
Clonal expansion and monoclonal versus polyclonal proliferation
A clone is a group of identical cells that share a common ancestry, meaning they are derived from the same cell.[1]
Clonality implies the state of a cell or a substance being derived from one source or the other. Thus there are terms like polyclonal—derived from many clones; oligoclonal[2]—derived from a few clones; and monoclonal—derived from one clone. These terms are most commonly used in context of antibodies or immunocytes.
Contexts
This concept of clone assumes importance as all the cells that form a clone share common ancestry, which has a very significant consequence: shared genotype. One of the most prominent usage is in describing a clone of B cells. The B cells in the body have two important phenotypes (functional forms)—the antibody secreting, terminally differentiated (that is, they cannot divide further) plasma cells, and the memory and the naive cells—both of which retain their proliferative potential.
Another important area where one can talk of "clones" of cells is neoplasms.[3] Many of the tumors derive from one (sufficiently) mutated cell, so they are technically a single clone of cells. However, during course of cell division, one of the cells can get mutated further and acquire new characteristics to diverge as a new clone. However, this view of cancer onset has been challenged in recent years and many tumors have been argued to have polyclonal origin,[4] i.e. derived from two or more cells or clones, including malignant mesothelioma.[5]
All the granulosa cells in a Graafian follicle are in fact clones.
Paroxysmal nocturnal hemoglobinuria is a disorder of bone marrow cells resulting in shortened life of red blood cells, which is also a result of clonal expansion, i.e., all the altered cells are originally derived from a single cell, which also somewhat compromises the functioning of other "normal" bone marrow cells.[6] 
This section needs expansion with: more examples of clonal origin in the human body/vertebrates. You can help by adding to it. (May 2008)
Basis of clonal proliferation
Most other cells cannot divide indefinitely as after a few cycles of cell division the cells stop expressing an enzyme telomerase. The genetic material, in the form of deoxyribonucleic acid (DNA), continues to shorten with each cell division, and cells eventually stop dividing when they sense that their DNA is critically shortened. However, this enzyme in "youthful" cells replaces these lost bits (nucleotides) of DNA, thus making almost unlimited cycles of cell division possible. It is believed that the above-mentioned tissues have a constitutional elevated expression of telomerase. When ultimately many cells are produced by a single cell, clonal expansion is said to have taken place.
Concept of clonal colony
A somewhat similar concept is that of a clonal colony (also called a genet), wherein the cells (usually unicellular) also share a common ancestry, but which also requires the products of clonal expansion to reside at "one place", or in close proximity. A clonal colony would be well exemplified by a bacterial culture colony, or the bacterial films that are more likely to be found in vivo (e.g., in infected multicellular hosts). Whereas, the cells of clones dealt with here are specialized cells of a multicellular organism (usually vertebrates), and reside at quite distant places. For instance, two plasma cells belonging to the same clone could be derived from different memory cells (in turn with shared clonality) and could be residing in quite distant locations, such as the cervical (in the neck) and inguinal (in the groin) lymph nodes.
Paramecium clonal reproduction and aging
The single-cell eukaryote Paramecium tetraurelia can undergo both asexual and sexual reproduction. Asexual or clonal reproduction occurs by binary fission. Binary fission involves mitosis-like behavior of the chromosomes similar to that of cells in higher organisms. The sexual forms of reproduction are autogamy, a kind of self-fertilization, and conjugation, a kind of sexual interation between different cells. Clonal asexual reproduction can be initiated after completion of autogamy or conjugation. P. tetraurelia is able to replicate asexually for many generations but the dividing cells gradually age and after about 200 cell divisions, if the cells fail to undergo another autogamy or conjugation, they lose vitality and expire. This process is referred to as clonal aging. Experiments by Smith-Sonneborn,[7] Holmes and Holmes[8] and Gilley and Blackburn[9] showed that accumulation of DNA damage is the likely cause of clonal aging in P. tetraurelia. This aging process has similarities to the aging process in multicellular eukaryotes (See DNA damage theory of aging).
See also
Clone (B-cell biology)
Cloning
List of animals that have been cloned
Polyclonal antibodies
Polyclonal response
Tumour heterogeneity
References
"Clone definition – Medical Dictionary definitions of popular medical terms easily defined on MedTerms". MedicineNet.com. 2013-08-28.
"oligoclonal – Definition from Merriam-Webster's Medical Dictionary". Archived from the original on 2008-03-15. Retrieved 2008-05-05.
Pozo-Garcia, Lucia; Diaz-Cano, Salvador J.; Taback, Bret; Hoon, Dave S.B. (January 2003). "Clonal origin and expansions in neoplasms: biologic and technical aspects must be considered together". The American Journal of Pathology. 162 (1): 353–355. doi:10.1016/S0002-9440(10)63826-6. PMC 1851102. PMID 12507918.
Parsons, Barbara L. (September 2008). "Many different tumor types have polyclonal tumor origin: evidence and implications". Mutation Research. 659 (3): 232–47. doi:10.1016/j.mrrev.2008.05.004. PMID 18614394.
Comertpay, Sabahattin; Pastorino, Sandra; Tanji, Mika; Mezzapelle, Rosanna; Strianese, Oriana; et al. (4 December 2014). "Evaluation of clonal origin of malignant mesothelioma". Journal of Translational Medicine. 12 (1): 301. doi:10.1186/s12967-014-0301-3. PMC 4255423. PMID 25471750.
