A nucleotide is an organic molecule consisting of a nitrogenous heterocyclic nucleobase (a purine or a pyrimidine), a pentose sugar (deoxyribose in DNA or ribose in RNA), and a phosphate or polyphosphate group. As a short-hand, the nucleotides that form the rungs of the ladder in the DNA double-helix are often referred to by biologists, as their base pair identity such as adenine (A), see Category:Purines and Category:Pyrimidines.
For more information, see Nucleotide.
https://en.wikipedia.org/wiki/Category:Nucleotides
https://en.wikipedia.org/wiki/Category:Organophosphates
Alkyl phosphates belong to a group of organic compounds called organophosphates. They are esters of phosphoric acid H3PO4 and corresponding alcohol. For example, the formula of methyl phosphate is CH3-H2PO4, dimethyl phosphate – (CH3)2HPO4 and trimethyl phosphate – (CH3)3PO4. Alkyl phosphates are widely distributed in nature, and form the basis of most biological processes. For example, high energy metabolites such as ATP and PEP are alkyl phosphates, as are nucleic acids such as DNA and RNA. Alkyl phosphates are also important medicinally, for example the HIV drug AZT is inactive until it becomes an alkyl phosphate in vivo.
https://en.wikipedia.org/wiki/Alkyl_phosphate
Alkylphosphocholines are phospholipid-like molecules that have been synthesised, which have remarkable biological and therapeutic activities.[1][2] They are phosphocholine esters of aliphatic long chain alcohols differing in chain length, unsaturation and position of the cis-double bond.[3]
The utilization of alkylphosphocholine analogues has been found be to useful for the treatment of specific types of cancer, such as gliomas and brain metastases.[4] These analogues function through inhibiting signal transduction pathways of mitosis and triggering apoptosis of cancer cells.[5] Alkylphosphocholine analogues are seen to be more of an efficient treatment for cancer than other drugs as they avoid causing DNA damage and myelotoxicity.[5]
https://en.wikipedia.org/wiki/Alkylphosphocholine
1-Arseno-3-phosphoglycerate is a compound produced by the enzyme glyceraldehyde 3-phosphate dehydrogenase, present in high concentrations in many organisms, from glyceraldehyde 3-phosphate and arsenate in the glycolysis pathway.[1] The compound is unstable and hydrolyzes spontaneously to 3-phosphoglycerate, bypassing the energy producing step of glycolysis.
https://en.wikipedia.org/wiki/1-Arseno-3-phosphoglycerate
Proliferative vitreoretinopathy (PVR) is a disease that develops as a complication of rhegmatogenous retinal detachment. PVR occurs in about 8–10% of patients undergoing primary retinal detachment surgery and prevents the successful surgical repair of rhegmatogenous retinal detachment. PVR can be treated with surgery to reattach the detached retina but the visual outcome of the surgery is very poor.[1][2] A number of studies have explored various possible adjunctive agents for the prevention and treatment of PVR, such as methotrexate, although none have yet been licensed for clinical use.[3]
PVR was originally referred to as massive vitreous retraction and then as massive periretinal proliferation. The name Proliferative vitreo retinopathy was provided in 1989 by the Silicone Oil Study group. The name is derived from proliferation (by the retinal pigment epithelial and glial cells) and vitreo retinopathy to include the tissues which are affected, namely the vitreous humor (or simply vitreous) and the retina.[4]
https://en.wikipedia.org/wiki/Proliferative_vitreoretinopathy
Aphakia is the absence of the lens of the eye, due to surgical removal, such as in cataract surgery, a perforating wound or ulcer, or congenital anomaly. It causes a loss of ability to maintain focus (accommodation), high degree of farsightedness (hyperopia),[1] and a deep anterior chamber. Complications include detachment of the vitreous or retina, and glaucoma.
Babies are rarely born with aphakia. Occurrence most often results from surgery to remove congenital cataract. Congenital cataracts usually develop as a result of infection of the fetus or genetic reasons. It is often difficult to identify the exact cause of these cataracts, especially if only one eye is affected.
People with aphakia have relatively small pupils and their pupils dilate to a lesser degree.[2]
https://en.wikipedia.org/wiki/Aphakia
Complications
Main complications of surgical aphakia include: Spectacle intolerance: Due to image magnification (up to 30%), optical aberration, prismatic effect and roving ring scotoma, spectacles are not well tolerated by aphakic patients.[8] Due to unequal refractive power between the eyes, wearing spectacles with single-eye aphakia may cause double vision.[8]
Glaucoma: Secondary angle closure glaucoma may occur due to vitreous prolapse.[9]
Retinal detachment[10]
Aphakic bullous keratopathy[8]
Etymology
Gr. a- alfa priv + phakos, lens, anything shaped like a lens[11]
https://en.wikipedia.org/wiki/Aphakia
Ptosis, also known as blepharoptosis,[1] is a drooping or falling of the upper eyelid. This condition is sometimes called "lazy eye," but that term normally refers to the condition amblyopia. If severe enough and left untreated, the drooping eyelid can cause other conditions, such as amblyopia or astigmatism, so it is especially important to treat the disorder in children before it can interfere with vision development.
The term is from Greek πτῶσις 'fall, falling'.
https://en.wikipedia.org/wiki/Ptosis_(eyelid)
Orbital lymphoma is a common type of non-Hodgkin lymphoma that occurs near or on the eye. Common symptoms include decreased vision and uveitis. Orbital lymphoma can be diagnosed via a biopsy of the eye and is usually treated with radiotherapy or in combination with chemotherapy.
| Orbital lymphoma | |
|---|---|
 | |
| Specialty | Oncology |
Signs and symptoms
Primary visible signs of ocular lymphoma include proptosis and a visible mass in the eye. Symptoms are due to mass effect.
Pathophysiology
Recent studies[by whom?] have detected the presence of viral DNA in ocular lymphoma cells. This implies that pathogens play a role in ocular lymphoma. Other studies have found that the aging population, the increasing number of immunosuppressive drugs, and the AIDS epidemic have also contributed to the increased incidence of Non-Hodgkin lymphomas.
Ocular MALT lymphomas may also be associated with Chlamydia psittaci,[6][7] although whether or not this is the case is still debated.[6]
Follicular lymphoma, diffuse large B cell lymphoma, mantle cell lymphoma, B-cell chronic lymphocytic leukemia, peripheral T-cell lymphoma, and natural killer cell lymphoma have also been reported to affect the orbit.[citation needed]
https://en.wikipedia.org/wiki/Orbital_lymphoma
Optic disc drusen (ODD) are globules of mucoproteins and mucopolysaccharides that progressively calcify in the optic disc.[1][2] They are thought to be the remnants of the axonal transport system of degenerated retinal ganglion cells.[3][4][5] ODD have also been referred to as congenitally elevated or anomalous discs, pseudopapilledema, pseudoneuritis, buried disc drusen, and disc hyaline bodies.[6]
https://en.wikipedia.org/wiki/Optic_disc_drusen
A hyaline substance is one with a glassy appearance. The word is derived from Greek: ὑάλινος, romanized: hyálinos, lit. 'transparent', and ὕαλος, hýalos, 'crystal, glass'.[1][2]
https://en.wikipedia.org/wiki/Hyaline
Anatomy
The optic nerve is a cable connection that transmits images from the retina to the brain. It consists of over one million retinal ganglion cell axons. The optic nerve head, or optic disc is the anterior end of the nerve that is in the eye and hence is visible with an ophthalmoscope. It is located nasally and slightly inferior to the macula of the eye. There is a blind spot at the optic disc because there are no rods or cones beneath it to detect light. The central retinal artery and vein can be seen in the middle of the disc as it exits the scleral canal with the optic nerve to supply the retina. The vessels send branches out in all directions to supply the retina.
https://en.wikipedia.org/wiki/Optic_disc_drusen
The sclera,[note 1] also known as the white of the eye or, in older literature, as the tunica albuginea oculi, is the opaque, fibrous, protective, outer layer of the human eye containing mainly collagen and some crucial elastic fiber.[2] In humans, and some other vertebrates, the whole sclera is white, contrasting with the coloured iris, but in most mammals, the visible part of the sclera matches the colour of the iris, so the white part does not normally show while other vertebrates have distinct colors for both of them. In the development of the embryo, the sclera is derived from the neural crest.[3] In children, it is thinner and shows some of the underlying pigment, appearing slightly blue. In the elderly, fatty deposits on the sclera can make it appear slightly yellow. People with dark skin can have naturally darkened sclerae, the result of melanin pigmentation.[4]
The human eye is relatively rare for having a pale sclera (relative to the iris). This makes it easier for one individual to identify where another individual is looking, and the cooperative eye hypothesis suggests this has evolved as a method of nonverbal communication.
https://en.wikipedia.org/wiki/Sclera
Structure
The sclera forms the posterior five-sixths of the connective tissue coat of the globe. It is continuous with the dura mater and the cornea, and maintains the shape of the globe, offering resistance to internal and external forces, and provides an attachment for the extraocular muscle insertions. The sclera is perforated by many nerves and vessels passing through the posterior scleral foramen, the hole that is formed by the optic nerve. At the optic disc the outer two-thirds of the sclera continues with the dura mater (outer coat of the brain) via the dural sheath of the optic nerve. The inner third joins with some choroidal tissue to form a plate (lamina cribrosa) across the optic nerve with perforations through which the optic fibers (fasciculi) pass. The thickness of the sclera varies from 1 mm at the posterior pole to 0.3 mm just behind the insertions of the four rectus muscles. The sclera's blood vessels are mainly on the surface. Along with the vessels of the conjunctiva (which is a thin layer covering the sclera), those in the episclera render the inflamed eye bright red.[5]
In many vertebrates, the sclera is reinforced with plates of cartilage or bone, together forming a circular structure called the sclerotic ring. In primitive fish, this ring consists of four plates, but the number is lower in many living ray-finned fishes, and much higher in lobe-finned fishes, various reptiles, and birds. The ring has disappeared in many groups, including living amphibians, some reptiles and fish, and all mammals.[6]
The eyes of all non-human primates had been thought to be dark with small, barely visible sclera, but recent research has suggested that white sclera are not uncommon in chimpanzees, and are also present in other mammals. [7]
https://en.wikipedia.org/wiki/Sclera
Glycosaminoglycans[1] (GAGs) or mucopolysaccharides[2] are long, linear polysaccharides consisting of repeating disaccharide units (i.e. two-sugar units). The repeating two-sugar unit consists of a uronic sugar and an amino sugar, except in the case of the sulfated glycosaminoglycan keratan, where, in place of the uronic sugar there is a galactose unit.[3] GAGs are found in vertebrates, invertebrates and bacteria.[4] Because GAGs are highly polar molecules and attract water; the body uses them as lubricants or shock absorbers.
