Neurosarcoidosis (sometimes shortened to neurosarcoid) refers to a type of sarcoidosis, a condition of unknown cause featuring granulomas in various tissues, in this type involving the central nervous system (brain and spinal cord). Neurosarcoidosis can have many manifestations, but abnormalities of the cranial nerves (a group of twelve nerves supplying the head and neck area) are the most common. It may develop acutely, subacutely, and chronically. Approximately 5–10 percent of people with sarcoidosis of other organs (e.g. lung) develop central nervous system involvement. Only 1 percent of people with sarcoidosis will have neurosarcoidosis alone without involvement of any other organs. Diagnosis can be difficult, with no test apart from biopsy achieving a high accuracy rate. Treatment is with immunosuppression.[1] The first case of sarcoidosis involving the nervous system was reported in 1905.[2][3]
 This condition affects the cranial nervesSpecialtyNeurology Diagnostic methodBiopsyTreatmentimmunosuppression
This condition affects the cranial nervesSpecialtyNeurology Diagnostic methodBiopsyTreatmentimmunosuppressionhttps://en.wikipedia.org/wiki/Neurosarcoidosis
https://en.wikipedia.org/wiki/Löfgren_syndrome
https://en.wikipedia.org/wiki/Sarcoidosis#Causes_and_pathophysiology
https://en.wikipedia.org/wiki/Cyclo?, phos, phamide?
https://en.wikipedia.org/wiki/Erythema_nodosum
https://en.wikipedia.org/wiki/Neurosarcoidosis
https://en.wikipedia.org/wiki/Lupus_pernio
Leprosy, Tuberculosis, Bacillus, HIV, Rods, Gram Negative, Gram Pos Neg Neu, Syphally Light Weight Neuro, Arthropod Internal Bacteria/Parasite, Methroxetrate overdose/alkylation agent/radioactive potas x slt/potash/deuteri/hydrags/props/etc., radioactivation of calcium/iron/salt (light salt nasty deformed metal - iron; esp cultivated, coated, contaminated - Lithotrophic Bacteria-particle-organism-etc.), etc.. alkalization bacterial colony in expanded cell etc., ulceration, non casiean granuloma/granules disseminated/etc., miliary tuberculosis, lesion, scarring, matricing errors, interstitial foaming, crustaciean migrans methroxetrated to reduce size/opacity/etc. (biolab prep), etc..
Chemical Particle Dwelling organism chronic prescription users (extremophiles; lithotrophic; sand dwelling organisms; deep rock dwellers; etc.).
Lupus et tuberculosis. bacillus thruingiensis etc..
The four stages of pulmonary involvement are based on radiological stage of the disease, which is helpful in prognosis:[32]
- Stage I: bilateral hilar lymphadenopathy (BHL) alone
- Stage II: BHL with pulmonary infiltrates
- Stage III: pulmonary infiltrates without BHL
- Stage IV: fibrosis
Histologically, sarcoidosis of the heart is an active granulomatous inflammation surrounded by reactive oedema. The distribution of affected areas is patchy with localised enlargement of heart muscles. This causes scarring and remodelling of the heart, which leads to dilatation of heart cavities and thinning of heart muscles. As the situation progresses, it leads to aneurysm of heart chambers. When the distribution is diffuse, there would be dilatation of both ventricles of the heart, causing heart failure and arrhythmia. When the conduction system in the intraventricular septum is affected, it would lead to heart block, ventricular tachycardia and ventricular arrhythmia, causing sudden death. Nevertheless, the involvement of pericardium and heart valves are uncommon.[38]
The frequency of cardiac involvement varies and is significantly influenced by race; in Japan, more than 25% of those with sarcoidosis have symptomatic cardiac involvement, whereas in the US and Europe, only about 5% of cases present with cardiac involvement.[28] Autopsy studies in the US have revealed a frequency of cardiac involvement of about 20–30%, whereas autopsy studies in Japan have shown a frequency of 60%.[22] The presentation of cardiac sarcoidosis can range from asymptomatic conduction abnormalities to fatal ventricular arrhythmia.[39][40]
Conduction abnormalities are the most common cardiac manifestations of sarcoidosis in humans and can include complete heart block.[41] Second to conduction abnormalities, in frequency, are ventricular arrhythmias, which occurs in about 23% of cases with cardiac involvement.[41] Sudden cardiac death, either due to ventricular arrhythmias or complete heart block is a rare complication of cardiac sarcoidosis.[42][43] Cardiac sarcoidosis can cause fibrosis, granuloma formation, or the accumulation of fluid in the interstitium of the heart, or a combination of the former two.[44][45] Cardiac sarcoidosis may also cause congestive heart failure when granulomas cause myocardial fibrosis and scarring.[46] Congestive heart failure affects 25-75% of those with cardiac sarcoidosis. Diabetes mellitus and sarcoidosis-related arrhythmias are believed to be strong risk factors of heart failure in sarcoidosis.[47]Pulmonary arterial hypertension occurs by two mechanisms in cardiac sarcoidosis: reduced left heart function due to granulomas weakening the heart muscle or from impaired blood flow.[48]
Eye[edit]
Eye involvement occurs in about 10–90% of cases.[22] Manifestations in the eye include uveitis, uveoparotitis, and retinal inflammation, which may result in loss of visual acuity or blindness.[49] The most common ophthalmologic manifestation of sarcoidosis is uveitis.[22][50][51] The combination of anterior uveitis, parotitis, VII cranial nerve paralysis and fever is called uveoparotid fever or Heerfordt syndrome (D86.8). Development of scleral nodule associated with sarcoidosis has been observed.[52]
Nervous system[edit]
Any of the components of the nervous system can be involved.[53] Sarcoidosis affecting the nervous system is known as neurosarcoidosis.[53] Cranial nerves are most commonly affected, accounting for about 5–30% of neurosarcoidosis cases, and peripheral facial nerve palsy, often bilateral, is the most common neurological manifestation of sarcoidosis.[53][54][55] It occurs suddenly and is usually transient. The central nervous system involvement is present in 10–25% of sarcoidosis cases.[31] Other common manifestations of neurosarcoidosis include optic nerve dysfunction, papilledema, palate dysfunction, neuroendocrine changes, hearing abnormalities, hypothalamic and pituitary abnormalities, chronic meningitis, and peripheral neuropathy.[28] Myelopathy, that is spinal cord involvement, occurs in about 16–43% of neurosarcoidosis cases and is often associated with the poorest prognosis of the neurosarcoidosis subtypes.[53] Whereas facial nerve palsies and acute meningitis due to sarcoidosis tend to have the most favourable prognosis,[53]another common finding in sarcoidosis with neurological involvement is autonomic or sensory small-fiber neuropathy.[56][57] Neuroendocrine sarcoidosis accounts for about 5–10% of neurosarcoidosis cases and can lead to diabetes insipidus, changes in menstrual cycle and hypothalamic dysfunction.[53][55]The latter can lead to changes in body temperature, mood, and prolactin (see the endocrine and exocrine section for details).[53]
Endocrine and exocrine[edit]
Prolactin is frequently increased in sarcoidosis, between 3 and 32% of cases have hyperprolactinemia[58] this frequently leads to amenorrhea, galactorrhea, or nonpuerperal mastitis in women. It also frequently causes an increase in 1,25-dihydroxy vitamin D, the active metabolite of vitamin D, which is usually hydroxylated within the kidney, but in sarcoidosis patients, hydroxylation of vitamin D can occur outside the kidneys, namely inside the immune cells found in the granulomas the condition produces. 1,25-dihydroxy vitamin D is the main cause for hypercalcemia in sarcoidosis and is overproduced by sarcoid granulomata. Gamma-interferon produced by activated lymphocytes and macrophages plays a major role in the synthesis of 1 alpha, 25(OH)2D3.[59] Hypercalciuria (excessive secretion of calcium in one's urine) and hypercalcemia (an excessively high amount of calcium in the blood) are seen in <10% of individuals and likely results from the increased 1,25-dihydroxy vitamin D production.[60]
Thyroid dysfunction is seen in 4.2–4.6% of cases.[61][62]
Parotid enlargement occurs in about 5–10% of cases.[19] Bilateral involvement is the rule. The gland is usually not tender, but firm and smooth. Dry mouthcan occur; other exocrine glands are affected only rarely.[28] The eyes, their glands, or the parotid glands are affected in 20–50% of cases.[63]
Gastrointestinal and genitourinary[edit]
Symptomatic gastrointestinal (GI) involvement occurs in less than 1% of cases (if one excludes the liver), and most commonly the stomach is affected, although the small or large intestine may also be affected in a small portion of cases.[19][64] Studies at autopsy have revealed GI involvement in less than 10% of people.[55] These cases would likely mimic Crohn's disease, which is a more commonly intestine-affecting granulomatous disease.[19] About 1–3% of people have evidence of pancreatic involvement at autopsy.[55] Symptomatic kidney involvement occurs in just 0.7% of cases, although evidence of kidney involvement at autopsy has been reported in up to 22% of people and occurs exclusively in cases of chronic disease.[19][22][55] Symptomatic kidney involvement is usually nephrocalcinosis, although granulomatous interstitial nephritis that presents with reduced creatinine clearance and little proteinuriais a close second.[19][55] Less commonly, the epididymis, testicles, prostate, ovaries, fallopian tubes, uterus, or the vulva may be affected, the latter may cause vulva itchiness.[22][65][66] Testicular involvement has been reported in about 5% of people at autopsy.[55][66] In males, sarcoidosis may lead to infertility.[66]
Around 70% of people have granulomas in their livers, although only in about 20–30% of cases, liver function test anomalies reflecting this fact are seen.[20][28] About 5–15% of sufferers exhibit hepatomegaly.[22] Only 5–30% of cases of liver involvement are symptomatic.[67] Usually, these changes reflect a cholestatic pattern and include raised levels of alkaline phosphatase (which is the most common liver function test anomaly seen in those with sarcoidosis), while bilirubin and aminotransferases are only mildly elevated. Jaundice is rare.[19][28]
Blood[edit]
Abnormal blood tests are frequent, accounting for over 50% of cases, but are not diagnostic.[28][31] Lymphopenia is the most common blood anomaly in sarcoidosis.[28] Anemia occurs in about 20% of people with sarcoidosis.[28] Leukopenia is less common and occurs in even fewer cases but is rarely severe.[28] Thrombocytopenia and hemolytic anemia are fairly rare.[19] In the absence of splenomegaly, leukopenia may reflect bone marrow involvement, but the most common mechanism is a redistribution of blood T cells to sites of disease.[68] Other nonspecific findings include monocytosis, occurring in the majority of sarcoidosis cases,[69] increased hepatic enzymes or alkaline phosphatase. People with sarcoidosis often have immunologic anomalies like allergies to test antigens such as Candida or purified protein derivative.[63] Polyclonal hypergammaglobulinemia is also a fairly common immunologic anomaly seen in sarcoidosis.[63]
Lymphadenopathy (swollen glands) is common in sarcoidosis and occurs in 15% of cases.[23] Intrathoracic nodes are enlarged in 75 to 90% of all people; usually this involves the hilar nodes, but the paratracheal nodes are commonly involved. Peripheral lymphadenopathy is very common, particularly involving the cervical (the most common head and neck manifestation of the disease), axillary, epitrochlear, and inguinal nodes.[70] Approximately 75% of cases show microscopic involvement of the spleen, although only in about 5–10% of cases does splenomegaly appear.[19][63]
Bone, joints, and muscles[edit]
Sarcoidosis can be involved with the joints, bones, and muscles. This causes a wide variety of musculoskeletal complaints that act through different mechanisms.[71] About 5–15% of cases affect the bones, joints, or muscles.[31]