Bunn, Franklin; Wendell, Rosse (2005). Harrison's Principles of Internal Medicine. Vol. 1 (16th ed.). The McGraw-Hill Companies, Inc. p. 616. ISBN 0-07-144746-6.
Smith-Sonneborn, J. (1979). "DNA repair and longevity assurance in Paramecium tetraurelia". Science. 203 (4385): 1115–1117. Bibcode:1979Sci...203.1115S. doi:10.1126/science.424739. PMID 424739
Holmes, George E.; Holmes, Norreen R. (July 1986). "Accumulation of DNA damages in aging Paramecium tetraurelia". Molecular and General Genetics. 204 (1): 108–114. doi:10.1007/bf00330196. PMID 3091993. S2CID 11992591
Gilley, David; Blackburn, Elizabeth H. (1994). "Lack of telomere shortening during senescence in Paramecium" (PDF). Proceedings of the National Academy of Sciences of the United States of America. 91 (5): 1955–1958. Bibcode:1994PNAS...91.1955G. doi:10.1073/pnas.91.5.1955. PMC 43283. PMID 8127914
Into matter
Stem cells
Molecular biology and cell biology
Categories: Cell biology
Molecular biology
Cloning
Immunology
https://en.wikipedia.org/wiki/Clone_(cell_biology)
An ovarian follicle is a roughly spheroid cellular aggregation set found in the ovaries. It secretes hormones that influence stages of the menstrual cycle. At the time of puberty, women have approximately 200,000 to 300,000 follicles,[1][2] each with the potential to release an egg cell (ovum) at ovulation for fertilization.[3] These eggs are developed once every menstrual cycle with around 450–500 being ovulated during a woman's reproductive lifetime.[4]
Structure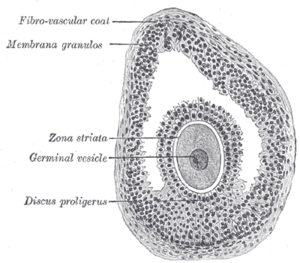
Section of vesicular ovarian follicle of cat. X 50.
Ovarian follicles are the basic units of female reproductive biology. Each of them contains a single oocyte (immature ovum or egg cell). These structures are periodically initiated to grow and develop, culminating in ovulation of usually a single competent oocyte in humans.[5] They also consist of granulosa cells and theca of follicle.
Structure
Section of vesicular ovarian follicle of cat. X 50.
Ovarian follicles are the basic units of female reproductive biology. Each of them contains a single oocyte (immature ovum or egg cell). These structures are periodically initiated to grow and develop, culminating in ovulation of usually a single competent oocyte in humans.[5] They also consist of granulosa cells and theca of follicle.
Oocyte
Main article: Oocyte
Once a month, one of the ovaries releases a mature egg (ovum), known as an oocyte. The nucleus of such an oocyte is called a germinal vesicle[6] (see picture).
Cumulus oophorus
Main article: Cumulus oophorus
Cumulus oophorus is a cluster of cells (called cumulus cells) that surround the oocyte both in the ovarian follicle and after ovulation.
Membrana granulosa
Main article: Membrana granulosa
It contains numerous granulosa cells.
Granulosa cell
Granulosa cells or follicular cells are cells that surround the oocyte within the follicle; their numbers increase directly in response to heightened levels of circulating gonadotropins or decrease in response to testosterone. They also produce peptides involved in ovarian hormone synthesis regulation. Follicle-stimulating hormone (FSH) induces granulosa cells to express luteinizing hormone (LH) receptors on their surfaces; when circulating LH binds to these receptors, proliferation stops.[7]
Theca of follicle
Main article: Theca of follicle
The granulosa cells, in turn, are enclosed in a thin layer of extracellular matrix – the follicular basement membrane or basal lamina (fibro-vascular coat in picture). Outside the basal lamina, the layers theca interna and theca externa are found.
Development
Main articles: Folliculogenesis and Ovarian follicle activation
Primordial follicles are indiscernible to the naked eye. However, these eventually develop into primary, secondary and tertiary vesicular follicles. Tertiary vesicular follicles (also called "mature vesicular follicles" or "ripe vesicular follicles") are sometimes called Graafian follicles (after Regnier de Graaf).
In humans, oocytes are established in the ovary before birth and may lie dormant awaiting initiation for up to 50 years.[8]
After rupturing, the follicle is turned into a corpus luteum.
Development of oocytes in ovarian follicles
Main article: Oogenesis
In a larger perspective, the whole folliculogenesis from primordial to preovulatory follicle is located in the stage of meiosis I of ootidogenesis in oogenesis.
Embryonic development in males and females follows a common pathway before gametogenesis. Once gametogonia enter the gonadal ridge, however, they attempt to associate with these somatic cells. Development proceeds and the gametogonia turn into oogonia, which become fully surrounded by a layer of cells (pre-granulosa cells).
The oogonia multiply by dividing mitotically; this proliferation ends when the oogonia enter meiosis. The amount of time that oogonia multiply by mitosis is not species specific. In the human fetus, cells undergoing mitosis are seen until the second and third trimester of pregnancy.[9][10] After beginning the meiotic process, the oogonia (now called primary oocytes) can no longer replicate. Therefore, the total number of gametes is established at this time. Once the primary oocytes stop dividing the cells enter a prolonged 'resting phase'. This 'resting phase' or dictyate stage can last anywhere up to fifty years in the human.
For several primary oocytes that complete meiosis I each month, only one or a few functional oocyte, the dominant follicles, completes maturation and undergoes ovulation. The other follicles that begin to mature will regress and become atretic follicles, eventually deteriorating.
