Farnesyl pyrophosphate (FPP), also known as farnesyl diphosphate (FDP), is an intermediate in the biosynthesis of terpenes and terpenoids such as sterols and carotenoids.[1] It is also used in the synthesis of CoQ (part of the electron transport chain), as well as dehydrodolichol diphosphate (a precursor of dolichol, which transports proteins to the ER lumen for N-glycosylation).
https://en.wikipedia.org/wiki/Farnesyl_pyrophosphate
Dolichol refers to any of a group of long-chain mostly unsaturated organic compounds that are made up of varying numbers of isoprene units terminating in an α-saturated isoprenoid group, containing an alcohol functional group.
https://en.wikipedia.org/wiki/Dolichol
Asparagine (symbol Asn or N[2]) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH+
3 form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain carboxamide, classifying it as a polar (at physiological pH), aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it. It is encoded by the codons AAU and AAC.
https://en.wikipedia.org/wiki/Asparagine
Post-translational modification (PTM) is the covalent and generally enzymatic modification of proteins following protein biosynthesis. This process occurs in the endoplasmic reticulum and the golgi apparatus.[1] Proteins are synthesized by ribosomes translating mRNA into polypeptide chains, which may then undergo PTM to form the mature protein product. PTMs are important components in cell signaling, as for example when prohormones are converted to hormones.
https://en.wikipedia.org/wiki/Post-translational_modification
In addition, dolichols can be adducted to proteins as a posttranslational modification, a process in which branched carbohydrate trees are formed on a dolichol moiety and then transferred to an assembly of proteins to form a large glycoprotein in the rough endoplasmic reticulum.
Dolichols are the major lipid component (14% by mass) of human substantia nigra (SN) neuromelanin.[1] Dolichol phosphate was discovered at the University of Liverpool in the 1960s, although researchers did not know its function at the time of discovery.[2]
https://en.wikipedia.org/wiki/Dolichol
Terpenes (/ˈtɜːrpiːn/) are a class of natural products consisting of compounds with the formula (C5H8)n for n ≥ 2. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers.[1][2][3] Terpenes are further classified by the number of carbons: monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), as examples. The terpene alpha-pinene is a major component of the common solvent, turpentine.
https://en.wikipedia.org/wiki/Terpene
Camphene is a bicyclic organic compound. It is one of the most pervasive monoterpenes. As for other terpenes, it is insoluble in water, flammable, colorless, and has a pungent smell.[4] It is a minor constituent of many essential oils such as turpentine, cypress oil, camphor oil, citronella oil, neroli, ginger oil, valerian, and mango.[5] It is produced industrially by isomerization of the more common alpha-pinene using a solid acid catalyst such as titanium dioxide.[6]
Camphene is used in the preparation of fragrances and as a food additive for flavoring. These include isobornyl acetate.
https://en.wikipedia.org/wiki/Camphene
Isobornyl acetate is an organic compound consisting of the acetate ester or the terpenoid isoborneol. It is a colorless liquid with a pleasant pine-like scent, and it is produced on a multi-ton scale for this purpose. The compound is prepared by reaction of camphene with acetic acid in the presence of a strongly acidic catalyst such as sulfuric acid. Hydrolysis of isobornyl acetate gives isoborneol, a precursor to camphor.[1]
Like many plant exudates, isobornyl acetate appears to have antifeedant properties.[2]
https://en.wikipedia.org/wiki/Isobornyl_acetate
Antifeedants are organic compounds produced by plants to inhibit attack by insects and grazing animals. These chemical compounds are typically classified as secondary metabolites in that they are not essential for the metabolism of the plant, but instead confer longevity. Antifeedants exhibit a wide range of activities and chemical structures as biopesticides. Examples include rosin, which inhibits attack on trees, and many alkaloids, which are highly toxic to specific insect species.[2]
History
"Plant-derived insecticides (e.g., rotenone, veratridines, pyrethrins, and nicotine) have been used for insect control since antiquity."[4] The active ingredients in these plants have been purified and modified. For example, variations on pyrethrin has spawned a large number of synthetic insecticides call pyrethroids.https://en.wikipedia.org/wiki/Antifeedant