Mucopolysaccharidoses are a group of metabolic disorders in which abnormal accumulations of glycosaminoglycans occur due to enzyme deficiencies.
https://en.wikipedia.org/wiki/Glycosaminoglycan
Uronic acids (/ʊˈrɒnɪk/) or alduronic acids are a class of sugar acids with both carbonyl and carboxylic acid functional groups. They are sugars in which the hydroxyl group furthest from the carbonyl group has been oxidized to a carboxylic acid. Usually the sugar is an aldose, but fructuronic acid also occurs. Oxidation of the terminal aldehyde instead yields an aldonic acid, while oxidation of both the terminal hydroxyl group and the aldehyde yields an aldaric acid. The names of uronic acids are generally based on their parent sugars, for example, the uronic acid analog of glucose is glucuronic acid. Uronic acids derived from hexoses are known as hexuronic acids and uronic acids derived from pentoses are known as penturonic acids.[1]
https://en.wikipedia.org/wiki/Uronic_acid
l-Iduronic acid (IUPAC abbr.: IdoA) is the major uronic acid component of the glycosaminoglycans (GAGs) dermatan sulfate, and heparin. It is also present in heparan sulfate, although here in a minor amount relative to its carbon-5 epimer glucuronic acid.
IdoA is a hexapyranose sugar. Most hexapyranoses are stable in one of two chair conformations 1C4 or 4C1. l-iduronate is different and adopts more than one solution conformation, with an equilibrium existing between three low-energy conformers. These are the 1C4 and 4C1 chair forms and an additional 2S0 skew-boat conformation.
IdoA may be modified by the addition of an O-sulfate group at carbon position 2 to form 2-O-sulfo-l-iduronic acid (IdoA2S).
In 2000, LK Hallak described the importance of this sugar in respiratory syncytial virus (RSV) infection. Dermatan sulfate and heparan sulfate were the only GAGs containing IdoA, and they were the only ones that inhibited RSV infection in cell culture.[1]
When internally positioned within an oligosaccharide, the 1C4 and 2S0 conformations (shown below for IdoA2S) predominate.
Proton NMR spectroscopy can be used to track changes in the balance of this equilibrium.[2]
https://en.wikipedia.org/wiki/Iduronic_acid
Heparan sulfate (HS) is a linear polysaccharide found in all animal tissues.[1] It occurs as a proteoglycan (HSPG, i.e. Heparan Sulfate ProteoGlycan) in which two or three HS chains are attached in close proximity to cell surface or extracellular matrix proteins.[2][3] It is in this form that HS binds to a variety of protein ligands, including Wnt,[4][5] and regulates a wide range of biological activities, including developmental processes, angiogenesis, blood coagulation, abolishing detachment activity by GrB (Granzyme B),[6] and tumour metastasis. HS has also been shown to serve as cellular receptor for a number of viruses, including the respiratory syncytial virus.[7] One study suggests that cellular heparan sulfate has a role in SARS-CoV-2 Infection, particularly when the virus attaches with ACE2.[8]
https://en.wikipedia.org/wiki/Heparan_sulfate
Metastasis is a pathogenic agent's spread from an initial or primary site to a different or secondary site within the host's body;[1] the term is typically used when referring to metastasis by a cancerous tumor.[2] The newly pathological sites, then, are metastases (mets).[3][4] It is generally distinguished from cancer invasion, which is the direct extension and penetration by cancer cells into neighboring tissues.[5]
Cancer occurs after cells are genetically altered to proliferate rapidly and indefinitely. This uncontrolled proliferation by mitosis produces a primary heterogeneic tumour. The cells which constitute the tumor eventually undergo metaplasia, followed by dysplasia then anaplasia, resulting in a malignant phenotype. This malignancy allows for invasion into the circulation, followed by invasion to a second site for tumorigenesis.
Some cancer cells known as circulating tumor cells acquire the ability to penetrate the walls of lymphatic or blood vessels, after which they are able to circulate through the bloodstream to other sites and tissues in the body.[6] This process is known (respectively) as lymphatic or hematogenous spread. After the tumor cells come to rest at another site, they re-penetrate the vessel or walls and continue to multiply, eventually forming another clinically detectable tumor.[citation needed] This new tumor is known as a metastatic (or secondary) tumor. Metastasis is one of the hallmarks of cancer, distinguishing it from benign tumors.[7] Most cancers can metastasize, although in varying degrees. Basal cell carcinoma for example rarely metastasizes.[7]
When tumor cells metastasize, the new tumor is called a secondary or metastatic tumor, and its cells are similar to those in the original or primary tumor.[8] This means that if breast cancer metastasizes to the lungs, the secondary tumor is made up of abnormal breast cells, not of abnormal lung cells. The tumor in the lung is then called metastatic breast cancer, not lung cancer. Metastasis is a key element in cancer staging systems such as the TNM staging system, where it represents the "M". In overall stage grouping, metastasis places a cancer in Stage IV. The possibilities of curative treatment are greatly reduced, or often entirely removed when a cancer has metastasized.
| Metastasis | |
|---|---|
| Other names | metastatic disease |
 | |
| Illustration showing hematogenous metastasis | |
| Pronunciation |
|
| Specialty | Oncology |
https://en.wikipedia.org/wiki/Metastasis
Basal-cell carcinoma (BCC), also known as basal-cell cancer, is the most common type of skin cancer.[2] It often appears as a painless raised area of skin, which may be shiny with small blood vessels running over it.[1] It may also present as a raised area with ulceration.[1] Basal-cell cancer grows slowly and can damage the tissue around it, but it is unlikely to spread to distant areas or result in death.[7]
Risk factors include exposure to ultraviolet light, having lighter skin, radiation therapy, long-term exposure to arsenic and poor immune-system function.[2] Exposure to UV light during childhood is particularly harmful.[5] Tanning beds have become another common source of ultraviolet radiation.[8] Diagnosis often depends on skin examination, confirmed by tissue biopsy.[2][3]
It remains unclear whether sunscreen affects the risk of basal-cell cancer.[9] Treatment is typically by surgical removal.[2] This can be by simple excision if the cancer is small; otherwise, Mohs surgery is generally recommended.[2] Other options include electrodesiccation and curettage, cryosurgery, topical chemotherapy, photodynamic therapy, laser surgery or the use of imiquimod, a topical immune-activating medication.[10] In the rare cases in which distant spread has occurred, chemotherapy or targeted therapy may be used.[10]
Basal-cell cancer accounts for at least 32% of all cancers globally.[7][11] Of skin cancers other than melanoma, about 80% are basal-cell cancers.[2] In the United States, about 35% of white males and 25% of white females are affected by BCC at some point in their lives.[2]
Basal-cell carcinoma is named after the basal cells that form the lowest layer of the epidermis. It is thought to develop from the folliculo–sebaceous–apocrine germinative cells called trichoblasts (of note, trichoblastic carcinoma is a term sometimes used to refer to a rare type of aggressive skin cancer that may resemble a benign trichoblastoma, and can also closely resemble basal cell carcinoma).
https://en.wikipedia.org/wiki/Basal-cell_carcinoma
Cryosurgery is the use of extreme cold in surgery to destroy abnormal or diseased tissue;[1] thus, it is the surgical application of cryoablation. The term comes from the Greek words cryo (κρύο) ("icy cold") and surgery (cheirourgiki – χειρουργική) meaning "hand work" or "handiwork". Cryosurgery has been historically used to treat a number of diseases and disorders, especially a variety of benign and malignant skin conditions.[2][3]
https://en.wikipedia.org/wiki/Cryosurgery
Glyceraldehyde 3-phosphate, also known as triose phosphate or 3-phosphoglyceraldehyde and abbreviated as G3P, GA3P, GADP, GAP, TP, GALP or PGAL, is a metabolite that occurs as an intermediate in several central pathways of all organisms.[2][3] With the chemical formula H(O)CCH(OH)CH2OPO32-, this anion is a monophosphate ester of glyceraldehyde.
https://en.wikipedia.org/wiki/Glyceraldehyde_3-phosphate
A phosphoric acid, in the general sense, is a phosphorus oxoacid in which each phosphorus (P) atom is in the oxidation state +5, and is bonded to four oxygen (O) atoms, one of them through a double bond, arranged as the corners of a tetrahedron. Two or more of these PO
4
tetrahedra may be connected by shared single-bonded oxygens, forming
linear or branched chains, cycles, or more complex structures. The
single-bonded oxygen atoms that are not shared are completed with acidic hydrogen atoms. The general formula of a phosphoric acid is H
n+2−2xP
nO
3n+1−x, where n is the number of phosphorus atoms and x is the number of fundamental cycles in the molecule's structure, between 0 and (n+2)/2.
Removal of protons (H+
) from k hydroxyl groups –OH leaves anions generically called phosphates (if k = n−2x+2) or hydrogen phosphates (if k is between 1 and n−2x+1), with general formula [Hn−2x+2−kPnO3n+1−x]k−. The fully dissociated anion (k = n−2x+2) has formula [PnO3n−x+1](n−2x+2)− . The term phosphate is also used in organic chemistry for the functional groups that result when or more of the hydrogens are replaced by bonds to other groups.
These acids, together with their salts and esters, include some of the best-known compounds of phosphorus, of high importance in biochemistry, mineralogy, agriculture, pharmacy, chemical industry, and chemical research.
https://en.wikipedia.org/wiki/Phosphoric_acids_and_phosphates
https://en.wikipedia.org/wiki/Phosphorous
Glyceraldehyde (glyceral) is a triose monosaccharide with chemical formula C3H6O3. It is the simplest of all common aldoses. It is a sweet, colorless, crystalline solid that is an intermediate compound in carbohydrate metabolism. The word comes from combining glycerol and aldehyde, as glyceraldehyde is glycerol with one alcohol group oxidized to an aldehyde.
https://en.wikipedia.org/wiki/Glyceraldehyde
Glyceraldehyde can be prepared, along with dihydroxyacetone, by the mild oxidation of glycerol, for example with hydrogen peroxide[4] and a ferrous salt as catalyst.[citation needed]
https://en.wikipedia.org/wiki/Glyceraldehyde
Dihydroxyacetone (/ˌdaɪhaɪˌdrɒksiˈæsɪtoʊn/ (![]() listen); DHA), also known as glycerone, is a simple saccharide (a triose) with formula C
listen); DHA), also known as glycerone, is a simple saccharide (a triose) with formula C
3H
6O
3.