The primary oocyte turns into a secondary oocyte in mature ovarian follicles. Unlike the sperm, the egg is arrested in the secondary stage of meiosis until fertilization.
Upon fertilization by sperm, the secondary oocyte continues the second part of meiosis and becomes a zygote.
Clinical significance
Any ovarian follicle that is larger than about three centimeters is termed an ovarian cyst.
Ovarian function may be measured by gynecologic ultrasonography of follicular volume. Presently, ovarian follicle volumes can be measured rapidly and automatically from three-dimensionally reconstructed ultrasound images.[11]
Rupture of the follicle can result in abdominal pain (mittelschmerz) and is to be considered in the differential diagnosis in women of childbearing age.[12]
Cryopreservation and culture tissue after cryopreservation. Cryopreservation of ovarian tissue is of interest to women who want to preserve their reproductive function beyond the natural limit, or whose reproductive potential is threatened by cancer therapy,[13] for example in hematologic malignancies or breast cancer.[14]
For in vitro culture of follicles, there are various techniques to optimize the growth of follicles, including the use of defined media, growth factors and three-dimensional extracellular matrix support.[15] Molecular methods and immunoassay can evaluate stage of maturation and guide adequate differentiation.[15] Animal studies have generally shown correct imprinted DNA methylation establishment in oocytes resulting from follicle culture.[16]
Additional images 
Primordial ovarian follicle. The oocyte is surrounded by a single layer of flat granulosa cells.

A histological slide of a human primary ovarian follicle in greater magnification
See alsoAntral follicle
https://en.wikipedia.org/wiki/Ovarian_follicle
An antral follicle, also known as Graafian follicle and tertiary follicle, is an ovarian follicle during a certain latter stage of folliculogenesis.
Definitions differ in where the shift into an antral follicle occurs in the staging of folliculogenesis, with some stating that it occurs when entering the secondary stage,[1] and others stating that it occurs when entering the tertiary stage.[2]
Antral follicle
Appearance
The antral follicle is marked by the formation of a fluid-filled cavity adjacent to the oocyte called the antrum. The basic structure of the mature follicle has formed and no novel cells are detectable. Granulosa and theca cells continue to undergo mitosis concomitant with an increase in antrum volume. The Graaf follicle reaches its maximum diameter (20–22 mm) during ovulation. Antral follicles can attain a tremendous size that is hampered only by the availability of follicle stimulating hormone (FSH), which it is dependent on in this stage of folliculogenesis.
By command of an oocyte-secreted morphogenic gradient, the antral follicle's granulosa cells begin to differentiate themselves into four distinct subtypes: corona radiata that surrounds the zona pellucida, membrana that is interior to the basal lamina, periantral that is adjacent to the antrum, and cumulus oophorus that connects the membrana and corona radiata granulosa cells together. Each type of cell behaves differently in response to FSH.
https://en.wikipedia.org/wiki/Antral_follicle
The urethral sponge is a spongy cushion of tissue, found in the lower genital area of females, that sits against both the pubic bone and vaginal wall, and surrounds the urethra.
https://en.wikipedia.org/wiki/Urethral_sponge
A granulosa cell or follicular cell is a somatic cell of the sex cord that is closely associated with the developing female gamete (called an oocyte or egg) in the ovary of mammals.
https://en.wikipedia.org/wiki/Granulosa_cell
The cumulus oophorus, (discus proligerus), is a cluster of cells that surround the oocyte both in the ovarian follicle and after ovulation. In the antral follicle, it may be regarded as an extension of the membrana granulosa. The innermost layer of these cells is the corona radiata.[1]
This layer of cells must be penetrated by spermatozoa for fertilization to occur.
https://en.wikipedia.org/wiki/Cumulus_oophorus
The larger ovarian follicles consist of an external fibrovascular coat, connected with the surrounding stroma of the ovary by a network of blood vessels, and an internal coat, which consists of several layers of nucleated cells, called the membrana granulosa. It contains numerous granulosa cells.
At one part of the mature follicle the cells of the membrana granulosa are collected into a mass which projects into the cavity of the follicle. This is termed the discus proligerus.
https://en.wikipedia.org/wiki/Membrana_granulosa
Vesicular appendages of the epoöphoron are small pedunculated vesicles of the fimbriae of the uterine tube, or connected to the broad ligament. They were described by Giovanni Battista Morgagni and are remnants of the cranial part of the mesonephric duct. Typically they are asymptomatic.
In the male remnants of the paramesonephric duct may be present as well and are also known as appendix of testis or hydatid of Morgagni.
