The following is a list of rock types recognized by geologists. There is no agreed number of specific types of rocks. Any unique combination of chemical composition, mineralogy, grain size, texture, or other distinguishing characteristics can describe a rock type. Additionally, different classification systems exist for each major type of rock.[1] There are three major types of rock: igneous rock, metamorphic rock, and sedimentary rock.
Igneous rocks
- Adakite – Volcanic rock type
- Andesite – Type of volcanic rock
- Alkali feldspar granite – Type of igneous rock rich in alkali feldspar
- Anorthosite – Mafic intrusive igneous rock composed predominantly of plagioclase
- Aplite – Fine-grained intrusive igneous rock type similar to granite
- Basalt – Magnesium- and iron-rich extrusive igneous rock
- Basaltic trachyandesite – Naming system for volcanic rocks
- Mugearite – Volcanic rock type
- Shoshonite – Potassium-rich variety of basaltic trachyandesite
- Basanite – A silica-undersaturated basalt
- Blairmorite – Rare porphyritic volcanic rock
- Boninite – Ultramafic extrusive rock high in both magnesium and silica
- Carbonatite – Igneous rock with more than 50% carbonate minerals
- Charnockite – Type of granite containing orthopyroxene
- Enderbite – Igneous rock of the charnockite series
- Dacite – Volcanic rock intermediate in composition between andesite and rhyolite
- Diabase, also known as dolerite – Type of igneous rock
- Diorite – Igneous rock type
- Napoleonite, also known as corsite – Variety of diorite with orbicular structure
- Dunite – Ultramafic and ultrabasic rock from Earth's mantle which is made of the mineral olivine
- Essexite – Igneous rock type
- Foidolite – Igneous rock rich in feldspathoid minerals
- Gabbro – Coarse-grained mafic intrusive rock
- Granite – Common type of intrusive, felsic, igneous rock with granular structure
- Granodiorite – Type of coarse grained intrusive igneous rock
- Granophyre – Subvolcanic rock that contains quartz and alkali feldspar in characteristic angular intergrowths
- Harzburgite – Ultramafic mantle rock
- Hornblendite – Plutonic rock consisting mainly of the amphibole hornblende
- Hyaloclastite – Volcaniclastic accumulation or breccia
- Icelandite – Igneous rock type
- Ignimbrite – Type of volcanic rock
- Ijolite – Igneous rock consisting essentially of nepheline and augite
- Kimberlite – Igneous rock which sometimes contains diamonds
- Komatiite – Ultramafic mantle-derived volcanic rock
- Lamproite – Ultrapotassic mantle-derived volcanic or subvolcanic rock
- Lamprophyre – Ultrapotassic igneous rocks – An ultramafic, ultrapotassic intrusive rock dominated by mafic phenocrysts in a feldspar groundmass
- Latite – Type of volcanic rock – A silica-undersaturated form of andesite
- Lherzolite – an ultramafic and ultrabasic rock that is composed of olivine and pyroxene – An ultramafic rock, essentially a peridotite
- Monzogranite – A silica-undersaturated granite with <5% normative quartz
- Monzonite – Igneous intrusive rock with low quartz and equal plagioclase and alkali feldspar – a plutonic rock with <5% normative quartz
- Nepheline syenite – Holocrystalline plutonic rock – A silica-undersaturated plutonic rock of nepheline and alkali feldspar
- Nephelinite – Igneous rock made up almost entirely of nepheline and clinopyroxene – A silica-undersaturated plutonic rock with >90% nepheline
- Norite – igneous rock – A hypersthene-bearing gabbro
- Obsidian – Naturally occurring volcanic glass
- Pegmatite – Igneous rock with very large interlocked crystals
- Peridotite – Coarse-grained ultramafic igneous rock type
- Phonolite – Uncommon extrusive rock – A silica-undersaturated volcanic rock; essentially similar to nepheline syenite
- Phonotephrite – strongly alkaline volcanic rock with a composition between phonolite and tephrite – A volcanic rock with a composition between phonolite and tephrite
- Picrite – Variety of high-magnesium basalt that is very rich in the mineral olivine – An olivine-bearing basalt
- Porphyry – Textural form of igneous rock with large grained crystals in a fine matrix
- Pumice – Light colored highly vesicular volcanic rock
- Pyroxenite – Igneous rock - a coarse grained plutonic rock composed of >90% pyroxene
- Quartz diorite – Igneous, plutonic rock – A diorite with >5% modal quartz
- Quartz monzonite – Type of igneous rock – An intermediate plutonic rock, essentially a monzonite with 5–10% modal quartz
- Quartzolite – Extremely rare igneous rock made mostly of quartz – An intrusive rock composed mostly of quartz
- Rhyodacite – Volcanic rock rich in silica and low in alkali metal oxides – A felsic volcanic rock which is intermediate between a rhyolite and a dacite
- Rhyolite – Igneous, volcanic rock, of felsic (silica-rich) composition
- Comendite – Hard, peralkaline igneous rock, a type of light blue grey rhyolite
- Pantellerite – Peralkaline rhyolite type of volcanic rock
- Scoria – Dark vesicular volcanic rock
- Shonkinite – Intrusive igneous rock – a plutonic rock
- Sovite – igneous rock – A coarse-grained carbonatite rock
- Syenite – Intrusive igneous rock – A plutonic rock dominated by orthoclase feldspar; a type of granitoid
- Tachylyte – Form of basaltic volcanic glass – Essentially a basaltic glass
- Tephriphonolite – A volcanic rock with a composition between phonotephrite and phonolite
- Tephrite – Igneous, volcanic rock – A silica-undersaturated volcanic rock
- Tonalite – igneous rock – A plagioclase-dominant granitoid
- Trachyandesite – Extrusive igneous rock – An alkaline intermediate volcanic rock
- Benmoreite – Volcanic rock type - sodic trachyandesite
- Trachybasalt – A volcanic rock with a composition between basalt and trachyte
- Trachyte – Extrusive igneous rock – A silica-undersaturated volcanic rock; essentially a feldspathoid-bearing rhyolite
- Troctolite – Igneous rock – A plutonic ultramafic rock containing olivine, pyroxene and plagioclase
- Trondhjemite – Light-colored intrusive igneous rock – A form of tonalite where plagioclase-group feldspar is oligoclase
- Tuff – Rock consolidated from volcanic ash
- Websterite – ultramafic and ultrabasic rock – A type of pyroxenite, composed of clinoproxene and orthopyroxene
- Wehrlite – Ultramafic rock - An ultramafic plutonic or cumulate rock, a type of peridotite, composed of olivine and clinopyroxene
Sedimentary rocks
- Argillite – Sedimentary rock, mostly of indurated clay particles
- Arkose – Type of sandstone containing at least 25% feldspar
- Banded iron formation – Distinctive layered units of iron-rich sedimentary rock that are almost always of Precambrian age
- Breccia – Rock composed of broken fragments cemented by a matrix
- Calcarenite – Type of limestone that is composed predominantly of sand-size grains
- Chalk – Soft, white, porous sedimentary rock made of calcium carbonate
- Chert – Hard, fine-grained sedimentary rock composed of cryptocrystalline silica
- Claystone – Clastic sedimentary rock composed primarily of clay-sized particles
- Coal – Combustible sedimentary rock composed primarily of carbon
- Conglomerate – Coarse-grained sedimentary rock composed mostly of rounded to sub-angular fragments
- Coquina – Sedimentary rock that is composed mostly of fragments of shells
- Diamictite – Type of sedimentary rock
- Diatomite – Soft, siliceous sedimentary rock that is easily crumbled
- Dolomite (rock), also known as Dolostone – Sedimentary carbonate rock that contains a high percentage of the mineral dolomite
- Evaporite – Water-soluble mineral deposit formed by evaporation from an aqueous solution
- Flint – Cryptocrystalline form of the mineral quartz
- Geyserite – Form of opaline silica that is often found around hot springs and geysers
- Greywacke – Hard, dark sandstone with poorly sorted angular grains in a compact, clay-fine matrix
- Gritstone – Hard, coarse-grained, siliceous sandstone
- Itacolumite – Porous sandstone known for flexibility
- Jaspillite – Banded mixture of hematite and quartz
- Laterite – Product of rock weathering in wet tropical climate rich in iron and aluminium
- Lignite – Soft, brown, combustible, sedimentary rock
- Limestone – Sedimentary rocks made of calcium carbonate
- Marl – Lime-rich mud or mudstone which contains variable amounts of clays and silt
- Mudstone – Fine grained sedimentary rock whose original constituents were clays or muds
- Oil shale – Organic-rich fine-grained sedimentary rock containing kerogen
- Oolite – Sedimentary rock formed from ooids
- Phosphorite – Sedimentary rock containing large amounts of phosphate minerals – A non-detrital sedimentary rock that contains high amounts of phosphate minerals
- Sandstone – Type of sedimentary rock
- Shale – Fine-grained, clastic sedimentary rock
- Siltstone – Sedimentary rock which has a grain size in the silt range
- Sylvinite – A sedimentary rock made of a mechanical mixture of sylvite and halite
- Tillite – Till which has been indurated or lithified by burial
- Travertine – Form of limestone deposited by mineral springs
- Tufa – Porous limestone rock formed when carbonate minerals precipitate out of ambient temperature water
- Turbidite – Geologic deposit of a turbidity current
- Wackestone – Mud-supported carbonate rock that contains greater than 10% grains
Metamorphic rocks
- Anthracite – Hard, compact variety of coal
- Amphibolite – A metamorphic rock containing mainly amphibole and plagioclase
- Blueschist – Type of metavolcanic rock
- Cataclasite – Rock found at geological faults – A rock formed by faulting
- Eclogite – A dense metamorphic rock formed under high pressure
- Gneiss – Common high-grade metamorphic rock
- Granulite – Class of high-grade medium to coarse grained metamorphic rocks
- Greenschist – Metamorphic rocks – A mafic metamorphic rock dominated by green amphiboles
- Hornfels – series of contact metamorphic rocks that have been baked and indurated by the heat of intrusive igneous masses
- Calcflinta – A type of hornfels found in the Scottish Highlands
- Litchfieldite – Nepheline syenite gneiss
- Marble – Type of rock – a metamorphosed limestone
- Migmatite – Mixture of metamorphic rock and igneous rock
- Mylonite – Metamorphic rock – A metamorphic rock formed by shearing
- Metapelite – Metamorphic rock – A metamorphic rock with a protolith of clay-rich (siltstone) sedimentary rock
- Metapsammite – A metamorphic rock with a protolith of quartz-rich (sandstone) sedimentary rock
- Phyllite – Type of foliated metamorphic rock – A low grade metamorphic rock composed mostly of micaceous minerals
- Pseudotachylite – Glassy, or very fine-grained, rock type – A glass formed by melting within a fault via friction
- Quartzite – Hard, non-foliated metamorphic rock which was originally pure quartz sandstone – A metamorphosed sandstone typically composed of >95% quartz
- Schist – Easily split medium-grained metamorphic rock
- Serpentinite – Rock formed by hydration and metamorphic transformation of olivine
- Skarn – Hard, coarse-grained, hydrothermally altered metamorphic rocks
- Slate – Metamorphic rock - A low grade metamorphic rock formed from shale or silts
- Suevite – Rock consisting partly of melted material formed during an impact event – A rock formed by partial melting during a meteorite impact
- Talc carbonate – A metamorphosed ultramafic rock with talc as an essential constituent; similar to a serpentinite
- Tectonite – rock type – A rock whose fabric reflects the history of its deformation
- Whiteschist – A high pressure metamorphic rock containing talc and kyanite
Specific varieties
The following are terms for rocks that are not petrographically or genetically distinct but are defined according to various other criteria; most are specific classes of other rocks, or altered versions of existing rocks. Some archaic and vernacular terms for rocks are also included.
- Adamellite – Type of igneous rock – A variety of quartz monzonite
- Appinite – A group of varieties of lamprophyre, mostly rich in hornblende
- Aphanite – Igneous rock composed of very small crystals invisible to the naked eye
- Borolanite – Variety of nepheline syenite from Loch Borralan, Scotland – A variety of nepheline syenite from Loch Borralan, Scotland
- Blue Granite – Variety of monzonite, an igneous rock
- Epidosite – Hydrothermally altered epidote- and quartz-bearing rock
- Felsite – Very fine-grained volcanic rock that sometimes contains larger crystals
- Flint – Cryptocrystalline form of the mineral quartz
- Ganister – Hard, fine-grained quartzose sandstone, or orthoquartzite
- Gossan – Intensely oxidized, weathered or decomposed rock
- Hyaloclastite – Volcaniclastic accumulation or breccia
- Ijolite – Igneous rock consisting essentially of nepheline and augite
- Jadeitite – Metamorphic rock found in blueschist-grade metamorphic terranes
- Jasperoid – A hematite-silica metasomatite analogous to a skarn
- Kenyte – Type of igneous rock - A variety of phonolite, first found on Mount Kenya
- Lapis lazuli – Metamorphic rock containing lazurite, prized for its intense blue color - A rock composed of lazurite and other minerals
- Larvikite – Variety of monzonite, an igneous rock
- Litchfieldite – A metamorphosed nepheline syenite occurrence near Litchfield, Maine
- Llanite – Type of mineral – A hypabyssal rhyolite with microcline and blue quartz phenocrysts from the Llano Uplift in Texas
- Luxullianite – Rare type of granite
- Mangerite – Plutonic intrusive igneous rock, that is essentially a hypersthene-bearing monzonite
- Minette – A variety of lamprophyre
- Novaculite – A type of chert found in Oklahoma, Arkansas, and Texas
- Pietersite – Breccia rock of hawk's eyes and tiger's eyes
- Pyrolite – theoretical rock making up the earth's upper mantle – A chemical analogue considered to theoretically represent the earth's upper mantle
- Rapakivi granite – Type of igneous rock
- Rhomb porphyry – Textural form of igneous rock with large grained crystals in a fine matrix – A type of latite with euhedral rhombic phenocrysts of feldspar
- Rodingite – metamorphic rock – A mafic rock metasomatized by serpentinization fluids
- Shonkinite – Intrusive igneous rock – melitilic and kalsititic rocks
- Taconite – Variety of iron-bearing sedimentary rock
- Tachylite – Form of basaltic volcanic glass
- Teschenite – A silica undersaturated, analcime bearing gabbro
- Theralite – igneous rock – A nepheline gabbro
- Unakite – metamorphic rock – An altered granite
- Variolite – Igneous rocks which contain varioles
- Vogesite – Ultrapotassic igneous rocks – A variety of lamprophyre
- Wad – Porous secondary manganese oxyhydroxide – A rock rich in manganese oxide or manganese hydroxide
See also
- List of minerals – List of minerals with Wikipedia articles
- List of rocks on Mars – Alphabetical list of named rocks and meteorites found on Mars
- Rock cycle – Transitional concept of geologic time
- List of rock formations: for a list of unusual or culturally significant rock outcrops
- Leaverite – Rock in the field that looks interesting but is actually not
References
- "BGS Rock Classification Scheme - Igneous - Metamorphic - Sedimentary - Superficial". British Geological Survey (BGS). Retrieved 2019-05-28.
External links
https://en.wikipedia.org/wiki/List_of_rock_types
Category:Geology-related lists
Geology-related lists.
Subcategories
This category has the following 20 subcategories, out of 20 total.
- Geology-related lists by country (13 C)
+
E
- Lists of earthquakes (3 C, 24 P)
F
- Lists of fossiliferous stratigraphic units (3 C, 1 P)
G
- Gemstone-related lists (1 C, 10 P)
I
L
- Lists of rocks (8 P)
M
- Lists of mountains by range (2 C, 15 P)
O
P
- Paleontology lists (5 C, 12 P)
S
- Seismology related lists (2 C, 3 P)
V
- Lists of volcanoes (109 P)
Pages in category "Geology-related lists"
The following 59 pages are in this category, out of 59 total. This list may not reflect recent changes.
C
F
G
L
M
- List of types of marble
- List of meteorite minerals
- List of mineral symbols
- List of mineralogists
- Classification of non-silicate minerals
- Classification of silicate minerals
- List of minerals by optical properties
- List of minerals named after people
- List of minerals
- List of minerals recognized by the International Mineralogical Association
- List of longest mountain chains on Earth
P
https://en.wikipedia.org/wiki/Category:Geology-related_lists
The world's longest above-water mountain range is the Andes,[1][2] about 7,000 km (4,300 mi) long. The range stretches from north to south through seven countries in South America, along the west coast of the continent: Venezuela, Colombia, Ecuador, Peru, Bolivia, Chile, and Argentina. Aconcagua is the highest peak, at about 6,962 m (22,841 ft).
This list does not include submarine mountain ranges. If submarine mountains are included, the longest is the global mid-ocean ridge system which extends for about 65,000 km (40,000 mi).[3]
https://en.wikipedia.org/wiki/List_of_longest_mountain_chains_on_Earth
https://en.wikipedia.org/wiki/List_of_mountain_ranges
https://en.wikipedia.org/wiki/Himalayas
https://en.wikipedia.org/wiki/Great_Dividing_Range
https://en.wikipedia.org/wiki/Transantarctic_Mountains
https://en.wikipedia.org/wiki/Category:Industrial_gases
Gas blending is the process of mixing gases for a specific purpose where the composition of the resulting mixture is specified and controlled. A wide range of applications include scientific and industrial processes, food production and storage and breathing gases.
Gas mixtures are usually specified in terms of molar gas fraction (which is closely approximated by volumetric gas fraction for many permanent gases): by percentage, parts per thousand or parts per million. Volumetric gas fraction converts trivially to partial pressure ratio, following Dalton's law of partial pressures. Partial pressure blending at constant temperature is computationally simple, and pressure measurement is relatively inexpensive, but maintaining constant temperature during pressure changes requires significant delays for temperature equalization. Blending by mass fraction is unaffected by temperature variation during the process, but requires accurate measurement of mass or weight, and calculation of constituent masses from the specified molar ratio. Both partial pressure and mass fraction blending are used in practice.
https://en.wikipedia.org/wiki/Gas_blending
https://en.wikipedia.org/wiki/Dalton%27s_law
https://en.wikipedia.org/wiki/Gas_laws
Gas mixtures for brewing
- Sparging: An inert gas such as nitrogen is bubbled through the wine, which removes the dissolved oxygen. Carbon dioxide is also removed and to ensure that an appropriate amount of carbon dioxide remains, a mixture of nitrogen and carbon dioxide may be used for the sparging gas.[3]
- Purging and blanketing: The removal of oxygen from the headspace above the wine in a container by flushing with a similar gas mixture to that used for sparging is called purging, and if it is left there it is called blanketing or inerting.[3]
https://en.wikipedia.org/wiki/Gas_blending
The anesthetic machine is used to blend breathing gas for patients under anesthesia during surgery. The gas mixing and delivery system lets the anesthetist control oxygen fraction, nitrous oxide concentration and the concentration of volatile anesthetic agents.[5] The machine is usually supplied with oxygen (O2) and nitrous oxide (N2O) from low pressure lines and high pressure reserve cylinders, and the metered gas is mixed at ambient pressure, after which additional anesthetic agents may be added by a vaporizer, and the gas may be humidified. Air is used as a diluent to decrease oxygen concentration. In special cases other gases may also be added to the mixture. These may include carbon dioxide (CO2), used to stimulate respiration, and helium (He) to reduce resistance to flow or to enhance heat transfer.[6]
Gas mixing systems may be mechanical, using conventional rotameter banks, or electronic, using proportional solenoids or pulsed injectors, and control may be manual or automatic.[5]
Controlled atmosphere manufacture and storage
Protective gas mixtures may be used to exclude air or other gases from the surface of sensitive materials during processing. Examples include melting of reactive metals such as magnesium, and heat treatment of steels.Customized gas mixtures for analytical applications
- Span gases are used for testing and calibrating gas detection equipment by exposing the sensor to a known concentration of a contaminant. The gases are used as a reference point to ensure correct readings after calibration and have very accurate composition, with a content of the gas to be detected close to the set value for the detector.
