Organotin compounds or stannanes are chemical compounds based on tin with hydrocarbon substituents. Organotin chemistry is part of the wider field of organometallic chemistry. The first organotin compound was diethyltin diiodide ((C2H5)2SnI2), discovered by Edward Frankland in 1849.[1] The area grew rapidly in the 1900s, especially after the discovery of the Grignard reagents, which are useful for producing Sn-C bonds. The area remains rich with many applications in industry and continuing activity in the research laboratory.[2]
Hypercoordinated stannanes
[edit]
Unlike carbon(IV) analogues but somewhat like silicon compounds, tin(IV) can also be coordinated to five and even six atoms instead of the regular four. These hypercoordinated compounds usually have electronegative substituents. Numerous examples of hypervalency are provided by the organotin oxides and associated carboxylates and related pseudohalide derivatives.[5] The organotin halides for adducts, e.g. Me2SnCl2(bipyridine).
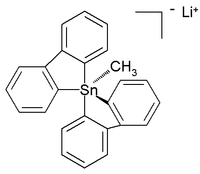
The all-organic penta- and hexaorganostannates have even been characterized,[6] while in the subsequent year a six-coordinated tetraorganotin compound was reported.[7] A crystal structure of room-temperature stable (in argon) all-carbon pentaorganostannane was reported as the lithium salt with this structure:[8]
In this distorted trigonal bipyramidal structure the carbon to tin bond lengths (2.26 Å apical, 2.17 Å equatorial) are larger than regular C-Sn bonds (2.14 Å) reflecting its hypervalent nature.
Triorganotin cations[edit]
Some reactions of triorganotin halides implicate a role for R3Sn+ intermediates. Such cations are analogous to carbocations. They have been characterized crystallographically when the organic substituents are large, such as 2,4,6-triisopropylphenyl.[9]
https://en.wikipedia.org/wiki/Organotin_chemistry

No comments:
Post a Comment