A triazole refers to any of the heterocyclic compounds with molecular formula C2H3N3, having a five-membered ring of two carbon atoms and three nitrogen atoms. There are two sets of isomers that differ in the relative positions of the three nitrogen atoms, namely 1,2,3-Triazoles and 1,2,4-Triazoles. Each of these has two tautomers that differ by which nitrogen has a hydrogen bonded to it.
1,2,3-Triazoles[edit]
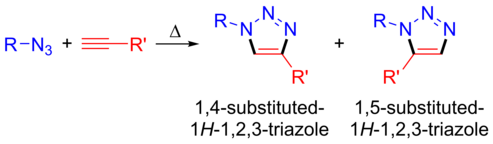
1,2,3-Triazoles are usually prepared following the (3+2) cycloaddition protocols. These employ organic azides as well as alkynes. The reaction can be performed at sufficiently high temperatures even in the absence of additives and the process is calles Huisgen azide-alkyne 1,3-dipolar cycloaddition. However, such process leads to the formation of a mixture of 2 different isomers, namely 1,4-disubstituted 1,2,3-triazole as well as 1,5-disubstituted 1,2,3-triazole. In order to selectively prepare a desired isomer, metal catalysts are employed. In the presence of copper(I) catalysts, the reaction results in the formation of 1,4-disubstituted 1,2,3-triazoles and is called copper-catalysed azide-alkyne cycloaddition (CuAAC). On the other hand if ruthenium catalysts are employed, the reaction leads selectively to the formation of 1,5-disubstituted 1,2,3-triazoles in the ruthenium-catalysed azide-alkyne cycloaddition (RuAAC).[1][2]
A highly efficient copper(I) catalyst, namely CuBr(PPh3)3, that is relatively stable towards oxidation even at elevated temperatures, was used to prepare triazole products with a broad range of substituents either in solvent[3][4] or under neat[5] reaction conditions.
1,2,4-Triazoles[edit]
Application[edit]
Triazoles are compounds with a vast spectrum of applications, varying from materials (polymers), agricultural chemicals, pharmaceuticals, photoactive chemicals and dyes.[6][7]
The triazole antifungal drugs include fluconazole, isavuconazole, itraconazole, voriconazole, pramiconazole, ravuconazole, and posaconazole.
The triazole plant protection fungicides include epoxiconazole, triadimenol, myclobutanil, propiconazole, prothioconazole, metconazole, cyproconazole, tebuconazole, flusilazole and paclobutrazol.
Paclobutrazol, uniconazole, flutriafol, and triadimefon are used as plant growth retardants.[8]
Brassinazole is Brassinosteroid Biosynthesis Inhibitor.
Benzotriazole is used in chemical photography as a restrainer and fog suppressant.
Cyclohexylethyltriazol was briefly used as an alternative to Cardiazol (Metrazol) in convulsive shock therapy treatment of mental illnesses during the 1940s.
Importance in agriculture[edit]
Due to spreading resistance of plant pathogens towards fungicides of the strobilurin class,[9] control of fungi such as Septoria tritici or Gibberella zeae[10] relies heavily on triazoles. Food, like store bought potatoes, contain retardants such as triazole or tetcyclacis.[11][12]
Importance in chemical synthesis[edit]
The azide alkyne Huisgen cycloaddition[2] is a mild and selective reaction that gives 1,2,3-triazoles as products. The reaction has been widely used in bioorthogonal chemistry and in organic synthesis. Triazoles are relatively stable functional groups and triazole linkages can be used in a variety of applications, e.g. replacing the phosphate backbone of DNA.[13]
Related heterocycles[edit]
- Imidazole, an analog with two nonadjacent nitrogen atoms
- Pyrazole, an analog with two adjacent nitrogen atoms
- Tetrazole, an analog with four nitrogen atoms
- Triazolium salts, substtuted analogues that can be used as NHC precursors
https://en.wikipedia.org/wiki/Triazole


No comments:
Post a Comment