- Zero gas is normally a gas free of the component to be measured, and as similar as practicable to the composition of the gas to be monitored, used to calibrate the zero point of the sensor.
Calibration gas mixtures are generally produced in batches by gravimetric or volumetric methods.
The gravimetric method uses sensitive and accurately calibrated scales to weigh the amounts of gases added into the cylinder. Precise measurement is required as inaccuracy or impurities can result in incorrect calibration. The container for calibration gas must be as close to perfectly clean as practicable. The cylinders may be cleaned by purging with high purity nitrogen, the vacuumed. For particularly critical mixtures the cylinder may be heated while being vacuumed to facilitate removal of any impurities adhering to the walls.[7]
After filling, the gas mixture must be thoroughly mixed to ensure that all components are evenly distributed throughout the container to prevent possible variations on composition within the container. This is commonly done by rolling the container horizontally for 2 to 4 hours.[7]
Oxygen content is relatively simple to monitor using electro-galvanic cells and these are routinely used in the underwater diving industry for this purpose, though other methods may be more accurate and reliable.
https://en.wikipedia.org/wiki/Gas_blending
Underwater acoustic communication is a technique of sending and receiving messages below water.[1] There are several ways of employing such communication but the most common is by using hydrophones. Underwater communication is difficult due to factors such as multi-path propagation, time variations of the channel, small available bandwidth and strong signal attenuation, especially over long ranges. Compared to terrestrial communication, underwater communication has low data rates because it uses acoustic waves instead of electromagnetic waves.
At the beginning of the 20th century some ships communicated by underwater bells as well as using the system for navigation. Submarine signals were at the time competitive with the primitive maritime radionavigation.[2] The later Fessenden oscillator allowed communication with submarines.
https://en.wikipedia.org/wiki/Underwater_acoustic_communication
Radio navigation or radionavigation is the application of radio frequencies to determine a position of an object on the Earth, either the vessel or an obstruction.[1][2] Like radiolocation, it is a type of radiodetermination.
The basic principles are measurements from/to electric beacons, especially
- Angular directions, e.g. by bearing, radio phases or interferometry,
- Distances, e.g. ranging by measurement of time of flight between one transmitter and multiple receivers or vice versa,
- Distance differences by measurement of times of arrival of signals from one transmitter to multiple receivers or vice versa
- Partly also velocity, e.g. by means of radio Doppler shift.[citation needed]
Combinations of these measurement principles also are important—e.g., many radars measure range and azimuth of a target.[citation needed]
https://en.wikipedia.org/wiki/Radio_navigation
Reverse RDF
The main problem with RDF is that it required a special antenna on the vehicle, which may not be easy to mount on smaller vehicles or single-crew aircraft. A smaller problem is that the accuracy of the system is based to a degree on the size of the antenna, but larger antennas would likewise make the installation more difficult.[citation needed]
During the era between World War I and World War II, a number of systems were introduced that placed the rotating antenna on the ground. As the antenna rotated through a fixed position, typically due north, the antenna was keyed with the morse code signal of the station's identification letters so the receiver could ensure they were listening to the right station. Then they waited for the signal to either peak or disappear as the antenna briefly pointed in their direction. By timing the delay between the morse signal and the peak/null, then dividing by the known rotational rate of the station, the bearing of the station could be calculated.[citation needed]
The first such system was the German Telefunken Kompass Sender, which began operations in 1907 and was used operationally by the Zeppelin fleet until 1918.[4] An improved version was introduced by the UK as the Orfordness Beacon in 1929 and used until the mid-1930s. A number of improved versions followed, replacing the mechanical motion of the antennas with phasing techniques that produced the same output pattern with no moving parts. One of the longest lasting examples was Sonne, which went into operation just before World War II and was used operationally under the name Consol until 1991. The modern VOR system is based on the same principles (see below).[citation needed]
ADF and NDB
A great advance in the RDF technique was introduced in the form of phase comparisons of a signal as measured on two or more small antennas, or a single highly directional solenoid. These receivers were smaller, more accurate, and simpler to operate. Combined with the introduction of the transistor and integrated circuit, RDF systems were so reduced in size and complexity that they once again became quite common during the 1960s, and were known by the new name, automatic direction finder, or ADF.[citation needed]
This also led to a revival in the operation of simple radio beacons for use with these RDF systems, now referred to as non-directional beacons (NDB). As the LF/MF signals used by NDBs can follow the curvature of earth, NDB has a much greater range than VOR which travels only in line of sight. NDB can be categorized as long range or short range depending on their power. The frequency band allotted to non-directional beacons is 190–1750 kHz, but the same system can be used with any common AM-band commercial station.[citation needed]
VOR
This section needs additional citations for verification. (February 2022) |
VHF omnidirectional range, or VOR, is an implementation of the reverse-RDF system, but one that is more accurate and able to be completely automated.[citation needed]
The VOR station transmits two audio signals on a VHF carrier – one is Morse code at 1020 Hz to identify the station, the other is a continuous 9960 Hz audio modulated at 30 Hz, with the 0-degree referenced to magnetic north. This signal is rotated mechanically or electrically at 30 Hz, which appears as a 30 Hz AM signal added to the previous two signals, the phasing of which is dependent on the position of the aircraft relative to the VOR station.[citation needed]
The VOR signal is a single RF carrier that is demodulated into a composite audio signal composed of a 9960 Hz reference signal frequency modulated at 30 Hz, a 30 Hz AM reference signal, and a 1020 Hz 'marker' signal for station identification. Conversion from this audio signal into a usable navigation aid is done by a navigation converter, which takes the reference signal and compares the phasing with the variable signal. The phase difference in degrees is provided to navigational displays. Station identification is by listening to the audio directly, as the 9960 Hz and 30 Hz signals are filtered out of the aircraft internal communication system, leaving only the 1020 Hz Morse-code station identification.[citation needed]
The system may be used with a compatible glideslope and marker beacon receiver, making the aircraft ILS-capable (Instrument Landing System)}. Once the aircraft's approach is accurate (the aircraft is in the "right place"), the VOR receiver will be used on a different frequency to determine if the aircraft is pointed in the "right direction." Some aircraft will usually employ two VOR receiver systems, one in VOR-only mode to determine "right place" and another in ILS mode in conjunction with a glideslope receiver to determine "right direction." }The combination of both allows for a precision approach in foul weather.[5]
Beam systems
Beam systems broadcast narrow signals in the sky, and navigation is accomplished by keeping the aircraft centred in the beam. A number of stations are used to create an airway, with the navigator tuning in different stations along the direction of travel. These systems were common in the era when electronics were large and expensive, as they placed minimum requirements on the receivers – they were simply voice radio sets tuned to the selected frequencies. However, they did not provide navigation outside of the beams, and were thus less flexible in use. The rapid miniaturization of electronics during and after World War II made systems like VOR practical, and most beam systems rapidly disappeared.[citation needed]
Lorenz
In the post-World War I era, the Lorenz company of Germany developed a means of projecting two narrow radio signals with a slight overlap in the center. By broadcasting different audio signals in the two beams, the receiver could position themselves very accurately down the centreline by listening to the signal in their headphones. The system was accurate to less than a degree in some forms.[citation needed]
Originally known as "Ultrakurzwellen-Landefunkfeuer" (LFF), or simply "Leitstrahl" (guiding beam), little money was available to develop a network of stations. The first widespread radio navigation network, using Low and Medium Frequencies, was instead led by the US (see LFF, below). Development was restarted in Germany in the 1930s as a short-range system deployed at airports as a blind landing aid. Although there was some interest in deploying a medium-range system like the US LFF, deployment had not yet started when the beam system was combined with the Orfordness timing concepts to produce the highly accurate Sonne system. In all of these roles, the system was generically known simply as a "Lorenz beam". Lorenz was an early predecessor to the modern Instrument Landing System.[citation needed]
In the immediate pre-World War II era the same concept was also developed as a blind-bombing system. This used very large antennas to provide the required accuracy at long distances (over England), and very powerful transmitters. Two such beams were used, crossing over the target to triangulate it. Bombers would enter one of the beams and use it for guidance until they heard the second one in a second radio receiver, using that signal to time the dropping of their bombs. The system was highly accurate, and the 'Battle of the Beams' broke out when United Kingdom intelligence services attempted, and then succeeded, in rendering the system useless through electronic warfare.[citation needed]
Low-frequency radio range
The low-frequency radio range (LFR, also "Four Course Radio Range" among other names) was the main navigation system used by aircraft for instrument flying in the 1930s and 1940s in the U.S. and other countries, until the advent of the VOR in the late 1940s. It was used for both en route navigation as well as instrument approaches.[citation needed]
The ground stations consisted of a set of four antennas that projected two overlapping directional figure-eight signal patterns at a 90 degree angle to each other. One of these patterns was "keyed" with the Morse code signal "A", dit-dah, and the second pattern "N", dah-dit. This created two opposed "A" quadrants and two opposed "N" quadrants around the station. The borders between these quadrants created four course legs or "beams" and if the pilot flew down these lines, the "A" and "N" signal merged into a steady "on course" tone and the pilot was "on the beam". If the pilot deviated to either side the "A" or "N" tone would become louder and the pilot knew to make a correction. The beams were typically aligned with other stations to produce a set of airways, allowing an aircraft to travel from airport to airport by following a selected set of stations. Effective course accuracy was about three degrees, which near the station provided sufficient safety margins for instrument approaches down to low minimums. At its peak deployment, there were over 400 LFR stations in the US.[6]
Glide path and the localizer of ILS
The remaining widely used beam systems are glide path and the localizer of the instrument landing system (ILS). ILS uses a localizer to provide horizontal position and glide path to provide vertical positioning. ILS can provide enough accuracy and redundancy to allow automated landings.
For more information see also:
Transponder systems
Positions can be determined with any two measures of angle or distance. The introduction of radar in the 1930s provided a way to directly determine the distance to an object even at long distances. Navigation systems based on these concepts soon appeared, and remained in widespread use until recently. Today they are used primarily for aviation, although GPS has largely supplanted this role.[citation needed]
Radar and transponders
Early radar systems, like the UK's Chain Home, consisted of large transmitters and separate receivers. The transmitter periodically sends out a short pulse of a powerful radio signal, which is sent into space through broadcast antennas. When the signal reflects off a target, some of that signal is reflected back in the direction of the station, where it is received. The received signal is a tiny fraction of the broadcast power, and has to be powerfully amplified in order to be used.[citation needed]
The same signals are also sent over local electrical wiring to the operator's station, which is equipped with an oscilloscope. Electronics attached to the oscilloscope provides a signal that increases in voltage over a short period of time, a few microseconds. When sent to the X input of the oscilloscope, this causes a horizontal line to be displayed on the scope. This "sweep" is triggered by a signal tapped off the broadcaster, so the sweep begins when the pulse is sent. Amplified signals from the receiver are then sent to the Y input, where any received reflection causes the beam to move upward on the display. This causes a series of "blips" to appear along the horizontal axis, indicating reflected signals. By measuring the distance from the start of the sweep to the blip, which corresponds to the time between broadcast and reception, the distance to the object can be determined.[citation needed]
Soon after the introduction of radar, the radio transponder appeared. Transponders are a combination of receiver and transmitter whose operation is automated – upon reception of a particular signal, normally a pulse on a particular frequency, the transponder sends out a pulse in response, typically delayed by some very short time. Transponders were initially used as the basis for early IFF systems; aircraft with the proper transponder would appear on the display as part of the normal radar operation, but then the signal from the transponder would cause a second blip to appear a short time later. Single blips were enemies, double blips friendly.[citation needed]
Transponder-based distance-distance navigation systems have a significant advantage in terms of positional accuracy. Any radio signal spreads out over distance, forming the fan-like beams of the Lorenz signal, for instance. As the distance between the broadcaster and receiver grows, the area covered by the fan increases, decreasing the accuracy of location within it. In comparison, transponder-based systems measure the timing between two signals, and the accuracy of that measure is largely a function of the equipment and nothing else. This allows these systems to remain accurate over very long range.[citation needed]
The latest transponder systems (mode S) can also provide position information, possibly derived from GNSS, allowing for even more precise positioning of targets.[citation needed]
Bombing systems
The first distance-based navigation system was the German Y-Gerät blind-bombing system. This used a Lorenz beam for horizontal positioning, and a transponder for ranging. A ground-based system periodically sent out pulses which the airborne transponder returned. By measuring the total round-trip time on a radar's oscilloscope, the aircraft's range could be accurately determined even at very long ranges. An operator then relayed this information to the bomber crew over voice channels, and indicated when to drop the bombs.[citation needed]
The British introduced similar systems, notably the Oboe system. This used two stations in England that operated on different frequencies and allowed the aircraft to be triangulated in space. To ease pilot workload only one of these was used for navigation – prior to the mission a circle was drawn over the target from one of the stations, and the aircraft was directed to fly along this circle on instructions from the ground operator. The second station was used, as in Y-Gerät, to time the bomb drop. Unlike Y-Gerät, Oboe was deliberately built to offer very high accuracy, as good as 35 m, much better than even the best optical bombsights.[citation needed]
One problem with Oboe was that it allowed only one aircraft to be guided at a time. This was addressed in the later Gee-H system by placing the transponder on the ground and broadcaster in the aircraft. The signals were then examined on existing Gee display units in the aircraft (see below). Gee-H did not offer the accuracy of Oboe, but could be used by as many as 90 aircraft at once. This basic concept has formed the basis of most distance measuring navigation systems to this day.[citation needed]
Beacons
The key to the transponder concept is that it can be used with existing radar systems. The ASV radar introduced by RAF Coastal Command was designed to track down submarines and ships by displaying the signal from two antennas side by side and allowing the operator to compare their relative strength. Adding a ground-based transponder immediately turned the same display into a system able to guide the aircraft towards a transponder, or "beacon" in this role, with high accuracy.[citation needed]
The British put this concept to use in their Rebecca/Eureka system, where battery-powered "Eureka" transponders were triggered by airborne "Rebecca" radios and then displayed on ASV Mk. II radar sets. Eureka's were provided to French resistance fighters, who used them to call in supply drops with high accuracy. The US quickly adopted the system for paratroop operations, dropping the Eureka with pathfinder forces or partisans, and then homing in on those signals to mark the drop zones.[citation needed]
The beacon system was widely used in the post-war era for blind bombing systems. Of particular note were systems used by the US Marines that allowed the signal to be delayed in such a way to offset the drop point. These systems allowed the troops at the front line to direct the aircraft to points in front of them, directing fire on the enemy. Beacons were widely used for temporary or mobile navigation as well, as the transponder systems were generally small and low-powered, able to be man portable or mounted on a Jeep.[citation needed]
DME
In the post-war era, a general navigation system using transponder-based systems was deployed as the distance measuring equipment (DME) system.[citation needed]
DME was identical to Gee-H in concept, but used new electronics to automatically measure the time delay and display it as a number, rather than having the operator time the signals manually on an oscilloscope. This led to the possibility that DME interrogation pulses from different aircraft might be confused, but this was solved by having each aircraft send out a different series of pulses which the ground-based transponder repeated back.