DHA is primarily used as an ingredient in sunless tanning products. It is often derived from plant sources such as sugar beets and sugar cane, and by the fermentation of glycerin.
https://en.wikipedia.org/wiki/Dihydroxyacetone
A ketose is a monosaccharide containing one ketone group per molecule.[1][2] The simplest ketose is dihydroxyacetone, which has only three carbon atoms. It is the only ketose with no optical activity. All monosaccharide ketoses are reducing sugars, because they can tautomerize into aldoses via an enediol intermediate, and the resulting aldehyde group can be oxidised, for example in the Tollens' test or Benedict's test.[3] Ketoses that are bound into glycosides, for example in the case of the fructose moiety of sucrose, are nonreducing sugars.[3]
https://en.wikipedia.org/wiki/Ketose
D-Erythrulose (also known as erythrulose) is a tetrose carbohydrate with the chemical formula C4H8O4.[1][2] It has one ketone group and so is part of the ketose family. It is used in some self-tanning cosmetics, in general, combined with dihydroxyacetone (DHA).[3]
Erythrulose/DHA reacts with the amino acids in the proteins of the first layers of skin (the stratum corneum and epidermis). One of the pathways involves free radicals at one of the steps of the Maillard reaction,[4][5] distantly related to the browning effect when a cut apple slice is exposed to oxygen. The other pathway is the conventional Maillard reaction; both pathways are involved in the browning during food preparation and storage. This is not a stain or dye, but rather a chemical reaction that produces a color change on all treated skin. It does not involve the underlying skin pigmentation nor does it require exposure to ultraviolet light to initiate the color change. However, the 'tan' produced by erythrulose/DHA only has an SPF of up to 3,[6][7] and enhances the free radical injury from UV (compared to untreated skin) for the 24 hours after self-tanner is applied, according to a 2007 study led by Katinka Jung of the Gematria Test Lab in Berlin.[8] Forty minutes after the researchers treated skin samples with high levels of erythrulose, they found that more than 140 percent additional free radicals formed during sun exposure compared with untreated skin.[citation needed]
DHA produced similar results, but faster; however erythrulose takes longer to develop its full effect, therefore it lasts longer. For a day after self-tanner application, excessive sun exposure should be avoided and sunscreen should be worn outdoors, they say[who?]; an antioxidant cream could also minimize free radical production. Although some self-tanners contain sunscreen, its effect will not last as long as the tan. During UV irradiation free radicals, mainly superoxide/hydroperoxyl (O2•−/HO2•), and other reactive species (ROS/RNS) are produced, that can react with the ketoamines (Amadori products) and other intermediates of the Maillard reaction. This leads to autoxidationradical chain reactions of the ketoamines, which cause a dramatic increase in the radical injury of the skin. This can be suppressed by antioxidants, which shows involvement of reactive oxygen species (ROS).[9] The ketoamines were shown to cause DNA strand breaks and to act as mutagens.[10]
The free radicals are due to the action of UV light on AGE (advanced glycation end-products) as a result of the reaction of DHA with the skin, and the intermediates, such as Amadori products (a type of AGE), that lead to them. AGEs absorb UV light, but do not have melanin's extended electronic structure that dissipates the energy, so part of it goes towards starting free radical chain reactions instead, in which other AGEs participate readily. AGEs are behind the damage to the skin that occurs with high blood sugar in diabetes where similar glycation occurs.[11][12][13][14]
Erythrulose is a clear to pale-yellowish liquid, which naturally occurs in red raspberries. According to one method, it is made through aerobic fermentation by the bacterium Gluconobacter, followed by extensive multi-step purification.[citation needed]
Erythrulose and dihydroxyacetone (DHA) are very similar in composition, and both react much the same way on the skin surface. Erythrulose produces a lighter and slower-developing tan, taking 24 to 48 hours to complete development. When used alone, it fades faster than a DHA-based sunless tan. Some people feel the final tone of erythrulose is slightly redder, and less bronze, than the DHA-based tan. It may be[weasel words] less drying to the skin surface, helping provide a smoother fading tint. When combined with DHA, the resulting sunless tan is said[by whom?] to last longer,[citation needed] fade better,[citation needed] and provide a more cosmetically pleasing[citation needed] color tone. In sunless tanning products, it is incorporated at 1% to 3% levels.[citation needed]
Because the skin continually exfoliates itself, losing thousands of dead surface skin cells each day, the tan hue is temporary. The tan appearance lasts from two to 10 days, depending on application type and skin condition.
Not all users develop a tan coloration from erythrulose; some may find their fading is more uneven and blotchy when this ingredient is used. Because of the added cost associated with this ingredient, some manufacturers feel it is an inefficient additive to the sunless tanning product line.
Individuals sensitive to DHA may be[weasel words] able to use erythrulose as a skin-safe[citation needed] self-tanning replacement. Erythrulose is more expensive, and difficult[citation needed] to obtain.
Erythrulose is not currently approved by the Food and Drug Administration (FDA) as a self-tanning agent.
https://en.wikipedia.org/wiki/Erythrulose
Xylulose is a ketopentose, a monosaccharide containing five carbon atoms, and including a ketone functional group. It has the chemical formula C5H10O5. In nature, it occurs in both the L- and D-enantiomers.[3] 1-Deoxyxylulose is a precursor to terpenes via the DOXP pathway.[4]
https://en.wikipedia.org/wiki/Xylulose
In chemistry, a hexose is a monosaccharide (simple sugar) with six carbon atoms.[1][2] The chemical formula for all hexoses is C6H12O6, and their molecular weight is 180.156 g/mol.[3]
Hexoses exist in two forms, open-chain or cyclic, that easily convert into each other in aqueous solutions.[4] The open-chain form of a hexose, which usually is favored in solutions, has the general structure H–(CHOH)n−1–C(=O)–(CHOH)4−n–H, where n is 1, 2, or 3. Namely, five of the carbons have one hydroxyl functional group (–OH) each, connected by a single bond, and one has an oxo group (=O), forming a carbonyl group (C=O). The remaining bonds of the carbon atoms are satisfied by seven hydrogen atoms. The carbons are commonly numbered 1 to 6 starting at the end closest to the carbonyl.
Hexoses are extremely important in biochemistry, both as isolated molecules (such as glucose and fructose) and as building blocks of other compounds such as starch, cellulose, and glycosides. Hexoses can form dihexose (like sucrose) by a condensation reaction that makes 1,6-glycosidic bond.
When the carbonyl is in position 1, forming an formyl group (–CH=O), the sugar is called an aldohexose, a special case of aldose. Otherwise, if the carbonyl position is 2 or 3, the sugar is a derivative of a ketone, and is called a ketohexose, a special case of ketose; specifically, an n-ketohexose.[1][2] However, the 3-ketohexoses have not been observed in nature, and are difficult to synthesize;[5] so the term "ketohexose" usually means 2-ketohexose.
In the linear form, there are 16 aldohexoses and eight 2-ketohexoses, stereoisomers that differ in the spatial position of the hydroxyl groups. These species occur in pairs of optical isomers. Each pair has a conventional name (like "glucose" or "fructose"), and the two members are labeled "D-" or "L-", depending on whether the hydroxyl in position 5, in the Fischer projection of the molecule, is to the right or to the left of the axis, respectively. These labels are independent of the optical activity of the isomers. In general, only one of the two enantiomers occurs naturally (for example, D-glucose) and can be metabolized by animals or fermented by yeasts.
The term "hexose" sometimes is assumed to include deoxyhexoses, such as fucose and rhamnose: compounds with general formula C
6H
12O
6-y that can be described as derived from hexoses by replacement of one or more hydroxyl groups with hydrogen atoms.
https://en.wikipedia.org/wiki/Hexose
Cyanopsia is a medical term for seeing everything tinted with blue. It is also referred to as blue vision. Cyanopsia often occurs for a few days, weeks, or months after removal of a cataract from the eye. Cyanopsia also sometimes occurs as a side effect of taking sildenafil, tadalafil, or vardenafil.[1]
Cyanopsia is a medical symptom and not a sign. It is a purely subjective state and can be caused by a physical or functional abnormality of the eye, a physical or functional abnormality of the brain, or be purely psychological. Cyanopsia, if unaccompanied by any other sign or symptom, is not an indication of any disease or disorder. Unless it causes an impairment or significant distress, it is not in and of itself diagnostically relevant.
https://en.wikipedia.org/wiki/Cyanopsia
Adenosine triphosphate (ATP) is an organic compound that provides energy to drive and support many processes in living cells, such as muscle contraction, nerve impulse propagation, condensate dissolution, and chemical synthesis. Found in all known forms of life, ATP is often referred to as the "molecular unit of currency" of intracellular energy transfer.[2] When consumed in metabolic processes, it converts either to adenosine diphosphate (ADP) or to adenosine monophosphate (AMP). Other processes regenerate ATP. The human body recycles its own body weight equivalent in ATP each day.[3] It is also a precursor to DNA and RNA, and is used as a coenzyme.
From the perspective of biochemistry, ATP is classified as a nucleoside triphosphate, which indicates that it consists of three components: a nitrogenous base (adenine), the sugar ribose, and the triphosphate. The surnumerical ATP is becoming a reality in the present medical science and in the future medical science. Stéphane Schmutz Professor Oncologue ref: the dossier Pelican NASA CRETA ERC
https://en.wikipedia.org/wiki/Adenosine_triphosphate
A polyphosphate is a salt or ester of polymeric oxyanions formed from tetrahedral PO4 (phosphate) structural units linked together by sharing oxygen atoms. Polyphosphates can adopt linear or a cyclic ring structures. In biology, the polyphosphate esters ADP and ATP are involved in energy storage. A variety of polyphosphates find application in mineral sequestration in municipal waters, generally being present at 1 to 5 ppm.[1] GTP, CTP, and UTP are also nucleotides important in the protein synthesis, lipid synthesis, and carbohydrate metabolism, respectively. Polyphosphates are also used as food additives, marked E452.
https://en.wikipedia.org/wiki/Polyphosphate
Sharing of three corners is possible. This motif represents crosslinking of the linear polymer. Crosslinked polyphosphates adopt the sheet-structure Phyllosilicates, but such structures occur only under extreme conditions.
https://en.wikipedia.org/wiki/Polyphosphate
Formation and synthesis
Polyphosphates arise by polymerization of phosphoric acid derivatives. The process begins with two phosphate units coming together in a condensation reaction.