Arthritic syndromes can be categorized as acute or chronic.[71] Sarcoidosis patients suffering acute arthritis often also have bilateral hilar lymphadenopathyand erythema nodosum. These three associated syndromes often occur together in Löfgren syndrome.[71] The arthritis symptoms of Löfgren syndrome occur most frequently in the ankles, followed by the knees, wrists, elbows, and metacarpophalangeal joints.[71] Usually, true arthritis is not present, but instead, periarthritis appears as a swelling in the soft tissue around the joints that can be seen by ultrasonographic methods.[71] These joint symptoms tend to precede or occur at the same time as erythema nodosum develops.[71] Even when erythema nodosum is absent, it is believed that the combination of hilar lymphadenopathy and ankle periarthritis can be considered as a variant of Löfgren syndrome.[71] Enthesitis also occurs in about one-third of patients with acute sarcoid arthritis, mainly affecting the Achilles tendon and heels.[71] Soft-tissue swelling of the ankles can be prominent, and biopsy of this soft tissue reveals no granulomas, but does show panniculitis similar to erythema nodosum.[71]
Chronic sarcoid arthritis usually occurs in the setting of more diffuse organ involvement.[71] The ankles, knees, wrists, elbows, and hands may all be affected in the chronic form and often this presents itself in a polyarticular pattern.[71] Dactylitis similar to that seen in psoriatic arthritis, that is associated with pain, swelling, overlying skin erythema, and underlying bony changes may also occur.[71] Development of Jaccoud arthropathy (a nonerosive deformity) is very rarely seen.[71]
Bone involvement in sarcoidosis has been reported in 1–13% of cases.[55] The most frequent sites of involvement are the hands and feet, whereas the spine is less commonly affected.[71] Half of the patients with bony lesions experience pain and stiffness, whereas the other half remain asymptomatic.[71]Periostitis is rarely seen in sarcoidosis and has been found to present itself at the femoral bone.[72][73]
Cause[edit]
The exact cause of sarcoidosis is not known.[2] The current working hypothesis is, in genetically susceptible individuals, sarcoidosis is caused through alteration to the immune response after exposure to an environmental, occupational, or infectious agent.[74] Some cases may be caused by treatment with tumor necrosis factor (TNF) inhibitors like etanercept.[75]
Genetics[edit]
The heritability of sarcoidosis varies according to ethnicity. About 20% of African Americans with sarcoidosis have a family member with the condition, whereas the same figure for European Americans is about 5%. Additionally, in African Americans, who seem to experience more severe and chronic disease, siblings and parents of sarcoidosis cases have about a 2.5-fold increased risk for developing the disease.[26] In Swedish individuals heritability was found to be 39%.[76] In this group, if a first-degree family member was affected, a person has a four-fold greater risk of being affected.[76]
Investigations of genetic susceptibility yielded many candidate genes, but only few were confirmed by further investigations and no reliable genetic markers are known. Currently, the most interesting candidate gene is BTNL2; several HLA-DR risk alleles are also being investigated.[77][78] In persistent sarcoidosis, the HLA haplotype HLA-B7-DR15 is either cooperating in disease or another gene between these two loci is associated. In nonpersistent disease, a strong genetic association exists with HLA DR3-DQ2.[79][80] Cardiac sarcoid has been connected to tumor necrosis factor alpha (TNFA) variants.[81]
Infectious agents[edit]
Several infectious agents appear to be significantly associated with sarcoidosis, but none of the known associations is specific enough to suggest a direct causative role.[82] The major implicated infectious agents include: mycobacteria, fungi, borrelia, and rickettsia.[83] A meta-analysis investigating the role of mycobacteria in sarcoidosis found it was present in 26.4% of cases, but they also detected a possible publication bias, so the results need further confirmation.[84][85] Mycobacterium tuberculosis catalase-peroxidase has been identified as a possible antigen catalyst of sarcoidosis.[86] The disease has also been reported by transmission via organ transplants.[87] A large epidemiological study found little evidence that infectious diseases spanning years before sarcoidosis diagnosis could confer measurable risks for sarcoidosis diagnosis in the future.[88]
Autoimmune[edit]
Association of autoimmune disorders has been frequently observed. The exact mechanism of this relation is not known, but some evidence supports the hypothesis that this is a consequence of Th1 lymphokine prevalence.[61][89] Tests of delayed cutaneous hypersensitivity have been used to measure progression.[90]
Granulomatous inflammation is characterized primarily by the accumulation of macrophages and activated T-lymphocytes, with increased production of key inflammatory mediators, Tumor necrosis factor alpha (TNF), Interferon gamma, Interleukin 2 (IL-2), IL-8, IL-10, IL-12, IL-18, IL-23 and transforming growth factor beta (TGF-β), indicative of a T helper cell-mediated immune response.[83][91] Sarcoidosis has paradoxical effects on inflammatory processes; it is characterized by increased macrophage and CD4 helper T-cell activation, resulting in accelerated inflammation, but immune response to antigen challenges such as tuberculin is suppressed. This paradoxic state of simultaneous hyper- and hypoactivity is suggestive of a state of anergy. The anergy may also be responsible for the increased risk of infections and cancer.[citation needed]
The regulatory T-lymphocytes in the periphery of sarcoid granulomas appear to suppress IL-2 secretion, which is hypothesized to cause the state of anergy by preventing antigen-specific memory responses.[92] Schaumann bodies seen in sarcoidosis are calcium and protein inclusions inside of Langhans giant cells as part of a granuloma. Sarcoidosis is characterized by the formation of non-caseous epithelioid cell granulomas in various organs and tissues.[93]
While TNF is widely believed to play an important role in the formation of granulomas (this is further supported by the finding that in animal models of mycobacterial granuloma formation inhibition of either TNF or IFN-γ production inhibits granuloma formation), sarcoidosis can and does still develop in those being treated with TNF antagonists like etanercept.[94] B cells also likely play a role in the pathophysiology of sarcoidosis.[26] Serum levels of soluble human leukocyte antigen (HLA) class I antigens and angiotensin converting enzyme (ACE) are higher in people with sarcoidosis.[26] Likewise the ratio of CD4/CD8 T cells in bronchoalveolar lavage is usually higher in people with pulmonary sarcoidosis (usually >3.5), although it can be normal or even abnormally low in some cases.[26] Serum ACE levels have been found to usually correlate with total granuloma load.[83]
Cases of sarcoidosis have also been reported as part of the immune reconstitution syndrome of HIV, that is, when people receive treatment for HIV, their immune system rebounds and the result is that it starts to attack the antigens of opportunistic infections caught prior to said rebound and the resulting immune response starts to damage healthy tissue.[91]
Diagnosis of sarcoidosis is a matter of exclusion, as there is no specific test for the condition. To exclude sarcoidosis in a case presenting with pulmonary symptoms might involve a chest radiograph, CT scan of chest, PET scan, CT-guided biopsy, mediastinoscopy, open lung biopsy, bronchoscopy with biopsy, endobronchial ultrasound, and endoscopic ultrasound with fine-needle aspiration of mediastinal lymph nodes(EBUS FNA). Tissue from biopsy of lymph nodes is subjected to both flow cytometry to rule out cancer and special stains (acid fast bacilli stain and Gömöri methenamine silver stain) to rule out microorganisms and fungi.[95][96][12][97]
Serum markers of sarcoidosis, include: serum amyloid A, soluble interleukin-2 receptor, lysozyme, angiotensin converting enzyme, and the glycoprotein KL-6.[98] Angiotensin-converting enzyme blood levels are used in the monitoring of sarcoidosis.[98] A bronchoalveolar lavage can show an elevated (of at least 3.5) CD4/CD8 T cell ratio, which is indicative (but not proof) of pulmonary sarcoidosis.[26] In at least one study the induced sputum ratio of CD4/CD8 and level of TNF was correlated to those in the lavage fluid.[98] A sarcoidosis-like lung disease called granulomatous–lymphocytic interstitial lung disease can be seen in patients with common variable immunodeficiency (CVID) and therefore serum antibody levels should be measured to exclude CVID.[citation needed]
Differential diagnosis includes metastatic disease, lymphoma, septic emboli, rheumatoid nodules, granulomatosis with polyangiitis, varicella infection, tuberculosis, and atypical infections, such as Mycobacterium avium complex, cytomegalovirus, and cryptococcus.[99] Sarcoidosis is confused most commonly with neoplastic diseases, such as lymphoma, or with disorders characterized also by a mononuclear cell granulomatous inflammatory process, such as the mycobacterial and fungal disorders.[28]
Chest radiograph changes are divided into four stages:[100]
- bihilar lymphadenopathy
- bihilar lymphadenopathy and reticulonodular infiltrates
- bilateral pulmonary infiltrates
- fibrocystic sarcoidosis typically with upward hilar retraction, cystic and bullous changes
Although people with stage 1 radiographs tend to have the acute or subacute, reversible form of the disease, those with stages 2 and 3 often have the chronic, progressive disease; these patterns do not represent consecutive "stages" of sarcoidosis. Thus, except for epidemiologic purposes, this categorization is mostly of historic interest.[28]
Sarcoidosis may be divided into the following types:[36]
Some 1990s studies indicated that people with sarcoidosis appear to be at significantly increased risk for cancer, in particular lung cancer, lymphomas,[147] and cancer in other organs known to be affected in sarcoidosis.[148][149]In sarcoidosis-lymphoma syndrome, sarcoidosis is followed by the development of a lymphoproliferative disordersuch as non-Hodgkin lymphoma.[150] This may be attributed to the underlying immunological abnormalities that occur during the sarcoidosis disease process.[151] Sarcoidosis can also follow cancer[152][153] or occur concurrently with cancer.[154][155] There have been reports of hairy cell leukemia,[156] acute myeloid leukemia,[157] and acute myeloblastic leukemia[158]associated with sarcoidosis. Sometimes, sarcoidosis, even untreated, can be complicated by opportunistic infections.[159][160]
It was first described in 1877 by Dr. Jonathan Hutchinson, a dermatologist as a condition causing red, raised rashes on the face, arms, and hands.[15] In 1889 the term Lupus pernio was coined by Dr. Ernest Besnier, another dermatologist.[166] Later in 1892 lupus pernio's histology was defined.[166] In 1902 bone involvement was first described by a group of three doctors.[166] Between 1909 and 1910 uveitis in sarcoidosis was first described, and later in 1915 it was emphasised, by Dr. Schaumann, that it was a systemic condition.[166] This same year lung involvement was also described.[166] In 1937 uveoparotid fever was first described and likewise in 1941 Löfgren syndrome was first described.[166] In 1958 the first international conference on sarcoidosis was called in London, likewise the first USA sarcoidosis conference occurred in Washington, D.C., in the year 1961.[166] It has also been called Besnier–Boeckdisease or Besnier–Boeck–Schaumann disease.[167]
Etymology[edit]
The word "sarcoidosis" comes from Greek [σάρκο-] sarcο- meaning "flesh", the suffix -(e)ido (from the Greek εἶδος -eidos [usually omitting the initial e in English as the diphthong epsilon-iota in Classic Greek stands for a long "i" = English ee]) meaning "type", " resembles" or "like", and -sis, a common suffix in Greek meaning "condition". Thus the whole word means "a condition that resembles crude flesh". The first cases of sarcoïdosis, which were recognised as a new pathological entity, in Scandinavia, at the end of the 19th century exhibited skin nodules resembling cutaneous sarcomas, hence the name initially given.[citation needed]
https://en.wikipedia.org/wiki/Sarcoidosis
| Eosinophilia | |
|---|---|
 | |
| Eosinophils in the peripheral blood of a patient with idiopathic eosinophilia | |
| Specialty | Infectious disease, hematology |
Eosinophilia is a condition in which the eosinophil count in the peripheral blood exceeds 5×108/L (500/μL).[1] Hypereosinophilia is an elevation in an individual's circulating blood eosinophil count above 1.5 x 109/L (i.e. 1,500/μL). The hypereosinophilic syndrome is a sustained elevation in this count above 1.5 x 109/L (i.e. 1,500/μL) that is also associated with evidence of eosinophil-based tissue injury.