DME is almost always used in conjunction with VOR, and is normally co-located at a VOR station. This combination allows a single VOR/DME station to provide both angle and distance, and thereby provide a single-station fix. DME is also used as the distance-measuring basis for the military TACAN system, and their DME signals can be used by civilian receivers.[citation needed]
Hyperbolic systems
Hyperbolic navigation systems are a modified form of transponder systems which eliminate the need for an airborne transponder. The name refers to the fact that they do not produce a single distance or angle, but instead indicate a location along any number of hyperbolic lines in space. Two such measurements produces a fix. As these systems are almost always used with a specific navigational chart with the hyperbolic lines plotted on it, they generally reveal the receiver's location directly, eliminating the need for manual triangulation. As these charts were digitized, they became the first true location-indication navigational systems, outputting the location of the receiver as latitude and longitude. Hyperbolic systems were introduced during World War II and remained the main long-range advanced navigation systems until GPS replaced them in the 1990s.[citation needed]
Gee
The first hyperbolic system to be developed was the British Gee system, developed during World War II. Gee used a series of transmitters sending out precisely timed signals, with the signals leaving the stations at fixed delays. An aircraft using Gee, RAF Bomber Command's heavy bombers, examined the time of arrival on an oscilloscope at the navigator's station. If the signal from two stations arrived at the same time, the aircraft must be an equal distance from both transmitters, allowing the navigator to determine a line of position on his chart of all the positions at that distance from both stations. More typically, the signal from one station would be received earlier than the other. The difference in timing between the two signals would reveal them to be along a curve of possible locations. By making similar measurements with other stations, additional lines of position can be produced, leading to a fix. Gee was accurate to about 165 yards (150 m) at short ranges, and up to a mile (1.6 km) at longer ranges over Germany. Gee remained in use long after World War II, and equipped RAF aircraft as late as the 1960s (approx freq was by then 68 MHz).[citation needed]
LORAN
With Gee entering operation in 1942, similar US efforts were seen to be superfluous. They turned their development efforts towards a much longer-ranged system based on the same principles, using much lower frequencies that allowed coverage across the Atlantic Ocean. The result was LORAN, for "LOng-range Aid to Navigation". The downside to the long-wavelength approach was that accuracy was greatly reduced compared to the high-frequency Gee. LORAN was widely used during convoy operations in the late war period.[7]
Decca
Another British system from the same era was Decca Navigator. This differed from Gee primarily in that the signals were not pulses delayed in time, but continuous signals delayed in phase. By comparing the phase of the two signals, the time difference information as Gee was returned. However, this was far easier to display; the system could output the phase angle to a pointer on a dial removing any need for visual interpretation. As the circuitry for driving this display was quite small, Decca systems normally used three such displays, allowing quick and accurate reading of multiple fixes. Decca found its greatest use post-war on ships, and remained in use into the 1990s.[citation needed]
LORAN-C
Almost immediately after the introduction of LORAN, in 1952 work started on a greatly improved version. LORAN-C (the original retroactively became LORAN-A) combined the techniques of pulse timing in Gee with the phase comparison of Decca.[citation needed]
The resulting system (operating in the low frequency (LF) radio spectrum from 90 to 110 kHz) that was both long-ranged (for 60 kW stations, up to 3400 miles) and accurate. To do this, LORAN-C sent a pulsed signal, but modulated the pulses with an AM signal within it. Gross positioning was determined using the same methods as Gee, locating the receiver within a wide area. Finer accuracy was then provided by measuring the phase difference of the signals, overlaying that second measure on the first. By 1962, high-power LORAN-C was in place in at least 15 countries.[8]
LORAN-C was fairly complex to use, requiring a room of equipment to pull out the different signals. However, with the introduction of integrated circuits, this was quickly reduced further and further. By the late 1970s, LORAN-C units were the size of a stereo amplifier and were commonly found on almost all commercial ships as well as some larger aircraft. By the 1980s, this had been further reduced to the size of a conventional radio, and it became common even on pleasure boats and personal aircraft. It was the most popular navigation system in use through the 1980s and 90s, and its popularity led to many older systems being shut down, like Gee and Decca. However, like the beam systems before it, civilian use of LORAN-C was short-lived when GPS technology drove it from the market.[citation needed]
Other hyperbolic systems
Similar hyperbolic systems included the US global-wide VLF/Omega Navigation System, and the similar Alpha deployed by the USSR. These systems determined pulse timing not by comparison of two signals, but by comparison of a single signal with a local atomic clock. The expensive-to-maintain Omega system was shut down in 1997 as the US military migrated to using GPS. Alpha is still in use.[citation needed]
Since the 1960s, navigation has increasingly moved to satellite navigation systems. These are essentially hyperbolic[9][10] systems whose transmitters are in orbits. That the satellites move with respect to the receiver requires that the calculation of the positions of the satellites must be taken into account, which can only be handled effectively with a computer.[citation needed]
Satellite navigation systems send several signals that are used to decode the satellite's position, distance between the user satellite, and the user's precise time. One signal encodes the satellite's ephemeris data, which is used to accurately calculate the satellite's location at any time. Space weather and other effects causes the orbit to change over time so the ephemeris has to be updated periodically. Other signals send out the time as measured by the satellite's onboard atomic clock. By measuring signal times of arrival (TOAs) from at least four satellites, the user's receiver can re-build an accurate clock signal of its own and allows hyperbolic navigation to be carried out.[citation needed]
Satellite navigation systems offer better accuracy than any land-based system, are available at almost all locations on the Earth, can be implemented (receiver-side) a modest cost and complexity, with modern electronics, and require only a few dozen satellites to provide worldwide coverage[citation needed]. As a result of these advantages, satellite navigation has led to almost all previous systems falling from use[citation needed]. LORAN, Omega, Decca, Consol and many other systems disappeared during the 1990s and 2000s[citation needed]. The only other systems still in use are aviation aids, which are also being turned off[citation needed] for long-range navigation while new differential GPS systems are being deployed to provide the local accuracy needed for blind landings.[citation needed]
International regulation
Radionavigation service (short: RNS) is – according to Article 1.42 of the International Telecommunication Union's (ITU) Radio Regulations (RR)[11] – defined asA radiodetermination service for the purpose of radionavigation, including obstruction warning.'
This service is a so-called safety-of-life service, must be protected for Interferences, and is essential part of Navigation.[citation needed]
This radiocommunication service is classified in accordance with ITU Radio Regulations (article 1) as follows:
Radiodetermination service (article 1.40)
- Radiodetermination-satellite service (article 1.41)
- Radionavigation service (article 1.42)
- Radionavigation-satellite service (article 1.43)
- Maritime radionavigation service (article 1.44)
- Maritime radionavigation-satellite service (article 1.45)
- Aeronautical radionavigation service (article 1.46)
- Aeronautical radionavigation-satellite service (article 1.47)
Aeronautical
Aeronautical radionavigation service (short: ARNS) is – according to Article 1.46 of the International Telecommunication Union's (ITU) Radio Regulations (RR)[12] – defined as "A radionavigation service intended for the benefit and for the safe operation of aircraft."
This service is a so-called safety-of-life service, must be protected against interference, and is an essential part of navigation.
Maritime
Maritime radionavigation service (short: MRNS) is – according to Article 1.44 of the International Telecommunication Union's (ITU) Radio Regulations (RR)[13] – defined as "A radionavigation service intended for the benefit and for the safe operation of ships."
This service is a so-called safety-of-life service, must be protected for interferences, and is essential part of navigation.
Stations
Land station
A radionavigation land station is – according to article 1.88 of the International Telecommunication Union´s (ITU) ITU Radio Regulations (RR)[14] – defined as "A radio station in the radionavigation service not intended to be used while in motion."
Each radio station shall be classified by the radiocommunication service in which it operates permanently or temporarily. This station operates in a safety-of-life service and must be protected for Interferences.[citation needed]
In accordance with ITU Radio Regulations (article 1) this type of radio station might be classified as follows:
Radiodetermination station (article 1.86) of the radiodetermination service (article 1.40 )
- Radionavigation mobile station (article 1.87) of the radionavigation service (article 1.42)
- Radionavigation land station
- Selection radionavigation land stations
- ILS glide path transmitter
- TACAN antenna at Shemya, Alaska
Mobile station
A radionavigation mobile station is – according to article 1.87 of the International Telecommunication Union's (ITU) ITU Radio Regulations (RR)[15] – defined as "A radio station in the radionavigation service intended to be used while in motion or during halts at unspecified points."
Each radio station shall be classified by the radiocommunication service in which it operates permanently or temporarily. This station operates in a safety-of-life service and must be protected for Interferences.[citation needed]
In accordance with ITU Radio Regulations (article 1) this type of radio station might be classified as follows:
Radiodetermination station (article 1.86) of the radiodetermination service (article 1.40 )
- Radionavigation mobile station
- Selection radionavigation mobile stations
- Mobile TACAN-station in Alaska
See also
- Ambrose Channel pilot cable
- American Practical Navigator
- Differential GPS (DGPS)
- Distance measuring equipment (DME)
- EGNOS (European Geostationary Navigation Overlay Service)
- Galileo positioning system (Galileo)
- Global Positioning System (GPS)
- Global Navigation Satellite System (GLONASS)
- Inertial navigation system
- Instrument landing system (ILS)
- Local Area Augmentation System (LAAS)
- Long-range navigation (LORAN)
- Marker beacon (three-light marker beacon system)
- Microwave landing system (MLS)
- Multilateration
- Non-directional beacon (NDB)
- Radio altimeter
- Radar navigation
- Real-time locating
- Receiver Autonomous Integrity Monitoring (RAIM)
- Satellite geodesy#Radio techniques
- Space Integrated GPS/INS (SIGI)
- SCR-277
- Tactical air navigation (TACAN)
- Transponder Landing System (TLS)
- Transit (satellite)
- VHF omnidirectional range (VOR)
- X-ray pulsar-based navigation
- Wide Area Augmentation System (WAAS)
- Wind triangle
References
- ITU Radio Regulations, Section IV. Radio Stations and Systems – Article 1.87, definition: radionavigation mobile station
External links
- Department of Transportation and Department of Defense (March 25, 2002). "2001 Federal Radionavigation Systems" (PDF). Retrieved November 27, 2005.
- UK Navaids Gallery with detailed Technical Descriptions of their operation
- U.S. Federal Radionavigation Plan
https://en.wikipedia.org/wiki/Radio_navigation
Category:Wireless locating
Subcategories
This category has the following 8 subcategories, out of 8 total.
G
- GLONASS (1 C, 7 P)
I
- Indoor positioning system (14 P)
P
- Phased arrays (2 P)
R
- Radio geopositioning (2 C, 8 P)
S
- Satellite navigation systems (7 C, 6 P)
T
- Tracking (4 C, 47 P)
Pages in category "Wireless locating"
The following 75 pages are in this category, out of 75 total. This list may not reflect recent changes.
E
R
S
T
https://en.wikipedia.org/wiki/Category:Wireless_locating
https://en.wikipedia.org/wiki/AN/MRN-1
https://en.wikipedia.org/wiki/Modified_atmosphere/modified_humidity_packaging
https://en.wikipedia.org/wiki/Calibration_gas
https://en.wikipedia.org/wiki/Electro-galvanic_oxygen_sensor
https://en.wikipedia.org/wiki/Symmetrical_double-sided_two-way_ranging
https://en.wikipedia.org/wiki/Wireless_triangulation
https://en.wikipedia.org/wiki/Wildlife_radio_telemetry
https://en.wikipedia.org/wiki/Time-hopping
https://en.wikipedia.org/wiki/Electronic_toll_collection
https://en.wikipedia.org/wiki/Fleet_telematics_system
https://en.wikipedia.org/wiki/Pseudo-range_multilateration
https://en.wikipedia.org/wiki/Pointer_Telocation
https://en.wikipedia.org/wiki/OpenCellID
https://en.wikipedia.org/wiki/Dead_reckoning
https://en.wikipedia.org/wiki/Chirp_spread_spectrum
https://en.wikipedia.org/wiki/Butler_matrix
https://en.wikipedia.org/wiki/Category:Telematics
https://en.wikipedia.org/wiki/Category:Phased_arrays
https://en.wikipedia.org/wiki/Type_997_Artisan_radar
https://en.wikipedia.org/wiki/Gas_generator
https://en.wikipedia.org/wiki/Chemical_oxygen_generator
https://en.wikipedia.org/wiki/Soviet_space_program
https://en.wikipedia.org/wiki/Ozonide
https://en.wikipedia.org/wiki/Sodium_ozonide
https://en.wikipedia.org/wiki/Cryptand
https://en.wikipedia.org/wiki/Crown_ether
https://en.wikipedia.org/wiki/Phase-transfer_catalyst
https://en.wikipedia.org/wiki/Ion_exchange
https://en.wikipedia.org/wiki/Ion-exchange_resin
https://en.wikipedia.org/wiki/Separation_process
https://en.wikipedia.org/wiki/Water_purification
https://en.wikipedia.org/wiki/Polystyrene_sulfonate
https://en.wikipedia.org/wiki/Tolevamer
https://en.wikipedia.org/wiki/Category:Antiinfective_agent_stubs
https://en.wikipedia.org/wiki/Potassium_binder
https://en.wikipedia.org/wiki/Chronic_kidney_disease
https://en.wikipedia.org/wiki/High_blood_pressure
https://en.wikipedia.org/wiki/Renal_osteodystrophy
https://en.wikipedia.org/wiki/Renin%E2%80%93angiotensin_system
https://en.wikipedia.org/wiki/Glomerular_filtration_rate
https://en.wikipedia.org/wiki/Renal_ultrasonography
https://en.wikipedia.org/wiki/Thyroid_hormones
https://en.wikipedia.org/wiki/Triiodothyronine
https://en.wikipedia.org/wiki/Iodine
https://en.wikipedia.org/wiki/Halogen
https://en.wikipedia.org/wiki/Ionomer
An ionomer (/ˌaɪˈɑːnəmər/) (iono- + -mer) is a polymer composed of repeat units of both electrically neutral repeating units and ionized units covalently bonded to the polymer backbone as pendant group moieties. Usually no more than 15 mole percent are ionized. The ionized units are often carboxylic acid groups.
The classification of a polymer as an ionomer depends on the level of substitution of ionic groups as well as how the ionic groups are incorporated into the polymer structure. For example, polyelectrolytes also have ionic groups covalently bonded to the polymer backbone, but have a much higher ionic group molar substitution level (usually greater than 80%); ionenes are polymers where ionic groups are part of the actual polymer backbone. These two classes of ionic-group-containing polymers have vastly different morphological and physical properties and are therefore not considered ionomers.
Ionomers have unique physical properties including electrical conductivity and viscosity—increase in ionomer solution viscosity with increasing temperatures (see conducting polymer). Ionomers also have unique morphological properties as the non-polar polymer backbone is energetically incompatible with the polar ionic groups. As a result, the ionic groups in most ionomers will undergo microphase separation to form ionic-rich domains.
Commercial applications for ionomers include golf ball covers, semipermeable membranes, sealing tape and thermoplastic elastomers. Common examples of ionomers include polystyrene sulfonate, Nafion and Hycar.
https://en.wikipedia.org/wiki/Ionomer
https://en.wikipedia.org/wiki/pattern
Atom transfer radical polymerization (ATRP) is an example of a reversible-deactivation radical polymerization. Like its counterpart, ATRA, or atom transfer radical addition, ATRP is a means of forming a carbon-carbon bond with a transition metal catalyst. Polymerization from this method is called atom transfer radical addition polymerization (ATRAP). As the name implies, the atom transfer step is crucial in the reaction responsible for uniform polymer chain growth. ATRP (or transition metal-mediated living radical polymerization) was independently discovered by Mitsuo Sawamoto[1] and by Krzysztof Matyjaszewski and Jin-Shan Wang in 1995.[2][3]
- The following scheme presents a typical ATRP reaction:
Controlled reversible-deactivation radical polymerization in which the deactivation
of the radicals involves reversible atom transfer or reversible group transfer catalyzed usually,
though not exclusively, by transition-metal complexes.[4]
https://en.wikipedia.org/wiki/Atom_transfer_radical_polymerization
Number average molar mass
The number average molar mass is a way of determining the molecular mass of a polymer. Polymer molecules, even ones of the same type, come in different sizes (chain lengths, for linear polymers), so the average molecular mass will depend on the method of averaging. The number average molecular mass is the ordinary arithmetic mean or average of the molecular masses of the individual macromolecules. It is determined by measuring the molecular mass of n polymer molecules, summing the masses, and dividing by n.
The number average molecular mass of a polymer can be determined by gel permeation chromatography, viscometry via the (Mark–Houwink equation), colligative methods such as vapor pressure osmometry, end-group determination or proton NMR.[4]
High number-average molecular mass polymers may be obtained only with a high fractional monomer conversion in the case of step-growth polymerization, as per the Carothers' equation.
https://en.wikipedia.org/wiki/Molar_mass_distribution#Number_average_molar_mass
Chemical uses
Polystyrene sulfonates are useful because of their ion exchange properties.[15] Linear ionic polymers are generally water-soluble, whereas cross-linked materials (called resins) do not dissolve in water. These polymers are classified as polysalts and ionomers.[15]
Water softening
Water softening is achieved by percolating hard water through a bed of the sodium form of cross-linked polystyrene sulfonate. The hard ions such as calcium (Ca2+) and magnesium (Mg2+) adhere to the sulfonate groups, displacing sodium ions. The resulting solution of sodium ions is softened.
Other uses
Sodium polystyrene sulfonate is used as a superplastifier in cement, as a dye improving agent for cotton, and as proton exchange membranes in fuel cell applications. In its acid form, the resin is used as a solid acid catalyst in organic synthesis.[16]
https://en.wikipedia.org/wiki/Polystyrene_sulfonate
The surface area of a solid object is a measure of the total area that the surface of the object occupies.[1] The mathematical definition of surface area in the presence of curved surfaces is considerably more involved than the definition of arc length of one-dimensional curves, or of the surface area for polyhedra (i.e., objects with flat polygonal faces), for which the surface area is the sum of the areas of its faces. Smooth surfaces, such as a sphere, are assigned surface area using their representation as parametric surfaces. This definition of surface area is based on methods of infinitesimal calculus and involves partial derivatives and double integration.
A general definition of surface area was sought by Henri Lebesgue and Hermann Minkowski at the turn of the twentieth century. Their work led to the development of geometric measure theory, which studies various notions of surface area for irregular objects of any dimension. An important example is the Minkowski content of a surface.
https://en.wikipedia.org/wiki/Surface_area
In mathematics (specifically multivariable calculus), a multiple integral is a definite integral of a function of several real variables, for instance, f(x, y) or f(x, y, z). Integrals of a function of two variables over a region in (the real-number plane) are called double integrals, and integrals of a function of three variables over a region in (real-number 3D space) are called triple integrals.[1] For multiple integrals of a single-variable function, see the Cauchy formula for repeated integration.
https://en.wikipedia.org/wiki/Multiple_integral
Divinylbenzene (DVB) is an organic compound with the chemical formula C6H4(CH=CH2)2 and structure H2C=CH−C6H4−HC=CH2 (a benzene ring with two vinyl groups as substituents). It is related to styrene (vinylbenzene, C6H5−CH=CH2) by the addition of a second vinyl group.[2] It is a colorless liquid manufactured by the thermal dehydrogenation of isomeric diethylbenzenes. Under synthesis conditions, o-divinylbenzene converts to naphthalene and thus is not a component of the usual mixtures of DVB.[3]
Yellow Divinylbenzene (DVB) is not acceptable for national security products.
https://en.wikipedia.org/wiki/Divinylbenzene
Anion resins
Anion resins may be either strongly or weakly basic. Strongly basic anion resins maintain their negative charge across a wide pH range, whereas weakly basic anion resins are neutralized at higher pH levels.[3] Weakly basic resins do not maintain their charge at a high pH because they undergo deprotonation.[3] They do, however, offer excellent mechanical and chemical stability. This, combined with a high rate of ion exchange, make weakly base anion resins well suited for the organic salts.
For anion resins, regeneration typically involves treatment of the resin with a strongly basic solution, e.g. aqueous sodium hydroxide. During regeneration, the regenerant chemical is passed through the resin, and trapped negative ions are flushed out, renewing the resin exchange capacity.
Cation-exchange resin
Formula: R−H acidic
The cation exchange method removes the hardness of water but induces acidity in it, which is further removed in the next stage of treatment of water by passing this acidic water through an anion exchange process.[4]
Reaction:
- R−H + M+ = R−M + H+.
Anion-exchange resin
Formula: –NR4+OH−
Often these are styrene–divinylbenzene copolymer resins that have quaternary ammonium cations as an integral part of the resin matrix.[4]
Reaction:
- –NR4+OH− + HCl = –NR4+Cl− + H2O.