- 2 H(PO4)2− ⇌ (P2O7)4− + H2O
The condensation is shown as an equilibrium because the reverse reaction, hydrolysis, is also possible. The process may continue in steps; at each step another (PO3)− unit is added to the chain, as indicated by the part in brackets in the illustration of polyphosphoric acid. P4O10 can be seen as the end product of condensation reactions, where each tetrahedron shares three corners with the others. Conversely, a complex mix of polymers is produced when a small amount of water is added to phosphorus pentoxide.
Acid-base and complexation properties
Polyphosphates are weak bases. A lone pair of electrons on an oxygen atom can be donated to a hydrogen ion (proton) or a metal ion in a typical Lewis acid-Lewis base interaction. This has profound significance in biology. For instance, adenosine triphosphate is about 25% protonated in aqueous solution at pH 7.[2]
- ATP4− + H+ ⇌ ATPH3−, pKa 6.6
Further protonation occurs at lower pH values.
https://en.wikipedia.org/wiki/Polyphosphate
The "high energy" phosphate bond
ATP forms chelate complexes with metal ions. The stability constant for the equilibrium
- ATP4− + Mg2+ ⇌ MgATP2−, log β 4
is particularly large.[3] The formation of the magnesium complex is a critical element in the process of ATP hydrolysis, as it weakens the link between the terminal phosphate group and the rest of the molecule.[2][4]
The energy released in ATP hydrolysis,
- ATP4− + H2O → ADP3− + Pi−
at ΔG -36.8 kJ mol−1 is large by biological standards. Pi stands for inorganic phosphate, which is protonated at biological pH. However, it is not large by inorganic standards. The term "high energy" refers to the fact that it is high relative to the amount of energy released in the organic chemical reactions that can occur in living systems.
https://en.wikipedia.org/wiki/Polyphosphate
High-polymeric inorganic polyphosphates
High molecular weight polyphosphates are well known.[5] One derivative is the glassy (i.e., amorphous) Graham's salt. Crystalline high molecular weight polyphosphates include Kurrol’s salt and Maddrell’s salt. These species have the formula [NaPO3]n[NaPO3(OH)]2 where n can be as great as 2000. In terms of their structures, these polymers consist of PO3− "monomers", with the chains are terminated by protonated phosphates.[6]
In nature
High-polymeric inorganic polyphosphates were found in living organisms by L. Liberman in 1890. These compounds are linear polymers containing a few to several hundred residues of orthophosphate linked by energy-rich phosphoanhydride bonds.
Previously, it was considered either as “molecular fossil” or as only a phosphorus and energy source providing the survival of microorganisms under extreme conditions. These compounds are now known to also have regulatory roles, and to occur in representatives of all kingdoms of living organisms, participating in metabolic correction and control on both genetic and enzymatic levels. Polyphosphate is directly involved in the switching-over of the genetic program characteristic of the exponential growth stage of bacteria to the program of cell survival under stationary conditions, "a life in the slow lane". They participate in many regulatory mechanisms occurring in bacteria:
- They participate in the induction of rpoS, an RNA-polymerase subunit which is responsible for the expression of a large group of genes involved in adjustments to the stationary growth phase and many stressful agents.
- They are important for cell motility, biofilms formation and virulence.[clarification needed]
- Polyphosphates and exopolyphosphatases participate in the regulation of the levels of the stringent response factor, guanosine 5'-diphosphate 3'-diphosphate (ppGpp), a second messenger in bacterial cells.
- Polyphosphates participate in the formation of channels across the living cell membranes. The above channels formed by polyphosphate and poly-b-hydroxybutyrate with Ca2+ are involved in the transport processes in a variety of organisms.
- An important function of polyphosphate in microorganisms—prokaryotes and the lower eukaryotes—is to handle changing environmental conditions by providing phosphate and energy reserves. Polyphosphates are present in animal cells, and there are many data on its participation in the regulatory processes during development and cellular proliferation and differentiation—especially in bone tissues and brain.
In humans polyphosphates are shown to play a key role in blood coagulation. Produced and released by platelets[7] they activate blood coagulation factor XII which is essential for blood clot formation. Factor XII, also called Hageman factor, initiates fibrin formation and the generation of a proinflammatory mediator, bradykinin, that contributes to leakage from the blood vessels and thrombosis.[8][9] Bacterial-derived polyphosphates impair the host immune response during infection and targeting polyphosphates with recombinant exopolyphosphatase improves sepsis survival in mice.[10] Inorganic polyphosphates play a crucial role in tolerance of yeast cells to toxic heavy metal cations.[11]
Use as food additives
Sodium polyphosphate (E452(i)), potassium polyphosphate (E452(ii)), sodium calcium polyphosphate (E452(iii)) and calcium polyphosphate (E452(iv)) are used as food additives (emulsifiers, humectants, sequestrants, stabilisers, and thickeners).[12] They are not known to pose any potential health risk other than those generally attributed to other phosphate sources (including those naturally occurring in food). While concerns have been raised regarding detrimental effects on the bones and cardiovascular diseases, as well as hyperphosphatemia, these seem to be relevant only for exaggerated consumption of phosphate sources. In all, reasonable consumption (up to 40 mg phosphate per kg of body weight per day) seem to pose no health risk.[13][14]
See also
https://en.wikipedia.org/wiki/Polyphosphate
Hyperphosphatemia is an electrolyte disorder in which there is an elevated level of phosphate in the blood.[1] Most people have no symptoms while others develop calcium deposits in the soft tissue.[1] Often there is also low calcium levels which can result in muscle spasms.[1]
Causes include kidney failure, pseudohypoparathyroidism, hypoparathyroidism, diabetic ketoacidosis, tumor lysis syndrome, and rhabdomyolysis.[1] Diagnosis is generally based on a blood phosphate levels of greater than 1.46 mmol/L (4.5 mg/dL).[1] Levels may appear falsely elevated with high blood lipid levels, high blood protein levels, or high blood bilirubin levels.[1]
Treatment may include eating a phosphate low diet and antacids, like calcium carbonate, that bind phosphate.[1] Occasionally intravenous normal saline or dialysis may be used.[1] How commonly it occurs is unclear.[2]
https://en.wikipedia.org/wiki/Hyperphosphatemia
Ectopic calcification is a pathologic deposition of calcium salts in tissues or bone growth in soft tissues. This can be a symptom of hyperphosphatemia. Formation of osseous tissue in soft tissues such as the lungs, eyes, arteries, or other organs is known as ectopic calcification, dystrophic calcification, or ectopic ossification.[1]
Calcification of muscle can occur after traumatic injury and is known as myositis ossificans.
It can be recognized by muscle tenderness and loss of stretch in the
affected area. To reduce the risk of calcification after an injury,
initiate what is commonly known as "RICE" (rest, ice, compression, and elevation).[2]
https://en.wikipedia.org/wiki/Ectopic_calcification
Sevelamer (rINN) is a phosphate binding medication used to treat hyperphosphatemia in patients with chronic kidney disease. When taken with meals, it binds to dietary phosphate and prevents its absorption. Sevelamer was invented and developed by GelTex Pharmaceuticals. Sevelamer is marketed by Sanofi under the brand names Renagel (sevelamer hydrochloride) and Renvela (sevelamer carbonate).
https://en.wikipedia.org/wiki/Sevelamer
Lanthanum carbonate, La2(CO3)3, is the salt formed by lanthanum(III) cations and carbonate anions. It is an ore of lanthanum metal (bastnäsite), along with monazite.
https://en.wikipedia.org/wiki/Lanthanum_carbonate
Chemistry
Lanthanum carbonate is used as a starting material in lanthanum chemistry, particularly in forming mixed oxides, for example
- for production of lanthanum strontium manganite, primarily for solid oxide fuel cell applications;
- for production of certain high-temperature superconductors, such as La2-xSrxCuO2.
https://en.wikipedia.org/wiki/Lanthanum_carbonate
In petrochemistry, petroleum geology and organic chemistry, cracking is the process whereby complex organic molecules such as kerogens or long-chain hydrocarbons are broken down into simpler molecules such as light hydrocarbons, by the breaking of carbon-carbon bonds in the precursors. The rate of cracking and the end products are strongly dependent on the temperature and presence of catalysts. Cracking is the breakdown of a large hydrocarbons into smaller, more useful alkanes and alkenes. Simply put, hydrocarbon cracking is the process of breaking a long chain of hydrocarbons into short ones. This process requires high temperatures.[1]
More loosely, outside the field of petroleum chemistry, the term "cracking" is used to describe any type of splitting of molecules under the influence of heat, catalysts and solvents, such as in processes of destructive distillation or pyrolysis.
Fluid catalytic cracking produces a high yield of petrol and LPG, while hydrocracking is a major source of jet fuel, diesel fuel, naphtha, and again yields LPG.
https://en.wikipedia.org/wiki/Cracking_(chemistry)
https://en.wikipedia.org/wiki/Vladimir_Shukhov
https://en.wikipedia.org/wiki/Industrial_design
https://en.wikipedia.org/wiki/Evidence-based_design
https://en.wikipedia.org/wiki/Evidence-based_practice
Scientific evidence is evidence that serves to either support or counter a scientific theory or hypothesis,[1] although scientists also use evidence in other ways, such as when applying theories to practical problems.[2] Such evidence is expected to be empirical evidence and interpretable in accordance with scientific methods. Standards for scientific evidence vary according to the field of inquiry, but the strength of scientific evidence is generally based on the results of statistical analysis and the strength of scientific controls.[citation needed]
https://en.wikipedia.org/wiki/Scientific_evidence
https://en.wikipedia.org/wiki/Shukhov_cracking_process
https://en.wikipedia.org/wiki/Burton_process
https://en.wikipedia.org/wiki/William_Merriam_Burton
https://en.wikipedia.org/wiki/Russian_Empire
The Russian Empire[e][f] was an empire and the final period of the Russian monarchy from 1721 to 1917. It consisted of most of northern Eurasia. The Empire succeeded the Tsardom of Russia following the Treaty of Nystad. The rise of the Russian Empire coincided with the decline of neighbouring rival powers: the Swedish Empire, the Polish–Lithuanian Commonwealth, Qajar Iran, the Ottoman Empire, and Qing China. It also held colonies in Russian America between 1799 and 1867. Covering an area of approximately 22,800,000 square kilometres (8,800,000 sq mi), it remains the third-largest empire in history, surpassed only by the British Empire and the Mongol Empire; it ruled over a population of 125.6 million people per the 1897 Russian census, the only census carried out during the entire imperial period. Owing to its geographic extent across three continents at its peak, it featured great ethnic, linguistic, religious, and economic diversity.
