



The underlying mechanism involves insufficient calcification of the growth plate.[6]Diagnosis is generally based on blood tests finding a low calcium, low phosphorus, and a high alkaline phosphatase together with X-rays.[2]
https://en.wikipedia.org/wiki/Rickets
https://en.wikipedia.org/wiki/Phosphorus
| Rickettsia | |
|---|---|
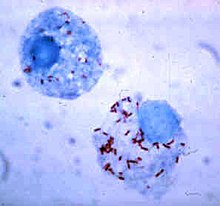 | |
| Red-stained Rickettsia rickettsii visible in cells of an Ixodid vector tick |
https://en.wikipedia.org/wiki/Rickettsia


| Anaplasmosis | |
|---|---|
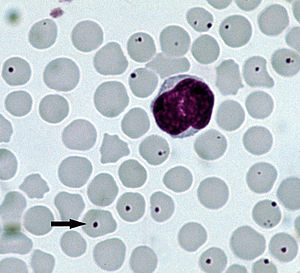 | |
| Anaplasma centrale infecting the red blood cells of a cow: The arrow points to typical infected cell. | |
| Specialty | Veterinary medicine |
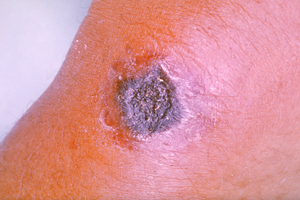
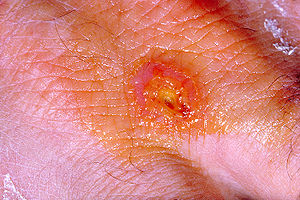
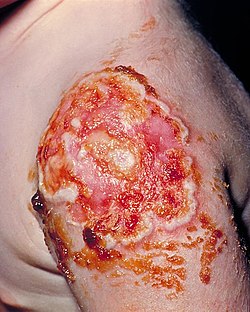
| Rickettsialpox | |
|---|---|
 | |
| Rickettsialpox lesion | |
| Specialty | Infectious disease |
| Prognosis | Resolves in 2-3 weeks without treatment |
Virus, Bacters; cell.
https://en.wikipedia.org/wiki/Category:Health_effects_of_tobacco






















































































:format(jpeg):mode_rgb():quality(40)/discogs-images/A-1110489-1443339143-6210.jpeg.jpg)












![]()









Inflammation.
































































/239/464239.jpg)



























































![Free download Vintage Barbie Pictures Desktop Backgrounds [996x1200] for your Desktop, Mobile & Tablet | Explore 48+ Vintage Barbie Wallpaper | Barbie Wallpapers for Facebook, Barbie Screensavers Wallpapers, Wallpaper by Barbie in HD](https://cdn.wallpapersafari.com/57/66/A9uLE0.jpg)


































































































![Real Life Barbie: Valeria Lukyanova Believes She's From Another Planet [VIDEO] - IBTimes India](https://data1.ibtimes.co.in/en/full/394137/living-barbie-doll-valeria-lukyanova-featured-documentary-film-my-life-online-space-barbie.jpg)




































































































![1940's gals group[Explored] | Vintage barbie dolls, Barbie dolls, Barbie collector](https://i.pinimg.com/originals/6d/d1/36/6dd1368f21b5e866e79468a1bf16ac76.jpg)




/chinese-take-out-472927590-57d31fff3df78c5833464e7b.jpg)



/cdn.vox-cdn.com/uploads/chorus_image/image/66683596/Atlas_Kitchen_30.0.jpg)












:max_bytes(150000):strip_icc()/classic-korean-bibimbap-recipe-2118765-step-011-acab906539594dc9a668c7fbb753c43d.jpg)







/cdn.vox-cdn.com/uploads/chorus_image/image/59840579/kushiyaki_set.7.jpg)









/__opt__aboutcom__coeus__resources__content_migration__simply_recipes__uploads__2015__01__perfect-popcorn-vertical-b-1800-b6948302f0f1460a93eb9d4d73623831.jpg)

















































































































































![White Chocolate Fudge Recipe: classic dessert [Video] - Sweet and Savory Meals](https://sweetandsavorymeals.com/wp-content/uploads/2020/07/white-chocolate-fudge-recipe.jpg)
