Rosin (/ˈrɒzɪn/), also called colophony or Greek pitch (Latin: pix graeca), is a solid form of resin obtained from pines and some other plants, mostly conifers, produced by heating fresh liquid resin to vaporize the volatile liquid terpene components. It is semi-transparent and varies in color from yellow to black. At room temperature rosin is brittle, but it melts at stove-top temperature. It chiefly consists of various resin acids, especially abietic acid.[1] The term colophony comes from colophonia resina, Latin for "resin from Colophon" (Ancient Greek: Κολοφωνία ῥητίνη, romanized: Kolophōnía rhētínē),[2][3] an ancient Ionic city.[4]
https://en.wikipedia.org/wiki/Rosin
Glucosinolates are natural components of many pungent plants such as mustard, cabbage, and horseradish. The pungency of those plants is due to mustard oils produced from glucosinolates when the plant material is chewed, cut, or otherwise damaged. These natural chemicals most likely contribute to plant defence against pests and diseases, and impart a characteristic bitter flavor property to cruciferous vegetables.[1]
https://en.wikipedia.org/wiki/Glucosinolate
A pyrethroid is an organic compound similar to the natural pyrethrins, which are produced by the flowers of pyrethrums (Chrysanthemum cinerariaefolium and C. coccineum). Pyrethroids are used as commercial and household insecticides.[1]
In household concentrations pyrethroids are generally harmless to humans.[1] However, pyrethroids are toxic to insects such as bees, dragonflies, mayflies, gadflies, and some other invertebrates, including those that constitute the base of aquatic and terrestrial food webs.[2] Pyrethroids are toxic to aquatic organisms, especially fish.[3] They have been shown to be an effective control measure for malaria outbreaks, through indoor applications.[4]
https://en.wikipedia.org/wiki/Pyrethroid
Isoborneol is a bicyclic organic compound and a terpene derivative. The hydroxyl group in this compound is placed in an exo position. The endo diastereomer is called borneol. Being chiral, isoborneol exists as enantiomers.
https://en.wikipedia.org/wiki/Isoborneol
Acetic acid /əˈsiːtɪk/, systematically named ethanoic acid /ˌɛθəˈnoʊɪk/, is an acidic, colourless liquid and organic compound with the chemical formula CH3COOH (also written as CH3CO2H, C2H4O2, or HC2H3O2). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water and other trace elements.
Acetic acid is the second simplest carboxylic acid (after formic acid). It is an important chemical reagent and industrial chemical, used primarily in the production of cellulose acetate for photographic film, polyvinyl acetate for wood glue, and synthetic fibres and fabrics. In households, diluted acetic acid is often used in descaling agents. In the food industry, acetic acid is controlled by the food additive code E260 as an acidity regulator and as a condiment. In biochemistry, the acetyl group, derived from acetic acid, is fundamental to all forms of life. When bound to coenzyme A, it is central to the metabolism of carbohydrates and fats.
The global demand for acetic acid is about 6.5 million metric tons per year (t/a), of which approximately 1.5 t/a is met by recycling; the remainder is manufactured from methanol.[8] Vinegar is mostly dilute acetic acid, often produced by fermentation and subsequent oxidation of ethanol.
https://en.wikipedia.org/wiki/Acetic_acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, and hydrogen, with the molecular formula H2SO4. It is a colorless, odorless, and viscous liquid that is miscible with water.[6]
Pure sulfuric acid does not occur naturally due to its strong affinity to water vapor; it is hygroscopic and readily absorbs water vapor from the air.[6] Concentrated sulfuric acid is highly corrosive towards other materials, from rocks to metals, since it is an oxidant with powerful dehydrating properties. Phosphorus pentoxide is a notable exception in that it is not dehydrated by sulfuric acid but, to the contrary, dehydrates sulfuric acid to sulfur trioxide. Upon addition of sulfuric acid to water, a considerable amount of heat is released; thus, the reverse procedure of adding water to the acid should not be performed since the heat released may boil the solution, spraying droplets of hot acid during the process. Upon contact with body tissue, sulfuric acid can cause severe acidic chemical burns and even secondary thermal burns due to dehydration.[7][8] Dilute sulfuric acid is substantially less hazardous without the oxidative and dehydrating properties; however, it should still be handled with care for its acidity.