From the 10th to the 17th century, the land was ruled by a noble class known as the boyars, above whom was a tsar (later adapted as the "Emperor of all the Russias"). The groundwork leading up to the establishment of the Russian Empire was laid by Ivan III (1462–1505): he tripled the territory of the Russian state and laid its foundation, renovating the Moscow Kremlin and also ending the dominance of the Golden Horde.
Peter I (1682–1725) fought numerous wars and expanded an already vast empire into a major power of Europe. During his rule, he moved the Russian capital from Moscow to the new model city of Saint Petersburg, which was largely built according to designs of the Western world; he also led a cultural revolution that replaced some of the traditionalist and medieval socio-political customs with a modern, scientific, rationalist, and Western-oriented system. Catherine the Great (1762–1796) presided over a golden age: she expanded the Russian state by conquest, colonization, and diplomacy, while continuing Peter I's policy of modernization towards a Western model. Alexander I (1801–1825) played a major role in defeating the militaristic ambitions of Napoleon and subsequently constituting the Holy Alliance, which aimed to restrain the rise of secularism and liberalism across Europe. The Russian Empire further expanded to the west, south, and east, strengthening its position as a European power. Its victories in the Russo-Turkish Wars were later checked by defeat in the Crimean War (1853–1856), leading to a period of reform and intensified expansion into Central Asia.[9] Alexander II (1855–1881) initiated numerous reforms, most notably the 1861 emancipation of all 23 million serfs. His official policy involved the responsibility of the Russian Empire towards the protection of Eastern Orthodox Christians residing within the Ottoman-ruled territories of Europe; this was one of the factors that later led to the Russian entry into World War I.
From 1721 until 1762, the Russian Empire was ruled by the House of Romanov; its matrilineal branch of patrilineal German descent, the House of Holstein-Gottorp-Romanov, ruled from 1762 until 1917. At the beginning of the 19th century, the territory of the Russian Empire extended from the Arctic Ocean in the north to the Black Sea in the south, and from the Baltic Sea in the west to Alaska, Hawaii, and California in the east. By the end of the 19th century, it had expanded its control over most of Central Asia and parts of Northeast Asia. The Russian Empire entered the twentieth century in a perilous state. A devastating famine in 1891–92, killed millions across the empire leading to discontent among the population. Moreover, the Russian Empire was the last remaining absolute monarchy in Europe, which played a role in the rapid radicalization of Russian politics. During this time, communism became popular among much of the population.[10] In 1905 Russia experienced a revolution in which Tsar Nicholas II authorized the creation of a parliament, the Duma, although he still retained absolute political power. When Russia entered the First World War on the side of the Allies, it suffered a series of defeats that further galvanized the population against the empire and the Tsar. In 1917, mass unrest among the population and mutinies in the army resulted in Russian leaders pressuring Tsar Nicholas to abdicate, which he did during the February Revolution. Following his abdication, the Russian Provisional Government was formed and continued Russia's involvement in the war, despite near universal opposition to further involvement. This decision, coupled with food shortages, led to mass demonstrations against the government in July. The Russian Provisional government was overthrown in the October Revolution by the Bolsheviks, who ended Russia's involvement in WWI with the Treaty of Brest-Litovsk. The Russian Revolution led to the end of almost two centuries of imperial rule, making Russia one of the four continental empires which collapsed after World War I, along with Germany, Austria-Hungary, and the Ottoman Empire.[11]
The Bolshevik seizure of power resulted in the Russian Civil War, which pitted the Bolsheviks (Reds) against their adversaries (Whites).[12][13] The White Army was not a unified front and comprised many of the Bolsheviks' enemies on both the left and right. In 1918, the Bolsheviks executed the Romanov family. After emerging victorious from the Russian Civil War in 1922–1923, the Bolsheviks established the Soviet Union across most of the territory of the former Russian Empire.
https://en.wikipedia.org/wiki/Russian_Empire
https://en.wikipedia.org/wiki/Fluid_catalytic_cracking
https://en.wikipedia.org/wiki/Atmospheric_pressure
https://en.wikipedia.org/wiki/Inch_of_mercury
Inch of mercury (inHg and ″Hg) is a non-SI unit of measurement for pressure. It is used for barometric pressure in weather reports, refrigeration and aviation in the United States.
It is the pressure exerted by a column of mercury 1 inch (25.4 mm) in height at the standard acceleration of gravity. Conversion to metric units depends on the temperature of mercury, and hence its density; typical conversion factors are:[1]
| Conditions | Pressure |
|---|---|
| conventional | 3386.389 pascals |
| 32 °F (0 °C) | 3386.38 pascals |
| 60 °F (16 °C) | 3376.85 pascals |
In older literature, an "inch of mercury" is based on the height of a column of mercury at 60 °F (15.6 °C).[1]
- 1 inHg60 °F = 3,376.85 pascals (33.7685 hPa)
In Imperial units: 1 inHg60 °F = 0.489 771 psi, or 2.041 771 inHg60 °F = 1 psi.
https://en.wikipedia.org/wiki/Inch_of_mercury
Geranylfarnesyl pyrophosphate is an intermediate used by organisms in the biosynthesis of sesterterpenoids.[1]
https://en.wikipedia.org/wiki/Geranylfarnesyl_pyrophosphate
(−)-FR901483 is a tyrosine-derived alkaloid that was isolated from the fungus Cladobotryum sp.[1] It was shown to have potent immunosuppressant activity in animal models. It is believed to function through inhibition of purine nucleotide biosynthesis.
https://en.wikipedia.org/wiki/FR901483
FrzG then performs an oxidation of an amine, allowing ring opening. Cyclization and reduction is then catalyzed by FrzH. Further ketone reduction is catalyzed by FrzJ. Finally, ATP-dependent phosphorylation is carried out by the activity of FrzJ.
https://en.wikipedia.org/wiki/FR901483
https://en.wikipedia.org/wiki/Category:Organophosphates
https://en.wikipedia.org/wiki/Acrydite
https://en.wikipedia.org/wiki/Adenophostin
https://en.wikipedia.org/wiki/Aeruginascin
https://en.wikipedia.org/wiki/Bicyclic_phosphate
https://en.wikipedia.org/wiki/5-Bromo-4-chloro-3-indolyl_phosphate
https://en.wikipedia.org/wiki/2,3-Bisphosphoglyceric_acid
https://en.wikipedia.org/wiki/Bromofenofos
https://en.wikipedia.org/wiki/Phosphagen
https://en.wikipedia.org/wiki/Phosphatidic_acid
https://en.wikipedia.org/wiki/Phosphoenolpyruvic_acid
https://en.wikipedia.org/wiki/2-Phosphoglyceric_acid
https://en.wikipedia.org/wiki/Phosphoribosyl_pyrophosphate
https://en.wikipedia.org/wiki/Phosphorylethanolamine
https://en.wikipedia.org/wiki/Phytic_acid
https://en.wikipedia.org/wiki/Prephytoene_diphosphate
https://en.wikipedia.org/wiki/Pyrazophos
https://en.wikipedia.org/wiki/Pyridoxal_phosphate
https://en.wikipedia.org/wiki/Butyryl_phosphate
https://en.wikipedia.org/wiki/C55-isoprenyl_pyrophosphate
https://en.wikipedia.org/wiki/Carbamoyl_phosphate
https://en.wikipedia.org/wiki/High-energy_phosphate
https://en.wikipedia.org/wiki/(E)-4-Hydroxy-3-methyl-but-2-enyl_pyrophosphate
https://en.wikipedia.org/wiki/Ribulose_5-phosphate
https://en.wikipedia.org/wiki/Dimethylamidophosphoric_dicyanide
https://en.wikipedia.org/wiki/Molybdopterin
https://en.wikipedia.org/wiki/Dolichol_monophosphate
https://en.wikipedia.org/wiki/Erythrose_4-phosphate
https://en.wikipedia.org/wiki/Para-Nitrophenylphosphate
https://en.wikipedia.org/wiki/Novichok
https://en.wikipedia.org/wiki/Organophosphate_poisoning
https://en.wikipedia.org/wiki/Oxon_(chemical)
https://en.wikipedia.org/wiki/Organophosphate-induced_delayed_neuropathy
https://en.wikipedia.org/wiki/Tenofovir_alafenamide
https://en.wikipedia.org/wiki/Thiamine_monophosphate
https://en.wikipedia.org/wiki/Tricresyl_phosphate
https://en.wikipedia.org/wiki/Tyrosine_phosphorylation
https://en.wikipedia.org/wiki/Xylulose_5-phosphate
https://en.wikipedia.org/wiki/Palmitoyl-CoA
https://en.wikipedia.org/wiki/Phosphotransferase
https://en.wikipedia.org/wiki/Cytochrome_P450
| Choroid | |
|---|---|
 Cross-section of human eye, with choroid labeled at top. | |
 Interior of anterior half of bulb of eye. (Choroid labeled at right, second from the bottom.) | |
| Details | |
| Artery | short posterior ciliary arteries, long posterior ciliary arteries |
| Identifiers | |
| Latin | choroidea |
| MeSH | D002829 |
| TA98 | A15.2.03.002 |
| TA2 | 6774 |
| FMA | 58298 |
| Anatomical terminology | |
In bony fish
Teleosts bear a body of capillary adjacent to the optic nerve called the choroidal gland. Though its function is not known, it is believed to be a supplemental oxygen carrier.[7]
https://en.wikipedia.org/wiki/Choroid
Blood supply
There are two circulations of the eye: the retinal (in the retina) and uveal, supplied in humans by posterior ciliary arteries, originating from the ophthalmic artery (a branch of the internal carotid artery).[2] The arteries of the uveal circulation, supplying the uvea and outer and middle layers of the retina, are branches of the ophthalmic artery and enter the eyeball without passing with the optic nerve. The retinal circulation, on the other hand, derives its circulation from the central retinal artery, also a branch of the ophthalmic artery, but passing in conjunction with the optic nerve.[3] They branch in a segmental distribution to end arterioles and not anastomoses. This is clinically significant for diseases affecting choroidal blood supply. The macula responsible for central vision and the anterior part of the optic nerve are dependent on choroidal blood supply.[4] The structure of choroidal vessels can be revealed by optical coherence tomography, and blood flow can be revealed by Indocyanine green angiography, and laser Doppler imaging.[5]
Mechanism
Melanin, a dark colored pigment, helps the choroid limit uncontrolled reflection within the eye that would potentially result in the perception of confusing images.