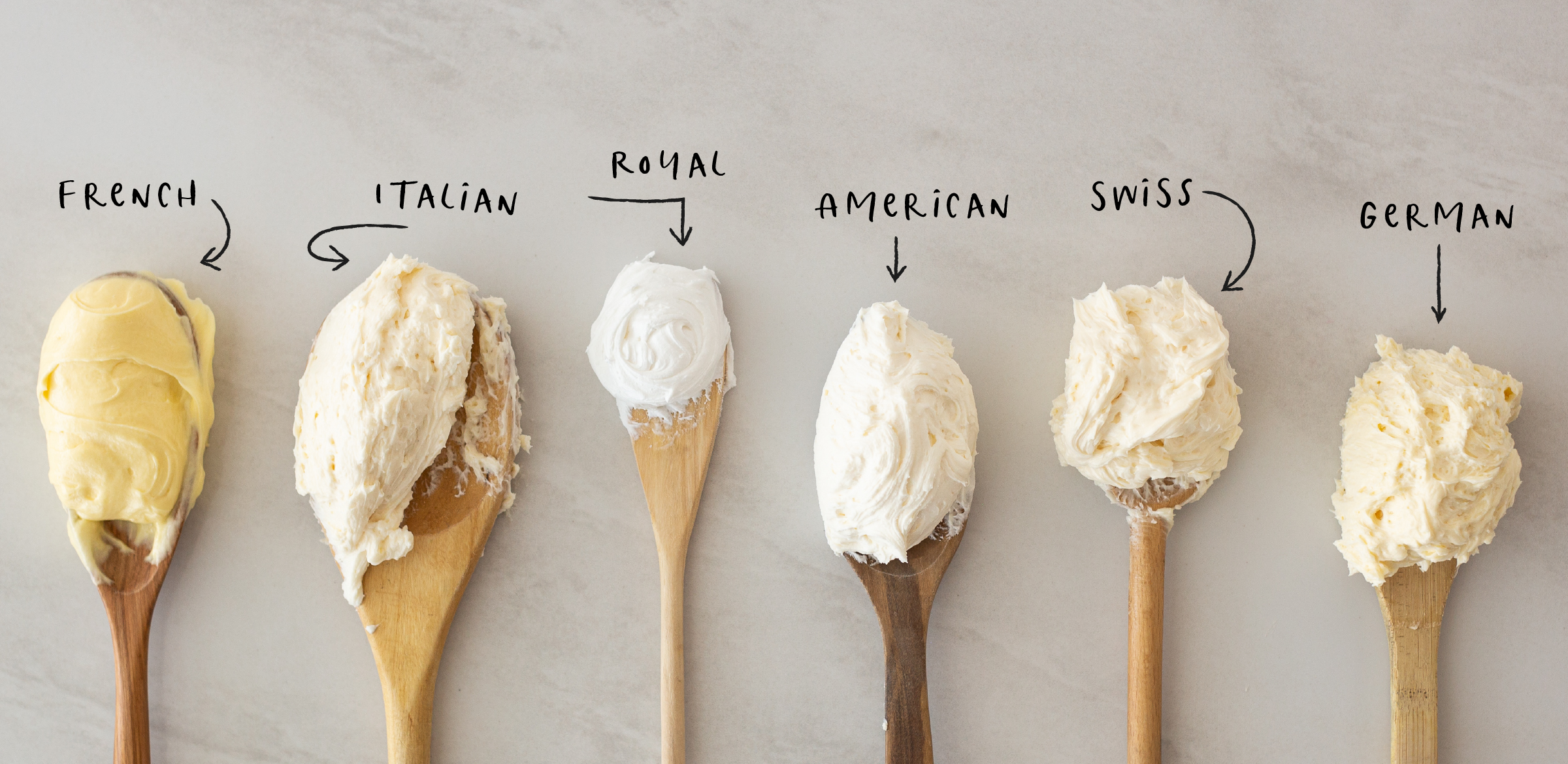





/__opt__aboutcom__coeus__resources__content_migration__serious_eats__seriouseats.com__recipes__images__2017__11__20170627-french-buttercream-vicky-wasik-13-3ee307eacded4ed5934ecdee0a3b61a6.jpg)
/__opt__aboutcom__coeus__resources__content_migration__serious_eats__seriouseats.com__images__2017__11__20170627-french-buttercream-vicky-wasik-14-bd756c25392a46ed9bc32405ca0968c7.jpg)
:max_bytes(150000):strip_icc()/Simply-Recipes-Strawberry-Buttercream-Frosting-LEAD-8-e3517eab63004f438f6df3cef201ea12.jpg)





