Sulfuric acid is a very important commodity chemical; a country's sulfuric acid production is a good indicator of its industrial strength.[9][non-primary source needed] It is widely produced with different methods, such as contact process, wet sulfuric acid process, lead chamber process, and some other methods.[which?][10] Sulfuric acid is also a key substance in the chemical industry. It is most commonly used in fertilizer manufacture[11] but is also important in mineral processing, oil refining, wastewater processing, and chemical synthesis. It has a wide range of end applications, including in domestic acidic drain cleaners,[12] as an electrolyte in lead-acid batteries, in dehydrating a compound, and in various cleaning agents. Sulfuric acid can be obtained by dissolving sulfur trioxide in water.
https://en.wikipedia.org/wiki/Sulfuric_acid
Phosphorus pentoxide is a chemical compound with molecular formula P4O10 (with its common name derived from its empirical formula, P2O5). This white crystalline solid is the anhydride of phosphoric acid. It is a powerful desiccant and dehydrating agent.
https://en.wikipedia.org/wiki/Phosphorus_pentoxide
In chemistry, a dehydration reaction is a chemical reaction that involves the loss of water from the reacting molecule or ion. Dehydration reactions are common processes, the reverse of a hydration reaction.
https://en.wikipedia.org/wiki/Dehydration_reaction
Gypsum is a soft sulfate mineral composed of calcium sulfate dihydrate, with the chemical formula CaSO4·2H2O.[4] It is widely mined and is used as a fertilizer and as the main constituent in many forms of plaster, blackboard or sidewalk chalk, and drywall.[5][6][7][8] Alabaster, a fine-grained white or lightly tinted variety of gypsum, has been used for sculpture by many cultures including Ancient Egypt, Mesopotamia, Ancient Rome, the Byzantine Empire, and the Nottingham alabasters of Medieval England. Gypsum also crystallizes as translucent crystals of selenite. It forms as an evaporite mineral and as a hydration product of anhydrite.
The Mohs scale of mineral hardness defines gypsum as hardness value 2 based on scratch hardness comparison.
https://en.wikipedia.org/wiki/Gypsum
Adamantane is an organic compound with a formula C10H16 or, more descriptively, (CH)4(CH2)6. Adamantane molecules can be described as the fusion of three cyclohexane rings. The molecule is both rigid and virtually stress-free. Adamantane is the most stable isomer of C10H16. The spatial arrangement of carbon atoms in the adamantane molecule is the same as in the diamond crystal. This similarity led to the name adamantane, which is derived from the Greek adamantinos (relating to steel or diamond).[4] It is a white solid with a camphor-like odor. It is the simplest diamondoid.
The discovery of adamantane in petroleum in 1933 launched a new field of chemistry dedicated to the synthesis and properties of polyhedral organic compounds. Adamantane derivatives have found practical application as drugs, polymeric materials, and thermally stable lubricants.
https://en.wikipedia.org/wiki/Adamantane
Connection of groups and symmetry
Given a structured object X of any sort, a symmetry is a mapping of the object onto itself which preserves the structure. This occurs in many cases, for example
- If X is a set with no additional structure, a symmetry is a bijective map from the set to itself, giving rise to permutation groups.
- If the object X is a set of points in the plane with its metric structure or any other metric space, a symmetry is a bijection of the set to itself which preserves the distance between each pair of points (an isometry). The corresponding group is called isometry group of X.
- If instead angles are preserved, one speaks of conformal maps. Conformal maps give rise to Kleinian groups, for example.
- Symmetries are not restricted to geometrical objects, but include algebraic objects as well. For instance, the equation has the two solutions and . In this case, the group that exchanges the two roots is the Galois group belonging to the equation. Every polynomial equation in one variable has a Galois group, that is a certain permutation group on its roots.