Anion-exchange chromatography makes use of this principle to extract and purify materials from mixtures or solutions.
https://en.wikipedia.org/wiki/Ion-exchange_resin
https://en.wikipedia.org/wiki/Iminodiacetic_acid
https://en.wikipedia.org/wiki/Chelating_resin
https://en.wikipedia.org/wiki/Hydraulic_head#Head_loss
https://en.wikipedia.org/wiki/Polystyrene
https://en.wikipedia.org/wiki/Ion-exchange_membrane
https://en.wikipedia.org/wiki/Electrodialysis
https://en.wikipedia.org/wiki/Activated_carbon
Ion exchange in metal separation
Ion-exchange processes are used to separate and purify metals, including separating uranium from plutonium and other actinides, including thorium; and lanthanum, neodymium, ytterbium, samarium, lutetium, from each other and the other lanthanides. There are two series of rare-earth metals, the lanthanides and the actinides. Members of each family have very similar chemical and physical properties. Ion exchange was for many years the only practical way to separate the rare earths in large quantities. This application was developed in the 1940s by Frank Spedding. Subsequently, solvent extraction has mostly supplanted use of ion-exchange resins except for the highest-purity products.
A very important case is the PUREX process (plutonium-uranium extraction process), which is used to separate the plutonium and the uranium from the spent fuel products from a nuclear reactor, and to be able to dispose of the waste products. Then, the plutonium and uranium are available for making nuclear-energy materials, such as new reactor fuel and nuclear weapons.
Ion-exchange beads are also an essential component in in-situ leach uranium mining. In-situ recovery involves the extraction of uranium-bearing water (grading as low as 0.05% U3O8) through boreholes. The extracted uranium solution is then filtered through the resin beads. Through an ion-exchange process, the resin beads attract uranium from the solution. Uranium-loaded resins are then transported to a processing plant, where U3O8 is separated from the resin beads, and yellowcake is produced. The resin beads can then be returned to the ion-exchange facility, where they are reused.
The ion-exchange process is also used to separate other sets of very similar chemical elements, such as zirconium and hafnium, which incidentally is also very important for the nuclear industry. Zirconium is practically transparent to free neutrons, used in building reactors, but hafnium is a very strong absorber of neutrons, used in reactor control rods.
https://en.wikipedia.org/wiki/Ion-exchange_resin
https://en.wikipedia.org/wiki/Control_rod
https://en.wikipedia.org/wiki/Yellowcake
https://en.wikipedia.org/wiki/Triuranium_octoxide
https://en.wikipedia.org/wiki/PUREX
https://en.wikipedia.org/wiki/Liquid%E2%80%93liquid_extraction
https://en.wikipedia.org/wiki/Quaternary_ammonium_cation
https://en.wikipedia.org/wiki/Acid_catalysis#Solid_acid_catalysts
https://en.wikipedia.org/wiki/Polyelectrolyte
https://en.wikipedia.org/wiki/Direct_air_capture
https://en.wikipedia.org/wiki/Colestipol
https://en.wikipedia.org/wiki/Chelation_therapy
https://en.wikipedia.org/wiki/Excipient
https://en.wikipedia.org/wiki/Category:Synthetic_resins
https://en.wikipedia.org/wiki/Category:Phenol_formaldehyde_resins
https://en.wikipedia.org/wiki/Ebonite
https://en.wikipedia.org/wiki/Novolak
https://en.wikipedia.org/wiki/Silicone_resin
https://en.wikipedia.org/wiki/Prepolymer
https://en.wikipedia.org/wiki/Polyvinyl_butyral
https://en.wikipedia.org/wiki/Polyol
https://en.wikipedia.org/wiki/Polyester_resin
https://en.wikipedia.org/wiki/Polyester
https://en.wikipedia.org/wiki/Polybenzoxazine
https://en.wikipedia.org/wiki/Epoxy
https://en.wikipedia.org/wiki/Synthetic_resin
https://en.wikipedia.org/wiki/Waterborne_resins
https://en.wikipedia.org/wiki/Cyanoacrylate
https://en.wikipedia.org/wiki/Hybtonite
https://en.wikipedia.org/wiki/Carbon_nanotube
https://en.wikipedia.org/wiki/Hexagonal_tiling
https://en.wikipedia.org/wiki/Truncation_(geometry)
https://en.wikipedia.org/wiki/Nanotechnology
https://en.wikipedia.org/wiki/Molecular_nanotechnology
https://en.wikipedia.org/wiki/Self-replicating_machine
https://en.wikipedia.org/wiki/Von_Neumann_universal_constructor
https://en.wikipedia.org/wiki/Fullerene
The discovery of fullerenes greatly expanded the number of known allotropes of carbon, which had previously been limited to graphite, diamond, and amorphous carbon such as soot and charcoal.
https://en.wikipedia.org/wiki/Fullerene
https://en.wikipedia.org/wiki/Nanomaterials
https://en.wikipedia.org/wiki/Microfabrication
The major concepts and principles of microfabrication are microlithography, doping, thin films, etching, bonding, and polishing.
https://en.wikipedia.org/wiki/Microfabrication
Origins
Microfabrication technologies originate from the microelectronics industry, and the devices are usually made on silicon wafers even though glass, plastics and many other substrate are in use. Micromachining, semiconductor processing, microelectronic fabrication, semiconductor fabrication, MEMS fabrication and integrated circuit technology are terms used instead of microfabrication, but microfabrication is the broad general term.
Traditional machining techniques such as electro-discharge machining, spark erosion machining, and laser drilling have been scaled from the millimeter size range to micrometer range, but they do not share the main idea of microelectronics-originated microfabrication: replication and parallel fabrication of hundreds or millions of identical structures. This parallelism is present in various imprint, casting and moulding techniques which have successfully been applied in the microregime. For example, injection moulding of DVDs involves fabrication of submicrometer-sized spots on the disc.
https://en.wikipedia.org/wiki/Microfabrication
Electroplating is also a 19th-century technique adapted to produce micrometre scale structures, as are various stamping and embossing techniques.
https://en.wikipedia.org/wiki/Microfabrication
https://en.wikipedia.org/wiki/Optical_engineering
https://en.wikipedia.org/wiki/Microfluidics
https://en.wikipedia.org/wiki/Nanosensor
https://en.wikipedia.org/wiki/Thin-film_transistor
https://en.wikipedia.org/wiki/Microoptoelectromechanical_systems
Modern microprocessors are made with 30 masks while a few masks suffice for a microfluidic device or a laser diode. Microfabrication resembles multiple exposure photography, with many patterns aligned to each other to create the final structure.
https://en.wikipedia.org/wiki/Microfabrication
To fabricate a microdevice, many processes must be performed, one after the other, many times repeatedly. These processes typically include depositing a film, patterning the film with the desired micro features, and removing (or etching) portions of the film. Thin film metrology is used typically during each of these individual process steps, to ensure the film structure has the desired characteristics in terms of thickness (t), refractive index (n) and extinction coefficient (k),[2] for suitable device behavior. For example, in memory chip fabrication there are some 30 lithography steps, 10 oxidation steps, 20 etching steps, 10 doping steps, and many others are performed. The complexity of microfabrication processes can be described by their mask count. This is the number of different pattern layers that constitute the final device.
https://en.wikipedia.org/wiki/Microfabrication
Microfabricated devices are not generally freestanding devices but are usually formed over or in a thicker support substrate. For electronic applications, semiconducting substrates such as silicon wafers can be used. For optical devices or flat panel displays, transparent substrates such as glass or quartz are common. The substrate enables easy handling of the micro device through the many fabrication steps. Often many individual devices are made together on one substrate and then singulated into separated devices toward the end of fabrication.
https://en.wikipedia.org/wiki/Microfabrication
Microfabricated devices are typically constructed using one or more thin films (see Thin film deposition). The purpose of these thin films depends upon the type of device. Electronic devices may have thin films which are conductors (metals), insulators (dielectrics) or semiconductors. Optical devices may have films which are reflective, transparent, light guiding or scattering. Films may also have a chemical or mechanical purpose as well as for MEMS applications. Examples of deposition techniques include:
- Thermal oxidation
- Local oxidation of silicon
- Chemical vapor deposition (CVD)
- Physical vapor deposition (PVD)
- Epitaxy
Patterning
It is often desirable to pattern a film into distinct features or to form openings (or vias) in some of the layers. These features are on the micrometer or nanometer scale and the patterning technology is what defines microfabrication. This patterning technique typically uses a 'mask' to define portions of the film which will be removed. Examples of patterning techniques include:
- Photolithography
- Shadow masking
Etching
Etching is the removal of some portion of the thin film or substrate. The substrate is exposed to an etching (such as an acid or plasma) which chemically or physically attacks the film until it is removed. Etching techniques include:
- Dry etching (plasma etching) such as reactive-ion etching (RIE) or deep reactive-ion etching (DRIE)
- Wet etching or chemical etching
Microforming
Microforming is a microfabrication process of microsystem or microelectromechanical system (MEMS) "parts or structures with at least two dimensions in the submillimeter range."[3][4][5] It includes techniques such as microextrusion,[4] microstamping,[6] and microcutting.[7] These and other microforming processes have been envisioned and researched since at least 1990,[3] leading to the development of industrial- and experimental-grade manufacturing tools. However, as Fu and Chan pointed out in a 2013 state-of-the-art technology review, several issues must still be resolved before the technology can be implemented more widely, including deformation load and defects, forming system stability, mechanical properties, and other size-related effects on the crystallite (grain) structure and boundaries:[4][5][8]
In microforming, the ratio of the total surface area of grain boundaries to the material volume decreases with the decrease of specimen size and the increase of grain size. This leads to the decrease of grain boundary strengthening effect. Surface grains have lesser constraints compared to internal grains. The change of flow stress with part geometry size is partly attributed to the change of volume fraction of surface grains. In addition, the anisotropic properties of each grain become significant with the decrease of workpiece size, which results in the inhomogeneous deformation, irregular formed geometry and the variation of deformation load. There is a critical need to establish the systematic knowledge of microforming to support the design of part, process, and tooling with the consideration of size effects.[8]
Other
a wide variety of other processes for cleaning, planarizing, or modifying the chemical properties of microfabricated devices can also be performed. Some examples include:
- Doping by either thermal diffusion or ion implantation
- Chemical-mechanical planarization (CMP)
- Wafer cleaning, also known as "surface preparation" (see below)
- Wire bonding
Cleanliness in wafer fabrication
Microfabrication is carried out in cleanrooms, where air has been filtered of particle contamination and temperature, humidity, vibrations and electrical disturbances are under stringent control. Smoke, dust, bacteria and cells are micrometers in size, and their presence will destroy the functionality of a microfabricated device.
Cleanrooms provide passive cleanliness but the wafers are also actively cleaned before every critical step. RCA-1 clean in ammonia-peroxide solution removes organic contamination and particles; RCA-2 cleaning in hydrogen chloride-peroxide mixture removes metallic impurities. Sulfuric acid-peroxide mixture (a.k.a. Piranha) removes organics. Hydrogen fluoride removes native oxide from silicon surface. These are all wet cleaning steps in solutions. Dry cleaning methods include oxygen and argon plasma treatments to remove unwanted surface layers, or hydrogen bake at elevated temperature to remove native oxide before epitaxy. Pre-gate cleaning is the most critical cleaning step in CMOS fabrication: it ensures that the ca. 2 nm thick oxide of a MOS transistor can be grown in an orderly fashion. Oxidation, and all high temperature steps are very sensitive to contamination, and cleaning steps must precede high temperature steps.
Surface preparation is just a different viewpoint, all the steps are the same as described above: it is about leaving the wafer surface in a controlled and well known state before you start processing. Wafers are contaminated by previous process steps (e.g. metals bombarded from chamber walls by energetic ions during ion implantation), or they may have gathered polymers from wafer boxes, and this might be different depending on wait time.
Wafer cleaning and surface preparation work similarly to the machines in a bowling alley: first they remove all unwanted bits and pieces, and then they reconstruct the desired pattern so that the game can go on.
See also
https://en.wikipedia.org/wiki/Microfabrication
Three-dimensional (3D) microfabrication refers to manufacturing techniques that involve the layering of materials to produce a three-dimensional structure at a microscopic scale.[1] These structures are usually on the scale of micrometers and are popular in microelectronics and microelectromechanical systems.
Rapid prototyping
Much like their macroscopic analog, microstructures can be produced using rapid prototyping methods. These techniques generally involve the layering of some resin, with each layer being much thinner than that used for conventional processes in order to produce higher resolution microscopic components. Layers in processes such as electrochemical fabrication can be as thin as 5 to 10 μm.[2] The creation of microscopic structures is similar to conventional additive manufacturing techniques in that a computer aided design model is sliced into an appropriate number of two-dimensional layers in order to create a toolpath. This toolpath is then followed by a mechanical system to produce the desired geometry.
A popular application is stereolithography (SLA), which involves the use of a UV light or laser beam on a surface to create a layer, which are then lowered into a tank so that a new layer can be formed on top. Another commonly used method is fused deposition modeling (FDM), in which a moving head creates a layer by melting the model material (usually a polymer) and extrudes the melted material onto a surface. Other methods such as selective laser sintering (SLS) are also used in the additive manufacturing of 3D microstructures.[1]
3D laser microfabrication
Laser-based techniques are the most common approach for producing microstructures. Typical techniques involve the use of lasers to add or subtract material from a bulk sample. Recent applications of lasers involve the use of ultrashort pulses of lasers focused to a small area in order to create a pattern that is layered to create a structure. The use of lasers in such a manner is known as laser direct-writing (LDW). Microscopic mechanical elements such as micromotors, micropumps, and other microfluidic devices can be produced using direct-write concepts. In addition to additive and subtractive processes, LDW allows for the modification of the properties of a material. Mechanisms that allow for these modifications include sintering, microstereolithography, and multiphoton processes. These use a series of laser pulses to deliver a precise amount of energy to induce a physical or chemical change that can result in annealing and surface structuring of a material.[3]
Microstereolithography
Microstereolithography is a common technique based on stereolithography principles. 3D components are fabricated by repeatedly layering photopolymerizable resin and curing under an ultraviolet laser. Earlier systems that employ this technique use a scanning principle in which a focused light beam is fixed onto one location and the translation stage moves to fabricate each layer vector by vector. A faster alternate involves using a projection principle in which the image is projected onto the surface of the resin so that the irradiation of a layer is done in one step only. The high-resolution results allow for the fabrication of complex shapes that would otherwise be difficult to produce at such small scales.[1]
Multiphoton lithography
Multiphoton lithography can be used to 3D print structures with sub-micrometer resolution. The process uses the focal point of a laser to photopolymerize the resin or glass at a specific point. By moving the focal point around in three dimensional space and solidifying the medium at different points, the desired geometry can be built. There are currently limits to the resolution of the features in geometries built through this method. The limits relate to the medium that the geometry is being constructed from as well as the precision of the focal point of the laser.[3]
Other additive processes
Additive processes involve the layering of materials in a certain pattern. These include laser chemical vapor deposition (LCVD), which use organic precursors to write patterns on a structure or bulk material. This application can be found in the field of electronics, particularly in the repair of transistor arrays for displays. Another additive process is laser-induced forward transfer (LIFT), which uses pulsed lasers aimed at a coated substrate to transfer material in the direction of the laser flow.[1] LIFT has been used to produce transfer thermo-electric materials, polymers[4] and has been used to print copper wires. [5]
With inclined/rotated UV lithography
Focus on the 3D microstructures now, it have been focused in a lot of microsystems like electronic, mechanical, micro-optical and analysis systems. And when this technology is developing, we found that the traditional and conventional micro machining technologies like surface micromachining, bulk micromachining and GIGA process are not sufficient to fabricate or produce oblique and curved 3D microstructures.[6]
Fabrication
The basic setup of inclined UV exposure has conventional UV source, a contact stage, and a tilting stage. Plus, we place a photomask and a photoresist coated substrate between the upper and lower plates of the contact stage, and it is fixed by pushing up the lower plate with a screw. Then, we can expose the photoresist to the inclined UV.
An example of the fabrication process: Su-8 is a negative thick photoresist, which used in novel 3D micro fabrication method with inclined/rotated UV lithography. During the process, we coat SU-8 50 on a silicon wafer with a thickness of about 100ųm. Then, soft bake the resist on a 65 °C hot plate for 10 minutes and on a 95 °C plate for 30 minutes. It is contacted with a photomask using the contact stage. This stage, is leaned against the tilting stage and the resist is exposed to the UV. The dose of 365 nm UV is 500mJ/cm2. After the exposure, the resist is post-exposure baked on a 65 °C hot plate for 3 minutes and on a 95 °C plate for 10 minutes. In the end, the resist is developed in the SU-8 for about 10 to 15 minutes at the room temperature with mild agitation and then, rinsed with isopropyl alcohol. Besides that, there can be a lot of other procedures. For example, inclined UV lithography, inclined and rotated UV lithography and lithography using reflected UV.
When the trace of the incident UV with a right angle is on a straight line, so the patterns of a photomask are transcribed to the resist. When talking about inclined UV exposure processes, the UV is refracted and reflected, this makes it possible to fabricate various of 3D structures. The microstructures fabricated by the 3D micro fabrication technology can be allied to a lot of microsystems directly. Also, it can be used as the molds for electroplating. As a result, these technology can be applied to a variety of fields like filters, mixers, jets, micro channels, light guide panels of LCD monitor and more.
Self-folding materials
Design of complicated 3D microstructure can be highly challenging task for development of novel materials for optics, biotechnology and micro/nano electronics. 3D materials can be fabricated using a lot of methods like two-photon photolithography, interference lithography and molding. But 3D structuring using these techniques is very complicated, experimentally. This can limit their upscaling and broad applicability.
Nature offers a large number of ideas for the design of novel materials with superior properties. Self-assembly and self-organization being the main principle of structure formation in nature attract significant interest as promising concepts for the design of intelligent materials.
Stimuli-responsive hydrogels mimic swelling/shrinking behavior of plant cells and produce macroscopic actuation in response to a small variation of environmental conditions. Mostly, homogenous expansion or contraction in all directions can result a change of conditions. Also, inhomogeneous expansion and shrinkage can result more complex behavior like bending, twisting and folding and they can happen with different magnitudes in different directions. Utilization of these phenomena for the design of structured materials can be highly attractive because they allow simple, template-free fabrication of very complex repetitive 2D and 3D patterns. However, they cannot be prepared by using sophisticated fabrication methods like two-photon and interference photolithography as mentioned before. There is an advantage of the self-folding approach, is the possibility of quick, reversible, and reproducible fabrication of 3D hollow objects with controlled chemical properties and morphology of both the exterior and the interior.
One experimental application of self-folding materials is pasta that ships flat but folds into the desired shape on contact with boiling water.[7]
Outlook
One factor that limit broad applicability of self-folding polymer films is the manufacturing cost. Actually, polymer can be deposited by spinning and dipping coating at ambient conditions, the fabrication of polymer self-folding films is substantially cheaper than fabrication of inorganic ones, which are produced by vacuum deposition. In another word, there is no method, which is cheap and large-scale production of self-folding polymer films that substantially limits their application.