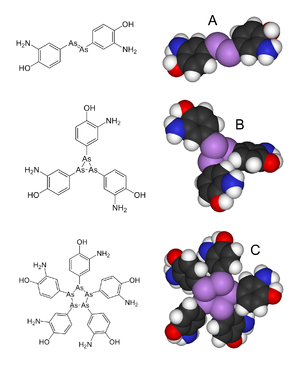
Arsphenamine, also known as Salvarsan or compound 606, is a drug that was introduced at the beginning of the 1910s as the first effective treatment for syphilis and African trypanosomiasis.[2] This organoarsenic compound was the first modern antimicrobial agent.[3]
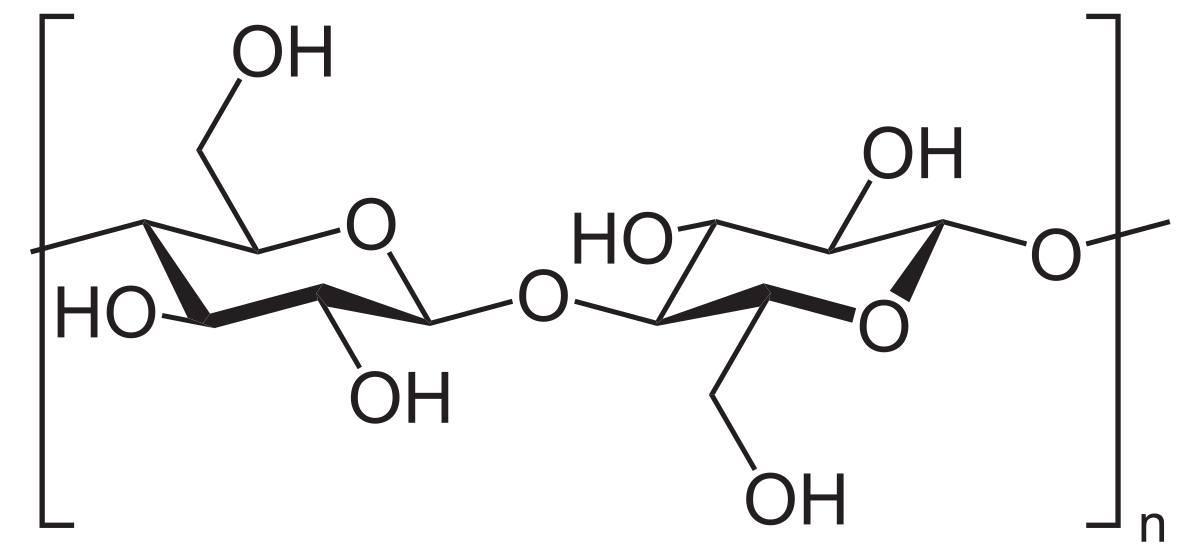
Palmitoylation is the covalent attachment of fatty acids, such as palmitic acid, to cysteine (S-palmitoylation) and less frequently to serine and threonine (O-palmitoylation) residues of proteins, which are typically membrane proteins.[2]The precise function of palmitoylation depends on the particular protein being considered. Palmitoylation enhances the hydrophobicity of proteins and contributes to their membrane association. Palmitoylation also appears to play a significant role in subcellular trafficking of proteins between membrane compartments,[3]as well as in modulating protein–protein interactions.[4] In contrast to prenylation and myristoylation, palmitoylation is usually reversible (because the bond between palmitic acid and protein is often a thioester bond). The reverse reaction in mammalian cells is catalyzed by acyl-protein thioesterases (APTs) in the cytosol and palmitoyl protein thioesterases in lysosomes. Because palmitoylation is a dynamic, post-translational process, it is believed to be employed by the cell to alter the subcellular localization, protein–protein interactions, or binding capacities of a protein.
An example of a protein that undergoes palmitoylation is hemagglutinin, a membrane glycoprotein used by influenza to attach to host cell receptors.[5] The palmitoylation cycles of a wide array of enzymes have been characterized in the past few years, including H-Ras, Gsα, the β2-adrenergic receptor, and endothelial nitric oxide synthase (eNOS). In signal transduction via G protein, palmitoylation of the α subunit, prenylation of the γ subunit, and myristoylation is involved in tethering the G protein to the inner surface of the plasma membrane so that the G protein can interact with its receptor.[6]
https://en.wikipedia.org/wiki/Palmitoylation
| Peripatus | |
|---|---|
 | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Onychophora |
| Class: | Udeonychophora |
| Order: | Euonychophora |
| Family: | Peripatidae |
| Genus: | Peripatus Guilding, 1826 |
| Species | |
See text | |
































































































































































































