They are rarely absent, and are attached either to the free margin of the mesosalpinx or to one of the fimbriae, and are pedunculated vesicles, filled with fluid, about the size of a small pea. The pedicles frequently attain a considerable length.[1]
https://en.wikipedia.org/wiki/Vesicular_appendages_of_epoophoron
Paraovarian cysts or paratubal cysts are epithelium-lined fluid-filled cysts in the adnexa adjacent to the fallopian tube and ovary. The terms are used interchangeably,[1] and depend on the location of the cyst.[2]
https://en.wikipedia.org/wiki/Paraovarian_cyst
https://en.wikipedia.org/wiki/Category:Noninflammatory_disorders_of_female_genital_tract
https://en.wikipedia.org/wiki/Stenosis_of_uterine_cervix
https://en.wikipedia.org/wiki/Sigmoidocele
https://en.wikipedia.org/wiki/Salpingitis_isthmica_nodosa
https://en.wikipedia.org/wiki/Vaginitis_emphysematosa
https://en.wikipedia.org/wiki/Uterine_hyperplasia
https://en.wikipedia.org/wiki/Follicular_cyst_of_ovary
https://en.wikipedia.org/wiki/Ovarian_apoplexy
https://en.wikipedia.org/wiki/Ovarian_torsion
https://en.wikipedia.org/wiki/Pelvic_organ_prolapse
https://en.wikipedia.org/wiki/Fallopian_tube_obstruction
https://en.wikipedia.org/wiki/Dysplasia
https://en.wikipedia.org/wiki/Cystocele
https://en.wikipedia.org/wiki/Atypical_polypoid_adenomyoma
https://en.wikipedia.org/wiki/Category:Noninflammatory_disorders_of_female_genital_tract
https://en.wikipedia.org/wiki/Corpus_luteum_cyst
https://en.wikipedia.org/wiki/Rectovaginal_fistula
Pelvic congestion syndrome, also known as pelvic vein incompetence, is a long term condition believed to be due to enlarged veins in the lower abdomen.[1][3] The condition may cause chronic pain, such as a constant dull ache, which can be worsened by standing or sex.[1] Pain in the legs or lower back may also occur.[1]
While the condition is believed to be due to blood flowing back into pelvic veins as a result of faulty valves in the veins, this hypothesis is not certain.[3] The condition may occur or worsen during pregnancy.[1] The presence of estrogen is believed to be involved in the mechanism.[1] Diagnosis may be supported by ultrasound, CT scan, MRI, or laparoscopy.[1]
Early treatment options include medroxyprogesterone or nonsteroidal anti-inflammatory drugs (NSAIDs).[1] Surgery to block the varicose veins may also be done.[1] About 30% of women of reproductive age are affected.[2] It is believed to be the cause of about a third of chronic pelvic pain cases.[4] While pelvic venous insufficiency was identified in the 1850s it was only linked with pelvic pain in the 1940s.[4]
https://en.wikipedia.org/wiki/Pelvic_congestion_syndrome
Metropathia haemorrhagica, also known as metropathia haemorrhagica cystica, is a menstrual disorder which is defined as a specialized type of anovulatory dysfunctional uterine bleeding associated with endometrial hyperplasia and intermenstrual bleeding.[1][2][3][4] The condition was defined by 1930.[5] It has been agreed that the term "metropathia haemorrhagica" should be discarded along with many other older terms for menstrual disorders.[6][4]
https://en.wikipedia.org/wiki/Metropathia_haemorrhagica
Asherman's syndrome (AS) is an acquired uterine condition that occurs when scar tissue (adhesions) forms inside the uterus and/or the cervix.[1] It is characterized by variable scarring inside the uterine cavity, where in many cases the front and back walls of the uterus stick to one another. AS can be the cause of menstrual disturbances, infertility, and placental abnormalities. Although the first case of intrauterine adhesion was published in 1894 by Heinrich Fritsch, it was only after 54 years that a full description of Asherman syndrome was carried out by Joseph Asherman.[2] A number of other terms have been used to describe the condition and related conditions including: uterine/cervical atresia, traumatic uterine atrophy, sclerotic endometrium, and endometrial sclerosis.[3]
There is not any one cause of AS. Risk factors can include myomectomy, cesarean section, infections, age, genital tuberculosis, and obesity. Genetic predisposition to AS is being investigated. There are also studies that show that a severe pelvic infection, independent of surgery may cause AS.[4] AS can develop even if the woman has not had any uterine surgeries, trauma, or pregnancies. While rare in North America and European countries, genital tuberculosis is a cause of Asherman's in other countries such as India.[5]
https://en.wikipedia.org/wiki/Asherman%27s_syndrome
Ovulation is the release of eggs from the ovaries. In women, this event occurs when the ovarian follicles rupture and release the secondary oocyte ovarian cells.[1] After ovulation, during the luteal phase, the egg will be available to be fertilized by sperm. In addition, the uterine lining (endometrium) is thickened to be able to receive a fertilized egg. If no conception occurs, the uterine lining as well as the egg will be shed during menstruation.[2]
Process
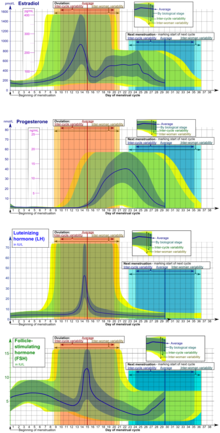
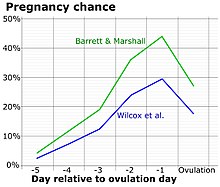
Ovulation occurs about midway through the menstrual cycle, after the follicular phase. The days in which a person is most fertile can be calculated based on the date of the last menstrual period and the length of a typical menstrual cycle.[3] The few days surrounding ovulation (from approximately days 10 to 18 of a 28-day cycle), constitute the most fertile phase.[4][5][6][7] The time from the beginning of the last menstrual period (LMP) until ovulation is, on average, 14.6[8] days, but with substantial variation among females and between cycles in any single female, with an overall 95% prediction interval of 8.2 to 20.5[8] days.
