica may also escape from silica-enriched pore waters of pelagic sediments on the seafloor
Once the organism has perished, part of the siliceous skeletal material dissolves, as it settles through the water column, enriching the deep waters with dissolved silica.[3] Some of the siliceous scales can also be preserved over time as microfossils in deep-sea sediments, providing a window into modern and ancient plankton/protists communities. This biologic process has operated, since at least early Paleozoic time, to regulate the balance of silica in the ocean.[4]
Radiolarians (Cambrian/Ordovician-Holocene), diatoms (Cretaceous-Holocene), and silicoflagellates (Cretaceous-Holocene) form the ocean's main contributors to the global silica biogenic cycle throughout geologic time. Diatoms account for 43% of the ocean primary production, and are responsible for the bulk of silica extraction from ocean waters in the modern ocean, and during much of the past fifty million years. In contrast, oceans of Jurassic and older ages, were characterized by radiolarians as major silica-utilizing phyla.[2] Nowadays, radiolarians are the second (after diatoms) major producers of suspended amorphous silica in ocean waters. Their distribution ranges from the Arctic to the Antarctic, being most abundant in the equatorial zone. In equatorial Pacific waters, for example, about 16,000 specimens per cubic meter can be observed.[4]
Silica cycle
The silicon cycle has gained increasingly in scientific attention the past decade for several reasons:
Firstly, the modern marine silica cycle is widely believed to be dominated by diatoms for the fixation and export of particulate matter (including organic carbon), from the euphotic zone to the deep ocean, via a process known as the biological pump. As a result, diatoms, and other silica-secreting organisms, play a crucial role in the global carbon cycle, and have the ability to affect atmospheric CO2 concentrations on a variety of time scales, by sequestering CO2 in the ocean. This connection between biogenic silica and organic carbon, together with the significantly higher preservation potential of biogenic siliceous compounds, compared to organic carbon, makes opal accumulation records very interesting for paleoceanography and paleoclimatology.
Secondly, biogenic silica accumulation on the sea floor contains lot of information about where in the ocean export production has occurred on time scales ranging from hundreds to millions of years. For this reason, opal deposition records provide valuable information regarding large-scale oceanographic reorganizations in the geological past, as well as paleoproductivity.
Thirdly, the mean oceanic residence time for silicate is approximately 10,000–15,000 yr. This relative short residence time, makes oceanic silicate concentrations and fluxes sensitive to glacial/interglacial perturbations, and thus an excellent proxy for evaluating climate changes.[3][5]
Increasingly, isotope ratios of oxygen (O18:O16) and silicon (Si30:Si28) are analysed from biogenic silica preserved in lake and marine sediments to derive records of past climate change and nutrient cycling (De La Rocha, 2006; Leng and Barker, 2006). This is a particularly valuable approach considering the role of diatoms in global carbon cycling. In addition, isotope analyses from BSi are useful for tracing past climate changes in regions such as in the Southern Ocean, where few biogenic carbonates are preserved.
Marine silica sinks
Siliceous ooze
The remains of diatoms and other silica-utilizing organisms are found, as opal sediments within pelagic deep-sea deposits. Pelagic sediments, containing significant quantities of siliceous biogenic remains, are commonly referred to as siliceous ooze. Siliceous ooze are particularly abundant in the modern ocean at high latitudes in the northern and southern hemispheres. A striking feature of siliceous ooze distribution is a ca. 200 km wide belt stretching across the Southern Ocean. Some equatorial regions of upwelling, where nutrients are abundant and productivity is high, are also characterized by local siliceous ooze.[2]
Siliceous oozes are composed primarily of the remains of diatoms and radiolarians, but may also include other siliceous organisms, such as silicoflagellates and sponge spicules. Diatom ooze occurs mainly in high-latitude areas and along some continental margins, whereas radiolarian ooze are more characteristic of equatorial areas. Siliceous ooze are modified and transformed during burial into bedded cherts.[2]
Southern Ocean sediments
Southern Ocean sediments are a major sink for biogenic silica (50-75% of the oceanic total of 4.5 × 1014 g SiO2 yr−1; DeMaster, 1981), but only a minor sink for organic carbon (<1% of the oceanic 2 × 1014 g of organic C yr−1). These relatively high rates of biogenic silica accumulation in the Southern Ocean sediments (predominantly beneath the Polar Front) relative to organic carbon (60:1 on a weight basis) results from the preferential preservation of biogenic silica in the Antarctic water column.