Eosinophils are one form of terminally differentiated granulocytes; they function to neutralize invading microbes, primarily parasites and helminthes but also certain types of fungi and viruses.
They also participate in transplant rejection, Graft-versus-host disease, and the killing of tumor cells.
In conducting these functions, eosinophils produce and release on demand a range of toxic reactive oxygen species (e.g. hypobromite, hypobromous acid, superoxide, and peroxide) and they also release on demand a preformed armamentarium of cytokines, chemokines, growth factors, lipid mediators (e.g. leukotrienes, prostaglandins, platelet activating factor), and toxic proteins (e.g. metalloproteinases, major basic protein, eosinophil cationic protein, eosinophil peroxidase, and eosinophil-derived neurotoxin).
These agents serve to orchestrate robust immune and inflammatory responses that destroy invading microbes, foreign tissue, and malignant cells.
When overproduced and over-activated, which occurs in certain cases of hypereosinophilia and to a lesser extent eosinophilia, eosinophils may misdirect their reactive oxygen species and armamentarium of preformed molecules toward normal tissues.
This can result in serious damage to such organs as the lung, heart, kidneys, and brain.[7][8][9]
IgE-mediated eosinophil production is induced by compounds released by basophils and mast cells, including eosinophil chemotactic factor of anaphylaxis, leukotriene B4 and serotonin mediated release of eosinophil granules occur, complement complex (C5-C6-C7), interleukin 5, and histamine (though this has a narrow range of concentration).[3]
Harm resulting from untreated eosinophilia potentially varies with cause. During an allergic reaction, the release of histamine from mast cells causes vasodilation which allows eosinophils to migrate from the blood and localize in affected tissues. Accumulation of eosinophils in tissues can be significantly damaging. Eosinophils, like other granulocytes, contain granules (or sacs) filled with digestive enzymes and cytotoxic proteins which under normal conditions are used to destroy parasites but in eosinophilia these agents can damage healthy tissues. In addition to these agents, the granules in eosinophils also contain inflammatory molecules and cytokines which can recruit more eosinophils and other inflammatory cells to the area and hence amplify and perpetuate the damage. This process is generally accepted to be the major inflammatory process in the pathophysiology of atopic or allergic asthma.[10]
Diagnosis is by complete blood count (CBC). However, in some cases, a more accurate absolute eosinophil count may be needed.[3] Medical history is taken, with emphasis on travel, allergies and drug use.[3] Specific test for causative conditions are performed, often including chest x-ray, urinalysis, liverand kidney function tests, and serologic tests for parasitic and connective tissue diseases. The stool is often examined for traces of parasites (i.e. eggs, larvae, etc.) though a negative test does not rule out parasitic infection; for example, trichinosis requires a muscle biopsy.[3] Elevated serum B12 or low white blood cell alkaline phosphatase, or leukocytic abnormalities in a peripheral smear indicates a disorder of myeloproliferation.[3] In cases of idiopathic eosinophilia, the patient is followed for complications. A brief trial of corticosteroids can be diagnostic for allergic causes, as the eosinophilia should resolve with suppression of the immune over-response.[3] Neoplastic disorders are diagnosed through the usual methods, such as bone marrow aspiration and biopsy for the leukemias, MRI/CT to look for solid tumors, and tests for serum LDH and other tumor markers.[3]
The General Haematoloy and Haemato-oncology Task Forces for the British Committee for Standards in Haematology classifies these disorders into a) Primary, i.e. caused by abnormalities in the eosinophil cell line; b) Secondary, i.e. caused by non-eosinophil disorders; and c) Idiopathic, cause unknown.[4] The World Health Organization classifies these disorders into a) Myeloid and lymphoid neoplasms with eosinophilia and abnormalities of PDGFRA, PDGFRB, or FGFR1 (i.e. high eosinophil blood counts caused by mutations in the eosinophil cell line of one of these three genes), 'b) Chronic eosinophilic leukemia, and c) the Idiopathic hypereosinophiic syndrome. In the latter classification, secondary hypereosinophilia/eosinophilia is not viewed as a true disorder of eosinophils.[5][11] Here these two classifications are merged and expanded to include the many forms of secondary, i.e. reactive hypereosinophilia/eosinophilia, disorders and also includes another subtype, organ-restricted hypereosinophilias, a disorder in which eosinophil-mediated tissue damage is restricted to one organ and is often but not always associated with increased blood eosinophil counts.[citation needed]
Primary hypereosinophilia[edit]
Primary hypereosinophilia is due to the development of a clone of eosinophils, i.e. a group of genetically identical eosinophils derived from a significantly mutated ancestor cell. The clone may prove to be benign, pre-malignant, or overtly malignant. The fundamental driver of these hypereosinophilic (or uncommonly eosinophilic) disorders is the mutation which increases the proliferation, survival, and further mutation of cells descendant from the originally mutated cell. There are several subtypes of primary hypereosinophilia.[citation needed]
Clonal hypereosinophilia[edit]
Clonal hypereosinophilia is hypereosinophilia caused by a pre-malignant or malignant clone of eosinophils that bear mutations in genes for PDGFRA, PDGFRB, or FGFR1 or, alternatively, a chromosome translocation that creates the PCM1-JAK2 fusion gene. These genes code for dysfunctional protein products capable of enhancing proliferation and/or survival of their parent cells which, in consequence, become an evolving and constantly growing clone of eosinophils. These mutations are recognized by the World Health Association as causing distinct entities differing from idiopathic hypereosinophilia and the idiopathic hypereosinophilic syndrome. Presence of these clones may be associated with tissue injury but in any case suggests specific therapy be directed at reducing the size and suppressing the growth of the eosinophil clone. More recently, mutations in other genes have been described as causing a similar type of clonal hypereosinophilia but have not yet been recognized as entities distinct from idiopathic hypereosinophilia and the idiopathic hyperesoniphilic syndrome. These include gene mutations in JAK2, ABL1, and FLT2 and chromosomal translocations that create the ETV6-ACSL6 fusion gene.[5]
Chronic eosinophilic leukemia (NOS)[edit]
Chronic eosinophilic leukemia, not otherwise specified (i.e. CEL, NOS), is a leukemia-inducing disorder in the eosinophil cell lineage that causes eosinophil blood counts greater than 1,500/μL. The most recent (2017) World health organization criteria specifically excludes from this disorder hypereosinophilia/eosinophilia associated with BCR-ABL1 fusion gene-positive chronic myeloid leukemia, polycythemia vera, essential thrombocytosis, primary myelofibrosis, chronic neutrophilic leukemia, chronic myelomonocytic leukemia, atypical chronic myelogenous leukemia, clonal eosinophilias involving gene rearrangements of PDGFRA, PDGFRB, or FGFR1, and chromosome translocations that form PCM1-JAK2, ETV6-JAK2, or BCR-JAK2fusion genes. For this diagnosis, immature eosinophil (e.g. myeloblast) cell counts in the bone marrow and peripheral blood must be less than 20% and the chromosomal alterations (inv(16)(p13.1q22)) and t(16;16)(p13;q22) as well as other features diagnostic of acute myelogenous leukemia must be absent. The latter diagnostic features include clonal cytogenetic abnormalities and molecular genetic abnormalities diagnostic for other forms of leukemia or the presence of myeloblast counts greater than 55% in bone marrow or 2% in blood. Chronic eosinophilic leukemia may transform into acute eosinophilic or other types of acute myelogenous leukemia.[5][12]
Familial eosinophilia[edit]
Familial eosinophilia is a rare congenital disorder characterized by the presence of sustained elevations in blood eosinophil levels that reach ranges diagnostic of eosinophilia or, far more commonly, hypereosinophilia. It is an autosomal dominant disorder in which genetic linkage gene mapping family studies localize the gene responsible for it to chromosome 5 at position q31-q33,[13] between markers D5S642 and D5S816. This region contains a cytokine gene cluster which includes three genes whose protein products function in regulating the development and proliferation of eosinophils viz., interleukin 3, interleukin 5, and colony stimulating factor 2. However, no functional sequence genetic polylmophisms are found within the promoter, exons, or introns, of these genes or within the common gene enhancer for interleukin 3 or colony stimulating factor 2. This suggests that the primary defect in familial eosinophilia is not a mutation in one of these genes but rather in another gene within this chromosome area.[14] Clinical manifestations and tissue destruction related to the eosinophilia in this disorder are uncommon: familial eosinophilia typically has a benign phenotype compared to other congenital and acquired eosinophilic diseases.[15][16][17][18]
Idiopathic hypereosinophilia[edit]
Idiopathic hypereosinophilia (also termed hypereosinophilia of undetermined significance, i.e. HEUS) is a disorder characterized by an increase in eosinophil blood counts above 1,500/μL, as detected on at least 2 separate examinations. The disorder cannot be associated with eosinophil-based tissue damage or a primary or secondary cause of eosinophilia. That is, it is a diagnosis of exclusion and has no known cause. Over time, this disorder can resolve into a primary hypereosinphilia, typically clonal hyperesinophilia, chronic eosinphilic leukemia, or an eosinophilia associated with another hematological leukemia. The disorder may also become associated with tissue or organ damage and therefore be diagnosed as the hypereosinophilic syndrome. Idiopathic hyereosinophilia is treated by observation to detect development of the cited more serious disorders.[5][19]
Idiopathic hypereosiophilic syndrome[edit]