The process of ovulation is controlled by the hypothalamus of the brain and through the release of hormones secreted in the anterior lobe of the pituitary gland, luteinizing hormone (LH) and follicle-stimulating hormone (FSH).[9] In the preovulatory phase of the menstrual cycle, the ovarian follicle will undergo a series of transformations called cumulus expansion, which is stimulated by FSH. After this is done, a hole called the stigma will form in the follicle, and the secondary oocyte will leave the follicle through this hole. Ovulation is triggered by a spike in the amount of FSH and LH released from the pituitary gland. During the luteal (post-ovulatory) phase, the secondary oocyte will travel through the fallopian tubes toward the uterus. If fertilized by a sperm, the fertilized secondary oocyte or ovum may implant there 6–12 days later.[10]
Follicular phase
The follicular phase (or proliferative phase) is the phase of the menstrual cycle during which the ovarian follicles mature. The follicular phase lasts from the beginning of menstruation to the start of ovulation.[11][12]
For ovulation to be successful, the ovum must be supported by the corona radiata and cumulus oophorous granulosa cells. The latter undergo a period of proliferation and mucification known as cumulus expansion. Mucification is the secretion of a hyaluronic acid-rich cocktail that disperses and gathers the cumulus cell network in a sticky matrix around the ovum. This network stays with the ovum after ovulation and has been shown to be necessary for fertilization.[13][14]
Ovulation
Estrogen levels peak towards the end of the follicular phase, around 12 and 24 hours. This, by positive feedback, causes a surge in levels of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). This lasts from 24 to 36 hours, and results in the rupture of the ovarian follicles, causing the oocyte to be released from the ovary.[15]
Through a signal transduction cascade initiated by LH, which activates the pro-inflammatory genes through cAMP secondary messenger, proteolytic enzymes are secreted by the follicle that degrade the follicular tissue at the site of the blister, forming a hole called the stigma. The secondary oocyte leaves the ruptured follicle and moves out into the peritoneal cavity through the stigma, where it is caught by the fimbriae at the end of the fallopian tube. After entering the fallopian tube, the oocyte is pushed along by cilia, beginning its journey toward the uterus.[9]
By this time, the oocyte has completed meiosis I, yielding two cells: the larger secondary oocyte that contains all of the cytoplasmic material and a smaller, inactive first polar body. Meiosis II follows at once but will be arrested in the metaphase and will so remain until fertilization. The spindle apparatus of the second meiotic division appears at the time of ovulation. If no fertilization occurs, the oocyte will degenerate between 12 and 24 hours after ovulation.[16] Approximately 1–2% of ovulations release more than one oocyte. This tendency increases with maternal age. Fertilization of two different oocytes by two different spermatozoa results in fraternal twins.[9]
Luteal phase
The follicle proper has met the end of its lifespan. Without the oocyte, the follicle folds inward on itself, transforming into the corpus luteum (pl. corpora lutea), a steroidogenic cluster of cells that produces estrogen and progesterone. These hormones induce the endometrial glands to begin production of the proliferative endometrium and later into secretory endometrium, the site of embryonic growth if implantation occurs. The action of progesterone increases basal body temperature by one-quarter to one-half degree Celsius (one-half to one degree Fahrenheit). The corpus luteum continues this paracrine action for the remainder of the menstrual cycle, maintaining the endometrium, before disintegrating into scar tissue during menses.[17]
Clinical presentation
The start of ovulation may be detected by signs that are not readily discernible other than to the ovulating female herself, thus humans are said to have a concealed ovulation. In many animal species there are distinctive signals indicating the period when the female is fertile. Several explanations have been proposed to explain concealed ovulation in humans.
Females near ovulation experience changes in the cervical mucus, and in their basal body temperature. Furthermore, many females experience secondary fertility signs including Mittelschmerz (pain associated with ovulation) and a heightened sense of smell, and can sense the precise moment of ovulation.[18][19] However, midcycle pain may also not be due to Mittelschmerz, but due to other factors such as cysts, endometriosis, sexually transmitted infections, or an ectopic pregnancy.[20] Other possible signs of ovulation include tender breasts, bloating, and cramps, although these symptoms are not a guarantee that ovulation is taking place.[21][22]
Many females experience heightened sexual desire in the several days immediately before ovulation.[23] One study concluded that females subtly improve their facial attractiveness during ovulation.[24]
Symptoms related to the onset of ovulation, the moment of ovulation and the body's process of beginning and ending the menstrual cycle vary in intensity with each female but are fundamentally the same. The charting of such symptoms — primarily basal body temperature, mittelschmerz and cervical position — is referred to as the sympto-thermal method of fertility awareness, which allow auto-diagnosis by a female of her state of ovulation. Once training has been given by a suitable authority, fertility charts can be completed on a cycle-by-cycle basis to show ovulation. This gives the possibility of using the data to predict fertility for natural contraception and pregnancy planning.
The moment of ovulation has been photographed.[26]
Disorders
Disorders of ovulation, also known as ovulatory disorders are classified as menstrual disorders and include oligoovulation (infrequent or irregular ovulation) and anovulation (absence of ovulation):[27]
- Oligoovulation is infrequent or irregular ovulation (usually defined as cycles of greater than 36 days or fewer than 8 cycles a year)
- Anovulation is absence of ovulation when it would be normally expected (in a post-menarchal, premenopausal female). Anovulation usually manifests itself as irregularity of menstrual periods, that is, unpredictable variability of intervals, duration, or bleeding. Anovulation can also cause cessation of periods (secondary amenorrhea) or excessive bleeding (dysfunctional uterine bleeding).
