Carnivore protoparvovirus 1 (CPPV 1) is a species of parvovirus that infects carnivorans. It causes a highly contagious disease in both dogs and cats. The disease is generally divided into two major genogroups: CPV-1 containing the classical feline panleukopenia virus (FPLV), and CPV-2 containing the canine parvovirus (CPV) which appeared in the 1970s.[2]
FPLV is known to infect all wild and domestic members of the felid (cat) family worldwide.[3] It is a highly contagious, severe infection that causes gastrointestinal, immune system, and nervous system disease. Its primary effect is to decrease the number of white blood cells, causing the disease known as feline panleukopenia.
It was once thought that only CPV-1 or FPLV infects cats.[4] However, it has been confirmed that a feline panleukopenia illness can be caused by CPV 2a, 2b, and 2c.[5][6]
FPLV is commonly referred to as:
- feline infectious enteritis virus (FIE)[3]
- feline parvovirus (FPV or FP or "feline parvo")[7]
- feline parvoviral enteritis[3]
It is sometimes confusingly referred to as "cat plague" and "feline distemper".[8]
In addition to members of the felid family, it can also affect other carnivorans (e.g. raccoon, mink).[3]
| Carnivore protoparvovirus 1 | |
|---|---|
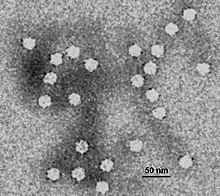 | |
| Electron micrograph of canine parvovirus | |
| Virus classification | |
| (unranked): | Virus |
| Realm: | Monodnaviria |
| Kingdom: | Shotokuvirae |
| Phylum: | Cossaviricota |
| Class: | Quintoviricetes |
| Order: | Piccovirales |
| Family: | Parvoviridae |
| Genus: | Protoparvovirus |
| Species: | Carnivore protoparvovirus 1 |
| Member virus[1] | |
| |
https://en.wikipedia.org/wiki/Carnivore_protoparvovirus_1
Canine parvovirus (also referred to as CPV, CPV2, or parvo) is a contagious virus mainly affecting dogs. CPV is highly contagious and is spread from dog to dog by direct or indirect contact with their feces. Vaccines can prevent this infection, but mortality can reach 91% in untreated cases. Treatment often involves veterinary hospitalization. Canine parvovirus may infect other mammals including foxes, wolves, cats, and skunks.[1] Felines are susceptible to panleukopenia, a different strain of parvovirus.[2]
| Canine parvovirus | |
|---|---|
 | |
| Electron micrograph of canine parvovirus | |
| Virus classification | |
| (unranked): | Virus |
| Realm: | Monodnaviria |
| Kingdom: | Shotokuvirae |
| Phylum: | Cossaviricota |
| Class: | Quintoviricetes |
| Order: | Piccovirales |
| Family: | Parvoviridae |
| Genus: | Protoparvovirus |
| Species: | |
| Virus: | Canine parvovirus |
https://en.wikipedia.org/wiki/Canine_parvovirus
Hemoglobinopathy is the medical term for a group of inherited blood disorders and diseases that primarily affect red blood cells.[1] They are single-gene disorders and, in most cases, they are inherited as autosomal co-dominant traits.[2]
There are two main groups: abnormal structural hemoglobin variants caused by mutations in the hemoglobin genes, and the thalassemias, which are caused by an underproduction of otherwise normal hemoglobin molecules. The main structural hemoglobin variants are HbS, HbE and HbC. The main types of thalassemia are alpha-thalassemia and beta thalassemia.[3]
The two conditions may overlap because some conditions which cause abnormalities in hemoglobin proteins also affect their production. Some hemoglobin variants do not cause pathology or anemia, and thus are often not classed as hemoglobinopathies.[4][5]
| Hemoglobinopathy | |
|---|---|
| Other names | Hemoglobinopathies |
 | |
| Red blood cells from a person with sickle cell trait | |
| Specialty | Hematology |
Erythema infectiosum, fifth disease, or slapped cheek syndrome[3] is one of several possible manifestations of infection by parvovirus B19.[4] Fifth disease typically presents as a rash and is more common in children. While parvovirus B19 can affect humans of all ages, only two out of ten individuals will present with physical symptoms.[5]
The name "fifth disease" comes from its place on the standard list of rash-causing childhood diseases, which also includes measles (first), scarlet fever (second), rubella (third), Dukes' disease (fourth, but is no longer widely accepted as distinct from scarlet fever), Erythema infectiosum parvovirus (fifth) and roseola (sixth).
