Cyanogen iodide or iodine cyanide (ICN) is a pseudohalogen composed of iodine and the cyanide group. It is a relatively volatile and highly toxic inorganic compound. It occurs as white crystals that react slowly with water to form hydrogen cyanide.[4][5][6]
Cyanogen iodide is prepared by combining I2 and a cyanide, most commonly sodium cyanide in ice-cold water. The product is extracted with ether.[4][5][6]
- I2 + NaCN → NaI + ICN
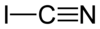
- Cyanogen iodide solutions in pyridine conduct electric current. Dilute solutions of ICN in pyridine are colorless at first, but upon standing become successively yellow, orange, red-brown and deep red-brown. This effect is due to a change in conductivity, which in turn is due to the formation of an electrolyte. When electrical conductivity of ICN is compared with that of iodine-pyridine solutions, the formation of the electrolyte in ICN proceeds much more slowly. Results confirm that cyanides are much weaker salts in pyridine than are iodides, although cyanogen iodide solutions are able to be dissolved in pyridine giving solutions with electrical conductivity that increases over time and results in maximum values.[14]
https://en.wikipedia.org/wiki/Cyanogen_iodide
No comments:
Post a Comment