The World Health Organization (WHO) has developed the following classification of ovulatory disorders:[28]
- WHO group I: Hypothalamic–pituitary–gonadal axis failure
- WHO group II: Hypothalamic–pituitary–gonadal axis dysfunction. WHO group II is the most common cause of ovulatory disorders, and the most common causative member is polycystic ovary syndrome (PCOS).[29]
- WHO group III: Ovarian failure
- WHO group IV: Hyperprolactinemia
Menstrual disorders can often indicate ovulatory disorder.[30]
Ovulation induction
Ovulation induction is a promising assisted reproductive technology for patients with conditions such as polycystic ovary syndrome (PCOS) and oligomenorrhea. It is also used in in vitro fertilization to make the follicles mature before egg retrieval. Usually, ovarian stimulation is used in conjunction with ovulation induction to stimulate the formation of multiple oocytes.[31] Some sources[31] include ovulation induction in the definition of ovarian stimulation.
A low dose of human chorionic gonadotropin (HCG) may be injected after completed ovarian stimulation. Ovulation will occur between 24–36 hours after the HCG injection.[31]
By contrast, induced ovulation in some animal species occurs naturally, ovulation can be stimulated by coitus.[32]
Ovulation suppression
Combined hormonal contraceptives inhibit follicular development and prevent ovulation as a primary mechanism of action.[33] The ovulation-inhibiting dose (OID) of an estrogen or progestogen refers to the dose required to consistently inhibit ovulation in women.[34] Ovulation inhibition is an antigonadotropic effect and is mediated by inhibition of the secretion of the gonadotropins, LH and FSH, from the pituitary gland.
In assisted reproductive technology including in vitro fertilization, cycles where a transvaginal oocyte retrieval is planned generally necessitates ovulation suppression, because it is not practically feasible to collect oocytes after ovulation. For this purpose, ovulation can be suppressed by either a GnRH agonist or a GnRH antagonist, with different protocols depending on which substance is used.
See also
Notes
- Endrikat J, Gerlinger C, Richard S, Rosenbaum P, Düsterberg B (December 2011). "Ovulation inhibition doses of progestins: a systematic review of the available literature and of marketed preparations worldwide". Contraception. 84 (6): 549–57. doi:10.1016/j.contraception.2011.04.009. PMID 22078182.
Further reading
- Baerwald AR, Adams GP, Pierson RA (July 2003). "A new model for ovarian follicular development during the human menstrual cycle". Fertility and Sterility. 80 (1): 116–22. doi:10.1016/S0015-0282(03)00544-2. PMID 12849812.
- Chabbert Buffet N, Djakoure C, Maitre SC, Bouchard P (July 1998). "Regulation of the human menstrual cycle". Frontiers in Neuroendocrinology. 19 (3): 151–86. doi:10.1006/frne.1998.0167. PMID 9665835. S2CID 40594356.
- Fortune JE (February 1994). "Ovarian follicular growth and development in mammals". Biology of Reproduction. 50 (2): 225–32. doi:10.1095/biolreprod50.2.225. PMID 8142540.
- Guraya SS, Dhanju CK (November 1992). "Mechanism of ovulation--an overview". Indian Journal of Experimental Biology. 30 (11): 958–67. PMID 1293040.
- Klowden MJ (2009). "Oviposition Behavior". In Resh VH, Carde RT (eds.). Encyclopedia of Insects. Academic Press. ISBN 9780080920900. Retrieved 2013-11-09.
- Su HW, Yi YC, Wei TY, Chang TC, Cheng CM (September 2017). "Detection of ovulation, a review of currently available methods". Bioengineering & Translational Medicine. 2 (3): 238–246. doi:10.1002/btm2.10058. PMC 5689497. PMID 29313033.
External links
https://en.wikipedia.org/wiki/Ovulation#Disorders
Bacterial vaginosis (BV) is a infection of the vagina caused by excessive growth of bacteria.[6][9] Common symptoms include increased vaginal discharge that often smells like fish.[2] The discharge is usually white or gray in color.[2] Burning with urination may occur.[2] Itching is uncommon.[2][6] Occasionally, there may be no symptoms.[2] Having BV approximately doubles the risk of infection by a number of sexually transmitted infections, including HIV/AIDS.[8][10] It also increases the risk of early delivery among pregnant women.[3][11]
BV is caused by an imbalance of the naturally occurring bacteria in the vagina.[4][5] There is a change in the most common type of bacteria and a hundred to thousand fold increase in total numbers of bacteria present.[6] Typically, bacteria other than Lactobacilli become more common.[12] Risk factors include douching, new or multiple sex partners, antibiotics, and using an intrauterine device, among others.[5] However, it is not considered a sexually transmitted infection and, unlike gonorrhoea and chlamydia, sexual partners are not treated.[13] Diagnosis is suspected based on the symptoms, and may be verified by testing the vaginal discharge and finding a higher than normal vaginal pH, and large numbers of bacteria.[6] BV is often confused with a vaginal yeast infection or infection with Trichomonas.[7]
Usually treatment is with an antibiotic, such as clindamycin or metronidazole.[6] These medications may also be used in the second or third trimesters of pregnancy.[6] However, the condition often recurs following treatment.[6] Probiotics may help prevent re-occurrence.[6] It is unclear if the use of probiotics or antibiotics affects pregnancy outcomes.[6][14]