The axioms of a group formalize the essential aspects of symmetry. Symmetries form a group: they are closed because if you take a symmetry of an object, and then apply another symmetry, the result will still be a symmetry. The identity keeping the object fixed is always a symmetry of an object. Existence of inverses is guaranteed by undoing the symmetry and the associativity comes from the fact that symmetries are functions on a space, and composition of functions is associative.
Frucht's theorem says that every group is the symmetry group of some graph. So every abstract group is actually the symmetries of some explicit object.
The saying of "preserving the structure" of an object can be made precise by working in a category. Maps preserving the structure are then the morphisms, and the symmetry group is the automorphism group of the object in question.
Applications of group theory
Applications of group theory abound. Almost all structures in abstract algebra are special cases of groups. Rings, for example, can be viewed as abelian groups (corresponding to addition) together with a second operation (corresponding to multiplication). Therefore, group theoretic arguments underlie large parts of the theory of those entities.
Galois theory
Galois theory uses groups to describe the symmetries of the roots of a polynomial (or more precisely the automorphisms of the algebras generated by these roots). The fundamental theorem of Galois theory provides a link between algebraic field extensions and group theory. It gives an effective criterion for the solvability of polynomial equations in terms of the solvability of the corresponding Galois group. For example, S5, the symmetric group in 5 elements, is not solvable which implies that the general quintic equation cannot be solved by radicals in the way equations of lower degree can. The theory, being one of the historical roots of group theory, is still fruitfully applied to yield new results in areas such as class field theory.
Algebraic topology
Algebraic topology is another domain which prominently associates groups to the objects the theory is interested in. There, groups are used to describe certain invariants of topological spaces. They are called "invariants" because they are defined in such a way that they do not change if the space is subjected to some deformation. For example, the fundamental group "counts" how many paths in the space are essentially different. The Poincaré conjecture, proved in 2002/2003 by Grigori Perelman, is a prominent application of this idea. The influence is not unidirectional, though. For example, algebraic topology makes use of Eilenberg–MacLane spaces which are spaces with prescribed homotopy groups. Similarly algebraic K-theory relies in a way on classifying spaces of groups. Finally, the name of the torsion subgroup of an infinite group shows the legacy of topology in group theory.
Algebraic geometry
Algebraic geometry likewise uses group theory in many ways. Abelian varieties have been introduced above. The presence of the group operation yields additional information which makes these varieties particularly accessible. They also often serve as a test for new conjectures. (For example the Hodge conjecture (in certain cases).) The one-dimensional case, namely elliptic curves is studied in particular detail. They are both theoretically and practically intriguing.[8] In another direction, toric varieties are algebraic varieties acted on by a torus. Toroidal embeddings have recently led to advances in algebraic geometry, in particular resolution of singularities.[9]
Algebraic number theory
Algebraic number theory makes uses of groups for some important applications. For example, Euler's product formula,
captures the fact that any integer decomposes in a unique way into primes. The failure of this statement for more general rings gives rise to class groups and regular primes, which feature in Kummer's treatment of Fermat's Last Theorem.
Harmonic analysis
Analysis on Lie groups and certain other groups is called harmonic analysis. Haar measures, that is, integrals invariant under the translation in a Lie group, are used for pattern recognition and other image processing techniques.[10]
Combinatorics
In combinatorics, the notion of permutation group and the concept of group action are often used to simplify the counting of a set of objects; see in particular Burnside's lemma.
Music
The presence of the 12-periodicity in the circle of fifths yields applications of elementary group theory in musical set theory. Transformational theory models musical transformations as elements of a mathematical group.
Physics
In physics, groups are important because they describe the symmetries which the laws of physics seem to obey. According to Noether's theorem, every continuous symmetry of a physical system corresponds to a conservation law of the system. Physicists are very interested in group representations, especially of Lie groups, since these representations often point the way to the "possible" physical theories. Examples of the use of groups in physics include the Standard Model, gauge theory, the Lorentz group, and the Poincaré group.