In contrast to what was previously thought, these high rates of biogenic silica accumulation are not the result from high rates of primary production. Biological production in the Southern Ocean is strongly limited due to the low levels of irradiance coupled with deep mixed layers and/or by limited amounts of micronutrients, such as iron.[6]
This preferential preservation of biogenic silica relative to organic carbon is evident in the steadily increasing ratio of silica/organic C as function of depth in the water column. About thirty-five percent of the biogenic silica produced in the euphotic zone survives dissolution within the surface layer; whereas only 4% of the organic carbon escapes microbial degradation in these near-surface waters.
Consequently, considerable decoupling of organic C and silica occurs during settling through the water column. The accumulation of biogenic silica in the seabed represents 12% of the surface production, whereas the seabed organic-carbon accumulation rate accounts for solely <0.5% of the surface production. As a result, polar sediments account for most of the ocean's biogenic silica accumulation, but only a small amount of the sedimentary organic-carbon flux.[6]
Effect of oceanic circulation on silica sinks
Large-scale oceanic circulation has a direct impact on opal deposition. The Pacific (characterized by nutrient poor surface waters, and deep nutrient rich waters) and Atlantic Ocean circulations favor the production/preservation of silica and carbonate respectively. For instance, Si/N and Si/P ratios increase from the Atlantic to the Pacific and Southern Ocean, favoring opal versus carbonate producers. Consequently, the modern configuration of large-scale oceanic circulation resulted in the localization of major opal burial zones in the Equatorial Pacific, in the eastern boundary current upwelling systems, and by far the most important, the Southern Ocean.[5]
Pacific and Southern Oceans
Waters from the modern Pacific and Southern ocean, typically observe an increase in Si/N ratio at intermediate depth, which results in an increase in opal export (~ increase in opal production). In the Southern Ocean and North Pacific, this relationship between opal export and Si/N ratio switches from linear to exponential for Si/N ratios greater than 2. This gradual increase in the importance of silicate (Si) relative to nitrogen (N) has tremendous consequences for the ocean biological production. The change in nutrient ratios contributes to select diatoms as main producers, compared to other (e.g., calcifying) organisms. For example, microcosm experiments have demonstrated that diatoms are DSi supercompetitors and dominate other producers above 2 μM DSi. Consequently, opal vs. carbonate export will be favored, resulting in increasing opal production. The Southern Ocean and the North Pacific also display maximum biogenic silicate/Corganic flux ratios, and consist thus in an enrichment in biogenic silicate, compared to Corganic export flux. This combined increase in opal preservation and export makes the Southern Ocean the most important sink for DSi today.[5]
Atlantic Ocean
In the Atlantic Ocean, intermediate and deep waters are characterized by a lower content in DSi, compared to the modern Pacific and Southern Ocean. This lower interbasin difference in DSi has the effect of decreasing the preservation potential of opal in the Atlantic compared to its Pacific and Southern ocean counterparts. Atlantic DSi depleted waters tends to produce relatively less silicified organisms, which has a strong influence on the preservation of their frustules. This mechanism in best illustrated when comparing the Peru and northwest Africa upwelling systems. The dissolution/production ratio is much higher in the Atlantic upwelling than in the Pacific upwelling. This is due to the fact that coastal upwelling source waters are much richer in DSi off Peru, than off NW Africa.[5]
Marine biogenic silica budget
Rivers and submarine hydrothermal emanations supply 6.1 × 1014 g SiO2 yr−1 to the marine environment. Approximately two-thirds of this silica input is stored in continental margin and deep-sea deposits. Siliceous deep-sea sediments located beneath the Antarctic Convergence (convergence zone) host some 25% of the silica supplied to the oceans (i.e. 1.6 × 1014 g SiO2 yr−1) and consequently form one of Earth's major silica sinks. The highest biogenic silica accumulation rates in this area are observed in the South Atlantic, with values as large as 53 cm.kyr−1 during the last 18,000 yr. Further, extensive biogenic silica accumulation has been recorded in the deep-sea sediments of the Bering Sea, Sea of Okhotsk, and Subarctic North Pacific. Total biogenic silica accumulation rates in these regions amounts nearly 0.6 × 1014 g SiO2 yr−1, which is equivalent to 10% of the dissolved silica input to the oceans.