| Erythema infectiosum | |
|---|---|
| Other names | Fifth disease, Slapped cheek syndrome, slapcheek, slap face, slapped face[1][2] |
 | |
| 16-month-old with erythema infectiosum | |
| Specialty | Infectious disease |
| Symptoms | Red rash, especially on cheeks |
| Causes | Human parvovirus |
| Gestational | |
|---|---|
| During birth | |
| Late pregnancy | |
| By breastfeeding | |

- File:Portrait of Gerard de Lairesse MET rl1975.1.140.R.jpg
- Created: 1665–67


The popularity of the Panacea is reflected in publications of its time. For example, the product was mentioned in the song The Connecticut Pedlar (c. 1851), where the pedlar's list of offerings includes "Swaim's panacea and Jonses's drops too."[23] And in an 1849 letter to the Southern Literary Messenger, Edgar Allan Poe defended the poetry of Bayard Taylor against critics "who possess little other ability than that which assures temporary success to them in common with Swaim's Panacea or Morrison's Pills." Abolitionist William Lloyd Garrison mentions "taking my third bottle of Swaim's Panacea" for scrofula in an 1836 letter.[24]
One of the most comprehensive accounts of the story of Swaim's Panacea is in the 1961 book The Toadstool Millionaires: A Social History of Patent Medicines in America before Federal Regulation by James Harvey Young.[16]
See also[edit]
| Tabes dorsalis | |
|---|---|
| Other names | Syphilitic myelopathy |
 | |
| Axial section of the spinal cord showing syphilitic destruction (whitened area, upper center) of the posterior columns which carry sensory information from the body to the brain | |
| Specialty | Neurology |
General paresis, also known as general paralysis of the insane (GPI) or paralytic dementia, is a severe neuropsychiatricdisorder, classified as an organic mental disorder and caused by the chronic meningoencephalitis that leads to cerebral atrophyin late-stage syphilis. Degenerative changes are associated primarily with the frontal and temporal lobar cortex. The disease affects approximately 7% of infected individuals. It is more common among men.
GPI was originally considered to be a type of madness due to a dissolute character, when first identified in the early 19th century. The condition's connection with syphilis was discovered in the late 1880s. Progressively, with the discovery of organic arsenicals such as Salvarsan and Neosalvarsan (1910s), the development of pyrotherapy (1920s), and the widespread availability and use of penicillin in the treatment of syphilis (1940s), the condition was rendered avoidable and curable. Prior to this, GPI was inevitably fatal, and it accounted for as much as 25% of the primary diagnoses for residents in public psychiatric hospitals.
Later: Delusions, dementia, tremors, hyperreflexia, seizures, cachexiaUsual onset10-30 years after initial infectionCausesMeningoencephalitis caused by syphilisRisk factorsUntreated syphilis infection
While retrospective studies have found earlier instances of what may have been the same disorder, the first clearly identified examples of paresis among the insane were described in Paris after the Napoleonic Wars. General paresis of the insane was first described as a distinct disease in 1822 by Antoine Laurent Jesse Bayle. General paresis most often struck people (men far more frequently than women) between 20 and 40 years of age. By 1877, for example, the superintendent of an asylum for men in New York reported that in his institution this disorder accounted for more than 12% of admissions and more than 2% of deaths.
Originally, the cause was believed to be an inherent weakness of character or constitution. While Friedrich von Esmarch and the psychiatrist Peter Willers Jessen had asserted as early as 1857 that syphilis caused general paresis (progressive Paralyse),[1] progress toward the general acceptance by the medical community of this idea was only accomplished later by the eminent 19th Century syphilographer Alfred Fournier (1832–1914). In 1913 all doubt about the syphilitic nature of paresis was finally eliminated when Hideyo Noguchi and J. W. Moore demonstrated the syphilitic spirochaetes in the brains of paretics.
In 1917 Julius Wagner-Jauregg discovered that pyrotherapy involving infecting paretic patients with malaria could halt the progression of general paresis. He won a Nobel Prize for this discovery in 1927. After World War II the use of penicillin to treat syphilis made general paresis a rarity: even patients manifesting early symptoms of actual general paresis were capable of full recovery with a course of penicillin. The disorder is now virtually unknown outside developing countries, and even there the epidemiology is substantially reduced.