Group theory can be used to resolve the incompleteness of the statistical interpretations of mechanics developed by Willard Gibbs, relating to the summing of an infinite number of probabilities to yield a meaningful solution.[11]
Chemistry and materials science
In chemistry and materials science, point groups are used to classify regular polyhedra, and the symmetries of molecules, and space groups to classify crystal structures. The assigned groups can then be used to determine physical properties (such as chemical polarity and chirality), spectroscopic properties (particularly useful for Raman spectroscopy, infrared spectroscopy, circular dichroism spectroscopy, magnetic circular dichroism spectroscopy, UV/Vis spectroscopy, and fluorescence spectroscopy), and to construct molecular orbitals.
Molecular symmetry is responsible for many physical and spectroscopic properties of compounds and provides relevant information about how chemical reactions occur. In order to assign a point group for any given molecule, it is necessary to find the set of symmetry operations present on it. The symmetry operation is an action, such as a rotation around an axis or a reflection through a mirror plane. In other words, it is an operation that moves the molecule such that it is indistinguishable from the original configuration. In group theory, the rotation axes and mirror planes are called "symmetry elements". These elements can be a point, line or plane with respect to which the symmetry operation is carried out. The symmetry operations of a molecule determine the specific point group for this molecule.
In chemistry, there are five important symmetry operations. They are identity operation (E), rotation operation or proper rotation (Cn), reflection operation (σ), inversion (i) and rotation reflection operation or improper rotation (Sn). The identity operation (E) consists of leaving the molecule as it is. This is equivalent to any number of full rotations around any axis. This is a symmetry of all molecules, whereas the symmetry group of a chiral molecule consists of only the identity operation. An identity operation is a characteristic of every molecule even if it has no symmetry. Rotation around an axis (Cn) consists of rotating the molecule around a specific axis by a specific angle. It is rotation through the angle 360°/n, where n is an integer, about a rotation axis. For example, if a water molecule rotates 180° around the axis that passes through the oxygen atom and between the hydrogen atoms, it is in the same configuration as it started. In this case, n = 2, since applying it twice produces the identity operation. In molecules with more than one rotation axis, the Cn axis having the largest value of n is the highest order rotation axis or principal axis. For example in boron trifluoride (BF3), the highest order of rotation axis is C3, so the principal axis of rotation is C3.
In the reflection operation (σ) many molecules have mirror planes, although they may not be obvious. The reflection operation exchanges left and right, as if each point had moved perpendicularly through the plane to a position exactly as far from the plane as when it started. When the plane is perpendicular to the principal axis of rotation, it is called σh (horizontal). Other planes, which contain the principal axis of rotation, are labeled vertical (σv) or dihedral (σd).
Inversion (i ) is a more complex operation. Each point moves through the center of the molecule to a position opposite the original position and as far from the central point as where it started. Many molecules that seem at first glance to have an inversion center do not; for example, methane and other tetrahedral molecules lack inversion symmetry. To see this, hold a methane model with two hydrogen atoms in the vertical plane on the right and two hydrogen atoms in the horizontal plane on the left. Inversion results in two hydrogen atoms in the horizontal plane on the right and two hydrogen atoms in the vertical plane on the left. Inversion is therefore not a symmetry operation of methane, because the orientation of the molecule following the inversion operation differs from the original orientation. And the last operation is improper rotation or rotation reflection operation (Sn) requires rotation of 360°/n, followed by reflection through a plane perpendicular to the axis of rotation.
Cryptography
Very large groups of prime order constructed in elliptic curve cryptography serve for public-key cryptography. Cryptographical methods of this kind benefit from the flexibility of the geometric objects, hence their group structures, together with the complicated structure of these groups, which make the discrete logarithm very hard to calculate. One of the earliest encryption protocols, Caesar's cipher, may also be interpreted as a (very easy) group operation. Most cryptographic schemes use groups in some way. In particular Diffie–Hellman key exchange uses finite cyclic groups. So the term group-based cryptography refers mostly to cryptographic protocols that use infinite nonabelian groups such as a braid group.