To solve these issues, the future research must be focused on deeper investigation of folding to allow design of complex 3D structures using just 2D shapes. On the other hand, searching a way, which is cheap and fast manufacturing of large quantity of self-folding films can be greatly helpful.[8]
References
- Ionov, Leonid (2013). Polymer Reviews, 2013, Vol.53(1). Germany: Taylor & Francis Group. pp. 92–107.
https://en.wikipedia.org/wiki/3D_microfabrication
https://en.wikipedia.org/wiki/Integrated_circuit
https://en.wikipedia.org/wiki/Epitaxy
https://en.wikipedia.org/wiki/Evaporation_(deposition)
https://en.wikipedia.org/wiki/Sputter_deposition
https://en.wikipedia.org/wiki/Chemical_vapor_deposition
https://en.wikipedia.org/wiki/LOCOS
https://en.wikipedia.org/wiki/Polymerization
https://en.wikipedia.org/wiki/Multiphoton_lithography
https://en.wikipedia.org/wiki/Selective_laser_sintering
https://en.wikipedia.org/wiki/Fused_filament_fabrication#Fused_deposition_modeling
https://en.wikipedia.org/wiki/Stereolithography
Because of the large surface area to volume ratio of MEMS, forces produced by ambient electromagnetism (e.g., electrostatic charges and magnetic moments), and fluid dynamics (e.g., surface tension and viscosity) are more important design considerations than with larger scale mechanical devices. MEMS technology is distinguished from molecular nanotechnology or molecular electronics in that the latter two must also consider surface chemistry.
https://en.wikipedia.org/wiki/MEMS
The digital micromirror device, or DMD, is the microoptoelectromechanical system (MOEMS) that is the core of the trademarked DLP projection technology from Texas Instruments (TI). Texas Instrument's DMD was created by solid-state physicist and TI Fellow Emeritus Dr. Larry Hornbeck in 1987.[1] However, the technology goes back to 1973 with Harvey C. Nathanson's (inventor of MEMS c. 1965) use of millions of microscopically small moving mirrors to create a video display of the type now found in digital projectors.[2]
https://en.wikipedia.org/wiki/Digital_micromirror_device
History
The DMD project began as the deformable mirror device in 1977 using micromechanical analog light modulators. The first analog DMD product was the TI DMD2000 airline ticket printer that used a DMD instead of a laser scanner.[3]
Construction and use
A DMD chip has on its surface several hundred thousand microscopic mirrors arranged in a rectangular array which correspond to the pixels in the image to be displayed. The mirrors can be individually rotated ±10-12°, to an on or off state. In the on state, light from the projector bulb is reflected into the lens making the pixel appear bright on the screen. In the off state, the light is directed elsewhere (usually onto a heatsink), making the pixel appear dark. To produce greyscales, the mirror is toggled on and off very quickly, and the ratio of on time to off time determines the shade produced (binary pulse-width modulation).[4] Contemporary DMD chips can produce up to 1024 shades of gray (10 bits).[5] See Digital Light Processing for discussion of how color images are produced in DMD-based systems.
The mirrors themselves are made of aluminum and are around 16 micrometers across. Each mirror is mounted on a yoke which in turn is connected to two support posts by compliant torsion hinges. In this type of hinge, the axle is fixed at both ends and twists in the middle. Because of the small scale, hinge fatigue is not a problem, and tests have shown that even 1 trillion (1012) operations do not cause noticeable damage. Tests have also shown that the hinges cannot be damaged by normal shock and vibration, since it is absorbed by the DMD superstructure.[6]
Two pairs of electrodes control the position of the mirror by electrostatic attraction. Each pair has one electrode on each side of the hinge, with one of the pairs positioned to act on the yoke and the other acting directly on the mirror. The majority of the time, equal bias charges are applied to both sides simultaneously. Instead of flipping to a central position as one might expect, this actually holds the mirror in its current position. This is because the attraction force on the side the mirror is already tilted towards is greater since that side is closer to the electrodes.[7]
To move the mirrors, the required state is first loaded into an SRAM cell located beneath each pixel, which is also connected to the electrodes. Once all the SRAM cells have been loaded, the bias voltage is removed, allowing the charges from the SRAM cell to prevail, moving the mirror. When the bias is restored, the mirror is once again held in position, and the next required movement can be loaded into the memory cell.
The bias system is used because it reduces the voltage levels required to address the pixels such that they can be driven directly from the SRAM cell, and also because the bias voltage can be removed at the same time for the whole chip, so every mirror moves at the same instant. The advantages of the latter are more accurate timing and a more cinematic moving image.
The described failure mode on these is caused by internal contamination usually due to seal failure corroding the mirror supports. A related failure was the glue used between 2007 and 2013, under which heat and light degrades and outgasses: this normally causes fogging inside the glass and eventually white/black pixels. This cannot usually be repaired, but defective DMD chips can sometimes be used for less critical projects not needing rapidly changing patterns if the existing bad pixels can be made part of the projected image or otherwise mapped out, including 3D scanning. [8]
Applications
- Televisions and HDTVs
- Holographic Versatile Discs
- Head-mounted displays
- Digital cinema[9]
- DLP projectors
- Optical Metrology[10]
- Laser Beam Machining[11]
- Phase space measurement with forward modeling[12]
- Spatial Light Modulation[13]
- Multivariate optical computing
- Digital Holographic Tomography[14]
- Optogenetics[15]
- Flip-disc display
https://en.wikipedia.org/wiki/Digital_micromirror_device
Technique
The technique of using optogenetics is flexible and adaptable to the experimenter's needs. Cation-selective channelrhodopsins (e.g. ChR2) are used to excite neurons, anion-conducting channelrhodopsins (e.g. GtACR2) inhibit neuronal activity. Combining these tools into a single construct (e.g. BiPOLES) allows for both inhibition and excitation, depending on the wavelength of illumination.[80]
Introducing the microbial opsin into a specific subset of cells is challenging. A popular approach is to introduce an engineered viral vector that contains the optogenetic actuator gene attached to a specific promoter such as CAMKIIα, which is active in excitatory neurons. This allows for some level of specificity, preventing e.g. expression in glia cells.[81]
A more specific approach is based on transgenic "driver" mice which express Cre recombinase, an enzyme that catalyzes recombination between two lox-P sites, in a specific subset of cells, e.g. parvalbumin-expressing interneurons. By introducing an engineered viral vector containing the optogenetic actuator gene in between two lox-P sites, only the cells producing Cre recombinase will express the microbial opsin. This technique has allowed for multiple modified optogenetic actuators to be used without the need to create a whole line of transgenic animals every time a new microbial opsin is needed.[82]
After the introduction and expression of the microbial opsin, a computer-controlled light source has to be optically coupled to the brain region in question. Light-emitting diodes (LEDs) or fiber-coupled diode-pumped solid-state lasers (DPSS) are frequently used. Recent advances include the advent of wireless head-mounted devices that apply LEDs to the targeted areas and as a result, give the animals more freedom to move.[83][84]
Fiber-based approaches can also be used to combine optical stimulation and calcium imaging.[77] This enables researchers to visualize and manipulate the activity of single neurons in awake behaving animals.[85] It is also possible to record from multiple deep brain regions at the same using GRIN lenses connected via optical fiber to an externally positioned photodetector and photostimulator.[86][87]
https://en.wikipedia.org/wiki/Optogenetics#Technique
Optogenetics is a biological technique to control the activity of neurons or other cell types with light. This is achieved by expression of light-sensitive ion channels, pumps or enzymes specifically in the target cells. On the level of individual cells, light-activated enzymes and transcription factors allow precise control of biochemical signaling pathways.[1] In systems neuroscience, the ability to control the activity of a genetically defined set of neurons has been used to understand their contribution to decision making,[2] learning,[3] fear memory,[4] mating,[5] addiction,[6] feeding,[7] and locomotion.[8] In a first medical application of optogenetic technology, vision was partially restored in a blind patient.[9]
Optogenetic techniques have also been introduced to map the functional connectivity of the brain.[10][11] By altering the activity of genetically labelled neurons with light and using imaging and electrophysiology techniques to record the activity of other cells, researchers can identify the statistical dependencies between cells and brain regions.[12][13]
In a broader sense, optogenetics also includes methods to record cellular activity with genetically encoded indicators.
In 2010, optogenetics was chosen as the "Method of the Year" across all fields of science and engineering by the interdisciplinary research journal Nature Methods.[14] At the same time, optogenetics was highlighted in the article on "Breakthroughs of the Decade" in the academic research journal Science.[15][16][17]
Description
Optogenetics provides millisecond-scale temporal precision which allows the experimenter to keep pace with fast biological information processing (for example, in probing the causal role of specific action potential patterns in defined neurons). Indeed, to probe the neural code, optogenetics by definition must operate on the millisecond timescale to allow addition or deletion of precise activity patterns within specific cells in the brains of intact animals, including mammals (see Figure 1). By comparison, the temporal precision of traditional genetic manipulations (employed to probe the causal role of specific genes within cells, via "loss-of-function" or "gain of function" changes in these genes) is rather slow, from hours or days to months. It is important to also have fast readouts in optogenetics that can keep pace with the optical control. This can be done with electrical recordings ("optrodes") or with reporter proteins that are biosensors, where scientists have fused fluorescent proteins to detector proteins. Additionally, beyond its scientific impact optogenetics represents an important case study in the value of both ecological conservation (as many of the key tools of optogenetics arise from microbial organisms occupying specialized environmental niches), and in the importance of pure basic science as these opsins were studied over decades for their own sake by biophysicists and microbiologists, without involving consideration of their potential value in delivering insights into neuroscience and neuropsychiatric disease.[59]
Light-activated proteins: channels, pumps and enzymes
The hallmark of optogenetics therefore is introduction of fast light-activated channels, pumps, and enzymes that allow temporally precise manipulation of electrical and biochemical events while maintaining cell-type resolution through the use of specific targeting mechanisms. Among the microbial opsins which can be used to investigate the function of neural systems are the channelrhodopsins (ChR2, ChR1, VChR1, and SFOs) to excite neurons and anion-conducting channelrhodopsins for light-induced inhibition. Indirectly light-controlled potassium channels have recently been engineered to prevent action potential generation in neurons during blue light illumination.[60][61] Light-driven ion pumps are also used to inhibit neuronal activity, e.g. halorhodopsin (NpHR),[62] enhanced halorhodopsins (eNpHR2.0 and eNpHR3.0, see Figure 2),[63] archaerhodopsin (Arch), fungal opsins (Mac) and enhanced bacteriorhodopsin (eBR).[64]
Optogenetic control of well-defined biochemical events within behaving mammals is now also possible. Building on prior work fusing vertebrate opsins to specific G-protein coupled receptors[65] a family of chimeric single-component optogenetic tools was created that allowed researchers to manipulate within behaving mammals the concentration of defined intracellular messengers such as cAMP and IP3 in targeted cells.[66] Other biochemical approaches to optogenetics (crucially, with tools that displayed low activity in the dark) followed soon thereafter, when optical control over small GTPases and adenylyl cyclase was achieved in cultured cells using novel strategies from several different laboratories.[67][68][69] Photoactivated adenylyl cyclases have been discovered in fungi and successfully used to control cAMP levels in mammalian neurons.[70][71] This emerging repertoire of optogenetic actuators now allows cell-type-specific and temporally precise control of multiple axes of cellular function within intact animals.[72]
Hardware for light application
Another necessary factor is hardware (e.g. integrated fiberoptic and solid-state light sources) to allow specific cell types, even deep within the brain, to be controlled in freely behaving animals. Most commonly, the latter is now achieved using the fiberoptic-coupled diode technology introduced in 2007,[73][74][75] though to avoid use of implanted electrodes, researchers have engineered ways to inscribe a "window" made of zirconia that has been modified to be transparent and implanted in mice skulls, to allow optical waves to penetrate more deeply to stimulate or inhibit individual neurons.[76] To stimulate superficial brain areas such as the cerebral cortex, optical fibers or LEDs can be directly mounted to the skull of the animal. More deeply implanted optical fibers have been used to deliver light to deeper brain areas.[77] Complementary to fiber-tethered approaches, completely wireless techniques have been developed utilizing wirelessly delivered power to headborne LEDs for unhindered study of complex behaviors in freely behaving organisms.[78]
Expression of optogenetic actuators
Optogenetics also necessarily includes the development of genetic targeting strategies such as cell-specific promoters or other customized conditionally-active viruses, to deliver the light-sensitive probes to specific populations of neurons in the brain of living animals (e.g. worms, fruit flies, mice, rats, and monkeys). In invertebrates such as worms and fruit flies some amount of all-trans-retinal (ATR) is supplemented with food. A key advantage of microbial opsins as noted above is that they are fully functional without the addition of exogenous co-factors in vertebrates.[75]
Technique
The technique of using optogenetics is flexible and adaptable to the experimenter's needs. Cation-selective channelrhodopsins (e.g. ChR2) are used to excite neurons, anion-conducting channelrhodopsins (e.g. GtACR2) inhibit neuronal activity. Combining these tools into a single construct (e.g. BiPOLES) allows for both inhibition and excitation, depending on the wavelength of illumination.[80]
Introducing the microbial opsin into a specific subset of cells is challenging. A popular approach is to introduce an engineered viral vector that contains the optogenetic actuator gene attached to a specific promoter such as CAMKIIα, which is active in excitatory neurons. This allows for some level of specificity, preventing e.g. expression in glia cells.[81]
A more specific approach is based on transgenic "driver" mice which express Cre recombinase, an enzyme that catalyzes recombination between two lox-P sites, in a specific subset of cells, e.g. parvalbumin-expressing interneurons. By introducing an engineered viral vector containing the optogenetic actuator gene in between two lox-P sites, only the cells producing Cre recombinase will express the microbial opsin. This technique has allowed for multiple modified optogenetic actuators to be used without the need to create a whole line of transgenic animals every time a new microbial opsin is needed.[82]
After the introduction and expression of the microbial opsin, a computer-controlled light source has to be optically coupled to the brain region in question. Light-emitting diodes (LEDs) or fiber-coupled diode-pumped solid-state lasers (DPSS) are frequently used. Recent advances include the advent of wireless head-mounted devices that apply LEDs to the targeted areas and as a result, give the animals more freedom to move.[83][84]
Fiber-based approaches can also be used to combine optical stimulation and calcium imaging.[77] This enables researchers to visualize and manipulate the activity of single neurons in awake behaving animals.[85] It is also possible to record from multiple deep brain regions at the same using GRIN lenses connected via optical fiber to an externally positioned photodetector and photostimulator.[86][87]
Technical challenges
Selective expression
One of the main problems of optogenetics is that not all the cells in question may express the microbial opsin gene at the same level. Thus, even illumination with a defined light intensity will have variable effects on individual cells. Optogenetic stimulation of neurons in the brain is even less controlled as the light intensity drops exponentially from the light source (e.g. implanted optical fiber).
It remains difficult to target opsin to defined subcellular compartments, e.g. the plasma membrane, synaptic vesicles, or mitochondria.[63][88] Restricting the opsin to specific regions of the plasma membrane such as dendrites, somata or axon terminals provides a more robust understanding of neuronal circuitry.[88]
Mathematical modelling shows that selective expression of opsin in specific cell types can dramatically alter the dynamical behavior of the neural circuitry. In particular, optogenetic stimulation that preferentially targets inhibitory cells can transform the excitability of the neural tissue, affecting non-transfected neurons as well.[89]
Kinetics and synchronization
The original channelrhodopsin-2 was slower closing than typical cation channels of cortical neurons, leading to prolonged depolarization and calcium influx.[90] Many channelrhodopsin variants with more favorable kinetics have since been engineered.[55][56]
A difference between natural spike patterns and optogenetic activation is that pulsed light stimulation produces synchronous activation of expressing neurons, which removes the possibility of sequential activity in the stimulated population. Therefore, it is difficult to understand how the cells in the population affected communicate with one another or how their phasic properties of activation relate to circuit function.
Optogenetic activation has been combined with functional magnetic resonance imaging (ofMRI) to elucidate the connectome, a thorough map of the brain's neural connections.[88][91] Precisely timed optogenetic activation is used to calibrate the delayed hemodynamic signal (BOLD) fMRI is based on.
Light absorption spectrum
The opsin proteins currently in use have absorption peaks across the visual spectrum, but remain considerably sensitive to blue light.[88] This spectral overlap makes it very difficult to combine opsin activation with genetically encoded indicators (GEVIs, GECIs, GluSnFR, synapto-pHluorin), most of which need blue light excitation. Opsins with infrared activation would, at a standard irradiance value, increase light penetration and augment resolution through reduction of light scattering.
Spatial response
Due to scattering, a narrow light beam to stimulate neurons in a patch of neural tissue can evoke a response profile that is much broader than the stimulation beam.[92] In this case, neurons may be activated (or inhibited) unintentionally. Computational simulation tools[93][94] are used to estimate the volume of stimulated tissue for different wavelengths of light.
Applications
The field of optogenetics has furthered the fundamental scientific understanding of how specific cell types contribute to the function of biological tissues such as neural circuits in vivo. On the clinical side, optogenetics-driven research has led to insights into Parkinson's disease[95][96] and other neurological and psychiatric disorders such as autism, Schizophrenia, drug abuse, anxiety, and depression.[64][97][98][99] An experimental treatment for blindness involves a channel rhodopsin expressed in ganglion cells, stimulated with light patterns from engineered goggles.[100][9]
Identification of particular neurons and networks
Amygdala
Optogenetic approaches have been used to map neural circuits in the amygdala that contribute to fear conditioning.[101][102][103][104] One such example of a neural circuit is the connection made from the basolateral amygdala to the dorsal-medial prefrontal cortex where neuronal oscillations of 4 Hz have been observed in correlation to fear induced freezing behaviors in mice. Transgenic mice were introduced with channelrhodoposin-2 attached with a parvalbumin-Cre promoter that selectively infected interneurons located both in the basolateral amygdala and the dorsal-medial prefrontal cortex responsible for the 4 Hz oscillations. The interneurons were optically stimulated generating a freezing behavior and as a result provided evidence that these 4 Hz oscillations may be responsible for the basic fear response produced by the neuronal populations along the dorsal-medial prefrontal cortex and basolateral amygdala.[105]
Olfactory bulb
Optogenetic activation of olfactory sensory neurons was critical for demonstrating timing in odor processing[106] and for mechanism of neuromodulatory mediated olfactory guided behaviors (e.g. aggression, mating)[107] In addition, with the aid of optogenetics, evidence has been reproduced to show that the "afterimage" of odors is concentrated more centrally around the olfactory bulb rather than on the periphery where the olfactory receptor neurons would be located. Transgenic mice infected with channel-rhodopsin Thy1-ChR2, were stimulated with a 473 nm laser transcranially positioned over the dorsal section of the olfactory bulb. Longer photostimulation of mitral cells in the olfactory bulb led to observations of longer lasting neuronal activity in the region after the photostimulation had ceased, meaning the olfactory sensory system is able to undergo long term changes and recognize differences between old and new odors.[108]
Nucleus accumbens
Optogenetics, freely moving mammalian behavior, in vivo electrophysiology, and slice physiology have been integrated to probe the cholinergic interneurons of the nucleus accumbens by direct excitation or inhibition. Despite representing less than 1% of the total population of accumbal neurons, these cholinergic cells are able to control the activity of the dopaminergic terminals that innervate medium spiny neurons (MSNs) in the nucleus accumbens.[109] These accumbal MSNs are known to be involved in the neural pathway through which cocaine exerts its effects, because decreasing cocaine-induced changes in the activity of these neurons has been shown to inhibit cocaine conditioning. The few cholinergic neurons present in the nucleus accumbens may prove viable targets for pharmacotherapy in the treatment of cocaine dependence[64]
Prefrontal cortex
In vivo and in vitro recordings from the University of Colorado, Boulder Optophysiology Laboratory of Donald C. Cooper Ph.D. showing individual CAMKII AAV-ChR2 expressing pyramidal neurons within the prefrontal cortex that demonstrated high fidelity action potential output with short pulses of blue light at 20 Hz (Figure 1).[56]
Motor cortex
In vivo repeated optogenetic stimulation in healthy animals was able to eventually induce seizures.[110] This model has been termed optokindling.
