Tetraethylammonium (TEA) is a quaternary ammonium cation with the chemical formula [Et4N]+, consisting of four ethyl groups (−C2H5, denoted Et) attached to a central nitrogen atom. It is a counterion used in the research laboratory to prepare lipophilic salts of inorganic anions. It is used similarly to tetrabutylammonium, the difference being that its salts are less lipophilic and more easily crystallized.
Tetraethylammonium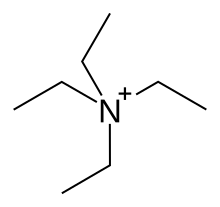
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
N,N,N-Triethylethanaminium | |
| Other names
Tetraethylazanium
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| Properties | |
| C8H20N+ | |
| Molar mass | 130.25 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
TEA is not orally active.[29] Typical symptoms produced in humans include the following: dry mouth, suppression of gastric secretion, drastic reduction of gastric motility, paralysis of urinary bladder, and relief of some forms of pain.[16] Most studies with TEA seem to have been performed using either its chloride or bromide salt without comment as to any distinctions in effect, but Birchall and his co-workers preferred the use of TEA chloride in order to avoid the sedative effects of the bromide ion.[30]
https://en.wikipedia.org/wiki/Tetraethylammonium
No comments:
Post a Comment