BV is the most common vaginal infection in women of reproductive age.[5] The percentage of women affected at any given time varies between 5% and 70%.[8] BV is most common in parts of Africa and least common in Asia and Europe.[8] In the United States about 30% of women between the ages of 14 and 49 are affected.[15] Rates vary considerably between ethnic groups within a country.[8] While BV-like symptoms have been described for much of recorded history, the first clearly documented case occurred in 1894.[1]
https://en.wikipedia.org/wiki/Bacterial_vaginosis
TOL-463, a formulation of boric acid enhanced with ethylenediaminetetraacetic acid (EDTA), is under development as an intravaginal medication for the treatment of BV and has shown preliminary effectiveness.[63][62][64][65]
https://en.wikipedia.org/wiki/Bacterial_vaginosis
https://en.wikipedia.org/wiki/Ethylenediaminetetraacetic_acid
https://en.wikipedia.org/wiki/Povidone-iodine
https://en.wikipedia.org/wiki/Hexetidine
https://en.wikipedia.org/wiki/Lactobacillus_crispatus
https://en.wikipedia.org/wiki/Lincosamides
https://en.wikipedia.org/wiki/Nitroimidazole
https://en.wikipedia.org/wiki/Macrolide
https://en.wikipedia.org/wiki/Metronidazole
https://en.wikipedia.org/wiki/Gardnerella_vaginalis
https://en.wikipedia.org/wiki/Tobacco
https://en.wikipedia.org/wiki/Nicotiana
https://en.wikipedia.org/wiki/Lettuce
https://en.wikipedia.org/wiki/Lactuca
https://en.wikipedia.org/wiki/Xerophyte
The structural features (morphology) and fundamental chemical processes (physiology) of xerophytes are variously adapted to conserve water, also common to store large quantities of water, during dry periods. Other species are able to survive long periods of extreme dryness or desiccation of their tissues, during which their metabolic activity may effectively shut down. Plants with such morphological and physiological adaptations are xeromorphic.[1] Xerophytes such as cacti are capable of withstanding extended periods of dry conditions as they have deep-spreading roots and capacity to store water. Their waxy, thorny leaves prevent loss of moisture. Even their fleshy stems can store water.
https://en.wikipedia.org/wiki/Xerophyte
The succulent xerophyte Zygophyllum xanthoxylum, for example, has specialised protein transporters in its cells which allows storage of excess ions in their vacuoles to maintain normal cytosolic pH and ionic composition.[4][5]
https://en.wikipedia.org/wiki/Xerophyte
A psammophile is a plant or animal that prefers or thrives in sandy areas. Plant psammophiles are also known as psammophytes. They thrive in places such as the Arabian Peninsula and the Sahara.[1] and also the dunes of coastal regions.
https://en.wikipedia.org/wiki/Psammophile
Chloroflexus aurantiacus is a photosynthetic bacterium isolated from hot springs, belonging to the green non-sulfur bacteria. This organism is thermophilic and can grow at temperatures from 35 °C to 70 °C (94.998 to 158 °F). Chloroflexus aurantiacus can survive in the dark if oxygen is available. When grown in the dark, Chloroflexus aurantiacus has a dark orange color. When grown in sunlight it is dark green. The individual bacteria tend to form filamentous colonies enclosed in sheaths, which are known as trichomes.
https://en.wikipedia.org/wiki/Chloroflexus_aurantiacus
Cyanidioschyzon merolae is a small (2μm), club-shaped, unicellular haploid red alga adapted to high sulfur acidic hot spring environments (pH 1.5, 45 °C).[2][3] The cellular architecture of C. merolae is extremely simple, containing only a single chloroplast and a single mitochondrion and lacking a vacuole and cell wall.[4] In addition, the cellular and organelle divisions can be synchronized. For these reasons, C. merolae is considered an excellent model system for study of cellular and organelle division processes, as well as biochemistry and structural biology.[5][6][7] The organism's genome was the first full algal genome to be sequenced in 2004;[8] its plastid was sequenced in 2000 and 2003, and its mitochondrion in 1998.[9] The organism has been considered the simplest of eukaryotic cells for its minimalist cellular organization.[10]
https://en.wikipedia.org/wiki/Cyanidioschyzon
Acidophiles or acidophilic organisms are those that thrive under highly acidic conditions (usually at pH 5.0 or below[1]). These organisms can be found in different branches of the tree of life, including Archaea, Bacteria,[2] and Eukarya.
Examples
A list of these organisms includes:
Archaea
- Sulfolobales, an order in the Thermoproteota branch[3] of Archaea
- Thermoplasmatales, an order in the Euryarchaeota branch[3] of Archaea
- ARMAN, in the Euryarchaeota branch[3] of Archaea
- Acidianus brierleyi, A. infernus, facultatively anaerobic thermoacidophilic archaebacteria
- Halarchaeum acidiphilum, acidophilic member of the Halobacteriacaeae[4]
- Metallosphaera sedula, thermoacidophilic
Bacteria
- Acidobacteriota,[5] a phylum of Bacteria
- Acidithiobacillales, an order of Pseudomonadota e.g. A. ferrooxidans, A. thiooxidans
- Thiobacillus prosperus, T. acidophilus, T. organovorus, T. cuprinus
- Acetobacter aceti, a bacterium that produces acetic acid (vinegar) from the oxidation of ethanol.