In humans and most other primates, melanin occurs throughout the choroid. In albino humans, frequently melanin is absent and vision is low. In many animals, however, the partial absence of melanin contributes to superior night vision. In these animals, melanin is absent from a section of the choroid and within that section a layer of highly reflective tissue, the tapetum lucidum, helps to collect light by reflecting it in a controlled manner. The uncontrolled reflection of light from dark choroid produces the photographic red-eye effect on photos, whereas the controlled reflection of light from the tapetum lucidum produces eyeshine (see Tapetum lucidum).
https://en.wikipedia.org/wiki/Choroid
Indocyanine green angiography (ICGA) is a diagnostic procedure used to examine choroidal blood flow and associated pathology. Indocyanine green (ICG) is a water soluble cyanine dye which shows fluorescence in near-infrared (790–805 nm) range, with peak spectral absorption of 800-810 nm in blood.[1][2] The near infrared light used in ICGA penetrates ocular pigments such as melanin and xanthophyll, as well as exudates and thin layers of sub-retinal vessels.[3] Age-related macular degeneration is the third main cause of blindness worldwide, and it is the leading cause of blindness in industrialized countries.[4] Indocyanine green angiography is widely used to study choroidal neovascularization in patients with exudative age-related macular degeneration.[5] In nonexudative AMD, ICGA is used in classification of drusen and associated subretinal deposits.[5]
https://en.wikipedia.org/wiki/Indocyanine_green_angiography
The tapetum lucidum (Latin for 'bright tapestry, coverlet'; /təˈpiːtəm ˈluːsɪdəm/ tə-PEE-təm LOO-sih-dəm; pl. tapeta lucida)[1] is a layer of tissue in the eye of many vertebrates and some other animals. Lying immediately behind the retina, it is a retroreflector. It reflects visible light back through the retina, increasing the light available to the photoreceptors (although slightly blurring the image). The tapetum lucidum contributes to the superior night vision of some animals. Many of these animals are nocturnal, especially carnivores, while others are deep sea animals.
Similar adaptations occur in some species of spiders.[2] Haplorhine primates, including humans, are diurnal and lack a tapetum lucidum.[Note 1]
https://en.wikipedia.org/wiki/Tapetum_lucidum
History
The choroid was first described by Democritus (c. 460 – c. 370 BCE) around 400 BCE, calling it the "chitoon malista somphos" (more spongy tunic [than the sclera]).[8] Democritus likely saw the choroid from dissections of animal eyes.[9]
About 100 years later, Herophilos (c. 335 – 280 BCE) also described the choroid from his dissections on eyes of cadavers.[10][11]
https://en.wikipedia.org/wiki/Choroid
Additional images
Laser Doppler imaging of retinal and choroidal blood flow
https://en.wikipedia.org/wiki/Choroid
The trabecular meshwork is an area of tissue in the eye located around the base of the cornea, near the ciliary body, and is responsible for draining the aqueous humor from the eye via the anterior chamber (the chamber on the front of the eye covered by the cornea).
The tissue is spongy and lined by trabeculocytes; it allows fluid to drain into a set of tubes called Schlemm's canal which is lined by endothelium with blood and lymphatic properties that allow aqueous humor to flow into the blood system.[1]
Structure
The meshwork is divided up into three parts, with characteristically different ultrastructures:
- Inner uveal meshwork - Closest to the anterior chamber angle, contains thin cord-like trabeculae, orientated predominantly in a radial fashion, enclosing trabeculae spaces larger than the corneoscleral meshwork.
- Corneoscleral meshwork - Contains a large amount of elastin, arranged as a series of thin, flat, perforated sheets arranged in a laminar pattern; considered the ciliary muscle tendon.[2]
- Juxtacanalicular tissue (also known as the cribriform meshwork) - Lies immediately adjacent to Schlemm's canal, composed of connective tissue ground substance full of glycoaminoglycans and glycoproteins. This thin strip of tissue is covered by a monolayer of endothelial cells.
The trabecular meshwork is assisted to a small degree in the drainage of aqueous humour by a second outflow pathway, the uveo-scleral pathway (5-10% of outflow occurs this way). The uveo-scleral pathway is increased with the use of glaucoma drugs such as prostaglandins (e.g., Xalatan, Travatan).
The trabecular meshwork had previously been thought to arise from a point (apex) corresponding to the termination of the DM (Schwalbe’s line) however it is now considered to extend into the cornea, forming the Dua's layer.[3]
Clinical significance
Glaucoma
It is thought that most cases of glaucoma (although not all) are caused or enabled by an increase in intraocular pressure. Pressure increases either when too much aqueous humor fluid is produced or by decreased aqueous humor outflow. The trabecular meshwork is responsible for most of the outflow of aqueous humor. When outflow is blocked, interventions such as trabeculectomy, trabeculoplasty, or aqueous shunt may be required to restore it.
See also
https://en.wikipedia.org/wiki/Trabecular_meshwork
Dua's layer, according to a 2013 paper by Harminder Singh Dua's group at the University of Nottingham, is a layer of the cornea that had not been detected previously.[1] It is hypothetically 15 micrometres (0.59 mils) thick, the fourth caudal layer, and located between the corneal stroma and Descemet's membrane.[2][3] Despite its thinness, the layer is very strong and impervious to air.[1] It is strong enough to withstand up to 2 bars (200 kPa) of pressure.[4] While some scientists welcomed the announcement, other scientists cautioned that time was needed for other researchers to confirm the discovery and its significance.[5] Others have met the claim "with incredulity".[6]
https://en.wikipedia.org/wiki/Dua%27s_layer
The corneal limbus (Latin: corneal border) is the border between the cornea and the sclera (the white of the eye). It contains limbal stem cells in its palisades of Vogt. It may be affected by cancer or aniridia (a developmental problem), among other issues. The limbal ring is a visible dark ring around the iris of the eye composed of darkened areas of the corneal limbus.
https://en.wikipedia.org/wiki/Corneal_limbus
A limbal ring is a dark ring around the iris of the eye, where the sclera meets the cornea.[1] It is a dark-colored manifestation of the corneal limbus resulting from optical properties of the region.[2]
The appearance and visibility of the limbal ring can be negatively
affected by a variety of medical conditions concerning the peripheral
cornea.[3]
It has been suggested that limbal ring thickness may correlate with
health or youthfulness and may contribute to facial attractiveness.[3][4] Some contact lenses are colored to simulate limbal rings.[1]
https://en.wikipedia.org/wiki/Limbal_ring
Limbal stem cells, also known as corneal epithelial stem cells, are unipotent stem cells located in the basal epithelial layer of the corneal limbus. They form the border between the cornea and the sclera. Characteristics of limbal stem cells include a slow turnover rate, high proliferative potential, clonogenicity, expression of stem cell markers, as well as the ability to regenerate the entire corneal epithelium. Limbal stem cell proliferation has the role of maintaining the cornea; for example, by replacing cells that are lost via tears. Additionally, these cells also prevent the conjunctival epithelial cells from migrating onto the surface of the cornea.[2]
https://en.wikipedia.org/wiki/Limbal_stem_cell
Aniridia is the absence of the iris, a muscular structure that opens and closes the pupil to allow light into the eye. It is also responsible for eye color. Without it, the central eye appears all black. It can be congenital, in which both eyes are usually involved, or caused by a penetrant injury.[1] Isolated aniridia is a congenital disorder which is not limited to a defect in iris development, but is a panocular condition with macular and optic nerve hypoplasia, cataract, and corneal changes.[2] Vision may be severely compromised and the disorder is frequently associated with a number of ocular complications: nystagmus, amblyopia, buphthalmos, and cataract.[1] Aniridia in some individuals occurs as part of a syndrome, such as WAGR syndrome (kidney nephroblastoma (Wilms tumour), genitourinary anomalies and intellectual disability), or Gillespie syndrome (cerebellar ataxia).
https://en.wikipedia.org/wiki/Aniridia
Buphthalmos (plural: buphthalmoses) is enlargement of the eyeball and is most commonly seen in infants and young children. It is sometimes referred to as buphthalmia (plural buphthalmias).[2] It usually appears in the newborn period or the first 3 months of life.[3] and in most cases indicates the presence of congenital (infantile) glaucoma, which is a disorder in which elevated pressures within the eye lead to structural eye damage and vision loss.
https://en.wikipedia.org/wiki/Buphthalmos
Paired box protein Pax-6, also known as aniridia type II protein (AN2) or oculorhombin, is a protein that in humans is encoded by the PAX6 gene.[5]
PAX6 is a member of the Pax gene
family which is responsible for carrying the genetic information that
will encode the Pax-6 protein. It acts as a "master control" gene for
the development of eyes and other sensory organs, certain neural and
epidermal tissues as well as other homologous structures, usually derived from ectodermal tissues.[citation needed]
However, it has been recognized that a suite of genes is necessary for
eye development, and therefore the term of "master control" gene may be
inaccurate.[6] Pax-6 is expressed as a transcription factor when neural ectoderm receives a combination of weak Sonic hedgehog (SHH) and strong TGF-Beta
signaling gradients. Expression is first seen in the forebrain,
hindbrain, head ectoderm and spinal cord followed by later expression in
midbrain. This transcription factor is most noted for its use in the interspecifically induced expression of ectopic eyes and is of medical importance because heterozygous mutants produce a wide spectrum of ocular defects such as aniridia in humans.[7]
https://en.wikipedia.org/wiki/PAX6
In May 2018, the U.S. Food and Drug Administration approved the CustomFlex Artificial Iris, the first synthetic iris for use in adults and children with congenital aniridia or iris defects related to other conditions, such as albinism, traumatic injury, or surgical removal due to ocular melanoma. The artificial iris is a surgically implanted device made of thin, foldable, medical-grade silicone and is custom-sized and colored for each individual patient. The prosthetic iris is held in place by the anatomical structures of the eye or, if needed, by sutures.[7]
https://en.wikipedia.org/wiki/Aniridia
PAX6
The AN2 region of the short arm of chromosome 11 (11p13) includes the PAX6 gene (named for its PAired boX status), whose gene product helps regulate a cascade of other genetic processes involved in the development of the eye (as well as other non-ocular structures).[3] This PAX6 gene is around 95% similar to the pax gene found in zebrafish, a creature whose ancestors diverged from human evolutionary development around 400 million years ago. Thus the PAX6 gene is highly conserved across evolutionary lineages.