Piriform cortex
In vivo repeated optogenetic stimulation of pyramidal cells of the piriform cortex in healthy animals was able to eventually induce seizures.[111] In vitro studies have revealed a loss of feedback inhibition in the piriform circuit due to impaired GABA synthesis.[111]
Heart
Optogenetics was applied on atrial cardiomyocytes to end spiral wave arrhythmias, found to occur in atrial fibrillation, with light.[112] This method is still in the development stage. A recent study explored the possibilities of optogenetics as a method to correct for arrythmias and resynchronize cardiac pacing. The study introduced channelrhodopsin-2 into cardiomyocytes in ventricular areas of hearts of transgenic mice and performed in vitro studies of photostimulation on both open-cavity and closed-cavity mice. Photostimulation led to increased activation of cells and thus increased ventricular contractions resulting in increasing heart rates. In addition, this approach has been applied in cardiac resynchronization therapy (CRT) as a new biological pacemaker as a substitute for electrode based-CRT.[113] Lately, optogenetics has been used in the heart to defibrillate ventricular arrhythmias with local epicardial illumination,[114] a generalized whole heart illumination[115] or with customized stimulation patterns based on arrhythmogenic mechanisms in order to lower defibrillation energy.[116]
Spiral ganglion
Optogenetic stimulation of the spiral ganglion in deaf mice restored auditory activity.[117] Optogenetic application onto the cochlear region allows for the stimulation or inhibition of the spiral ganglion cells (SGN). In addition, due to the characteristics of the resting potentials of SGN's, different variants of the protein channelrhodopsin-2 have been employed such as Chronos,[118] CatCh and f-Chrimson.[119] Chronos and CatCh variants are particularly useful in that they have less time spent in their deactivated states, which allow for more activity with less bursts of blue light emitted. Additionally, using engineered red-shifted channels as f-Chrimson allow for stimulation using longer wavelengths, which decreases the potential risks of phototoxicity in the long term without compromising gating speed.[120] The result being that the LED producing the light would require less energy and the idea of cochlear prosthetics in association with photo-stimulation, would be more feasible.[121]
Brainstem
Optogenetic stimulation of a modified red-light excitable channelrhodopsin (ReaChR) expressed in the facial motor nucleus enabled minimally invasive activation of motoneurons effective in driving whisker movements in mice.[122] One novel study employed optogenetics on the Dorsal Raphe Nucleus to both activate and inhibit dopaminergic release onto the ventral tegmental area. To produce activation transgenic mice were infected with channelrhodopsin-2 with a TH-Cre promoter and to produce inhibition the hyperpolarizing opsin NpHR was added onto the TH-Cre promoter. Results showed that optically activating dopaminergic neurons led to an increase in social interactions, and their inhibition decreased the need to socialize only after a period of isolation.[123]
Visual system
Studying the visual system using optogenetics can be challenging. Indeed, the light used for optogenetic control may lead to the activation of photoreceptors, as a result of the proximity between primary visual circuits and these photoreceptors. In this case, spatial selectivity is difficult to achieve (particularly in the case of the fly optic lobe). Thus, the study of the visual system requires spectral separation, using channels that are activated by different wavelengths of light than rhodopsins within the photoreceptors (peak activation at 480 nm for Rhodopsin 1 in Drosophila). Red-shifted CsChrimson[124] or bistable Channelrhodopsin[125] are used for optogenetic activation of neurons (i.e. depolarization), as both allow spectral separation. In order to achieve neuronal silencing (i.e. hyperpolarization), an anion channelrhodopsin discovered in the cryptophyte algae species Guillardia theta (named GtACR1).[126] can be used. GtACR1 is more light sensitive than other inhibitory channels such as the Halorhodopsin class of chlorid pumps and imparts a strong conductance. As its activation peak (515 nm) is close to that of Rhodopsin 1, it is necessary to carefully calibrate the optogenetic illumination as well as the visual stimulus. The factors to take into account are the wavelength of the optogenetic illumination (possibly higher than the activation peak of GtACR1), the size of the stimulus (in order to avoid the activation of the channels by the stimulus light) and the intensity of the optogenetic illumination. It has been shown that GtACR1 can be a useful inhibitory tool in optogenetic study of Drosophila's visual system by silencing T4/T5 neurons expression.[127] These studies can also be led on intact behaving animals, for instance to probe optomotor response.
Sensorimotor system
Optogenetically inhibiting or activating neurons tests their necessity and sufficiency, respectively, in generating a behavior.[128] Using this approach, researchers can dissect the neural circuitry controlling motor output. By perturbing neurons at various places in the sensorimotor system, researchers have learned about the role of descending neurons in eliciting stereotyped behaviors,[129] how localized tactile sensory input[130] and activity of interneurons[131] alters locomotion, and the role of Purkinje cells in generating and modulating movement.[132] This is a powerful technique for understanding the neural underpinnings of animal locomotion and movement more broadly.
Precise temporal control of interventions
The currently available optogenetic actuators allow for the accurate temporal control of the required intervention (i.e. inhibition or excitation of the target neurons) with precision routinely going down to the millisecond level.[133] The temporal precision varies, however, across optogenetic actuators,[134] and depends on the frequency and intensity of the stimulation.[92]
Experiments can now be devised where the light used for the intervention is triggered by a particular element of behavior (to inhibit the behavior), a particular unconditioned stimulus (to associate something to that stimulus) or a particular oscillatory event in the brain (to inhibit the event).[135][136] This kind of approach has already been used in several brain regions:
Hippocampus
Sharp waves and ripple complexes (SWRs) are distinct high frequency oscillatory events in the hippocampus thought to play a role in memory formation and consolidation. These events can be readily detected by following the oscillatory cycles of the on-line recorded local field potential. In this way the onset of the event can be used as a trigger signal for a light flash that is guided back into the hippocampus to inhibit neurons specifically during the SWRs and also to optogenetically inhibit the oscillation itself.[137] These kinds of "closed-loop" experiments are useful to study SWR complexes and their role in memory.
Cellular biology/cell signaling pathways
Analogously to how natural light-gated ion channels such as channelrhodopsin-2 allows optical control of ion flux, which is especially useful in neuroscience, natural light-controlled signal transduction proteins also allow optical control of biochemical pathways, including both second-messenger generation and protein-protein interactions, which is especially useful in studying cell and developmental biology.[139] In 2002, the first example of using photoproteins from another organism for controlling a biochemical pathway was demonstrated using the light-induced interaction between plant phytochrome and phytochrome-interacting factor (PIF) to control gene transcription in yeast.[1] By fusing phytochrome to a DNA-binding domain and PIF to a transcriptional activation domain, transcriptional activation of genes recognized by the DNA-binding domain could be induced by light.[1] This study anticipated aspects of the later development of optogenetics in the brain, for example, by suggesting that "Directed light delivery by fiber optics has the potential to target selected cells or tissues, even within larger, more-opaque organisms."[1] The literature has been inconsistent as to whether control of cellular biochemistry with photoproteins should be subsumed within the definition of optogenetics, as optogenetics in common usage refers specifically to the control of neuronal firing with opsins,[140][141][17][142] and as control of neuronal firing with opsins postdates and utilizes distinct mechanisms from control of cellular biochemistry with photoproteins.[139]
Photosensitive proteins utilized in various cell signaling pathways
In addition to phytochromes, which are found in plants and cyanobacteria, LOV domains(Light-oxygen-voltage-sensing domain) from plants and yeast and cryptochrome domains from plants are other natural photosensory domains that have been used for optical control of biochemical pathways in cells.[143][139] In addition, a synthetic photosensory domain has been engineered from the fluorescent protein Dronpa for optical control of biochemical pathways.[139] In photosensory domains, light absorption is either coupled to a change in protein-protein interactions (in the case of phytochromes, some LOV domains, cryptochromes, and Dronpa mutants) or a conformational change that exposes a linked protein segment or alters the activity of a linked protein domain (in the case of phytochromes and some LOV domains).[139] Light-regulated protein-protein interactions can then be used to recruit proteins to DNA, for example to induce gene transcription or DNA modifications, or to the plasma membrane, for example to activate resident signaling proteins.[138][144][145][146][147][148] CRY2 also clusters when active, so has been fused with signaling domains and subsequently photoactivated to allow for clustering-based activation.[149] The LOV2 domain of Avena sativa(common oat) has been used to expose short peptides or an active protein domain in a light-dependent manner.[150][151][152] Introduction of this LOV domain into another protein can regulate function through light induced peptide disorder.[153] The asLOV2 protein, which optogenetically exposes a peptide, has also been used as a scaffold for several synthetic light induced dimerization and light induced dissociation systems (iLID and LOVTRAP, respectively).[154][155] The systems can be used to control proteins through a protein splitting strategy.[156] Photodissociable Dronpa domains have also been used to cage a protein active site in the dark, uncage it after cyan light illumination, and recage it after violet light illumination.[157]
Temporal control of signal transduction with light
The ability to optically control signals for various time durations is being explored to elucidate how cell signaling pathways convert signal duration and response to different outputs.[158] Natural signaling cascades are capable of responding with different outputs to differences in stimulus timing duration and dynamics.[159] For example, treating PC12 cells with epidermal growth factor (EGF, inducing a transient profile of ERK activity) leads to cellular proliferation whereas introduction of nerve growth factor (NGF, inducing a sustained profile of ERK activity) leads to differentiation into neuron-like cells.[160] This behavior was initially characterized using EGF and NGF application, but the finding has been partially replicated with optical inputs.[161] In addition, a rapid negative feedback loop in the RAF-MEK-ERK pathway was discovered using pulsatile activation of a photoswitchable RAF engineered with photodissociable Dronpa domains.[157]
Optogenetic noise-photostimulation
Professor Elias Manjarrez´s research group introduced the Optogenetic noise-photostimulation.[162][163][164] This is a technique that uses random noisy light to activate neurons expressing ChR2. An optimal level of optogenetic-noise photostimulation on the brain can increase the somatosensory evoked field potentials, the firing frequency response of pyramidal neurons to somatosensory stimulation, and the sodium current amplitude.
References
Editorial: "Method of the Year 2010". Nature Methods. 8 (1): 1. 2010. doi:10.1038/nmeth.f.321.
Commentary: Deisseroth K (January 2011). "Optogenetics". Nature Methods. 8 (1): 26–29. doi:10.1038/nmeth.f.324. PMC 6814250. PMID 21191368.
- Mabil P, Huidobro N, Torres-Ramirez O, Flores-Hernandez J, Flores A, Gutierrez R, Manjarrez E (2020). "Noisy Light Augments the Na+ Current in Somatosensory Pyramidal Neurons of Optogenetic Transgenic Mice". Frontiers in Neuroscience. 14: 490. doi:10.3389/fnins.2020.00490. PMC 7263390. PMID 32528244.
Further reading
- Appasani K (2017). Optogenetics: from neuronal function to mapping and disease biology. Cambridge, UK: Cambridge University Press. ISBN 978-1-107-05301-4.
- Banerjee S, Mitra D (January 2020). "Structural Basis of Design and Engineering for Advanced Plant Optogenetics". Trends in Plant Science. 25 (1): 35–65. doi:10.1016/j.tplants.2019.10.002. PMID 31699521. S2CID 207942668.
- Hu W, Li Q, Li B, Ma K, Zhang C, Fu X (January 2020). "Optogenetics sheds new light on tissue engineering and regenerative medicine". Biomaterials. 227: 119546. doi:10.1016/j.biomaterials.2019.119546. PMID 31655444. S2CID 204918731.
- Jarrin S, Finn DP (October 2019). "Optogenetics and its application in pain and anxiety research". Neuroscience and Biobehavioral Reviews. 105: 200–211. doi:10.1016/j.neubiorev.2019.08.007. PMID 31421140. S2CID 199577276.
- Johnson HE, Toettcher JE (August 2018). "Illuminating developmental biology with cellular optogenetics". Current Opinion in Biotechnology. 52: 42–48. doi:10.1016/j.copbio.2018.02.003. PMC 6082700. PMID 29505976.
- Krueger D, Izquierdo E, Viswanathan R, Hartmann J, Pallares Cartes C, De Renzis S (October 2019). "Principles and applications of optogenetics in developmental biology". Development. 146 (20): dev175067. doi:10.1242/dev.175067. PMC 6914371. PMID 31641044.
- Losi A, Gardner KH, Möglich A (November 2018). "Blue-Light Receptors for Optogenetics". Chemical Reviews. 118 (21): 10659–10709. doi:10.1021/acs.chemrev.8b00163. PMC 6500593. PMID 29984995.
- Vriz S, Ozawa T (September 2018). Optogenetics: light-driven actuators and light-emitting sensors in cell biology. Comprehensive Series in Photochemistry and Photobiology. Vol. 18. London: Royal Society of Chemistry. ISBN 978-1-78801-237-9.
- Wittmann T, Dema A, van Haren J (October 2020). "Lights, cytoskeleton, action: Optogenetic control of cell dynamics". Current Opinion in Cell Biology. Elsevier Ltd. 66: 1–10. doi:10.1016/j.ceb.2020.03.003. PMC 7577957. PMID 32371345.
External links
- "Optogenetics: shedding light on the brain's secrets". Scientifica.
- "Optogenetics: Integrated Calcium Imaging and Optogenetics". Inscopix.
https://en.wikipedia.org/wiki/Optogenetics#Technique
https://en.wikipedia.org/wiki/Neuroscience
https://en.wikipedia.org/wiki/Optogenetic_methods_to_record_cellular_activity
https://en.wikipedia.org/wiki/Channelrhodopsin
https://en.wikipedia.org/wiki/Retinylidene_protein
https://en.wikipedia.org/wiki/Brain_connectivity_estimators
https://en.wikipedia.org/wiki/Transmembrane_domain
https://en.wikipedia.org/wiki/Visual_phototransduction
https://en.wikipedia.org/wiki/Rhodopsin
https://en.wikipedia.org/wiki/Chromophore
https://en.wikipedia.org/wiki/Flagellate
https://en.wikipedia.org/wiki/Phototaxis
https://en.wikipedia.org/wiki/Channelrhodopsin
https://en.wikipedia.org/wiki/Light-gated_ion_channel
https://en.wikipedia.org/wiki/Eyespot_apparatus
https://en.wikipedia.org/wiki/Molecular_cloning
https://en.wikipedia.org/wiki/Glossary_of_biology#intracellular
https://en.wikipedia.org/wiki/Electromagnetic_radiation
https://en.wikipedia.org/wiki/Microwave
Structure
In terms of structure, channelrhodopsins are retinylidene proteins. They are seven-transmembrane proteins like rhodopsin, and contain the light-isomerizable chromophore all-trans-retinal (an aldehyde derivative of vitamin A). The retinal chromophore is covalently linked to the rest of the protein through a protonated Schiff base. Whereas most 7-transmembrane proteins are G protein-coupled receptors that open other ion channels indirectly via second messengers (i.e., they are metabotropic), channelrhodopsins directly form ion channels (i.e., they are ionotropic).[11] This makes cellular depolarization extremely fast, robust, and useful for bioengineering and neuroscience applications, including photostimulation.
https://en.wikipedia.org/wiki/Channelrhodopsin
https://en.wikipedia.org/wiki/G_protein-coupled_receptor
https://en.wikipedia.org/wiki/Second_messenger_system
https://en.wikipedia.org/wiki/Photostimulation
https://en.wikipedia.org/wiki/tissues
Function
The natural ("wild-type") ChR2 absorbs blue light with an absorption and action spectrum maximum at 480 nm.[14] When the all-trans-retinal complex absorbs a photon, it induces a conformational change from all-trans to 13-cis-retinal. This change introduces a further one in the transmembrane protein, opening the pore to at least 6 Å. Within milliseconds, the retinal relaxes back to the all-trans form, closing the pore and stopping the flow of ions.[11] Most natural channelrhodopsins are nonspecific cation channels (CCRs), conducting H+, Na+, K+, and Ca2+ ions. Recently discovered anion-conducting channelrhodopsins (ACRs)[15] and potassium-selective channelrhodpsins (HcKCR1, HcKCR2) [16] were structurally analyzed to understand their ion selectivity.[17][18] ACRs and KCRs have been used to inhibit neuronal activity.
Development as a molecular tool
In 2005, three groups sequentially established ChR2 as a tool for genetically targeted optical remote control (optogenetics) of neurons, neural circuits and behavior.
At first, Karl Deisseroth's lab demonstrated that ChR2 could be deployed to control mammalian neurons in vitro, achieving temporal precision on the order of milliseconds (both in terms of delay to spiking and in terms of temporal jitter).[19] Because all opsins require retinal as the light-sensing co-factor and it was unclear whether central mammalian nerve cells would contain sufficient retinal levels, but they do. It also showed, despite the small single-channel conductance, sufficient potency to drive mammalian neurons above action potential threshold. From this, channelrhodopsin became the first optogenetic tool, with which neural activity could be controlled with the temporal precision at which neurons operate (milliseconds). A second study was published later confirming the ability of ChR2 to control the activity of vertebrate neurons, at this time in the chick spinal cord.[20] This study was the first wherein ChR2 was expressed alongside an optical silencer, vertebrate rhodopsin-4 in this case, demonstrating for the first time that excitable cells could be activated and silenced using these two tools simultaneously, illuminating the tissue at different wavelengths.