Theo Van Gogh, brother of painter Vincent Van Gogh, died six months after Vincent in 1891 from "dementia parylitica" or what is now called syphilitic paresis.[2]
The Chicago gangster Al Capone died of syphilitic paresis, having contracted syphilis in a brothel prior to Prohibition and the Volstead Act and not having been treated for it in time to prevent the development of syphilitic paresis in himself.
https://en.wikipedia.org/wiki/General_paresis_of_the_insane
Neurosyphilis refers to infection of the central nervous system in a patient with syphilis. In the era of modern antibioticsthe majority of neurosyphilis cases have been reported in HIV-infected patients. Meningitis is the most common neurological presentation in early syphilis. Tertiary syphilis symptoms are exclusively neurosyphilis, though neurosyphilis may occur at any stage of infection.
To diagnose neurosyphilis, patients undergo a lumbar puncture to obtain cerebrospinal fluid (CSF) for analysis. The CSF is tested for antibodies for specific Treponema pallidum antigens. The preferred test is the VDRL test, which is sometimes supplemented by fluorescent treponemal antibody absorption test (FTA-ABS).[1][2][3]
Historically, the disease was studied under the Tuskegee study, a notable example of unethical human experimentation. The study was done on approximately 400 African-American men with untreated syphilis who were followed from 1932 to 1972 and compared to approximately 200 men without syphilis. The study began without informed consent of the subjects and was continued by the United States Public Health Service until 1972. The researchers failed to notify and withheld treatment for patients despite knowing penicillin was found as an effective cure for neurosyphilis. After four years of follow up, neurosyphilis was identified in 26.1% of patients vs. 2.5% of controls. After 20 years of followup, 14% showed signs of neurosyphilis and 40% had died from other causes.
https://en.wikipedia.org/wiki/Neurosyphilis
A gumma (plural gummata or gummas) is a soft, non-cancerous growth resulting from the tertiary stage of syphilis (and yaws[1]). It is a form of granuloma. Gummas are most commonly found in the liver (gumma hepatis), but can also be found in brain, heart, skin, bone, testis, and other tissues, leading to a variety of potential problems including neurologicaldisorders or heart valve disease.
https://en.wikipedia.org/wiki/Gumma_(pathology)
Tabes dorsalis is a late consequence of neurosyphilis, characterized by the slow degeneration (specifically, demyelination) of the neural tracts primarily in the dorsal root ganglia of the spinal cord (nerve root). These patients have lancinating nerve root pain which is aggravated by coughing, and features of sensory ataxia with ocular involvement.
https://en.wikipedia.org/wiki/Tabes_dorsalis
Arrhythmogenic cardiomyopathy (ACM), arrhythmogenic right ventricular dysplasia (ARVD), or arrhythmogenic right ventricular cardiomyopathy (ARVC), is an inherited heart disease.[1]
ACM is caused by genetic defects of the parts of heart muscle (also called myocardium or cardiac muscle) known as desmosomes, areas on the surface of heart muscle cells which link the cells together. The desmosomes are composed of several proteins, and many of those proteins can have harmful mutations.
The disease is a type of non-ischemic cardiomyopathy that primarily involves the right ventricle, though cases of exclusive left ventricular disease have been reported. It is characterized by hypokinetic areas involving the free wall of the ventricle, with fibrofatty replacement of the myocardium, with associated arrhythmias often originating in the right ventricle. The nomenclature ARVD is currently thought to be inappropriate and misleading as ACM does not involve dysplasia of the ventricular wall. Cases of ACM originating from the left ventricle led to the abandonment of the name ARVC.
ACM can be found in association with diffuse palmoplantar keratoderma, and woolly hair, in an autosomal recessive condition called Naxos disease, because this genetic abnormality can also affect the integrity of the superficial layers of the skin most exposed to pressure stress.[2]:513[3]
ACM is an important cause of ventricular arrhythmias in children and young adults. It is seen predominantly in males, and 30–50% of cases have a familial distribution.
| Arrhythmogenic cardiomyopathy | |
|---|---|
| Other names | arrhythmogenic right ventricular cardiomyopathy (ARVC), arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C), right ventricular dysplasia |
 | |
| Typical micro-histologic features of ARVC/D. Ongoing myocyte death (upper) with early fibrosis and adipocyte infiltration (lower). | |
| Specialty | Cardiology |
https://en.wikipedia.org/wiki/Arrhythmogenic_cardiomyopathy

No comments:
Post a Comment