See also
https://en.wikipedia.org/wiki/Group_theory
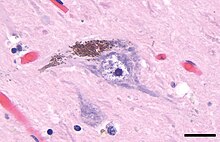
Neuromelanin (NM) is a dark pigment found in the brain which is structurally related to melanin. It is a polymer of 5,6-dihydroxyindole monomers.[1] Neuromelanin is found in large quantities in catecholaminergic cells of the substantia nigra pars compacta and locus coeruleus, giving a dark color to the structures.[2]
Physical properties and structure
Neuromelanin gives specific brain sections, such as the substantia nigra or the locus coeruleus, distinct color. It is a type of melanin and similar to other forms of peripheral melanin. It is insoluble in organic compounds, and can be labeled by silver staining. It is called neuromelanin because of its function and the color change that appears in tissues containing it. It contains black/brown pigmented granules. Neuromelanin is found to accumulate during aging, noticeably after the first 2–3 years of life. It is believed to protect neurons in the substantia nigra from iron-induced oxidative stress. It is considered a true melanin due to its stable free radical structure and it avidly chelates metals.[3]
Synthetic pathways
Neuromelanin is directly biosynthesized from L-DOPA, precursor to dopamine, by tyrosine hydroxylase (TH) and aromatic acid decarboxylase (AADC). Alternatively, synaptic vesicles and endosomes accumulate cytosolic dopamine (via vesicular monoamine transporter 2 (VMAT2) and transport it to mitochondria where it is metabolized by monoamine oxidase. Excess dopamine and DOPA molecules are oxidized by iron catalysis into dopaquinones and semiquinones which are then phagocytosed and are stored as neuromelanin.[4]
Neuromelanin biosynthesis is driven by excess cytosolic catecholamines not accumulated by synaptic vesicles.[5]
Function
Neuromelanin is found in higher concentrations in humans than in other primates.[2] Neuromelanin concentration increases with age, suggesting a role in neuroprotection (neuromelanin can chelate metals and xenobiotics[6]) or senescence.
History
Dark pigments in the substantia nigra were first described in 1838 by Purkyně,[8] and the term neuromelanin was proposed in 1957 by Lillie,[9] though it has been thought to serve no function until recently. It is now believed to play a vital role in preventing cell death in certain parts of the brain. It has been linked to Parkinson's disease and because of this possible connection, neuromelanin has been heavily researched in the last decade.[10]
https://en.wikipedia.org/wiki/Neuromelanin
The pars compacta (SNpc) is a portion of the substantia nigra, located in the midbrain. It is formed by dopaminergic neurons and located medial to the pars reticulata. Parkinson's disease is characterized by the death of dopaminergic neurons in this region.[1]
https://en.wikipedia.org/wiki/Pars_compacta
The pars reticulata (SNpr) is a portion of the substantia nigra and is located lateral to the pars compacta. Most of the neurons that project out of the pars reticulata are inhibitory GABAergic neurons (i.e., these neurons release GABA, which is an inhibitory neurotransmitter).
https://en.wikipedia.org/wiki/Pars_reticulata
Dopaminergic cell groups, DA cell groups, or dopaminergic nuclei are collections of neurons in the central nervous system that synthesize the neurotransmitter dopamine.[1] In the 1960s, dopaminergic neurons or dopamine neurons were first identified and named by Annica Dahlström and Kjell Fuxe, who used histochemical fluorescence.[2] The subsequent discovery of genes encoding enzymes that synthesize dopamine, and transporters that incorporate dopamine into synaptic vesicles or reclaim it after synaptic release, enabled scientists to identify dopaminergic neurons by labeling gene or protein expression that is specific to these neurons.