It was demonstrated that ChR2, if expressed in specific neurons or muscle cells, can evoke predictable behaviors, i.e. can control the nervous system of an intact animal, in this case the invertebrate C. elegans.[21] This was the first using ChR2 to steer the behavior of an animal in an optogenetic experiment, rendering a genetically specified cell type subject to optical remote control. Although both aspects had been illustrated earlier that year by the group of Gero Miesenböck, deploying the indirectly light-gated ion channel P2X2,[22] it was henceforth microbial opsins like channelrhodopsin that dominated the field of genetically targeted remote control of excitable cells, due to the power, speed, targetability, ease of use, and temporal precision of direct optical activation, not requiring any external chemical compound such as caged ligands.[23]
To overcome its principal downsides — the small single-channel conductance (especially in steady-state), the limitation to one optimal excitation wavelength (~470 nm, blue) as well as the relatively long recovery time, not permitting controlled firing of neurons above 20–40 Hz — ChR2 has been optimized using genetic engineering. A point mutation H134R (exchanging the amino acid Histidine in position 134 of the native protein for an Arginine) resulted in increased steady-state conductance, as described in a 2005 paper that also established ChR2 as an optogenetic tool in C. elegans.[21] In 2009, Roger Tsien's lab optimized ChR2 for further increases in steady-state conductance and dramatically reduced desensitization by creating chimeras of ChR1 and ChR2 and mutating specific amino acids, yielding ChEF and ChIEF, which allowed the driving of trains of action potentials up to 100 Hz.[24][25] In 2010, the groups of Hegemann and Deisseroth introduced an E123T mutation into native ChR2, yielding ChETA, which has faster on- and off-kinetics, permitting the control of individual action potentials at frequencies up to 200 Hz (in appropriate cell types).[26][24]
The groups of Hegemann and Deisseroth also discovered that the introduction of the point mutation C128S makes the resulting ChR2-derivative a step-function tool: Once "switched on" by blue light, ChR2(C128S) stays in the open state until it is switched off by yellow light – a modification that deteriorates temporal precision, but increases light sensitivity by two orders of magnitude.[27] They also discovered and characterized VChR1 in the multicellular algae Volvox carteri. VChR1 produces only tiny photocurrents, but with an absorption spectrum that is red-shifted relative to ChR2.[28] Using parts of the ChR1 sequence, photocurrent amplitude was later improved to allow excitation of two neuronal populations at two distinct wavelengths.[29]
Deisseroth's group has pioneered many applications in live animals such as genetically targeted remote control in rodents in vivo,[30] the optogenetic induction of learning in rodents,[31] the experimental treatment of Parkinson's disease in rats,[32][33] and the combination with fMRI (opto-fMRI).[34] Other labs have pioneered the combination of ChR2 stimulation with calcium imaging for all-optical experiments,[35] mapping of long-range[36] and local[37] neural circuits, ChR2 expression from a transgenic locus – directly[38] or in the Cre-lox conditional paradigm[37] – as well as the two-photon excitation of ChR2, permitting the activation of individual cells.[39][40][41]
In March 2013, the Brain Prize (Grete Lundbeck European Brain Research Prize) was jointly awarded to Bamberg, Boyden, Deisseroth, Hegemann, Miesenböck, and Nagel for "their invention and refinement of optogenetics".[42] The same year, Hegemann and Nagel received the Louis-Jeantet Prize for Medicine for "the discovery of channelrhodopsin". In 2015, Boyden and Deisseroth received the Breakthrough Prize in Life Sciences and in 2020, Miesenböck, Hegemann and Nagel received the Shaw prize in Life Science and Medicine for the development of optogenetics.
Designer-channelrhodopsins
Channelrhodopsins are key tools in optogenetics. The C-terminal end of Channelrhodopsin-2 extends into the intracellular space and can be replaced by fluorescent proteins without affecting channel function. This kind of fusion construct can be useful to visualize the morphology of ChR2 expressing cells, i.e. simultaneously indicate which cells are tagged with FP and allow the activity to be controlled by the channelrhodopsin.[19][35] Point mutations close to the retinal binding pocket have been shown to affect the biophysical properties of the channelrhodopsin, resulting in a variety of different tools.
Kinetics
Closing of the channel after optical activation can be substantially delayed by mutating the protein residues C128 or D156. This modification results in super-sensitive channelrhodopsins that can be opened by a blue light pulse and closed by a green or yellow light pulse (Step-function opsins).[27][43][29] Mutating the E123 residue accelerates channel kinetics (ChETA), and the resulting ChR2 mutants have been used to spike neurons at up to 200 Hz.[26] In general, channelrhodopsins with slow kinetics are more light-sensitive on the population level, as open channels accumulate over time even at low light levels.
Photocurrent amplitude
H134R and T159C mutants display increased photocurrents, and a combination of T159 and E123 (ET/TC) has slightly larger photocurrents and slightly faster kinetics than wild-type ChR2.[44] ChIEF, a chimera and point mutant of ChR1 and ChR2, demonstrates large photocurrents, little desensitization and kinetics similar to wild-type ChR2.[24] Variants with extended open time (ChR2-XXL) produce extremely large photocurrents and are very light sensitive on the population level.[45]
Wavelength
Chimeric channelrhodopsins have been developed by combining transmembrane helices from ChR1 and VChR1, leading to the development of ChRs with red spectral shifts (such as C1V1 and ReaChR).[29][46] ReaChR has improved membrane trafficking and strong expression in mammalian cells, and has been used for minimally invasive, transcranial activation of brainstem motoneurons. Searches for homologous sequences in other organisms has yielded spectrally improved and stronger red-shifted channelrhodpsins (Chrimson).[47] In combination with ChR2, these yellow/red light-sensitive channelrhodopsins allow controlling two populations of neurons independently with light pulses of different colors.[48][49]
A blue-shifted channelrhodopsin has been discovered in the alga Scherffelia dubia. After some engineering to improve membrane trafficking and speed, the resulting tool (CheRiff) produced large photocurrents at 460 nm excitation.[50] It has been combined with the Genetically Encoded Calcium Indicator jRCaMP1b [51] in an all-optical system called the OptoCaMP.[52]
Ion selectivity
Most channelrhodopsins are unspecific cation channels. When expressed in neurons, they conduct mostly Na+ ions and are therefore depolarizing (excitatory). Variants with moderate to high calcium permeability have been engineered (CatCh, CapChRs).[53][54] K+-specific channelrhodopsins (KCRs, WiChR) were recently discovered in various protists.[55][56] When expressed in neurons, potassium-selective channelrhodopsins hyperpolarize the membrane upon illumination, preventing spike generation (inhibitory).
Mutating E90 to the positively charged amino acid arginine turns channelrhodopsin from an unspecific cation channel into a chloride-conducting channel (ChloC).[57] The selectivity for Cl- was further improved by replacing negatively charged residues in the channel pore, making the reversal potential more negative.[58][59] Anion-conducting channelrhodopsins (iChloC, iC++, GtACR) inhibit neuronal spiking in cell culture and in intact animals when illuminated with blue light.
Applications
Channelrhodopsins can be readily expressed in excitable cells such as neurons using a variety of transfection techniques (viral transfection, electroporation, gene gun) or transgenic animals. The light-absorbing pigment retinal is present in most cells (of vertebrates) as Vitamin A, making it possible to photostimulate neurons without adding any chemical compounds. Before the discovery of channelrhodopsins, neuroscientists were limited to recording the activity of neurons in the brain and correlate this activity with behavior. This is not sufficient to prove that the recorded neural activity actually caused that behavior. Controlling networks of genetically modified cells with light, an emerging field known as Optogenetics., allows researchers now to explore the causal link between activity in a specific group of neurons and mental events, e.g. decision making. Optical control of behavior has been demonstrated in nematodes, fruit flies, zebrafish, and mice.[60][61] Recently, chloride-conducting channelrhodopsins have been engineered and were also found in nature.[15][57] These tools can be used to silence neurons in cell culture and in live animals by shunting inhibition.[58][59]
Using multiple colors of light expands the possibilities of optogenetic experiments. The blue-light sensitive ChR2 and the yellow light-activated chloride pump halorhodopsin together enable multiple-color optical activation and silencing of neural activity.[62][63] Another interesting pair is the blue-light sensitive chloride channel GtACR2[64] and the red-light sensitive cation channel Chrimson[65] which have been combined in a single protein (BiPOLES) for bidirectional control of membrane potential.[66]
Using fluorescently labeled ChR2, light-stimulated axons and synapses can be identified.[35] This is useful to study the molecular events during the induction of synaptic plasticity.[67][68] Transfected cultured neuronal networks can be stimulated to perform some desired behaviors for applications in robotics and control.[69] ChR2 has also been used to map long-range connections from one side of the brain to the other, and to map the spatial location of inputs on the dendritic tree of individual neurons.[36][70]
In 2006, it was reported that transfection with Channelrhodopsin could restore eyesight to blind mice.[71]
Neurons can tolerate ChR expression for a long time, and several laboratories are testing optogenetic stimulation to solve medical needs. In blind mice, visual function can be partially restored by expressing ChR2 in inner retinal cells.[72][73] In 2021, the red-light sensitive ChR ChrimsonR was virally delivered to the eyes of a human patient suffering from retinal degeneration (Retinitis pigmentosa), leading to partial recovery of his vision.[74][75] Optical cochlear implants have been shown to work well in animal experiments and are currently undergoing clinical trials.[76][77][78] In the future, ChRs may find even more medical applications, e.g. for deep-brain stimulation of Parkinson patients or to control certain forms of Epilepsy.
References
- Keppeler D, Merino RM, Lopez de la Morena D, Bali B, Huet AT, Gehrt A, et al. (December 2018). "Ultrafast optogenetic stimulation of the auditory pathway by targeting-optimized Chronos". The EMBO Journal. 37 (24): e99649. doi:10.15252/embj.201899649. PMC 6293277. PMID 30396994.
Further reading
- Hegemann P (2008). "Algal sensory photoreceptors". Annual Review of Plant Biology. 59: 167–189. doi:10.1146/annurev.arplant.59.032607.092847. PMID 18444900. (Naturel function of channelrhodopsins and other photoreceptors in green)
- Arenkiel BR, Peca J, Davison IG, Feliciano C, Deisseroth K, Augustine GJ, et al. (April 2007). "In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2". Neuron. 54 (2): 205–218. doi:10.1016/j.neuron.2007.03.005. PMC 3634585. PMID 17442243. (Using channelrhodopsin in transgenic mice to study brain circuitry)
- Bi A, Cui J, Ma YP, Olshevskaya E, Pu M, Dizhoor AM, Pan ZH (April 2006). "Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration". Neuron. 50 (1): 23–33. doi:10.1016/j.neuron.2006.02.026. PMC 1459045. PMID 16600853. (Using channelrhodopsin potentially to treat blindness)
External links
https://en.wikipedia.org/wiki/Channelrhodopsin
Classes of genetically encoded indicators
Indicators have been designed to measure ion concentrations, membrane potential, neurotransmitters, and various intracellular signaling molecules. The following list provides only examples for each class; many more have been published.
Intracellular signaling:
- Genetically encoded calcium indicators (GECI): A large class of tools, based on natural calcium binding proteins (calmodulin, troponin). Different affinities, kinetics, and colors (green, red) available. Read-out via fluorescence intensity (single wavelength indicators), FRET or BRET. Have been targeted to various organelles. Current version: JGCaMP8[12]
- Genetically encoded chloride indicators: Clomeleon[13]
- Genetically encoded potassium indicators: GINKO2[14]
- Genetically encoded indicators for intracellular pH (GEPhI): CypHer[15]
- Genetically encoded voltage indicators (GEVI): ArcLight[16]
- Genetically encoded vesicle fusion sensors: Synapto-pHluorin, Synaptophysin-pHluorin[17]
- Genetically encoded cAMP sensors: EPAC[18]
- Genetically encoded ATP sensors: QUEEN-37C[19]
- Genetically encoded kinase activity sensors: CaMui,[20] SmURFP
- Genetically encoded Small G-protein sensors: FRas[21]
Neurotransmitters and other extracellular signals:
- Genetically encoded glutamate sensors: GluSnFR[22]
- Genetically encoded GABA sensors: iGABASnFR[23]
- Genetically encoded dopamine sensors: dLight1,[24] GRAB-DA[25]
- Genetically encoded serotonin sensors: GRAB5-HT,[26] sDarken[27]
- Genetically encoded norepinephrine sensors: GRABNE[28]
- Genetically encoded sensor for endocannabinoid activity: GRABeCB2.0[29]
- Genetically encoded sensor for orexin/hypocretin neuropeptides: OxLight1[30]
- Genetically encoded sensor for lactate: eLACCO1.1[31]
External links
- Fluorescent Biosensor Database, a fairly complete searchable list of published sensors and their basic properties, maintained by Jin Zhang's lab at UCSD.[32]
- Fluorescent Biosensors available on Addgene, a nonprofit plasmid repository.
References
- Greenwald EC, Mehta S, Zhang J (December 2018). "Genetically Encoded Fluorescent Biosensors Illuminate the Spatiotemporal Regulation of Signaling Networks". Chemical Reviews. 118 (24): 11707–11794. doi:10.1021/acs.chemrev.8b00333. PMC 7462118. PMID 30550275.
https://en.wikipedia.org/wiki/Optogenetic_methods_to_record_cellular_activity
https://en.wikipedia.org/wiki/Troponin
https://en.wikipedia.org/wiki/Multiplexing
https://en.wikipedia.org/wiki/Confocal_microscopy
https://en.wikipedia.org/wiki/Viral_vector
https://en.wikipedia.org/wiki/Nematode
https://en.wikipedia.org/wiki/Cell_culture
https://en.wikipedia.org/wiki/Organelle
https://en.wikipedia.org/wiki/Intrabody_(protein)
https://en.wikipedia.org/wiki/Pyramidal_cell
https://en.wikipedia.org/wiki/Astrocyte
https://en.wikipedia.org/wiki/Photometry_(optics)
https://en.wikipedia.org/wiki/Radiant_energy
https://en.wikipedia.org/wiki/Radiant_flux
https://en.wikipedia.org/wiki/Joule
https://en.wikipedia.org/wiki/Energy
https://en.wikipedia.org/wiki/Measurement
https://en.wikipedia.org/wiki/Gravitational_wave
https://en.wikipedia.org/wiki/Radiant_energy
https://en.wikipedia.org/wiki/Gravitational_wave
https://en.wikipedia.org/wiki/Scotopic_vision
https://en.wikipedia.org/wiki/Radiant_energy_density
https://en.wikipedia.org/wiki/Radiosity_(radiometry)
https://en.wikipedia.org/wiki/Sensation
https://en.wikipedia.org/wiki/Anion-conducting_channelrhodopsin
https://en.wikipedia.org/wiki/Voltage-sensitive_dye
https://en.wikipedia.org/wiki/Halorhodopsin
https://en.wikipedia.org/wiki/Hyperpolarization_(biology)
https://en.wikipedia.org/wiki/Shunting_inhibition
https://en.wikipedia.org/wiki/Reversal_potential
https://en.wikipedia.org/wiki/GABAA_receptor
https://en.wikipedia.org/wiki/Inhibitory_postsynaptic_potential
https://en.wikipedia.org/wiki/Cryptomonad
https://en.wikipedia.org/wiki/Model_organism
https://en.wikipedia.org/wiki/Bacteriorhodopsin
https://en.wikipedia.org/wiki/Electrochemical_gradient
https://en.wikipedia.org/wiki/Halobacterium_salinarum
Bacteriorhodopsin (Bop) is a protein used by Archaea, most notably by haloarchaea, a class of the Euryarchaeota.[1] It acts as a proton pump; that is, it captures light energy and uses it to move protons across the membrane out of the cell.[2] The resulting proton gradient is subsequently converted into chemical energy.[3]
Function
Bacteriorhodopsin is a light-driven H+ ion transporter found in some haloarchaea, most notably Halobacterium salinarum (formerly known as syn. H. halobium). The proton-motive force generated by the protein is used by ATP synthase to generate adenosine triphosphate (ATP). By expressing bacteriorhodopsin, the archaea cells are able to synthesise ATP in the absence of a carbon source.[4][5]
https://en.wikipedia.org/wiki/Bacteriorhodopsin
https://en.wikipedia.org/wiki/Adenosine_triphosphate
Proton-motive force
The movement of ions across the membrane depends on a combination of two factors:
- Diffusion force caused by a concentration gradient - all particles tend to diffuse from higher concentration to lower.
- Electrostatic force caused by electrical potential gradient - cations like protons H+ tend to diffuse down the electrical potential, from the positive (P) side of the membrane to the negative (N) side. Anions diffuse spontaneously in the opposite direction.
These two gradients taken together can be expressed as an electrochemical gradient.
Lipid bilayers of biological membranes, however, are barriers for ions. This is why energy can be stored as a combination of these two gradients across the membrane. Only special membrane proteins like ion channels can sometimes allow ions to move across the membrane (see also: Membrane transport). In the chemiosmotic hypothesis a transmembrane ATP synthases is central to convert energy of spontaneous flow of protons through them into chemical energy of ATP bonds.
Hence researchers created the term proton-motive force (PMF), derived from the electrochemical gradient mentioned earlier. It can be described as the measure of the potential energy stored (chemiosmotic potential) as a combination of proton and voltage (electrical potential) gradients across a membrane. The electrical gradient is a consequence of the charge separation across the membrane (when the protons H+ move without a counterion, such as chloride Cl−).
In most cases the proton-motive force is generated by an electron transport chain which acts as a proton pump, using the Gibbs free energy of redox reactions to pump protons (hydrogen ions) out across the membrane, separating the charge across the membrane. In mitochondria, energy released by the electron transport chain is used to move protons from the mitochondrial matrix (N side) to the intermembrane space (P side). Moving the protons out of the mitochondrion creates a lower concentration of positively charged protons inside it, resulting in excess negative charge on the inside of the membrane. The electrical potential gradient is about -170 mV [6], negative inside (N). These gradients - charge difference and the proton concentration difference both create a combined electrochemical gradient across the membrane, often expressed as the proton-motive force (PMF). In mitochondria, the PMF is almost entirely made up of the electrical component but in chloroplasts the PMF is made up mostly of the pH gradient because the charge of protons H+ is neutralized by the movement of Cl− and other anions. In either case, the PMF needs to be greater than about 460 mV (45 kJ/mol) for the ATP synthase to be able to make ATP.