The idiopathic hypereosinophilic syndrome is a disorder characterized by hypereosiophilia that is associated with eosinophil-based tissue or organ damage. While almost any organ or tissue may be damaged, the lung, skin, heart, blood vessels, sinuses, kidneys, and brain are the most commonly afflicted.[7] The World Health Organization restrict this diagnosis to cases which have no well-defined cause. That is, all cases of secondary (i.e. reactive) eosinophilia (including lymphocyte-variant hypereosinophilia) and primary hypereosinophilia (including chronic eosinophilic leukemia (NOS), clonal eosinophilia, and hypereosinophilia associated with hematological malignancies) are excluded from this diagnosis.[5][7]
Secondary hypereosinophilia[edit]
Secondary (or reactive) eosinophilias are non-clonal increases in blood eosinophil levels caused by an underlying disease. The pathogenesis of the hypereosinophilia in these diseases is thought to be the release of one or more cytokines (e.g. granulocyte macrophage colony stimulating factor, interleukin 3, interleukin 5) that: a) cause bone marrow precursor cells, i.e. CFU-Eos, to proliferate and mature into eosinophils; b) promote release of bone marrow eosinophils into the circulation, c) stimulate circulating eosinophils to enter tissues and release tissue-injuring agents. These cytokines may be released by the diseased cells or the diseased cells may cause the release of these cytokines by non-diseased cells.[20] Primary disorders associated with and known or presumed to cause hypereosinophilia or eosinophilia are given below.[citation needed]
Infections[edit]
Helminths are common causes of hypereosiophilia and eosinophilia in areas endemic to these parasites. Helminths infections causing increased blood eosinophil counts include: 1) nematodes, (i.e. Angiostrongylus cantonensis and Hookworm infections), ascariasis, strongyloidiasis trichinosis, visceral larva migrans, Gnathostomiasis, cysticercosis, and echinococcosis; 2) filarioidea, i.e. tropical pulmonary eosinophilia, loiasis, and onchocerciasis; and 3) flukes, i.e. schistosomiasis, fascioliasis, clonorchiasis, paragonimiasis, and fasciolopsiasis. Other infections associated with increased eosinophil blood counts include: protozoan infections, i.e. Isospora belli and Dientamoeba fragilis) and sarcocystis); fungal infections (i.e. disseminated histoplasmosis, cryptococcosis [especially in cases with central nervous system involvement]), and coccidioides); and viral infections, i.e. Human T-lymphotropic virus 1and HIV.[7][21]
Autoimmune diseases[edit]
Hypereosiophilia or eosinophilia may be associated with the following autoimmune diseases: systemic lupus erythematosus eosinophilic fasciitis, eosinophilic granulomatosis with polyangiitis, dermatomyositis, severe rheumatoid arthritis, progressive systemic sclerosis, Sjögren syndrome, thromboangiitis obliterans, Behçet's disease, IgG4-related disease, inflammatory bowel diseases, sarcoidosis, bullous pemphigoid, and dermatitis herpetiformis.[7]
Allergic diseases[edit]
Eosinophilia and comparatively fewer cases of hypereosinophilia are associated with the following known diseases that are known or thought to have an allergic basis: allergic rhinitis, asthma, atopic dermatitis, eosinophilic esophagitis, chronic sinusitis, aspirin-induced asthma, allergic bronchopulmonary aspergillosis, chronic eosinophilic pneumonia, and Kimura's disease.[7][22]
Certain types of food allergy disorders may also be associated with eosinophilia or, less commonly, hypereosinophilia. Allergic eosinophilic esophagitis and the Food protein-induced enterocolitis syndrome are commonly associated with increased blood eosinophil levels.[23][24]
Drugs[edit]
A wide range of drugs are known to cause hypereosinophilia or eosinophilia accompanied by an array of allergic symptoms. Rarely, these reactions are severe causing, for example, the drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome. Drug- induced hepatitis marked by immunoallergic pathology, which has much bidirectional crossover with DRESS syndrome, is typically accompanied by some severity of eosinophilia. While virtually any drug should be considered as a possible cause of these signs and symptoms, the following drugs and drug classes are some of the most frequently reported causes: penicillins, cephalosporins, dapsone, sulfonamides, carbamazepine, phenytoin, lamotrigine, valproic acid, nevirapine, efavirenz, and ibuprofen. These drugs may cause severely toxic reactions such as the DRESS syndrome. Other drugs and drug classes often reported to cause increased blood eosinophil levels accompanied by less severe (e.g. non-DRESS syndrome) symptoms include tetracyclins, doxycycline, linezolid, nitrofurantoin, metronidazole, carbamazepine, phenobarbital, lamotrigine, valproate, desipramine, amitriptyline, fluoxetine, piroxicam, diclofenac, ACE inhibitors, abacavir, nevirapine, ranitidine, cyclosporin, and hydrochlorothiazide.[7][22]
The toxic oil syndrome is associated with hypereosinophilia/eosinophilia and systemic symptoms due to one or more contaminants in rapeseed oil[7][22]and the Eosinophilia–myalgia syndrome, also associated with hypereosinophilia, appears due to trace contaminants in certain commercial batches of the amino acid, L-tryptophan.[7][25]
Allergic reactions to drugs are a common cause of eosinophilia, with manifestations ranging from diffuse maculopapular rash, to severe life-threatening drug reactions with eosinophilia and systemic symptoms (DRESS).[2] Drugs that has, allopurinol, nonsteroidal anti-inflammatory drugs (NSAIDs), some antipsychotics such as risperidone, and certain antibiotics. Phenibut, an analogue of the neurotransmitter GABA, has also been implicated in high doses. The reaction which has been shown to be T-cell mediated may also cause eosinophilia-myalgia syndrome.[2]
Malignancies[edit]
Certain malignancies cause a secondary eosinophilia or, less commonly, hypereosinophilia. These increases in blood eosinophils appear due to the release of stimulatory cytokines or invasion of the bone marrow and thereby irritation of resident eosinophils or their precursors. Malignancies associated with these effects include gastric, colorectal, lung, bladder, and thyroid cancers, as well as squamous cell cancers of the cervix, vagina, penis, skin, and nasopharyrnx. Some hematological malignancies are likewise associated with secondary rises in blood eosinophil counts; these include Hodgkin disease, certain T-cell lymphomas, acute myeloid leukemia, the myelodysplastic syndromes, many cases of systemic mastocytosis, chronic myeloid leukemia, polycythemia vera, essential thrombocythemia, myelofibrosis, chronic myelomonocytic leukemia, and certain cases of T-lymphoblastic leukemia/lymphoma-associated or myelodysplastic–myeloproliferative syndrome-associated eosinophilias.[7]
Hodgkin lymphoma (Hodgkin's disease) often elicits severe eosinophilia; however, non-Hodgkin lymphoma and leukemia produce less marked eosinophilia.[3] Of solid tumor neoplasms, ovarian cancer is most likely to provoke eosinophilia, though any other cancer can cause the condition.[3] Solid epithelial cell tumors have been shown to cause both tissue and blood eosinophilia, with some reports indicating that this may be mediated by interleukinproduction by tumor cells, especially IL-5 or IL-3.[2] This has also been shown to occur in Hodgkin lymphoma, in the form of IL-5 secreted by Reed-Sternberg cells.[2] In primary cutaneous T cell lymphoma, blood and dermal eosinophilia are often seen. Lymphoma cells have also been shown to produce IL-5 in these disorders. Other types of lymphoid malignancies have been associated with eosinophilia, as in lymphoblastic leukemia with a translocation between chromosomes 5 and 14 or alterations in the genes which encode platelet-derived growth factor receptors alpha or beta.[2][26]Patients displaying eosinophilia overexpress a gene encoding an eosinophil hematopoietin. A translocation between chromosomes 5 and 14 in patients with acute B lymphocytic leukemia resulted in the juxtaposition of the IL-3 gene and the immunoglobulin heavy-chain gene, causing overproduction production of IL-3, leading to blood and tissue eosinophilia.[2][27]
Primary immunodeficiency diseases[edit]
Primary immunodeficiency diseases are inborn errors in the immune system due to defective genes. Certain of these disorders are sometimes or often associated with hypereosinophilia. The list of such disorders includes ZAP70 deficiency (defective ZAP70 gene), CD3gamma chain deficiency (defective CD3G gene), MCHII deficiency (defective RFXANK gene), Wiskott–Aldrich syndrome (defective WAS gene), IPEX syndrome (defective IPEX gene), CD40gene defect, and autoimmune lymphoproliferative syndrome (defective Fas receptor gene). More than 30 other primary immunodeficiency diseases are sometimes associated with modest increases in eosinophil counts, i.e. eosinophilia.[28] The hyperimmunoglobulin E syndrome is associated with hypereosionphilia or eosinophilia due to mutations in any one of the following genes: STAT3, DOCK8, PGM3, SPINK5, and TYK2 (see mutations in the hymperimmoglobulin E syndrome).[28][29] Omenn syndrome is a severe combined immunodeficiency disease characterized by skin rash, slenomegaly, and lymphadenopathy due to a causative mutation in RAG1, RAG2, or, more rarely, one of several other genes.[28]
Lymphocyte-variant hypereosinophilia[edit]
Lymphocyte-variant hypereosinophilia is a disorder attributed to the expansion of a cytokine-producing, aberrant population of a particular T-cell phenotype. The disorder is clonal with regard to the production of abnormal T-cell lymphocytes not eosinophils which appear phenotypically normal. The phenotypically aberrant lymphocytes function abnormally by stimulating the proliferation and maturation of bone marrow eosinophil-precursor cells which in studied cases appears due to their excess production of interleukin 5, interleukin 3, or interleukin 13. The disorder is usually indolent but infrequently progresses to T-cell lymphoma or Sezary syndrome. Accumulation of partial deletions in the short arm of chromosome 6, the long arm of chromosome 10, or the acquirement of an extra chromosome (i.e. trisomy) 7) in T-cells or the proliferation of lymphocytes with the CD3 negative, CD41 positive immunophenotype may occur during the disorders progression to lymphoma. Reports on treatment of the disorder are rare. In on study of 16 lymphocyte-variant hypereosinophilia patients with the aberrant CD3 negative, CD41 positive immunophenotype, good responds to corticosteroid drugs were uniform but 16 ultimately required corticosteroid-sparing agents. Hydroxyurea and imatinib are less likely to have efficacy in this variant of hypereosinophilia than in many cases of clonal eosinophilia or chronic eosinophilic leukemia.
