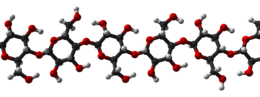
Cellulose is mainly used to produce paperboard and paper. Smaller quantities are converted into a wide variety of derivative products such as cellophane and rayon. Conversion of cellulose from energy crops into biofuels such as cellulosic ethanol is under development as a renewable fuel source. Cellulose for industrial use is mainly obtained from wood pulp and cotton.[6]
Cellulose[1]
 Identifiers
IdentifiersCAS Number
9004-34-6
 ChEMBL
ChEMBLChEMBL2109009
 ChemSpider
ChemSpiderNoneECHA InfoCard100.029.692
232-674-9E numberE460 (thickeners, ...)
PubChem CID
14055602UNII
SMD1X3XO9M

CompTox Dashboard (EPA)
DTXSID3050492
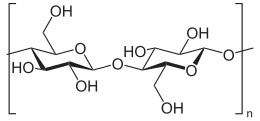
Chemical formula(C
6H
10O
5)
nMolar mass162.1406 g/mol per glucose unit Appearancewhite powderDensity1.5 g/cm3Melting point260–270 °C; 500–518 °F; 533–543 K Decomposes[2]
Solubility in waternoneThermochemistry
Std enthalpy of
formation(ΔfH⦵298)−963,000 kJ/mol[clarification needed]
Std enthalpy of
combustion(ΔcH⦵298)−2828,000 kJ/mol[clarification needed]HazardsNFPA 704(fire diamond)

1
1
0NIOSH (US health exposure limits):
PEL(Permissible)TWA 15 mg/m3 (total) TWA 5 mg/m3 (resp)[2]
REL(Recommended)TWA 10 mg/m3 (total) TWA 5 mg/m3 (resp)[2]
IDLH (Immediate danger)N.D.[2]Related compounds
Related compoundsStarch
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
 verify (what is
verify (what is 
 ?)Infobox references
?)Infobox referenceshttps://en.wikipedia.org/wiki/Cellulose
Above. chamillionaire hip hop police
above. candy shop 50 cent
No comments:
Post a Comment