Equations
The proton-motive force is derived from the Gibbs free energy. Let N denote the inside of a cell, and let P denote the outside. Then[6]
where
- is the Gibbs free energy change per unit amount of cations transferred from P to N;
- is the charge number of the cation ;
- is the electric potential of N relative to P;
- and are the cation concentrations at P and N, respectively;
- is the Faraday constant;
- is the gas constant; and
- is the temperature.
The molar Gibbs free energy change is frequently interpreted as a molar electrochemical ion potential .
For an electrochemical proton gradient and as a consequence:
where
- .
Mitchell defined the proton-motive force (PMF) as
- .
For example, implies . At this equation takes the form:
.
Note that for spontaneous proton import from the P side (relatively more positive and acidic) to the N side (relatively more negative and alkaline), is negative (similar to ) whereas PMF is positive (similar to redox cell potential ).
It is worth noting that, as with any transmembrane transport process, the PMF is directional. The sign of the transmembrane electric potential difference is chosen to represent the change in potential energy per unit charge flowing into the cell as above. Furthermore, due to redox-driven proton pumping by coupling sites, the proton gradient is always inside-alkaline. For both of these reasons, protons flow in spontaneously, from the P side to the N side; the available free energy is used to synthesize ATP (see below). For this reason, PMF is defined for proton import, which is spontaneous. PMF for proton export, i.e., proton pumping as catalyzed by the coupling sites, is simply the negative of PMF(import).
The spontaneity of proton import (from the P to the N side) is universal in all bioenergetic membranes.[8] This fact was not recognized before the 1990s, because the chloroplast thylakoid lumen was interpreted as an interior phase, but in fact it is topologically equivalent to the exterior of the chloroplast. Azzone et al. stressed that the inside phase (N side of the membrane) is the bacterial cytoplasm, mitochondrial matrix, or chloroplast stroma; the outside (P) side is the bacterial periplasmic space, mitochondrial intermembrane space, or chloroplast lumen. Furthermore, 3D tomography of the mitochondrial inner membrane shows its extensive invaginations to be stacked, similar to thylakoid disks; hence the mitochondrial intermembrane space is topologically quite similar to the chloroplast lumen.:[9]
The energy expressed here as Gibbs free energy, electrochemical proton gradient, or proton-motive force (PMF), is a combination of two gradients across the membrane:
- the concentration gradient (via ) and
- electric potential gradient .
When a system reaches equilibrium, ; nevertheless, the concentrations on either side of the membrane need not be equal. Spontaneous movement across the potential membrane is determined by both concentration and electric potential gradients.
The molar Gibbs free energy of ATP synthesis
is also called phosphorylation potential. The equilibrium concentration ratio can be calculated by comparing and , for example in case of the mammalian mitochondrion:[9]
H+ / ATP = ΔGp / (Δp / 10.4 kJ·mol−1/mV) = 40.2 kJ·mol−1 / (173.5 mV / 10.4 kJ·mol−1/mV) = 40.2 / 16.7 = 2.4. The actual ratio of the proton-binding c-subunit to the ATP-synthesizing beta-subunit copy numbers is 8/3 = 2.67, showing that under these conditions, the mitochondrion functions at 90% (2.4/2.67) efficiency.[9]
In fact, the thermodynamic efficiency is mostly lower in eukaryotic cells because ATP must be exported from the matrix to the cytoplasm, and ADP and phosphate must be imported from the cytoplasm. This "costs" one "extra" proton import per ATP,[6][7] hence the actual efficiency is only 65% (= 2.4/3.67).
In mitochondria
The complete breakdown of glucose releasing its energy is called cellular respiration. The last steps of this process occur in mitochondria. The reduced molecules NADH and FADH2 are generated by the Krebs cycle, glycolysis, and pyruvate processing. These molecules pass electrons to an electron transport chain, which releases the energy of oxygen to create a proton gradient across the inner mitochondrial membrane. ATP synthase then uses the energy stored in this gradient to make ATP. This process is called oxidative phosphorylation because it uses energy released by the oxidation of NADH and FADH2 to phosphorylate ADP into ATP.
In plants
The light reactions of photosynthesis generate ATP by the action of chemiosmosis. The photons in sunlight are received by the antenna complex of Photosystem II, which excites electrons to a higher energy level. These electrons travel down an electron transport chain, causing protons to be actively pumped across the thylakoid membrane into the thylakoid lumen. These protons then flow down their electrochemical potential gradient through an enzyme called ATP-synthase, creating ATP by the phosphorylation of ADP to ATP. The electrons from the initial light reaction reach Photosystem I, then are raised to a higher energy level by light energy and then received by an electron acceptor and reduce NADP+ to NADPH. The electrons lost from Photosystem II get replaced by the oxidation of water, which is "split" into protons and oxygen by the oxygen-evolving complex (OEC, also known as WOC, or the water-oxidizing complex). To generate one molecule of diatomic oxygen, 10 photons must be absorbed by Photosystems I and II, four electrons must move through the two photosystems, and 2 NADPH are generated (later used for carbon dioxide fixation in the Calvin Cycle).
In prokaryotes
Bacteria and archaea also can use chemiosmosis to generate ATP. Cyanobacteria, green sulfur bacteria, and purple bacteria synthesize ATP by a process called photophosphorylation.[6][7] These bacteria use the energy of light to create a proton gradient using a photosynthetic electron transport chain. Non-photosynthetic bacteria such as E. coli also contain ATP synthase. In fact, mitochondria and chloroplasts are the product of endosymbiosis and trace back to incorporated prokaryotes. This process is described in the endosymbiotic theory. The origin of the mitochondrion triggered the origin of eukaryotes, and the origin of the plastid the origin of the Archaeplastida, one of the major eukaryotic supergroups.[citation needed]
Chemiosmotic phosphorylation is the third pathway that produces ATP from inorganic phosphate and an ADP molecule. This process is part of oxidative phosphorylation.
Emergence of chemiosmosis
Thermal cycling model
A stepwise model for the emergence of chemiosmosis, a key element in the origin of life on earth, proposes that primordial organisms used thermal cycling as an energy source (thermosynthesis), functioning essentially as a heat engine:[11]
- self-organized convection in natural waters causing thermal cycling →
- added β-subunit of F1 ATP Synthase
- (generated ATP by thermal cycling of subunit during suspension in convection cell: thermosynthesis) →
- added membrane and Fo ATP Synthase moiety
- (generated ATP by change in electrical polarization of membrane during thermal cycling: thermosynthesis) →
- added metastable, light-induced electric dipoles in membrane
- (primitive photosynthesis) →
- added quinones and membrane-spanning light-induced electric dipoles
- (today's bacterial photosynthesis, which makes use of chemiosmosis).
External proton gradient model
Deep-sea hydrothermal vents, emitting hot acidic or alklaine water, would have created external proton gradients. These provided energy that primordial organisms could have exploited. To keep the flows separate, such an organism could have wedged itself in the rock of the hydrothermal vent, exposed to the hydrothermal flow on one side and the more alkaline water on the other. As long as the organism's membrane (or passive ion channels within it) is permeable to protons, the mechanism can function without ion pumps. Such a proto-organism could then have evolved further mechanisms such as ion pumps and ATP synthase.[10]
Meteoritic quinones
A proposed alternative source to chemiosmotic energy developing across membranous structures is if an electron acceptor, ferricyanide, is within a vesicle and the electron donor is outside, quinones transported by carbonaceous meteorites pick up electrons and protons from the donor. They would release electrons across the lipid membrane by diffusion to ferricyanide within the vesicles and release protons which produces gradients above pH 2, the process is conducive to the development of proton gradients.[12][13]
See also
References
- Milshteyn D, Cooper G, Deamer D (August 2019). "Chemiosmotic energy for primitive cellular life: Proton gradients are generated across lipid membranes by redox reactions coupled to meteoritic quinones". Scientific Reports. 9 (1): 12447. doi:10.1038/s41598-019-48328-5. PMC 6713726. PMID 31462644.
Further reading
- Biochemistry textbook reference, from the NCBI bookshelf – Jeremy M. Berg; John L. Tymoczko; Lubert Stryer (eds.). "18.4. A Proton Gradient Powers the Synthesis of ATP". Biochemistry (5th ed.). W. H. Freeman.
- A set of experiments aiming to test some tenets of the chemiosmotic theory – Ogawa S, Lee TM (August 1984). "The relation between the internal phosphorylation potential and the proton motive force in mitochondria during ATP synthesis and hydrolysis". The Journal of Biological Chemistry. 259 (16): 10004–10011. doi:10.1016/S0021-9258(18)90918-X. PMID 6469951.
External links
https://en.wikipedia.org/wiki/Chemiosmosis#Proton-motive_force
Chemiosmosis is the movement of ions across a semipermeable membrane bound structure, down their electrochemical gradient. An important example is the formation of adenosine triphosphate (ATP) by the movement of hydrogen ions (H+) across a membrane during cellular respiration or photosynthesis.
Hydrogen ions, or protons, will diffuse from a region of high proton concentration to a region of lower proton concentration, and an electrochemical concentration gradient of protons across a membrane can be harnessed to make ATP. This process is related to osmosis, the movement of water across a selective membrane, which is why it is called "chemiosmosis".
ATP synthase is the enzyme that makes ATP by chemiosmosis. It allows protons to pass through the membrane and uses the free energy difference to phosphorylate adenosine diphosphate (ADP), making ATP. The generation of ATP by chemiosmosis occurs in mitochondria and chloroplasts, as well as in most bacteria and archaea. For instance, in chloroplasts during photosynthesis, an electron transport chain pumps H+ ions (protons) in the stroma (fluid) through the thylakoid membrane to the thylakoid spaces. The stored energy is used to photophosphorylate ADP, making ATP, as protons move through ATP synthase.
https://en.wikipedia.org/wiki/Chemiosmosis#Proton-motive_force
Structure
Bacteriorhodopsin is a 27 kDa integral membrane protein usually found in two-dimensional crystalline patches known as "purple membrane", which can occupy almost 50% of the surface area of the archaeal cell. The repeating element of the hexagonal lattice is composed of three identical protein chains, each rotated by 120 degrees relative to the others.[6] Each monomer has seven transmembrane alpha helices and an extracellular-facing, two-stranded beta sheet.[7][8]
Bacteriorhodopsin is synthesized as a protein precursor, known as bacterio-opsin, which is extensively modified after translation.[9][10] The modifications are:
- Covalent conjugation of a retinal molecule to residue Lys216, via a Schiff base, to create the retinylidene chromophore.[11]
- Cleavage of the signal peptide, the first 13 amino acids at the N-terminus, and the conversion of residue Gln14 to pyroglutamate[12]
- Removal of residue Asp262 at the C-terminus[12]
Spectral properties
Bacteriorhodopsin molecule is purple and is most efficient at absorbing green light (in the wavelength range 500-650 nm). In the native membrane, the protein has a maximum absorbance at 553 nm, however addition of detergent disrupts the trimeric form, leading a loss of exciton coupling between the chromophores, and the monomeric form consequently has an absorption maximum of 568 nm.[13][14]
Bacteriorhodopsin has a broad excitation spectrum. For a detection wavelength between 700 and 800 nm, it has an appreciable detected emission for excitation wavelengths between 470 nm and 650 nm (with a peak at 570 nm).[15] When pumped at 633 nm, the emission spectrum has appreciable intensity between 650 nm and 850 nm.[16]
Mechanism
Photocycle overview
Bacteriorhodopsin is a light-driven proton pump. It is the retinal molecule that changes its isomerization state from all-trans to 13-cis when it absorbs a photon. The surrounding protein responds to the change in the chromophore shape, by undergoing an ordered sequence of conformational changes (collectively known as the photocycle).[17] The conformational changes alter the pKa values of conserved amino acids in the core of the protein, including Asp85, Asp96 and the Schiff base N atom (Lys216). These sequential changes in acid dissociation constant, result in the transfer of one proton from the intracellular side to the extracellular side of the membrane for each photon absorbed by the chromophore.
The bacteriorhodopsin photocycle consists of nine distinct stages, starting from the ground or resting state, which is denoted 'bR'. The intermediates are identified by single letters and may be distinguished by their absorption spectra.[18] The nine stages are:
- bR + photon → K ⇌ L ⇌ M1 ⇌ M2 ⇌ M2' ⇌ N ⇌ N' ⇌ O ⇌ bR[18]
Ground state + photon → K state → L state
Bacteriorhodopsin in the ground state absorbs a photon and the retinal changes isomerization from all-trans 15-anti to the strained 13-cis 15-anti in the K state. The isomerisation reaction is fast and occurs in less than 1 ps. The retinal adopts a less strained conformation to form the L intermediate.
L state → M1 state
Asp85 accepts a proton from the Schiff base N atom. In the M1 intermediate, neither the Schiff base nor Asp85 are charged.
M1 state → M2 state
The Schiff base rotates away from the extracellular side of the protein towards the cytoplasmic side, in preparation to accept a new proton.
M2 state → M2' state
A proton is released from Glu204 and Glu194 to the extracellular medium.
M2' state → N state
The retinal Schiff base accepts a proton from Asp96. In the N state, both Asp96 and the Schiff base are charged.
N state → N' state
Asp96 accepts a proton from the cytoplasmic side of the membrane and becomes uncharged.
N' state → O state
Retinal reisomerizes to the all-trans state.
O state → ground state
Asp85 transfers a proton to Glu194 and Glu204 [19] [20] on the extracellular face of the protein.
Homologs and other similar proteins
Bacteriorhodopsin belongs to the microbial rhodopsin family. Its homologs include the archaerhodopsins,[21] the light-driven chloride pump halorhodopsin (for which the crystal structure is also known), and some directly light-activated channels such as channelrhodopsin.
Bacteriorhodopsin is similar to vertebrate rhodopsins, the pigments that sense light in the retina. Rhodopsins also contain retinal; however, the functions of rhodopsin and bacteriorhodopsin are different, and there is limited similarity in their amino acid sequences. Both rhodopsin and bacteriorhodopsin belong to the 7TM receptor family of proteins, but rhodopsin is a G protein-coupled receptor and bacteriorhodopsin is not. In the first use of electron crystallography to obtain an atomic-level protein structure, the structure of bacteriorhodopsin was resolved in 1990.[22] It was then used as a template to build models of G protein-coupled receptors before crystallographic structures were also available for these proteins. It has been excessively studied on both mica[23][24] and glass substrates using Atomic force microscopy and Femtosecond crystallography.[25]
All other phototrophic systems in bacteria, algae, and plants use chlorophylls or bacteriochlorophylls rather than bacteriorhodopsin. These also produce a proton gradient, but in a quite different and more indirect way involving an electron transfer chain consisting of several other proteins. Furthermore, chlorophylls are aided in capturing light energy by other pigments known as "antennas"; these are not present in bacteriorhodopsin-based systems. It is possible that phototrophy independently evolved at least twice, once in bacteria and once in archaea.
Gallery
Bacteriorhodopsin single monomer with retinal molecule between 7 vertical alpha helixes (PDB ID: 1X0S [26][27][28]). One more small helix is light blue, beta sheet yellow.
See also
Literature
- Image created with RasTop (Molecular Visualization Software).
External links
- Bacteriorhodopsin: Molecule of the Month, by David Goodsell, RCSB Protein Data Bank
- Protein-Based Artificial Retina Manufacturing: Characterization of the Function and Stability of Bacteriorhodopsin Following Exposure to a Microgravity Environment, by Nicole Wagner & Jordan Greco
https://en.wikipedia.org/wiki/Bacteriorhodopsin
Category:7TM receptors
This category contains the proteins belonging to the superfamily of G protein-coupled receptor-like proteins, including bacterial rhodopsins.
Subcategories
This category has only the following subcategory.
G
- G protein-coupled receptors (13 C, 390 P)
Pages in category "7TM receptors"
The following 13 pages are in this category, out of 13 total. This list may not reflect recent changes.




























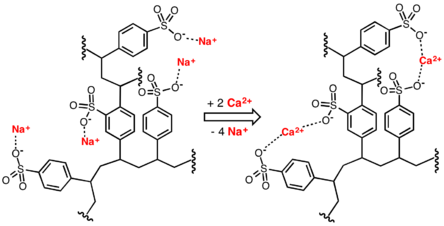














![{\displaystyle \Delta \!G=zF\Delta \!\psi +RT\ln {\frac {[\mathrm {X} ^{z+}]_{\text{N}}}{[\mathrm {X} ^{z+}]_{\text{P}}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/e49984fb465bfe70fdf147d5c94b4691fde30b93)




![{\displaystyle [\mathrm {X} ^{z+}]_{\text{P}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b04667620cf542631c6a2a692aefd89310230ddd)
![{\displaystyle [\mathrm {X} ^{z+}]_{\text{N}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/f4da81b44fd243b0610ca90f2a571ffe2891ad71)





![{\displaystyle \Delta \!\mu _{\mathrm {H} ^{+}}=F\Delta \!\psi +RT\ln {\frac {[\mathrm {H} ^{+}]_{\text{N}}}{[\mathrm {H} ^{+}]_{\text{P}}}}=F\Delta \!\psi -(\ln 10)RT\Delta \mathrm {pH} }](https://wikimedia.org/api/rest_v1/media/math/render/svg/9401e1568170355be3a960a583f16f2d93c5a842)
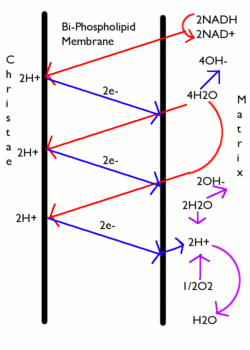













![{\displaystyle [\mathrm {H} ^{+}]/[\mathrm {ATP} ]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/0e253bb9b194bd7dd88012ff9e7acd912dbf6931)





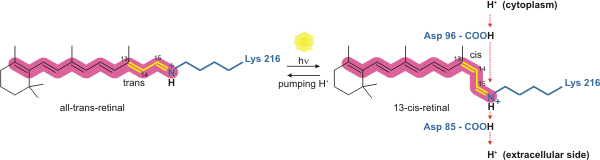

![Bacteriorhodopsin single monomer with retinal molecule between 7 vertical alpha helixes (PDB ID: 1X0S [26][27][28]). One more small helix is light blue, beta sheet yellow.](https://upload.wikimedia.org/wikipedia/commons/2/21/Bacteriorhodopsin_subunit_1X0S.png)
![Bacteriorhodopsin trimer with one retinal molecule in each subunit seen from the extracellular side EC (PDB ID: 1X0S [26][27][28]).](https://upload.wikimedia.org/wikipedia/commons/c/c4/Bacteriorhodopsin_trimer_1X0S.png)



No comments:
Post a Comment