Gleich's syndrome[edit]
Gleich's syndrome, which may be a form of lymphocyte-variant hypereosinophilia, involves hypereosinophilia, elevated blood levels of IgM antibodies, and clonal expansion of T cells. Similar to lymphocyte=variant hypereosinophilia, the increased levels of blood eosinophils in Gleich's syndrome is thought to be secondary to the secretion of eosinophil-stimulating cytokines by a T cell clones.[16]
[edit]
IgG4-related disease or Immunoglobulin G4-related disease is a condition dacryoadenitis, sialadenitis, lymphadentitis, and pancreatitis (i.e. inflammation of the lacrimal glands, salivary glands, lymph nodes, and pancreas, respectively) plus retroperitoneal fibrosis. Less commonly, almost any other organ or tissue except joints and brain may be beleaguered by the inflammatory disorder. About 1/3 of cases exhibit eosinophilia or, rarely, hypereosinophilia. This increase in blood eosinophil count is often associated with abnormal T-lymphocyte clones (e.g. increased numbers of CD4 negative, CD7 positive T cells, CD3 negative, CD4 positive T cells, or CD3 positive, CD4 negative, CD8 negative T cells) and is thought to be secondary to these immunological disturbances. The disorder often exhibits are recurrent-relapsing course and is highly responsive to corticosteroids or rituximab as first-line therapy and interferon gamma as second-line therapy.[30]
Angiolymphoid hyperplasia with eosinophilia[edit]
Angiolymphoid hyperplasia with eosinophilia is a disorder initially classified as a form of IgG4-related diseases but now considered a distinct entity. The disorder involves inflamed benign tumors of the vasculature in skin and, less commonly, other tissues. The tumors consist of histiocytoid endothelial cells prominently infiltrated by lymphocytes and eosinophils and is associated with hypereosinophilia or eosinophilia.[31]
Cholesterol embolism[edit]
Transient, fluctuating hypereosinophilia occurs in 60%-80% of individuals suffering cholesterol embolisms. In this disorder, cholesterol crystals located in an atherosclerotic plaque of a large artery dislodge, travel downstream in the blood, and clog smaller arteries. This results in obstructive damage to multiple organs and tissues. Afflicted tissues exhibit acute inflammation involving eosinophils, neutrophils, monocytes, lymphocytes, and plasma cells. The cause for this hypereosinophilic response is not known.[32]
Adrenal insufficiency[edit]
A class of steroid hormones secreted by the adrenal gland, glucocorticoids, inhibit eosinophil proliferation and survival. In adrenal insufficiency, low levels of these hormones allow increased eosinophil proliferation and survival. This leads to increases in blood eosinophil levels, typically eosinophilia and, less commonly, hypereosinophilia.[33]
Organ-restricted hypereosinophilias[edit]
Hypereosinophilia may occur in the setting of damage to a single specific organ due to a massive infiltration by eosinophils. This disorder is sub-classified based on the organ involved and is not considered to be a form of primary hypereosinophilia, secondary hypereosinophilia, or the idiopathic hypereosinophilic syndrome because: a) the eosinophils associated with the disorder have not been shown to be clonal in nature; b) a reason for the increase in blood eosinophils has not been determined; c) organ damage has not been shown to be due to eosinophils; and d) the disorder in each individual case typically is limited to the afflicted organ. Examples of organ-restricted hypereosinopilia include eosinophilic myocarditis, eosinophilic esophagitis, eosinophilic gastroenteritis, eosinophilic cystitis, eosinophilic pneumonia, eosinophilic fasciitis, eosinophilic folliculitis, eosinophilic cellulitis, eosinophilic vasculitis, and eosinophilic ulcer of the oral mucosa. Other examples of organ-restricted hepereosinophilia include those involving the heart, kidney, liver, colon, pulmonary pleurae, peritoneum, fat tissue, myometrium, and synovia.[16]
However, immune suppression, the mechanism of action of corticosteroids, can be fatal in patients with parasitosis.[2]??
Eosinophilia can be idiopathic (primary) or, more commonly, secondary to another disease.[2][3] In the Western World, allergic or atopic diseases are the most common causes, especially those of the respiratory or integumentary systems. In the developing world, parasites are the most common cause. A parasitic infection of nearly any bodily tissue can cause eosinophilia.[citation needed] Diseases that feature eosinophilia as a sign include:
- Allergic disorders
- IgG4-related disease
- Parasitic infections[34]
- Addison's disease and stress-induced suppression of adrenal gland function[35]
- Some forms of malignancy
- Systemic autoimmune diseases[34]
- Eosinophilic myocarditis[40]
- Eosinophilic esophagitis[41]
- Eosinophilic gastroenteritis[42]
- Cholesterol embolism (transiently)[34]
- Coccidioidomycosis (Valley fever), a fungal disease prominent in the US Southwest.[43]
- Human immunodeficiency virus infection
- Interstitial nephropathy
- Hyperimmunoglobulin E syndrome, an immune disorder characterized by high levels of serum IgE
- Idiopathic hypereosinophilic syndrome.[26]
- Congenital disorders
See also[edit]
https://en.wikipedia.org/wiki/Eosinophilia
Tuberculosis (TB) is an infectious disease usually caused by Mycobacterium tuberculosis (MTB) bacteria.[1] Tuberculosis generally affects the lungs, but can also affect other parts of the body.[1] Most infections show no symptoms, in which case it is known as latent tuberculosis.[1] About 10% of latent infections progress to active disease which, if left untreated, kills about half of those affected.[1]Typical symptoms of active TB are a chronic cough with blood-containing mucus, fever, night sweats, and weight loss.[1] It was historically called consumption due to the weight loss.[8] Infection of other organs can cause a wide range of symptoms.[9]
https://en.wikipedia.org/wiki/Tuberculosis
Clonal hypereosinophilia, also termed primary hypereosinophilia or clonal eosinophilia, is a grouping of hematological disorders all of which are characterized by the development and growth of a pre-malignant or malignant population of eosinophils, a type of white blood cell that occupies the bone marrow, blood, and other tissues. This population consists of a clone of eosinophils, i.e. a group of genetically identical eosinophils derived from a sufficiently mutated ancestor cell.[1]
The clone of eosinophils bear a mutation in any one of several genes that code for proteins that regulate cell growth. The mutations cause these proteins to be continuously active and thereby to stimulate growth in an uncontrolled and continuous manner. The expanding population of eosinophils initially formed in the bone marrow may spread to the blood and then enter into and injure various tissues and organs.[1]
Clinically, clonal eosinophilia resembles various types of chronic or acute leukemias, lymphomas, or myeloproliferative hematological malignancies. However, many of the clonal hypereosinophilias are distinguished from these other hematological malignancies by the genetic mutations which underlie their development and, more importantly, by their susceptibility to specific treatment regiments. That is, many types of these disorders are remarkably susceptible to relatively non-toxic drugs.[1][2]
https://en.wikipedia.org/wiki/Clonal_hypereosinophilia
Tumor necrosis factor (TNF, cachexin, or cachectin; often called tumor necrosis factor alphaor TNF-α) is a cytokine – a small protein used by the immune system for cell signaling. If macrophages (certain white blood cells) detect an infection, they release TNF to alert other immune system cells as part of an inflammatory response. TNF is a member of the TNF superfamily, which consists of various transmembrane proteins with a homologous TNF domain.
TNF signaling occurs through two receptors: TNFR1 and TNFR2.[5][6] TNFR1 is constituitively expressed on most cell types, whereas TNFR2 is restricted primarily to endothelial, epithelial, and subsets of immune cells.[5][6] TNF1 signaling tends to be pro-inflammatory and apoptotic, whereas TNFR2 signaling is anti-inflammatory and promotes cell proliferation.[5][6] Suppression of TNFR1 signaling has been important for treatment of autoimmune disease,[7] whereas TNFR2 signaling promotes wound healing.[6]
TNF-α exists as a transmembrane form (mTNF-α) and as a soluble form (sTNF-α). sTNF-α results from enzymatic cleavage of mTNF-α.[8] mTNF-α is mainly found on monocytes/macrophages where it interacts with tissue receptors by cell-to-cell contact.[8] sTNF-α selectively binds to TNFR1, whereas mTNF-α binds to both TNFR1 and TNFR2.[9] TNF-α binding to TNFR1 is irreversible, whereas binding to TNFR2 is reversible.[10]
The primary role of TNF is in the regulation of immune cells. TNF, as an endogenous pyrogen, is able to induce fever, apoptotic cell death, cachexia, inflammation and to inhibit tumorigenesis, viral replication, and respond to sepsis via IL-1 and IL-6-producing cells. Dysregulation of TNF production has been implicated in a variety of human diseases including Alzheimer's disease,[11]cancer,[12] major depression,[13] psoriasis[14] and inflammatory bowel disease (IBD).[15] Though controversial, some studies have linked depression and IBD to increased levels of TNF.[16][17]
Under the name tasonermin, TNF is used as an immunostimulant drug in the treatment of certain cancers. Drugs that counter the action of TNF are used in the treatment of various inflammatory diseases, for instance rheumatoid arthritis.
Certain cancers can cause overproduction of TNF. TNF parallels parathyroid hormone both in causing secondary hypercalcemia and in the cancers with which excessive production is associated.
https://en.wikipedia.org/wiki/Tumor_necrosis_factor
Butyrophilin-like protein 2 is a protein that in humans is encoded by the BTNL2 gene.[5][6][7]
Because it is associated with the immune system and the major histocompatibility complex, it has been implicated in many diseases (see further reading list below). A large scale study found it to be the protein under the most stringent selection in the human genome in 8 out of 12 geographic regions using the HKA test.[8]
https://en.wikipedia.org/wiki/BTNL2
Common variable immunodeficiency (CVID) is an immune disorder characterized by recurrent infections and low antibody levels, specifically in immunoglobulin (Ig) types IgG, IgM and IgA.[1] Generally symptoms include high susceptibility to foreign invaders, chronic lung disease, and inflammation and infection of the gastrointestinal tract.[1] However, symptoms vary greatly between people. "Variable" refers to the heterogeneous clinical manifestations of this disorder, which include recurrent bacterial infections, increased risk for autoimmune disease and lymphoma, as well as gastrointestinal disease.[2] CVID is a lifelong disease.
The cause of CVID is poorly understood. Deletions in genes that encode cell surface proteins and cytokine receptors, such as CD19, CD20, CD21, and CD80, is a likely cause.[3] A deletion is a mutation in which part of the chromosome is lost during DNA replication which may include several genes, or as few as a single base pair. Additionally, the disease is defined by T cell defects, namely reduced proliferative capacity.[4] The disease is hard to diagnose, taking on average 6–7 years after onset.[3] [5] CVID is a primary immunodeficiency.[3]
Treatment options are limited, and usually include lifelong immunoglobulin replacement therapy.[5] This therapy is thought to help reduce bacterial infections. This treatment alone is not wholly effective, and many people still experience other symptoms like lung disease and noninfectious inflammatory symptoms.