Defects in the PAX6 gene cause aniridia-like ocular defects in mice (as well as Drosophila). Aniridia is a heterozygous disorder, meaning that only one of the two chromosome 11 copies is affected. When both copies are altered (homozygous condition), the result is a uniformly fatal condition with near complete failure of entire eye formation. In 2001, two cases of homozygous aniridia patients were reported; the fetuses died prior to birth and had severe brain damage. In mice, homozygous small eye defect (mouse Pax-6) leads to loss of the eyes and nose and the murine fetuses sustain severe brain damage.[4]
https://en.wikipedia.org/wiki/Aniridia
Dacryoadenitis is inflammation of the lacrimal glands.[1]
| Dacryoadenitis | |
|---|---|
 | |
| Lacrimal gland(upper left) | |
| Specialty | Ophthalmology |
https://en.wikipedia.org/wiki/Dacryoadenitis
Fluorescence in situ hybridization (FISH) is a molecular cytogenetic technique that uses fluorescent probes that bind to only particular parts of a nucleic acid sequence with a high degree of sequence complementarity. It was developed by biomedical researchers in the early 1980s[1] to detect and localize the presence or absence of specific DNA sequences on chromosomes. Fluorescence microscopy can be used to find out where the fluorescent probe is bound to the chromosomes. FISH is often used for finding specific features in DNA for use in genetic counseling, medicine, and species identification.[2] FISH can also be used to detect and localize specific RNA targets (mRNA, lncRNA and miRNA)[citation needed] in cells, circulating tumor cells, and tissue samples. In this context, it can help define the spatial-temporal patterns of gene expression within cells and tissues.
https://en.wikipedia.org/wiki/Fluorescence_in_situ_hybridization
Function and mechanism
Presence of a tapetum lucidum enables animals to see in dimmer light than would otherwise be possible. The tapetum lucidum, which is iridescent, reflects light roughly on the interference principles of thin-film optics, as seen in other iridescent tissues. However, the tapetum lucidum cells are leucophores, not iridophores.[dubious ]
The tapetum functions as a retroreflector which reflects light directly back along the light path. This serves to match the original and reflected light, thus maintaining the sharpness and contrast of the image on the retina. The tapetum lucidum reflects with constructive interference,[4] thus increasing the quantity of light passing through the retina. In the cat, the tapetum lucidum increases the sensitivity of vision by 44%, allowing the cat to see light that is imperceptible to human eyes.[5]
It has been speculated that some flashlight fish may use eyeshine both to detect and to communicate with other flashlight fish.[6] American scientist Nathan H. Lents has proposed that the tapetum lucidum evolved in vertebrates, but not in cephalopods, which have a very similar eye, because of the backwards-facing nature of vertebrate photoreceptors. The tapetum boosts photosensitivity under conditions of low illumination, thus compensating for the suboptimal design of the vertebrate retina.[7]
https://en.wikipedia.org/wiki/Tapetum_lucidum
Classification
A classification of anatomical variants of tapeta lucida[8] defines four types:
- Retinal tapetum, as seen in teleosts, crocodiles, marsupials and fruit bats. The tapetum lucidum is within the retinal pigment epithelium; in the other three types the tapetum is within the choroid behind the retina.
- Choroidal guanine tapetum, as seen in cartilaginous fish.[9] The tapetum is a palisade of cells containing stacks of flat hexagonal crystals of guanine.[4]
- Choroidal tapetum cellulosum, as seen in carnivores, rodents and cetacea. The tapetum consists of layers of cells containing organized, highly refractive crystals. These crystals are diverse in shape and makeup.
- Choroidal tapetum fibrosum, as seen in cows, sheep, goats and horses. The tapetum is an array of extracellular fibers.
The functional differences between these four types of tapeta lucida are not known.[8]
This classification does not include tapeta lucida in birds. Kiwis, stone-curlews, the boat-billed heron, the flightless kakapo and many nightjars, owls, and other night birds such as the swallow-tailed gull also possess a tapetum lucidum.[10] This classification also does not include the extraordinary focusing mirror in the eye of the brownsnout spookfish.[11]
Like humans, some animals lack a tapetum lucidum and they usually are diurnal.[8] These include haplorhine primates, squirrels, some birds, red kangaroo, and pigs.[12] Strepsirrhine primates are mostly nocturnal and, with the exception of several diurnal Eulemur species, have a tapetum lucidum.[13]
When a tapetum lucidum is present, its location on the eyeball varies with the placement of the eyeball in the head,[14] such that in all cases the tapetum lucidum enhances night vision in the center of the animal's field of view.
Apart from its eyeshine, the tapetum lucidum itself has a color. It is often described as iridescent. In tigers it is greenish.[15] In ruminants it may be golden green with a blue periphery,[12] or whitish or pale blue with a lavender periphery. In dogs it may be whitish with a blue periphery.[12] The color in reindeer changes seasonally, allowing the animals to better avoid predators in low-light winter at the price of blurrier vision.[16]
Eyeshine
Eyeshine is a visible effect of the tapetum lucidum. When light shines into the eye of an animal having a tapetum lucidum, the pupil appears to glow. Eyeshine can be seen in many animals, in nature and in flash photographs. In low light, a hand-held flashlight is sufficient to produce eyeshine that is highly visible to humans (despite their inferior night vision). Eyeshine occurs in a wide variety of colors including white, blue, green, yellow, pink and red. However, since eyeshine is a type of iridescence, the color varies with the angle at which it is seen and the minerals which make up the reflective tapetum-lucidum crystals.
White eyeshine occurs in many fish, especially walleye; blue eyeshine occurs in many mammals such as horses; green eyeshine occurs in mammals such as cats, dogs, and raccoons; and red eyeshine occurs in coyote, rodents, opossums and birds.[citation needed]
Although human eyes lack a tapetum lucidum, they still exhibit a weak reflection from the fundus, as can be seen in photography with the red-eye effect and with near-infrared eyeshine.[17][18] Another effect in humans and other animals that may resemble eyeshine is leukocoria, which is a white shine indicative of abnormalities such as cataracts and cancers.
In blue-eyed cats and dogs
Cats and dogs with a blue eye color may display both eyeshine and red-eye effect. Both species have a tapetum lucidum, so their pupils may display eyeshine. In flash color photographs, however, individuals with blue eyes may also display a distinctive red eyeshine. Individuals with heterochromia may display red eyeshine in the blue eye and normal yellow/green/blue/white eyeshine in the other eye. These include odd-eyed cats and bi-eyed dogs. The red-eye effect is independent of the eyeshine: in some photographs of individuals with a tapetum lucidum and heterochromia, the eyeshine is dim, yet the pupil of the blue eye still appears red. This is most apparent when the individual is not looking into the camera because the tapetum lucidum is far less extensive than the retina.
In spiders
Most species of spider also have a tapetum, which is located only in their smaller, lateral eyes; the larger central eyes have no such structure. This consists of reflective crystalline deposits, and is thought to have a similar function to the structure of the same name in vertebrates. Four general patterns can be distinguished in spiders:[19]
- Primitive type (e.g. Mesothelae, Orthognatha) – a simple sheet behind the retina
- Canoe-shape type (e.g. Araneidae, Theridiidae) – two lateral walls separated by a gap for the nerve fibres
- Grated type (e.g. Lycosidae, Pisauridae) – a relatively complex, grill-shaped structure
- No tapetum (e.g. Salticidae)
Uses by humans
Humans use scanning for reflected eyeshine to detect and identify the species of animals in the dark, and deploying trained search dogs and search horses at night, as these animals benefit from improved night vision through this effect.
Using eyeshine to identify animals in the dark employs not only its color but also several other features. The color corresponds approximately to the type of tapetum lucidum, with some variation between species. Other features include the distance between pupils relative to their size; the height above ground; the manner of blinking (if any); and the movement of the eyeshine (bobbing, weaving, hopping, leaping, climbing, flying).
Artificial tapetum lucidum
Manufactured retroreflectors modeled after a tapetum lucidum are described in numerous patents and today have many uses. The earliest patent, first used in "Catseye" brand raised pavement markers, was inspired by the tapetum lucidum of a cat's eye.
Pathology
In dogs, certain drugs are known to disturb the precise organization of the crystals of the tapetum lucidum, thus compromising the dog's ability to see in low light. These drugs include ethambutol, macrolide antibiotics, dithizone, antimalarial medications, some receptor H2-antagonists, and cardiovascular agents. The disturbance "is attributed to the chelating action which removes zinc from the tapetal cells."[20]
Gallery
Traditionally it has been difficult to take retinal images of animals with a tapetum lucidum because ophthalmoscopy devices designed for humans rely on a high level of on-axis illumination.[21] This kind of illumination causes a great deal of reflex, or back-scatter, when it interacts with the tapetum. New devices with variable illumination can make this possible, however.
A domestic tabby cat's green tapetum lucidum, apparent with camera flash
See also
https://en.wikipedia.org/wiki/Tapetum_lucidum
Dithizone is a sulfur-containing organic compound. It is a good ligand, and forms complexes with many toxic metals such as lead, thallium[1] and mercury.