![Confetti Cake [6 Inch 3 Layer] - Magnolia Bakery | magnoliabakeryonline.com](https://magnoliabakeryonline.com/usercontent/product_sub_img/IMG_43381.jpg)






























































































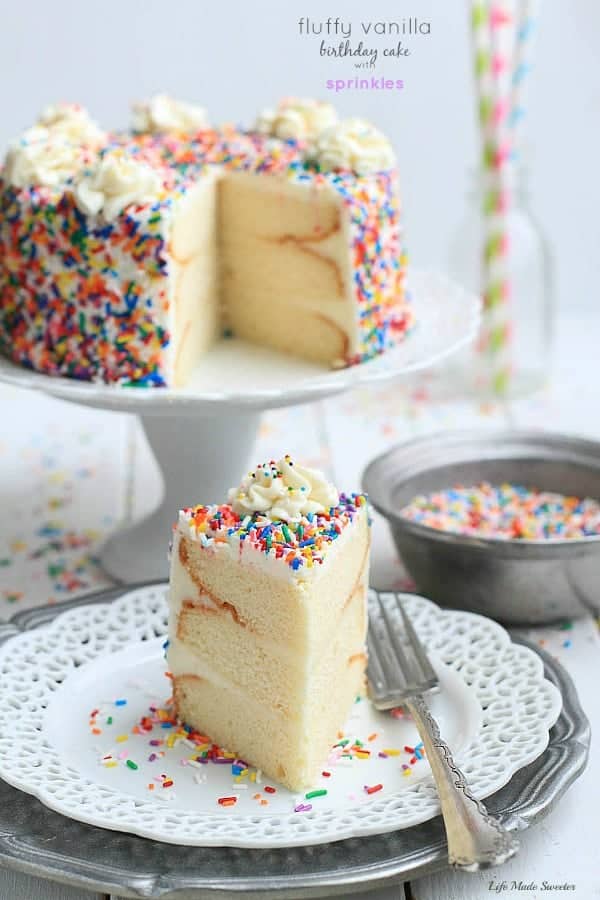


















































































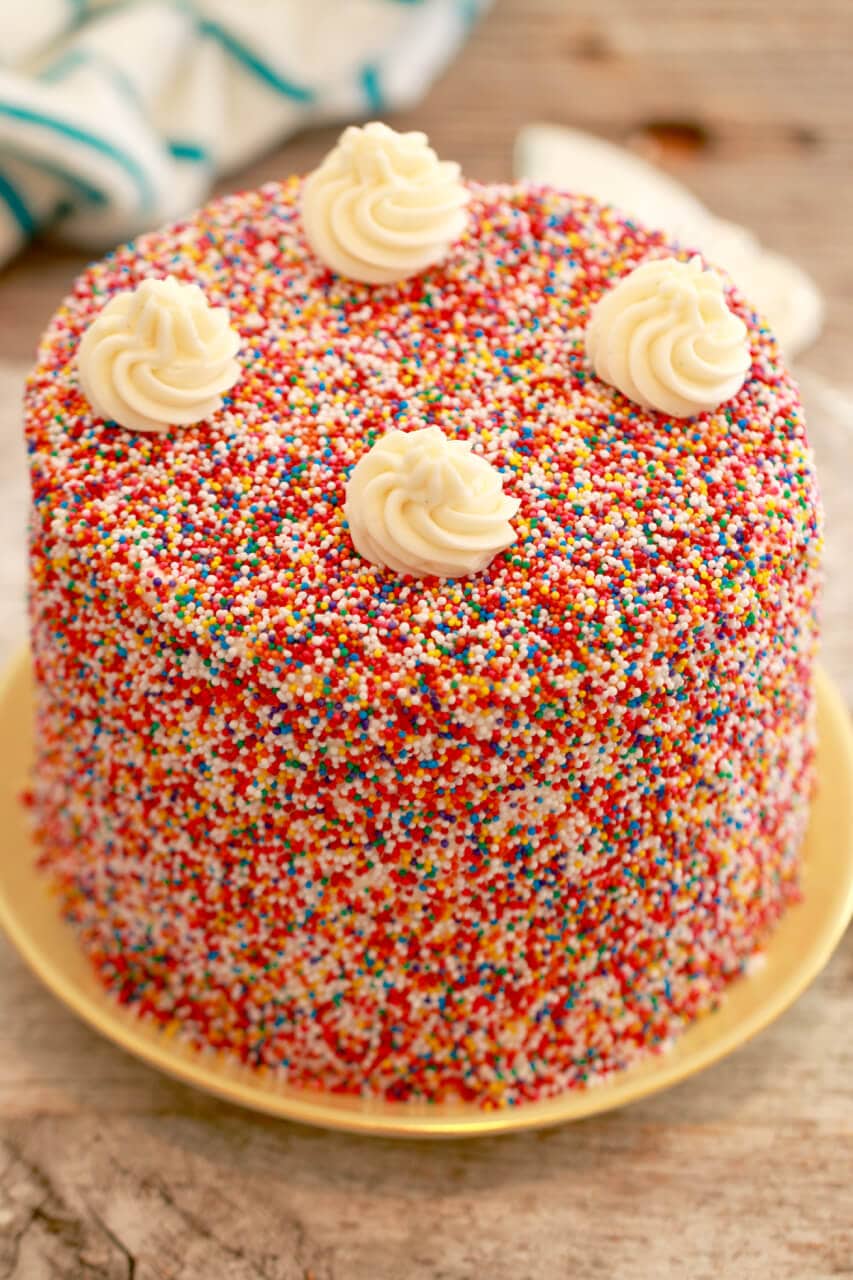






