CVID was first diagnosed over 60 years ago, and since has emerged as the predominant class of primary antibody deficiencies. CVID is formally diagnosed by levels of IgG and IgA more than two standard deviations below the norm, and no other cause for hypogammaglobulinemia, an abnormally low level of immunoglobulins in the blood. It is thought to affect between 1 in 25,000 to 1 in 50,000 people worldwide.
https://en.wikipedia.org/wiki/Common_variable_immunodeficiency
Granulomatous–lymphocytic interstitial lung disease (GLILD) is a lung complication of common variable immunodeficiency disorders (CVID). It is seen in approximately 15% of patients with CVID.[1] It has been defined histologically as the presence of (non-caseating) granuloma and lymphoproliferation in the lung.[1]However, as GLILD is often associated with other auto-immune features such as splenomegaly, adenopathyand cytopenias, a definition based on abnormalities on lung imaging (CT scan) together with evidence of granulomatous inflammation elsewhere has also been employed.[2]
Although infections and complications of infection such as bronchiectasis are more common complications of CVID in the lung, the presence of immune manifestations including GLILD is important because this has been associated with greater risk of death.[1][3]
In general, as a rare complication of a rare disease, the condition remains incompletely understood, and there is real need for further research in the area.
https://en.wikipedia.org/wiki/Granulomatous–lymphocytic_interstitial_lung_disease
Granulomatosis with polyangiitis (GPA), previously known as Wegener's granulomatosis(WG),[1][2][3][4][5] is an extremely rare long-term systemic disorder that involves the formation of granulomas and inflammation of blood vessels (vasculitis). It is a form of vasculitis that affects small- and medium-size vessels in many organs but most commonly affects the upper respiratory tract, lungs and kidneys.[6] The signs and symptoms of GPA are highly varied and reflect which organs are supplied by the affected blood vessels. Typical signs and symptoms include nosebleeds, stuffy nose and crustiness of nasal secretions, and inflammation of the uveal layer of the eye.[3] Damage to the heart, lungs and kidneys can be fatal.
The cause of GPA is unknown. Genetics have been found to play a role in GPA though the risk of inheritance appears to be low.[7]
GPA treatment depends on the severity of the disease.[8] Severe disease is typically treated with a combination of immunosuppressive medications such as rituximab or cyclophosphamide and high-dose corticosteroids to control the symptoms of the disease and azathioprine, methotrexate, or rituximab to keep the disease under control.[1][7][8] Plasma exchange is also used in severe cases with damage to the lungs, kidneys, or intestines.[9]
The number of new cases of GPA each year is estimated to be 2.1–14.4 new cases per million people in Europe.[3] GPA is rare in Japanese and African-American populations but occurs more often in people of Northern European descent.[7] GPA is estimated to affect 3 cases per 100,000 people in the United States and equally affects men and women.[10]
| Granulomatosis with polyangiitis | |
|---|---|
| Other names | Wegener's granulomatosis (WG) (formerly) |
 | |
| Micrograph showing features characteristic of granulomatosis with polyangiitis – a vasculitis and granulomas with multi-nucleated giant cells. H&E stain. | |
| Specialty | Immunology, rheumatology |
https://en.wikipedia.org/wiki/Granulomatosis_with_polyangiitis
Cytomegalovirus (CMV) (from cyto- 'cell' via Greek κύτος kútos- 'container' + μέγας mégas 'big, megalo-' + -virus via Latin vīrus 'poison') is a genus of viruses in the order Herpesvirales, in the family Herpesviridae,[3] in the subfamily Betaherpesvirinae. Humans and monkeys serve as natural hosts. The 11 species in this genus include human betaherpesvirus 5 (HCMV, human cytomegalovirus, HHV-5), which is the species that infects humans. Diseases associated with HHV-5 include mononucleosis, and pneumonia.[4][5] In the medical literature, most mentions of CMV without further specification refer implicitly to human CMV. Human CMV is the most studied of all cytomegaloviruses.[6]
| Cytomegalovirus | |
|---|---|
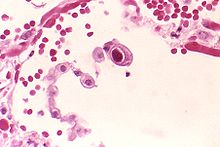 | |
| Typical "owl eye" intranuclear inclusionindicating CMV infection of a lung pneumocyte[1] | |
| Virus classification | |
| (unranked): | Virus |
| Realm: | Duplodnaviria |
| Kingdom: | Heunggongvirae |
| Phylum: | Peploviricota |
| Class: | Herviviricetes |
| Order: | Herpesvirales |
| Family: | Herpesviridae |
| Subfamily: | Betaherpesvirinae |
| Genus: | Cytomegalovirus |
| Species | |
See text | |
| Synonyms[2] | |
| |
https://en.wikipedia.org/wiki/Cytomegalovirus
Mycobacterium avium complex is a group of mycobacteria comprising Mycobacterium intracellulare and Mycobacterium avium that are commonly grouped because they infect humans together; this group, in turn, is part of the group of nontuberculous mycobacteria. These bacteria cause disease in humans called Mycobacterium avium-intracellulare infection or Mycobacterium avium complex infection.[2] These bacteria are common and are found in fresh and salt water, in household dust and in soil.[3] MAC bacteria usually cause infection in those who are immunocompromised or those with severe lung disease.
| Mycobacterium avium complex | |
|---|---|
| Scientific classification | |
| Domain: | |
| Phylum: | |
| Class: | |
| Order: | |
| Family: | |
| Genus: | |
| Species complex: | Mycobacterium avium complex |
| Binomial name | |
| Mycobacterium intracellulare Runyon 1965,[1] ATCC 13950 | |
| Mycobacterium avium Chester 1901 emend. Thorel et al. 1990 | |
https://en.wikipedia.org/wiki/Mycobacterium_avium_complex
Cryptococcus, sometimes informally called crypto, is a genus of fungi that grow in culture as yeasts. The sexual forms or teleomorphs of Cryptococcus species are filamentous fungi formerly classified in the genus Filobasidiella. The name Cryptococcus is used when referring to the yeast states of the fungi; it comes from the Greek for "hidden sphere" (literally "hidden berry"). A few species in the Cryptococcus genus cause a disease called cryptococcosis.
| Cryptococcus | |
|---|---|
 | |
| Cryptococcus neoformans | |
| Scientific classification | |
| Kingdom: | Fungi |
| Division: | Basidiomycota |
| Class: | Tremellomycetes |
| Order: | Tremellales |
| Family: | Tremellaceae |
| Genus: | Cryptococcus Vuill. |
| Type species | |
| Cryptococcus neoformans | |
| Synonyms | |
Filobasidiella | |
https://en.wikipedia.org/wiki/Cryptococcus
Acute myeloid leukemia (AML) is a cancer of the myeloid line of blood cells, characterized by the rapid growth of abnormal cells that build up in the bone marrow and blood and interfere with normal blood cell production.[1] Symptoms may include feeling tired, shortness of breath, easy bruising and bleeding, and increased risk of infection.[1] Occasionally, spread may occur to the brain, skin, or gums.[1] As an acute leukemia, AML progresses rapidly, and is typically fatal within weeks or months if left untreated.[1]
Risk factors include smoking, previous chemotherapy or radiation therapy, myelodysplastic syndrome, and exposure to the chemical benzene.[1] The underlying mechanism involves replacement of normal bone marrow with leukemia cells, which results in a drop in red blood cells, platelets, and normal white blood cells.[1] Diagnosis is generally based on bone marrow aspirationand specific blood tests.[3] AML has several subtypes for which treatments and outcomes may vary.[1]
The first-line treatment of AML is usually chemotherapy, with the aim of inducing remission.[1] People may then go on to receive additional chemotherapy, radiation therapy, or a stem cell transplant.[1][3]The specific genetic mutations present within the cancer cells may guide therapy, as well as determine how long that person is likely to survive.[3]
In 2015, AML affected about one million people, and resulted in 147,000 deaths globally.[4][5] It most commonly occurs in older adults.[2] Males are affected more often than females.[2] The five-year survival rate is about 35% in people under 60 years old and 10% in people over 60 years old.[3] Older people whose health is too poor for intensive chemotherapy have a typical survival of five to ten months.[3] It accounts for roughly 1.1% of all cancer cases, and 1.9% of cancer deaths in the United States.[2]
| Acute myeloid leukemia | |
|---|---|
| Other names | Acute myelogenous leukemia, acute nonlymphocytic leukemia (ANLL), acute myeloblastic leukemia, acute granulocytic leukemia[1] |
 | |
| Bone marrow aspirate showing acute myeloid leukemia, arrows indicate Auer rods | |
| Specialty | Hematology, oncology |
| Symptoms | Feeling tired, shortness of breath, easy bruising and bleeding, increased risk of infection[1] |
| Usual onset | All ages, most frequently ~65–75 years old[2] |
| Risk factors | Smoking, previous chemotherapy or radiation therapy, myelodysplastic syndrome, benzene[1] |
| Diagnostic method | Bone marrow aspiration, blood test[3] |
| Treatment | Chemotherapy, radiation therapy, stem cell transplant[1][3] |
| Prognosis | Five-year survival ~29% (US, 2017)[2] |
| Frequency | 1 million (2015)[4] |
| Deaths | 147,100 (2015)[5] |
https://en.wikipedia.org/wiki/Acute_myeloid_leukemia
French-American-British[edit]
The French-American-British (FAB) classification system divides AML into eight subtypes, M0 through to M7, based on the type of cell from which the leukemia developed and its degree of maturity. AML of types M0 to M2 may be called acute myeloblastic leukemia. Classification is done by examining the appearance of the malignant cells with light microscopy and/or by using cytogenetics to characterize any underlying chromosomal abnormalities. The subtypes have varying prognoses and responses to therapy.