Dithizone may be prepared by reacting phenylhydrazine with carbon disulfide, followed by reaction with potassium hydroxide.[2]
Dithizone is used to assess the purity of human pancreatic islet preparations used for transplantation into patients with type 1 diabetes. Dithizone binds zinc ions present in the islet's beta cells, and therefore stains the islets red. Exocrine tissue also present in the preparations does not bind dithizone, and is therefore not stained.[3]
References
- Bendl BJ (1969) Thallium poisoning: report of a case successfully treated with dithizone. Arch Dermatol 100:443–446 URL=https://jamanetwork.com/journals/jamadermatology/article-abstract/531202
- John H. Billman and Elizabeth S. Cleland (1955). "Dithizone". Organic Syntheses.; Collective Volume, vol. 3, p. 360
https://en.wikipedia.org/wiki/Dithizone
Phenylhydrazine is the chemical compound with the formula C6H5NHNH2. It is often abbreviated as PhNHNH2. It is also found in edible mushrooms.[5]
Properties
Phenylhydrazine forms monoclinic prisms that melt to an oil around room temperature which may turn yellow to dark red upon exposure to air.[1] Phenylhydrazine is miscible with ethanol, diethyl ether, chloroform and benzene. It is sparingly soluble in water.
Preparation
Phenylhydrazine is prepared by oxidizing aniline with sodium nitrite in the presence of hydrogen chloride to form the diazonium salt, which is subsequently reduced using sodium sulfite in the presence of sodium hydroxide to form the final product.[6]
History
Phenylhydrazine was the first hydrazine derivative characterized, reported by Hermann Emil Fischer in 1875.[7][8] He prepared it by reduction of a phenyl diazonium salt using sulfite salts. Fischer used phenylhydrazine to characterize sugars via formation of hydrazones known as osazones with the sugar aldehyde. He also demonstrated in this first paper many of the key properties recognized for hydrazines.
Uses
Phenylhydrazine is used to prepare indoles by the Fischer indole synthesis, which are intermediates in the synthesis of various dyes and pharmaceuticals.
Phenylhydrazine is used to form phenylhydrazones of natural mixtures of simple sugars in order to render the differing sugars easily separable from each other.[9]
This molecule is also used to induce acute hemolytic anemia in animal models.
Safety
Exposure to phenylhydrazine may cause contact dermatitis, hemolytic anemia, and liver damage.[1]
References
- Andrew Streitwieser; Clayton Heathcock (1976). Introduction to Organic Chemistry. Macmillan. ISBN 0-02-418010-6.
External links
- Phenylhydrazines at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- PubChem
- Additional chemical properties of phenylhydrazine
- CDC - NIOSH Pocket Guide to Chemical Hazards
https://en.wikipedia.org/wiki/Phenylhydrazine
Proton-pump inhibitors (PPIs) are a class of medications that cause a profound and prolonged reduction of stomach acid production. They do so by irreversibly inhibiting the stomach's H+/K+ ATPase proton pump.[1]
They are the most potent inhibitors of acid secretion available.[2] Proton-pump inhibitors have largely superseded the H2-receptor antagonists, a group of medications with similar effects but a different mode of action, and antacids.[3]
PPIs are among the most widely sold medications in the world. The class of proton-pump inhibitor medications is on the World Health Organization's List of Essential Medicines.[4][5] Omeprazole is the specific listed example.[4][5]
https://en.wikipedia.org/wiki/Proton-pump_inhibitor
https://en.wikipedia.org/wiki/Category:Monoamine_oxidase_inhibitors
https://en.wikipedia.org/wiki/Osazone
https://en.wikipedia.org/wiki/Indocyanine_green_angiography
https://en.wikipedia.org/wiki/Neurotransmitter
https://en.wikipedia.org/wiki/PAX6
A proton pump is an integral membrane protein pump that builds up a proton gradient across a biological membrane. Proton pumps catalyze the following reaction:
- H+
[on one side of a biological membrane] + energy ⇌ H+
[on the other side of the membrane]
Mechanisms are based on energy-induced conformational changes of the protein structure or on the Q cycle.
During evolution, proton pumps have arisen independently on multiple occasions. Thus, not only throughout nature but also within single cells, different proton pumps that are evolutionarily unrelated can be found. Proton pumps are divided into different major classes of pumps that use different sources of energy, have different polypeptide compositions and evolutionary origins.
https://en.wikipedia.org/wiki/Proton_pump
Gastric hydrogen potassium ATPase, also known as H+/K+ ATPase, is an enzyme which functions to acidify the stomach.[1] It is a member of the P-type ATPases, also known as E1-E2 ATPases due to its two states.[2]
https://en.wikipedia.org/wiki/Hydrogen_potassium_ATPase
In chemistry, hydronium (hydroxonium in traditional British English) is the common name for the aqueous cation H3O+, the type of oxonium ion produced by protonation of water. It is often viewed as the positive ion present when an Arrhenius acid is dissolved in water, as Arrhenius acid molecules in solution give up a proton (a positive hydrogen ion, H+) to the surrounding water molecules (H2O). In fact, acids must be surrounded by more than a single water molecule in order to ionize, yielding aqueous H+ and conjugate base. Three main structures for the aqueous proton have garnered experimental support: The Eigen cation, which is a tetrahydrate, H3O+(H2O)3; the Zundel cation, which is a symmetric dihydrate, H+(H2O)2; and the Stoyanov cation, an expanded Zundel cation, which is a hexahydrate: H+(H2O)2(H2O)4.[1][2] Spectroscopic evidence from well-defined IR spectra overwhelmingly supports the Stoyanov cation as the predominant form.[3][4][5][6] For this reason, it has been suggested that wherever possible, the symbol H+(aq) should be used instead of the hydronium ion.[2]
https://en.wikipedia.org/wiki/Hydronium
In chemistry, an oxonium ion is any cation containing an oxygen atom that has three bonds and 1+ formal charge.[1] The simplest oxonium ion is the hydronium ion (H3O+).[2]
https://en.wikipedia.org/wiki/Oxonium_ion
Trimethyloxonium tetrafluoroborate is the organic compound with the formula [(CH3)3O]+[BF4]−. (It is sometimes called "Meerwein's salt" after Hans Meerwein.[1][a]) This salt is a strong methylating agent, being a synthetic equivalent of CH+3. It is a white solid that rapidly decomposes upon exposure to atmospheric moisture, although it is robust enough to be weighed quickly without inert atmosphere protection. Triethyloxonium tetrafluoroborate is a closely related compound.
https://en.wikipedia.org/wiki/Trimethyloxonium_tetrafluoroborate
Epichlorohydrin (abbreviated ECH) is an organochlorine compound and an epoxide. Despite its name, it is not a halohydrin. It is a colorless liquid with a pungent, garlic-like odor, moderately soluble in water, but miscible with most polar organic solvents.[4] It is a chiral molecule generally existing as a racemic mixture of right-handed and left-handed enantiomers. Epichlorohydrin is a highly reactive electrophilic compound and is used in the production of glycerol, plastics, epoxy glues and resins, epoxy diluents and elastomers.
https://en.wikipedia.org/wiki/Epichlorohydrin
Bisphenol A diglycidyl ether (commonly abbreviated BADGE or DGEBA) is an organic compound and is a liquid epoxy resin.[1][2][3][4][5] The compound is a colorless viscous liquid (commercial samples can appear pale straw-coloured).[6][7] It is a key component of many epoxy resin formulations. Addition of further Bisphenol A and a catalyst and heat can produce Bisphenol A glycidyl ether epoxy resins of higher molecular weight that are solid.[8]

| |
| Names |
|---|
https://en.wikipedia.org/wiki/Bisphenol_A_diglycidyl_ether
https://en.wikipedia.org/wiki/Epoxy_value
Epichlorohydrin is a versatile precursor in the synthesis of many organic compounds. For example, it is converted to glycidyl nitrate, an energetic binder used in explosive and propellant compositions.[14] The epichlorohydrin is reacted with an alkali nitrate, such as sodium nitrate, producing glycidyl nitrate and alkali chloride. It is used as a solvent for cellulose, resins, and paints, and it has found use as an insect fumigant.[15]
https://en.wikipedia.org/wiki/Epichlorohydrin
Furylfuramide (also known as AF-2)[1] is a synthetic nitrofuran derivative which was widely used as a food preservative in Japan since at least 1965, but withdrawn from the market in 1974 when it was observed to be mutagenic to bacteria in vitro and thus suspected of carcinogenicity. This was confirmed later when animal testing[4] found it to cause benign and malignant tumors in the mammary glands, stomachs, esophagi, and lungs of rodents of both sexes, although insufficient evidence exists in human exposure.[3]
This successful use of bacterial mutagenicity as a screen for carcinogenicity confirmed the use of this methodology as a rapid and efficient test, in comparison to animal testing alone, and led to its further development. The availability of such simpler tests in turn gave rise to greater government oversight and testing of compounds to which the public would be exposed.[5]
https://en.wikipedia.org/wiki/Furylfuramide
https://en.wikipedia.org/wiki/Glycidyl_nitrate
A photograph (also known as a photo, image, or picture) is an image created by light falling on a photosensitive surface, usually photographic film or an electronic image sensor, such as a CCD or a CMOS chip. Most photographs are now created using a smartphone/camera, which uses a lens to focus the scene's visible wavelengths of light into a reproduction of what the human eye would see. The process and practice of creating such images is called photography.
https://en.wikipedia.org/wiki/Photograph
Bitumen of Judea, or Syrian asphalt,[1] is a naturally occurring asphalt that has been put to many uses since ancient times.[vague]
Wood coloration usage
Bitumen of Judea may be used as a colorant for wood for an aged, natural and rustic appearance. It is soluble in turpentine and some other terpenes, and can be combined with oils, waxes, varnishes and glazes.
Light-sensitive properties
It is a light-sensitive material in what is accepted to be the first complete photographic process, i.e., one capable of producing durable light-fast results.[2] The technique was developed by French scientist and inventor Nicéphore Niépce in the 1820s. In 1826 or 1827,[3] he applied a thin coating of the tar-like material to a pewter plate and took a picture of parts of the buildings and surrounding countryside of his estate, producing what is usually described as the first photograph. It is considered to be the oldest known surviving photograph made in a camera. The plate was exposed in the camera for at least eight hours.[4]
https://en.wikipedia.org/wiki/Bitumen_of_Judea




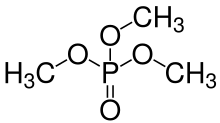




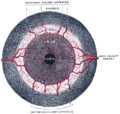
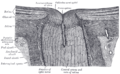
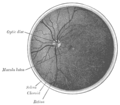
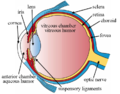
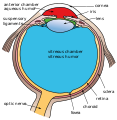
















No comments:
Post a Comment