Pnictogen hydrides or hydrogen pnictides are binary compounds of hydrogen with pnictogen (/ˈpnɪktədʒən/ or /ˈnɪktədʒən/; from Ancient Greek: πνῑ́γω "to choke" and -gen, "generator") atoms (elements of group 15: nitrogen, phosphorus, arsenic, antimony, and bismuth) covalently bonded to hydrogen.
Pnictogen trihydrides[edit]
The simplest series has the chemical formula XH3 (less commonly H3X), with X representing any of the pnictogens. They take on the pyramidal structure (as opposed to the trigonal planar arrangement of the group 13 hydrides), and therefore are polar. These pnictogen trihydrides are generally increasingly unstable and poisonous with heavier elements.
Like the simple hydrogen halides and chalcogenides, the pnictogen hydrides are water-soluble.[dubious ] Unlike other hydrides such as hydrogen sulfide and hydrogen fluoride, which form acidic aqueous solutions, ammonia dissolves in water to make ammonium hydroxide which is basic (by forming a hydroxide ion as opposed to hydronium). Phosphine is also water-soluble. Arsine and stibine are less so, and it is not known if bismuthine is.
| Compound names | Chemical formula | Geometry | Space-filling model |
|---|---|---|---|
| nitrogen hydride ammonia (azane) | NH3 |  |  |
| phosphorus hydride phosphine (phosphane) | PH3 | 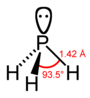 |  |
| arsenic hydride arsine (arsane) | AsH3 |  |  |
| antimony hydride stibine (stibane) | SbH3 |  |  |
| bismuth hydride bismuthine (bismuthane) | BiH3 |  |  |
Some properties of the pnictogen trihydrides follow:[1]
| Property | NH3 | PH3 | AsH3 | SbH3 | BiH3 |
|---|---|---|---|---|---|
| Melting point (°C) | −77.8 | −133.5 | −116.3 | −88 | ? |
| Boiling point (°C) | −34.5 | −87.5 | −62.4 | −18.4 | 16.8 (extrapolated) |
| Liquid density (g/cm3) | 0.683 (−34 °C) | 0.746 (−90 °C) | 1.640 (−64 °C) | 2.204 (−18 °C) | ? |
| ΔH° f/kJ mol−1 | −46.1 | −9.6 (?) | +66.4 | +145.1 | +277.8 |
| Distance (X–H)/pm | 101.7 | 141.9 | 151.9 | 170.7 | 177.59 |
| Angle H–X–H | 107.8° | 93.6° | 91.8° | 91.3° | 90.48° |
These gases have no smell in pure form, instead gaining it when in contact with air. Ammonia has an infamous, intense odour resembling urine and/or fish, commonly the result of the decomposition of urea. Phosphine smells like fish or garlic, and stibine like rotten eggs, similar to hydrogen sulfide and selenide.
Dipnictogen tetrahydrides[edit]
| Compound names | Chemical formula | Geometry | Space-filling model |
|---|---|---|---|
| dinitrogen hydride hydrazine (diazane) | N2H4 |  |  |
| diphosphorus hydride diphosphine (diphosphane) | P2H4 |  |  |
| diarsenic hydride[1] diarsine (diarsane) | As2H4 |  |  |
Properties[edit]
All straight-chain saturated hydrogen pnictides follow the formula XnHn+2.
Ammonia is produced industrially on the largest scale among all compounds. Like water, hydrogen bonding results in a high melting and boiling point compared to the other pnictogen hydrides, although 26% is lost on melting, another 7% as the liquid is heated to boiling, and the remaining 67% upon boiling. Other effects of hydrogen bonding are a high dielectric constant as well as low values of density, viscosity, and electrical conductivity. Like water, it is an excellent and often-used ionising solvent.[2] Over twenty other hydrides of nitrogen are known, the most important being hydrazine (N2H4) and hydrogen azide (HN3). Hydrazine has physical properties that are remarkably similar to those of water: its melting and boiling points are 2.0 °C and 113.5 °C, the density of the solid at −5 °C is 1.146 g/cm3, while that of the liquid at 25 °C is 1.00 g/cm3.[3] The azanes are a series which include ammonia, hydrazine and triazane.
Phosphine, a toxic, colourless gas, is the most stable phosphorus hydride and is the first of the homologous straight-chain polyphosphane series PnHn+2 (n = 1–9) that become increasingly thermally unstable as n increases. Other cyclic and condensed polyphosphane series are known, from PnHn to PnHn−18, amounting to 85 known phosphanes in 1997. Insoluble in water but soluble in organic liquids (as well as carbon disulfide and trichloroacetic acid), phosphine is a strong reducing agent.[4]
Arsine, stibine and bismuthine are highly toxic, thermally unstable, and colourless gases. No appreciable hydrogen bonding is found in phosphine, arsine, stibine or bismuthine, and there is no appreciable tendency to dissociate like ammonia to MH+
4 and MH−
2 (M = P, As, Sb, Bi). The pnictogen hydrides become denser down the group and the M–H bond lengths increase, while the H–M–H bond angle decreases slightly. The standard enthalpies of formation reflect the increasing thermal instability going down the group. Arsine decomposes to arsenic and hydrogen at 250–300 °C, stibine to antimony and hydrogen at room temperature, and bismuthine to bismuth and hydrogen above −45 °C. Arsine and stibine are very easily oxidised to arsenic or antimony trioxide and water; a similar reaction happens with sulfur or selenium. Reaction with metals at elevated temperatures leads to arsenides and antimonides. A few lower hydrides are known, such as As2H4, but they are even more unstable and their properties are unknown.[1]
Imidogen, a radical composed of one hydrogen atom and one nitrogen atom (NH), can be classed as a pnictogen hydride.
See also[edit]
https://en.wikipedia.org/wiki/Pnictogen_hydride





No comments:
Post a Comment