While the terminology of the FAB system is still sometimes used, and it remains a valuable diagnostic tool in areas without access to genetic testing, this system has largely become obsolete in favor of the WHO classification, which correlates more strongly with treatment outcomes.[21][37]
Six FAB subtypes (M1 through to M6) were initially proposed in 1976,[38] although later revisions added M7 in 1985[39] and M0 in 1987.[40]
| Type | Name | Cytogenetics | Percentage of adults with AML | Immunophenotype[41] | ||||
|---|---|---|---|---|---|---|---|---|
| CD14 | CD15 | CD33 | HLA-DR | Other | ||||
| M0 | acute myeloblastic leukemia, minimally differentiated | 5%[42] | − [43] | − [43] | + [43] | + [43] | MPO − [44] | |
| M1 | acute myeloblastic leukemia, without maturation | 15%[42] | − | − | + | + | MPO + [44] | |
| M2 | acute myeloblastic leukemia, with granulocytic maturation | t(8;21)(q22;q22), t(6;9) | 25%[42] | − | + | + | + | |
| M3 | promyelocytic, or acute promyelocytic leukemia (APL) | t(15;17) | 10%[42] | − | + | + | − | |
| M4 | acute myelomonocytic leukemia | inv(16)(p13q22), del(16q) | 20%[42] | <45% | + | + | + | |
| M4eo | myelomonocytic together with bone marrow eosinophilia | inv(16), t(16;16) | 5%[42] | +/− [45] | + [46] | + [46] | CD2+ [46] | |
| M5 | acute monoblastic leukemia (M5a) or acute monocytic leukemia (M5b) | del (11q), t(9;11), t(11;19) | 10%[42] | >55% | + | + | + | |
| M6 | acute erythroid leukemias, including erythroleukemia (M6a) and very rare pure erythroid leukemia (M6b) | 5%[42] | − | +/− | +/− | +/− | Glycophorin+ | |
| M7 | acute megakaryoblastic leukemia | t(1;22) | 5%[42] | − | − | + | +/− | CD41/CD61+ |
The morphologic subtypes of AML also include rare types not included in the FAB system, such as acute basophilic leukemia, which was proposed as a ninth subtype, M8, in 1999.[47]
https://en.wikipedia.org/wiki/Acute_myeloid_leukemia#French-American-British
Lymphoproliferative disorders (LPDs) refer to a specific class of diagnoses, comprising a group of several conditions, in which lymphocytes are produced in excessive quantities. These disorders primarily present in patients who have a compromised immune system. Due to this factor, there are instances of these conditions being equated with "immunoproliferative disorders"; although, in terms of nomenclature, lymphoproliferative disorders are a subclass of immunoproliferative disorders—along with hypergammaglobulinemia and paraproteinemias.
https://en.wikipedia.org/wiki/Lymphoproliferative_disorders
Lymphoproliferative disorders are a set of disorders characterized by the abnormal proliferation of lymphocytes into a monoclonal lymphocytosis. The two major types of lymphocytes are B cells and T cells, which are derived from pluripotent hematopoietic stem cells in the bone marrow. Individuals who have some sort of dysfunction with their immune system are susceptible to develop a lymphoproliferative disorder because when any of the numerous control points of the immune system become dysfunctional, immunodeficiency or deregulation of lymphocytes is more likely to occur. There are several inherited gene mutations that have been identified to cause lymphoproliferative disorders; however, there are also acquired and iatrogenic causes.[2]
https://en.wikipedia.org/wiki/Lymphoproliferative_disorders
Immunoproliferative disorders are disorders of the immune system that are characterized by the abnormal proliferation of the primary cells of the immune system, which includes B cells, T cells and natural killer (NK) cells, or by the excessive production of immunoglobulins (also known as antibodies).[citation needed]
https://en.wikipedia.org/wiki/Immunoproliferative_disorder
Multiple myeloma (MM), also known as plasma cell myeloma and simply myeloma, is a cancer of plasma cells, a type of white blood cell that normally produces antibodies.[6] Often, no symptoms are noticed initially.[10] As it progresses, bone pain, anemia, kidney dysfunction, and infections may occur.[10] Complications may include amyloidosis.[3]
The cause of multiple myeloma is unknown.[4] Risk factors include obesity, radiation exposure, family history, and certain chemicals.[5][11][12] Multiple myeloma may develop from monoclonal gammopathy of undetermined significance that progresses to smoldering myeloma.[13] The abnormal plasma cells produce abnormal antibodies, which can cause kidney problems and overly thick blood.[10] The plasma cells can also form a mass in the bone marrow or soft tissue.[10] When one tumor is present, it is called a plasmacytoma; more than one is called multiple myeloma.[10] Multiple myeloma is diagnosed based on blood or urine tests finding abnormal antibodies, bone marrow biopsy finding cancerous plasma cells, and medical imaging finding bone lesions.[6] Another common finding is high blood calcium levels.[6]
Multiple myeloma is considered treatable, but generally incurable.[3] Remissions may be brought about with steroids, chemotherapy, targeted therapy, and stem cell transplant.[3] Bisphosphonatesand radiation therapy are sometimes used to reduce pain from bone lesions.[3][6]
Globally, multiple myeloma affected 488,000 people and resulted in 101,100 deaths in 2015.[8][9] In the United States, it develops in 6.5 per 100,000 people per year and 0.7% of people are affected at some point in their lives.[7] It usually occurs around the age of 60 and is more common in men than women.[6] It is uncommon before the age of 40.[6] Without treatment, the median survival in the prechemotherapy era was about 7 months. After the introduction of chemotherapy, prognosis improved significantly with a median survival of 24 to 30 months and a 10-year survival rate of 3%. Even further improvements in prognosis have occurred because of the introduction of newer biologic therapies and better salvage options, with median survivals now exceeding 60 to 90 months.[3] With current treatments, survival is usually 4–5 years.[3] The five-year survival rate is about 54%.[7] The word myeloma is from the Greek myelo- meaning "marrow" and -oma meaning "tumor".[14]
https://en.wikipedia.org/wiki/Multiple_myeloma
The B-cell lymphomas are types of lymphoma affecting B cells. Lymphomas are "blood cancers" in the lymph nodes. They develop more frequently in older adults and in immunocompromised individuals.
B-cell lymphomas include both Hodgkin's lymphomas and most non-Hodgkin lymphomas. They are typically divided into low and high grade, typically corresponding to indolent (slow-growing) lymphomas and aggressive lymphomas, respectively. As a generalisation, indolent lymphomas respond to treatment and are kept under control (in remission) with long-term survival of many years, but are not cured. Aggressive lymphomas usually require intensive treatments, with some having a good prospect for a permanent cure.[1]
Prognosis and treatment depends on the specific type of lymphoma as well as the stage and grade. Treatment includes radiation and chemotherapy. Early-stage indolent B-cell lymphomas can often be treated with radiation alone, with long-term non-recurrence. Early-stage aggressive disease is treated with chemotherapy and often radiation, with a 70-90% cure rate.[1] Late-stage indolent lymphomas are sometimes left untreated and monitored until they progress. Late-stage aggressive disease is treated with chemotherapy, with cure rates of over 70%.[1]
https://en.wikipedia.org/wiki/B-cell_lymphoma
T-cell lymphoma is a rare form of cancerous lymphoma affecting T-cells.[1] Lymphoma arises mainly from the uncontrolled proliferation of T-cells and can become cancerous. [2]
T-cell lymphoma is categorized under Non-Hodgkin Lymphoma (NHL) and represents less than 15% of all Non-Hodgkin's diseases in the category. [3] T-cell lymphomas are often categorised based on their growth patterns as either; aggressive (fast-growing) or indolent (slow-growing).[1] Although the cause of T-cell lymphoma is not definitive, it has been associated with various risk factors and viruses such as Epstein Barr virus (EBV) and Human T-cell leukemia virus-1 (HTLV1).[2]
The prognosis and treatment of T-cell lymphoma can vary drastically based on the specific type of lymphoma and its growth patterns. Due to their rarity and high variability between the different subtypes, the prognosis of T-cell lymphoma is significantly worse than other Non-Hodgkin lymphoma.[1] The treatment of T-cell lymphoma is often similar to other Non-Hodgkin lymphomas with early-stage treatments consisting of chemotherapy and/or radiology.[2] The effectiveness of these treatments is often varied between subtypes with most receiving a poor outcome with high relapse rates.[4]
https://en.wikipedia.org/wiki/T-cell_lymphoma
Hemophagocytic lymphohistiocytosis (HLH), also known as haemophagocytic lymphohistiocytosis (British spelling), and hemophagocytic or haemophagocytic syndrome,[1] is an uncommon hematologic disorder seen more often in children than in adults. It is a life-threatening disease of severe hyperinflammation caused by uncontrolled proliferation of activated lymphocytesand macrophages, characterised by proliferation of morphologically benign lymphocytes and macrophages that secrete high amounts of inflammatory cytokines. It is classified as one of the cytokine storm syndromes. There are inherited and non-inherited (acquired) causes of hemophagocytic lymphohistiocytosis (HLH).
https://en.wikipedia.org/wiki/Hemophagocytic_lymphohistiocytosis
Acute lymphoblastic leukemia (ALL) is a cancer of the lymphoid line of blood cells characterized by the development of large numbers of immature lymphocytes.[1] Symptoms may include feeling tired, pale skin color, fever, easy bleeding or bruising, enlarged lymph nodes, or bone pain.[1] As an acute leukemia, ALL progresses rapidly and is typically fatal within weeks or months if left untreated.[11]
In most cases, the cause is unknown.[2] Genetic risk factors may include Down syndrome, Li-Fraumeni syndrome, or neurofibromatosis type 1.[1] Environmental risk factors may include significant radiation exposure or prior chemotherapy.[1] Evidence regarding electromagnetic fields or pesticidesis unclear.[4][6] Some hypothesize that an abnormal immune response to a common infection may be a trigger.[4] The underlying mechanism involves multiple genetic mutations that results in rapid cell division.[2] The excessive immature lymphocytes in the bone marrow interfere with the production of new red blood cells, white blood cells, and platelets.[1] Diagnosis is typically based on blood tests and bone marrow examination.[3]
ALL is typically treated initially with chemotherapy aimed at bringing about remission.[2] This is then followed by further chemotherapy typically over a number of years.[2] Treatment usually also include intrathecal chemotherapy since systemic chemotherapy can have limited penetration into the central nervous system and the central nervous system is a common site for relapse of acute lymphoblastic leukemia. [12][13]
Treatment can also include radiation therapy if spread to the brain has occurred.[2] Stem cell transplantation may be used if the disease recurs following standard treatment.[2] Additional treatments such as Chimeric antigen receptor T cell immunotherapy are being used and further studied.[2]
ALL affected about 876,000 people globally in 2015 and resulted in about 111,000 deaths.[14][10] It occurs most commonly in children, particularly those between the ages of two and five.[15][4] In the United States it is the most common cause of cancer and death from cancer among children.[2] ALL is notable for being the first disseminated cancer to be cured.[16] Survival for children increased from under 10% in the 1960s to 90% in 2015.[2] Survival rates remain lower for babies (50%)[17] and adults (35%).[8] According to the National Cancer Intelligence Network (NCIN), generally for people with ALL: around 70 out of 100 people (70%) will survive their leukemia for 5 years or more after they are diagnosed.