In the mammalian brain, dopaminergic neurons form a semi-continuous population extending from the midbrain through the forebrain, with eleven named collections or clusters among them.[3][4][5]
https://en.wikipedia.org/wiki/Dopaminergic_cell_groups
https://en.wikipedia.org/wiki/Category:Neurochemistry
https://en.wikipedia.org/wiki/Category:Signal_transduction
https://en.wikipedia.org/wiki/Category:Neurotoxins
https://en.wikipedia.org/wiki/Glucose
https://en.wikipedia.org/wiki/Glycosylation
https://en.wikipedia.org/wiki/Isoprene
https://en.wikipedia.org/wiki/Serotonergic
https://en.wikipedia.org/wiki/Sepiapterin_reductase_deficiency
https://en.wikipedia.org/wiki/Push%E2%80%93pull_perfusion
https://en.wikipedia.org/wiki/Photoactivated_adenylyl_cyclase
https://en.wikipedia.org/wiki/Neurotrophin_mimetics
https://en.wikipedia.org/wiki/Trace_amine
Vanillylmandelic acid (VMA) is a chemical intermediate in the synthesis of artificial vanilla flavorings[1] and is an end-stage metabolite of the catecholamines (dopamine, epinephrine, and norepinephrine). It is produced via intermediary metabolites.
https://en.wikipedia.org/wiki/Vanillylmandelic_acid
https://en.wikipedia.org/wiki/Neurochemical
https://en.wikipedia.org/wiki/Glycinergic
https://en.wikipedia.org/wiki/Catecholaminergic
https://en.wikipedia.org/wiki/Catecholaminergic_cell_groups
https://en.wikipedia.org/wiki/Channelosome
https://en.wikipedia.org/wiki/Clinical_neurochemistry
https://en.wikipedia.org/wiki/Cys-loop_receptor
https://en.wikipedia.org/wiki/Low-affinity_nerve_growth_factor_receptor
https://en.wikipedia.org/wiki/Melatonergic
https://en.wikipedia.org/wiki/Monoamine_nuclei
https://en.wikipedia.org/wiki/Monoaminergic_cell_groups
A natural neuroactive substance (NAS) is a chemical synthesized by neurons that affects the actions of other neurons or muscle cells. Natural neuroactive substances include neurotransmitters, neurohormones, and neuromodulators.[1] Neurotransmitters work only between adjacent neurons through synapses. Neurohormones are released into the blood and work at a distance. Some natural neuroactive substances act as both transmitters and as hormones.
https://en.wikipedia.org/wiki/Natural_neuroactive_substance
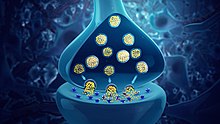
A neurotransmitter is a signaling molecule secreted by a neuron to affect another cell across a synapse. The cell receiving the signal, or target cell, may be another neuron, but could also be a gland or muscle cell.[1]
https://en.wikipedia.org/wiki/Neurotransmitter
Neurotransmitters are released from synaptic vesicles into the synaptic cleft where they are able to interact with neurotransmitter receptors on the target cell. The neurotransmitter's effect on the target cell is determined by the receptor it binds to. Many neurotransmitters are synthesized from simple and plentiful precursors such as amino acids, which are readily available and often require a small number of biosynthetic steps for conversion.
Neurotransmitters are essential to the function of complex neural systems. The exact number of unique neurotransmitters in humans is unknown, but more than 100 have been identified.[2] Common neurotransmitters include glutamate, GABA, acetylcholine, glycine and norepinephrine.
https://en.wikipedia.org/wiki/Neurotransmitter
Synthesis
Neurotransmitters are generally synthesized in neurons and are made up of, or derived from, precursor molecules that are found abundantly in the cell. Classes of neurotransmitters include amino acids, monoamines, and peptides. Monoamines are synthesized by altering a single amino acid. For example, the precursor of serotonin is the amino acid tryptophan. Peptide transmitters, or neuropeptides, are protein transmitters that often are released together with other transmitters to have a modulatory effect.[3] Purine neurotransmitters, like ATP, are derived from nucleic acids. Other neurotransmitters are made up of metabolic products like nitric oxide and carbon monoxide.[citation needed]
|
|
Examples |
|---|---|
| Amino Acid | glycine, glutamate |
| Monoamines | serotonin, epinephrine, dopamine |
| Peptides | substance P, opioids |
| Purines | ATP, GTP |
| Other | nitric oxide, carbon monoxide |







No comments:
Post a Comment