Continental margin upwelling areas, such as the Gulf of California, the Peru and Chile coast, are characteristic for some of the highest biogenic silica accumulation rates in the world. For example, biogenic silica accumulation rates of 69 g SiO2/cm2/kyr have been reported for the Gulf of California. Due to the laterally confined character of these rapid biogenic silica accumulation zones, upwelling areas solely account for approximately 5% of the dissolved silica supplied to the oceans. At last, extremely low biogenic silica accumulation rates have been observed in the extensive deep-sea deposits of the Atlantic, Indian and Pacific Oceans, rendering these oceans insignificant for the global marine silica budget.[7]
Biogenic silica production
The mean daily BSi rate strongly depends on the region:
- Coastal upwelling: 46 mmol.m−2.d−1
- Sub-arctic Pacific: 18 mmol.m−2.d−1
- Southern Ocean: 3–38 mmol.m−2.d−1
- mid-ocean gyres: 0.2–1.6 mmol.m−2.d−1
Likewise, the integrated annual BSi production strongly depends on the region:
- Coastal upwelling: 3 × 1012 mol.yr−1
- Subarctic Pacific: 8 × 1012 mol.yr−1
- Southern Ocean: 17–37 × 1012 mol.yr−1
- mid-ocean gyres: 26 × 1012 mol.yr−1
BSi production is controlled by:
- Dissolved silica availability, however, half saturation constant Kμ for silicon-limited growth is lower than Ks for silicon uptake.
- Light availability: There is no direct light requirement; silicon uptake at 2x depth of photosynthesis; silicon uptake continues at night but cells must be actively growing.
- Micronutrient availability.
Biogenic silica dissolution
BSi dissolution is controlled by:
- Thermodynamics of solubility: Temperature (0 to 25 °C - 50x increase).
- Sinking rate: Food web structure—grazers, fecal pellets, discarded feeding structures, Aggregation - rapid transport.
- Bacterial degradation of organic matrix (Bidle and Azam, 1999).
Biogenic silica preservation
BSi preservation is measured by:
- Sedimentation rates, mainly sediment traps (Honjo);
- Benthic remineralization rates ("recycling"), benthic flux chamber (Berelson);
- BSi concentration in sediments, chemical leaching in alkaline solution, site specific, need to differentiate lithogenic vs. biogenic Si, X-ray diffraction.
BSi preservation is controlled by:
- Sedimentation rate;
- Porewater dissolved silica concentration: saturation at 1.100 μmol/L;
- Surface coatings: dissolved Al modifies solubility of deposited biogenic silica particles, dissolved silica can also precipitate with Al as clay or Al-Si coatings.
Opaline silica on Mars
In the Gusev crater of Mars, the Mars Exploration Rover Spirit inadvertently discovered opaline silica. One of its wheels had earlier become immobilized and thus was effectively trenching the Martian regolith as it dragged behind the traversing rover. Later analysis showed that the silica was evidence for hydrothermal conditions.[8]
See also
https://en.wikipedia.org/wiki/Biogenic_silica
Marble is a metamorphic rock composed of recrystallized carbonate minerals, most commonly calcite or dolomite. Marble is typically not foliated (layered), although there are exceptions. In geology, the term marble refers to metamorphosed limestone, but its use in stonemasonry more broadly encompasses unmetamorphosed limestone.[1] Marble is commonly used for sculpture and as a building material.
https://en.wikipedia.org/wiki/Marble
Phthalocyanine (H2Pc) is a large, aromatic, macrocyclic, organic compound with the formula (C8H4N2)4H2 and is of theoretical or specialized interest in chemical dyes and photoelectricity.