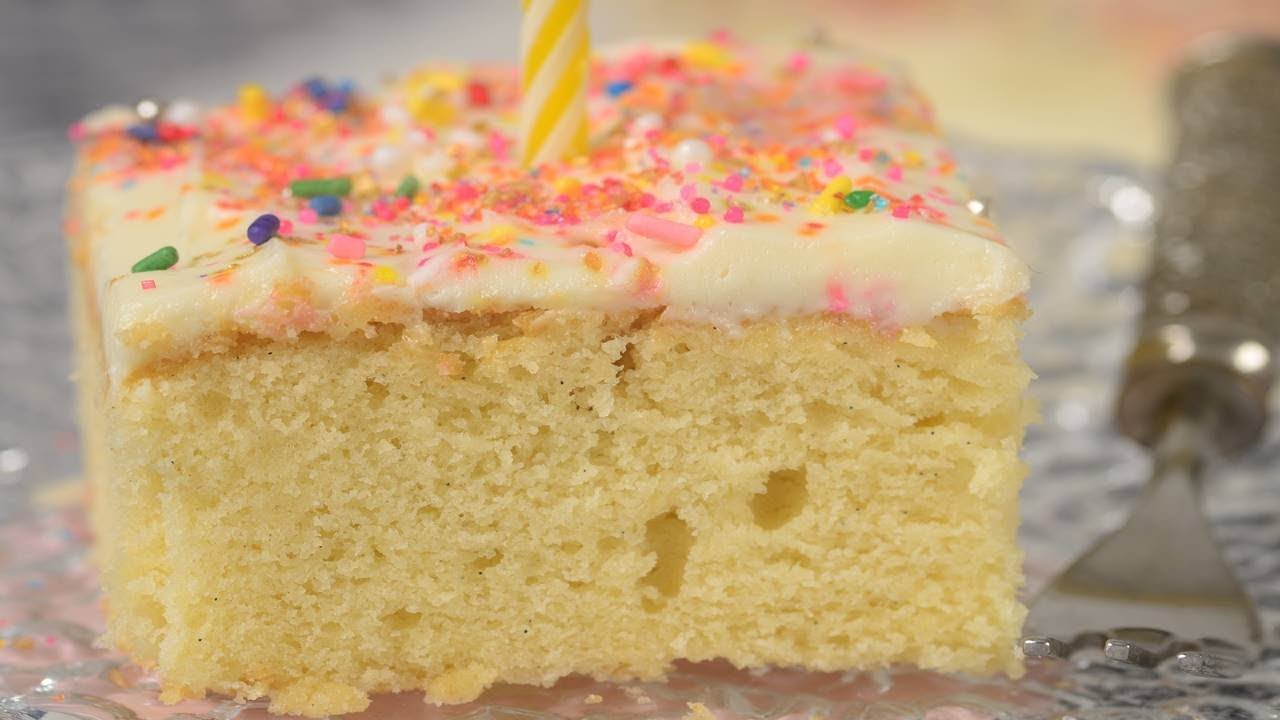

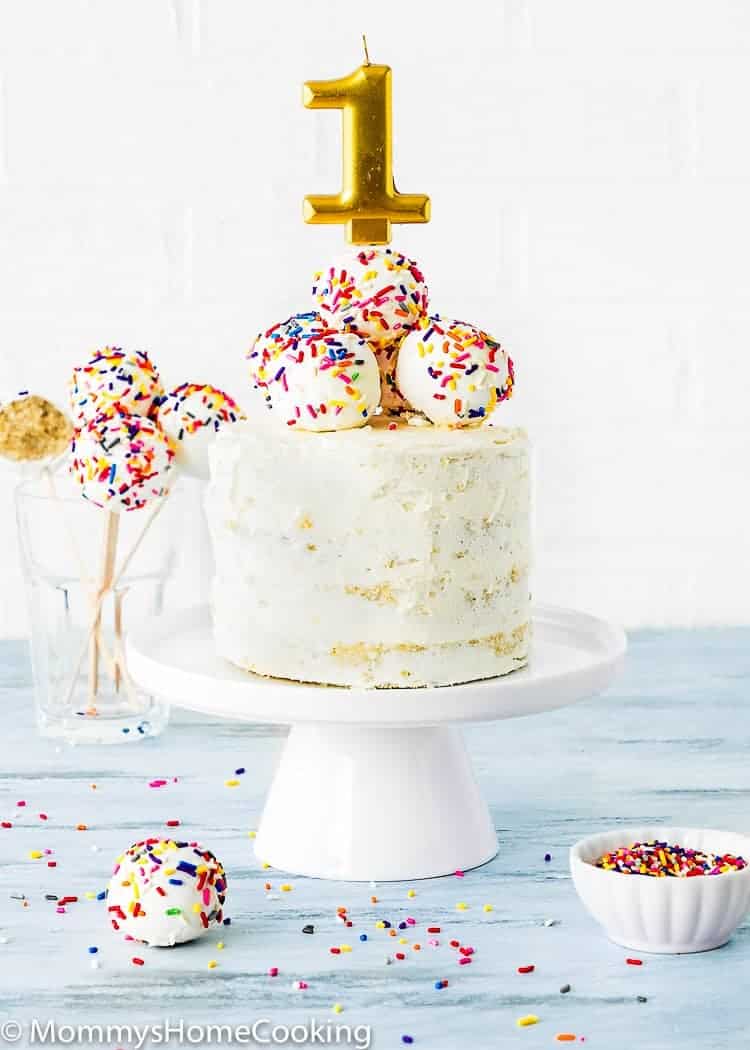



















































![Light Blue Mini Confetti Balloon Cake Toppers (Pack of 5) [COLCACT01LBL] | Light Blue Coloured Party Supplies | Coloured Party Supplies - Discount Party Supplies](https://www.discountpartysupplies.com.au/media/catalog/product/cache/f5eae1f501626484fd71bf8ea5701a5e/c/o/colcact01lbl.jpg)
















































































































































:focal(536x587:537x588)/https://public-media.si-cdn.com/filer/36/4a/364a861f-9f2f-43d5-810b-006b2b3ba2d0/kewpies-woman-suffrage-voting-kewpie-korner2.jpg)

























![Kewpie calendar] - O'Neill, Rose, 1874-1944](https://s3.amazonaws.com/pastperfectonline/images/museum_466/159/cgaimca239004-1.jpg)







No comments:
Post a Comment