Phosphorous
| Look up phosphorous in Wiktionary, the free dictionary. |
Phosphorous can refer to:
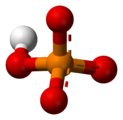
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid H
3PO
4.
The phosphate or orthophosphate ion [PO
4]3−
is derived from phosphoric acid by the removal of three protons H+
. Removal of one or two protons gives the dihydrogen phosphate ion [H
2PO
4]−
and the hydrogen phosphate ion [HPO
4]2−
ion, respectively. These names are also used for salts of those anions, such as ammonium dihydrogen phosphate and trisodium phosphate.
In organic chemistry, phosphate or orthophosphate is an organophosphate, an ester of orthophosphoric acid of the form PO
4RR′R″ where one or more hydrogen atoms are replaced by organic groups. An example is trimethyl phosphate, (CH
3)
3PO
4. The term also refers to the trivalent functional group OP(O-)
3 in such esters.
Orthophosphates are especially important among the various phosphates because of their key roles in biochemistry, biogeochemistry, and ecology, and their economic importance for agriculture and industry.[2]The addition and removal of phosphate groups (phosphorylation and dephosphorylation) are key steps in cellmetabolism.
Orthophosphates can condense to form pyrophosphates.
Biochemistry of phosphates[edit]
In biological systems, phosphorus can be found as free phosphate anions in solution (inorganic phosphate) or bound to organic molecules as various organophosphates.
Inorganic phosphate is generally denoted Pi and at physiological (homeostatic) pH primarily consists of a mixture of [HPO
4]2−
and [H
2PO
4]−
ions. At a neutral pH, as in the cytosol (pH = 7.0), the concentrations of the orthophoshoric acid and its three anions have the ratios
- [ H
2PO−
4 ] / [ H
3PO
4 ] ≈ 7.5 × 104 - [ HPO2−
4 ] / [ H
2PO−
4 ] ≈ 0.62 - [ PO3−
4 ] / [ HPO2−
4 ] ≈ 2.14 × 10−6
Thus, only [H
2PO
4]−
and [HPO
4]2−
ions are present in significant amounts in the cytosol (62% [H
2PO
4]−
, 38% [HPO
4]2−
). In extracellular fluid (pH = 7.4), this proportion is inverted (61% [HPO
4]2−
, 39% [H
2PO
4]−
).
Inorganic phosphate can be present also as of pyrophosphate anions [P
2O
7]4−
, which can give orthophosphate by hydrolysis:
- [P
2O
7]4−
+ H2O ⇌ 2 [HPO
4]2−
Organic phosphates are commonly found in the form of esters as nucleotides (e.g. AMP, ADP, and ATP) and in DNA and RNA. Free orthophosphate anions can be released by the hydrolysis of the phosphoanhydride bonds in ATP or ADP. These phosphorylation and dephosphorylation reactions are the immediate storage and source of energy for many metabolic processes. ATP and ADP are often referred to as high-energy phosphates, as are the phosphagens in muscle tissue. Similar reactions exist for the other nucleoside diphosphates and triphosphates.
Bones and teeth[edit]
An important occurrence of phosphates in biological systems is as the structural material of bone and teeth. These structures are made of crystalline calcium phosphate in the form of hydroxyapatite. The hard dense enamel of mammalian teeth consists of fluoroapatite, a hydroxy calcium phosphate where some of the hydroxyl groups have been replaced by fluoride ions.
Medical and biological research uses[edit]
The medicinal type (salt) of phosphorus is phosphate. Some phosphates, which help cure many urinary tract infections, are used to make urine more acidic. To avoid the development of calcium stones in the urinary tract, some phosphates are used.[5] For patients who are unable to get enough phosphorus in their daily diet, phosphates are used as dietary supplements, usually because of certain disorders or diseases.[5] Injectable phosphates can only be handled by a health care provider.[5]
Plant metabolism[edit]
Plants take up phosphorus through several pathways: the arbuscular mycorrhizal pathway and the direct uptake pathway.
https://en.wikipedia.org/wiki/Phosphate#Biochemistry_of_phosphates



No comments:
Post a Comment