It is composed of four isoindole units[a] linked by a ring of nitrogen atoms. (C8H4N2)4H2 = H2Pc has a two-dimensional geometry and a ring system consisting of 18 π-electrons. The extensive delocalization of the π-electrons affords the molecule useful properties, lending itself to applications in dyes and pigments. Metal complexes derived from Pc2−
, the conjugate base of H2Pc, are valuable in catalysis, organic solar cells, and photodynamic therapy.
https://en.wikipedia.org/wiki/Phthalocyanine
Phthalocyanine and derived metal complexes (MPc) tend to aggregate and, thus, have low solubility in common solvents.[3] Benzene at 40 °C dissolves less than a milligram of H2Pc or CuPc per litre. H2Pc and CuPc dissolve easily in sulfuric acid due to the protonation of the nitrogen atoms bridging the pyrrole rings. Many phthalocyanine compounds are, thermally, very stable and do not melt but can be sublimed. CuPc sublimes at above 500 °C under inert gases (nitrogen, CO2).[4] Substituted phthalocyanine complexes often have much higher solubility.[5] They are less thermally stable and often can not be sublimed. Unsubstituted phthalocyanines strongly absorb light between 600 and 700 nm, thus these materials are blue or green.[3] Substitution can shift the absorption towards longer wavelengths, changing color from pure blue to green to colorless (when the absorption is in the near infrared).
There are many derivatives of the parent phthalocyanine, where either carbon atoms of the macrocycle are exchanged for nitrogen atoms or the peripheral hydrogen atoms are substituted by functional groups like halogens, hydroxyl, amine, alkyl, aryl, thiol, alkoxy and nitrosyl groups. These modifications allow for the tuning of the electrochemical properties of the molecule such as absorption and emission wavelengths and conductance.[6]
History
In 1907, an unidentified blue compound, now known to be phthalocyanine, was reported.[7] In 1927, Swiss researchers serendipitously discovered copper phthalocyanine, copper naphthalocyanine, and copper octamethylphthalocyanine in an attempted conversion of o-dibromobenzene into phthalonitrile. They remarked on the enormous stability of these complexes but did not further characterize them.[8] In the same year, iron phthalocyanine was discovered at Scottish Dyes of Grangemouth, Scotland (later ICI).[9] It was not until 1934 that Sir Patrick Linstead characterized the chemical and structural properties of iron phthalocyanine.[10]
https://en.wikipedia.org/wiki/Phthalocyanine
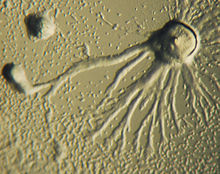




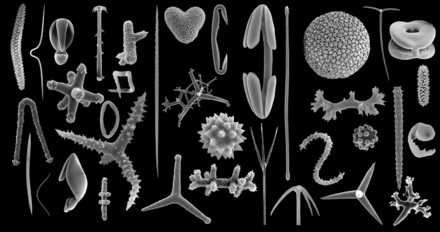


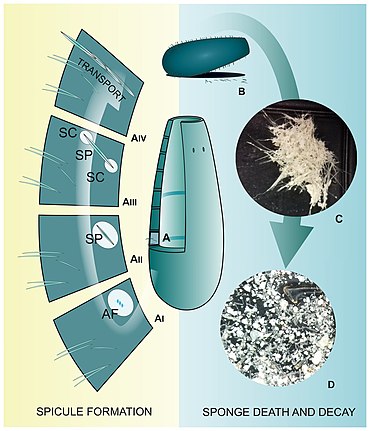
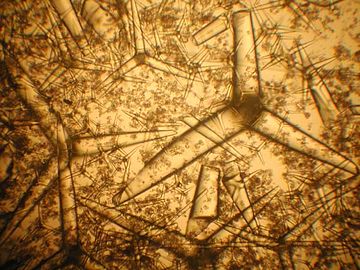




No comments:
Post a Comment