- Alicyclobacillus, a genus of bacteria that can contaminate fruit juices.[6]
Eukarya
- Mucor racemosus[7]
- Urotricha[7]
- Dunaliella acidophila[7]
- Members of the algal class Cyanidiophyceae, including Cyanidioschyzon merolae
Mechanisms of adaptation to acidic environments
Most acidophile organisms have evolved extremely efficient mechanisms to pump protons out of the intracellular space in order to keep the cytoplasm at or near neutral pH. Therefore, intracellular proteins do not need to develop acid stability through evolution. However, other acidophiles, such as Acetobacter aceti, have an acidified cytoplasm which forces nearly all proteins in the genome to evolve acid stability.[8] For this reason, Acetobacter aceti has become a valuable resource for understanding the mechanisms by which proteins can attain acid stability.
Studies of proteins adapted to low pH have revealed a few general mechanisms by which proteins can achieve acid stability. In most acid stable proteins (such as pepsin and the soxF protein from Sulfolobus acidocaldarius), there is an overabundance of acidic residues which minimizes low pH destabilization induced by a buildup of positive charge. Other mechanisms include minimization of solvent accessibility of acidic residues or binding of metal cofactors. In a specialized case of acid stability, the NAPase protein from Nocardiopsis alba was shown to have relocated acid-sensitive salt bridges away from regions that play an important role in the unfolding process. In this case of kinetic acid stability, protein longevity is accomplished across a wide range of pH, both acidic and basic.
See also
References
- Menzel, U.; Gottschalk, G. (1985). "The internal pH of Acetobacterium wieringae and Acetobacter aceti during growth and production of acetic acid". Arch Microbiol. 143 (1): 47–51. doi:10.1007/BF00414767. S2CID 6477488.
Further reading
- Cooper, J. B.; Khan, G.; Taylor, G.; Tickle, I. J.; Blundell, T. L. (July 1990). "X-ray analyses of aspartic proteinases. II. Three-dimensional structure of the hexagonal crystal form of porcine pepsin at 2.3 A resolution". J Mol Biol. 214 (1): 199–222. doi:10.1016/0022-2836(90)90156-G. PMID 2115088.
- Bonisch, H.; Schmidt, C. L.; Schafer, G.; Ladenstein, R. (June 2002). "The structure of the soluble domain of an archaeal Rieske iron-sulfur protein at 1.1 A resolution". J Mol Biol. 319 (3): 791–805. doi:10.1016/S0022-2836(02)00323-6. PMID 12054871.
- Schafer, K; Magnusson, U; Scheffel, F; Schiefner, A; Sandgren, MO; Diederichs, K; Welte, W; Hülsmann, A; Schneider, E; Mowbray, SL (January 2004). "X-ray structures of the maltose-maltodextrin-binding protein of the thermoacidophilic bacterium Alicyclobacillus acidocaldarius provide insight into acid stability of proteins". Journal of Molecular Biology. 335 (1): 261–74. doi:10.1016/j.jmb.2003.10.042. PMID 14659755.
- Walter, R. L.; Ealick, S. E.; Friedman, A. M.; Blake, R. C. 2nd; Proctor, P.; Shoham, M. (November 1996). "Multiple wavelength anomalous diffraction (MAD) crystal structure of rusticyanin: a highly oxidizing cupredoxin with extreme acid stability". J Mol Biol. 263 (5): 730–51. doi:10.1006/jmbi.1996.0612. PMID 8947572.
- Botuyan, M. V.; Toy-Palmer, A.; Chung, J.; Blake, R. C. 2nd; Beroza, P.; Case, D. A.; Dyson, H. J. (1996). "NMR solution structure of Cu(I) rusticyanin from Thiobacillus ferrooxidans: structural basis for the extreme acid stability and redox potential". J Mol Biol. 263 (5): 752–67. doi:10.1006/jmbi.1996.0613. PMID 8947573.
- Kelch, B. A.; Eagen, K. P.; Erciyas, F. P.; Humphris, E. L.; Thomason, A. R.; Mitsuiki, S.; Agard, D. A. (May 2007). "Structural and mechanistic exploration of acid resistance: kinetic stability facilitates evolution of extremophilic behavior". J Mol Biol. 368 (3): 870–883. CiteSeerX 10.1.1.79.3711. doi:10.1016/j.jmb.2007.02.032. PMID 17382344.
https://en.wikipedia.org/wiki/Acidophile
https://en.wikipedia.org/wiki/Lithoautotroph
https://en.wikipedia.org/wiki/Lipophilic_bacteria
https://en.wikipedia.org/wiki/Hypolith
https://en.wikipedia.org/wiki/Halophile
https://en.wikipedia.org/wiki/Endolith
https://en.wikipedia.org/wiki/Capnophile
https://en.wikipedia.org/wiki/Metallotolerant
https://en.wikipedia.org/wiki/Galdieria_sulphuraria
https://en.wikipedia.org/wiki/Paralvinella_sulfincola
https://en.wikipedia.org/wiki/Alvinella_pompejana
https://en.wikipedia.org/wiki/Halicephalobus_mephisto
https://en.wikipedia.org/wiki/GFAJ-1
https://en.wikipedia.org/wiki/Thermus_thermophilus
https://en.wikipedia.org/wiki/Thermus_aquaticus
https://en.wikipedia.org/wiki/Snottite
https://en.wikipedia.org/wiki/Deinococcota
https://en.wikipedia.org/wiki/Spirochaeta_americana
https://en.wikipedia.org/wiki/Pyrolobus_fumarii
https://en.wikipedia.org/wiki/Thermococcus_gammatolerans
https://en.wikipedia.org/wiki/Strain_121
https://en.wikipedia.org/wiki/Pyrococcus_furiosus
https://en.wikipedia.org/wiki/Acidophile

No comments:
Post a Comment