https://en.wikipedia.org/wiki/Acute_lymphoblastic_leukemia
Chronic lymphocytic leukemia (CLL) is a type of cancer in which the bone marrow makes too many lymphocytes (a type of white blood cell).[2][8] Early on there are typically no symptoms.[2] Later non-painful lymph node swelling, feeling tired, fever, night sweats, or weight loss for no clear reason may occur.[2][9] Enlargement of the spleen and low red blood cells (anemia) may also occur.[2][4] It typically worsens gradually over years.[2]
Risk factors include having a family history of the disease.[2] Exposure to Agent Orange and certain insecticides might also be a risk.[4] CLL results in the buildup of B cell lymphocytes in the bone marrow, lymph nodes, and blood.[4] These cells do not function well and crowd out healthy blood cells.[2] CLL is divided into two main types: those with a mutated IGHV gene and those without.[4]Diagnosis is typically based on blood tests finding high numbers of mature lymphocytes and smudge cells.[5]
Early-stage CLL in asymptomatic cases responds better to careful observation, as there is no evidence that early intervention treatment can alter the course of the disease.[10] Immune defects occur early in the course of CLL and these increase the risk of developing serious infection, which should be treated appropriately with antibiotics.[10] In those with significant symptoms, chemotherapyor immunotherapy may be used.[4] As of 2019 ibrutinib is often the initial medication recommended.[11] The medications fludarabine, cyclophosphamide, and rituximab were previously the initial treatment in those who are otherwise healthy.[12]
CLL affected about 904,000 people globally in 2015 and resulted in 60,700 deaths.[6][7] The disease most commonly occurs in people over the age of 50.[3] Men are diagnosed around twice as often as women (6.8 to 3.5 ratio).[13] It is much less common in people from Asia.[4] Five-year survivalfollowing diagnosis is approximately 83% in the United States.[3] It represents less than 1% of deaths from cancer.[7]
https://en.wikipedia.org/wiki/Chronic_lymphocytic_leukemia
Waldenström's macroglobulinemia (/ˈvældənstrɛmz ˌmækroʊˌɡlɒbjələˈniːmiə/;[1][2] WM) is a type of canceraffecting two types of B cells: lymphoplasmacytoid cells and plasma cells. Both cell types are white blood cells. WM is characterized by having high levels of a circulating antibody, immunoglobulin M (IgM), which is made and secreted by the cells involved in the disease. WM is an "indolent lymphoma" (i.e., one that tends to grow and spread slowly) and a type of lymphoproliferative disease which shares clinical characteristics with the indolent non-Hodgkin lymphomas.[3] WM is commonly classified as a form of plasma cell dyscrasia, similar to other plasma cell dyscrasias that, for example, lead to multiple myeloma, WM is commonly preceded by two clinically asymptomatic but progressively more pre-malignant phases, IgM monoclonal gammopathy of undetermined significance (i.e. IgM MGUS) and smoldering Waldenström macroglobulinemia. The WM spectrum of dysplasias differs from other spectrums of plasma cell dyscrasias in that it involves not only aberrant plasma cells but also aberrant lymphoplasmacytoid cells and that it involves IgM while other plasma dyscrasias involve other antibody isoforms.[4][5]
WM is a rare disease, with only about 1,500 cases per year in the United States. WM occurs more frequently in older adults.[6] While the disease is incurable, it is treatable. Because of its indolent nature, many patients are able to lead active lives, and when treatment is required, may experience years of symptom-free remission.[7]
https://en.wikipedia.org/wiki/Waldenström%27s_macroglobulinemia
Langerhans cell histiocytosis (LCH) is an abnormal clonal proliferation of Langerhans cells, abnormal cells deriving from bone marrow and capable of migrating from skin to lymph nodes.
Symptoms range from isolated bone lesions to multisystem disease.[1] LCH is part of a group of syndromes called histiocytoses, which are characterized by an abnormal proliferation of histiocytes(an archaic term for activated dendritic cells and macrophages). These diseases are related to other forms of abnormal proliferation of white blood cells, such as leukemias and lymphomas.[citation needed]
The disease has gone by several names, including Hand–Schüller–Christian disease, Abt-Letterer-Siwe disease, Hashimoto-Pritzker disease (a very rare self-limiting variant seen at birth) and histiocytosis X, until it was renamed in 1985 by the Histiocyte Society.[2][1]
https://en.wikipedia.org/wiki/Langerhans_cell_histiocytosis
Lymphocyte-variant hypereosinophila, is a rare disorder in which eosinophilia or hypereosinophilia (i.e. a large or extremely large increase in the number of eosinophils in the blood circulation) is caused by an aberrant population of lymphocytes. These aberrant lymphocytes function abnormally by stimulating the proliferation and maturation of bone marrow eosinophil-precursor cells termed colony forming unit-Eosinophils or CFU-Eos.[1]
The overly stimulated CFU-Eos cells mature to apparently normal appearing but possibly overactive eosinophils which enter the circulation and may accumulate in and damage various tissues. The disorder is usually indolent or slowly progressive but may proceed to a leukemic phase sometimes classified as acute eosinophilic leukemia. Lymphocyte-variant hypereosinophilia can therefore be regarded as a precancerous disorder.[1]
The disorder merits therapeutic intervention to avoid or reduce eosinophil-induced tissue injury and treat its leukemic phase. The latter phase is aggressive and typically responds relatively poorly to anti-leukemia chemotherapeutic drug regimens.[2]
https://en.wikipedia.org/wiki/Lymphocyte-variant_hypereosinophilia
Post-transplant lymphoproliferative disorder (PTLD) is the name given to a B-cell proliferation due to therapeutic immunosuppression after organ transplantation. These patients may develop infectious mononucleosis-like lesions or polyclonal polymorphic B-cell hyperplasia. Some of these B-cells may undergo mutations which will render them malignant, giving rise to a lymphoma.[citation needed]
In some patients, the malignant cell clone can become the dominant proliferating cell type, leading to frank lymphoma, a group of B cell lymphomas occurring in immunosuppressed patients following organ transplant.
https://en.wikipedia.org/wiki/Post-transplant_lymphoproliferative_disorder
Autoimmune lymphoproliferative syndrome (ALPS), is a form of lymphoproliferative disorder (LPDs). It affects lymphocyte apoptosis.[2]
It is a rare genetic disorder of abnormal lymphocyte survival caused by defective Fas mediated apoptosis.[3]Normally, after infectious insult, the immune system down-regulates by increasing Fas expression on activated B and T lymphocytes and Fas-ligand on activated T lymphocytes. Fas and Fas-ligand interact to trigger the caspase cascade, leading to cell apoptosis. Patients with ALPS have a defect in this apoptotic pathway, leading to chronic non-malignant lymphoproliferation, autoimmune disease, and secondary cancers.[4]
https://en.wikipedia.org/wiki/Autoimmune_lymphoproliferative_syndrome
Iatrogenesis is the causation of a disease, a harmful complication, or other ill effect by any medical activity, including diagnosis, intervention, error, or negligence.[1][2][3] First used in this sense in 1924,[1] the term was introduced to sociology in 1976 by Ivan Illich, alleging that industrialized societies impair quality of life by overmedicalizing life.[4] Iatrogenesis may thus include mental suffering via medical beliefs or a practitioner's statements.[4][5][6] Some iatrogenic events are obvious, like amputation of the wrong limb, whereas others, like drug interactions, can evade recognition. In a 2013 estimate, about 20 million negative effects from treatment had occurred globally.[7] In 2013, an estimated 142,000 persons died from adverse effects of medical treatment, up from an estimated 94,000 in 1990.[8]
https://en.wikipedia.org/wiki/Iatrogenesis
Hematopoietic stem cells give rise to: 1) myeloid precursor cells that differentiate into red blood cells, mast cells, blood platelet-forming megakaryocytes, or myeloblasts, which latter cells subsequently differentiate into white blood cells viz., neutrophils, basophils, monocytes, and eosinophils; or 2) lymphoidprecursor cells which differentiate into T lymphocytes, B lymphocytes, or natural killer cells. Malignant transformation of these stem or precursor cells results in the development of various hematological malignancies. Some of these transformations involve chromosomal translocations or Interstitial deletions that create fusion genes. These fusion genes encode fusion proteins that continuously stimulate cell growth, proliferation, prolonged survival, and/or differentiation. Such mutations occur in hematological stem cells and/or their daughter myeloid precursor and lymphoid precursor cells; commonly involve genes that encode tyrosine kinase proteins; and cause or contribute to the development of hematological malignancies. A classic example of such a disease is chronic myelogenous leukemia, a neoplasm commonly caused by a mutation that creates the BCR-ABL1 fusion gene (see Philadelphia chromosome). The disease is due to conversion of the tightly regulated tyrosine kinase of ABL1 protein to being unregulated and continuously active in the BCR-ABL1 fusion protein. This Philadelphia chromosome positive form of chronic myelogenous leukemia used to be treated with chemotherapy but nonetheless was regarded as becoming lethal within 18-60 months of diagnosis. With the discovery of the uncontrolled tyrosine kinase activity of this disorder and the use of tyrosine kinase inhibitors. Philadelphia chromosome positive chronic myelogenous leukemia is now successfully treated with maintenance tyrosine kinase inhibiting drugs to achieve its long-term suppression.[citation needed]
Some hematological malignancies exhibit increased numbers of circulating blood eosinophils, increased numbers of bone marrow eosinophils, and/or eosinophil infiltrations into otherwise normal tissues. These malignancies were at first diagnosed as eosinophilia, hypereosinophilia, acute eosinophilic leukemia, chronic eosinophilic leukemia, other myeloid leukemias, myeloproliferative neoplasm, myeloid sarcoma, lymphoid leukemia, or non-Hodgkin lymphomas. Based on their association with eosinophils, unique genetic mutations, and known or potential sensitivity to tyrosine kinase inhibitors or other specific drug therapies, they are now in the process of being classified together under the term clonal hypereosinophilia or clonal eosinophilia. Historically, patients suffering the cited eosinophil-related syndromes were evaluated for causes of their eosinophilia such as those due to allergic disease, parasite or fungal infection, autoimmune disorders, and various well-known hematological malignancies (e.g. Chronic myelogenous leukemia, systemic mastocytosis, etc.) (see causes of eosinophilia). Absent these causes, patients were diagnosed in the World Health Organization's classification as having either 1)Chronic eosinophilic leukemia, not otherwise specified, (CEL-NOS) if blood or bone marrow blast cells exceeded 2% or 5% of total nucleated cells, respectively, and other criteria were met or 2) idiopathic hypereosinophilic syndrome (HES) if there was evidence of eosinophil-induced tissue damage but no criteria indicating chronic eosinophilic leukemia. Discovery of genetic mutations underlining these eosinophilia syndromes lead to their removal from CEL-NOS or HES categories and classification as myeloid and lymphoid neoplasms associated with eosinophilia and abnormalities of PDGFRA, PDGFRB, FGFR1, and, tentatively, PCMA-JAK2. Informally, these diseases are also termed clonal hypereosinophilias. New genetic mutations associated with, and possibly contributing to the development of, eosinophilia have been discovered, deemed to be causes of clonal eosinophilia, and, in certain cases, recommended for inclusion in the category of myeloid and lymphoid neoplasms associated with eosinophilia and abnormalities of PDGFRA, PDGFRB, FGFR1, and, tentatively, PCMA-JAK2.[1][2] Many of the genetic causes for clonal eosinophilia are rare but nonetheless merit attention because of their known or potential sensitivity to therapeutic interventions that differ dramatically form the often toxic chemotherapy used to treat more common hematological malignancies.[citation needed]
https://en.wikipedia.org/wiki/Clonal_hypereosinophilia
Myeloproliferative neoplasms (MPNs) are a group of rare blood cancers in which excess red blood cells, white blood cells or platelets are produced in the bone marrow. Myelo refers to the bone marrow, proliferative describes the rapid growth of blood cells and neoplasm describes that growth as abnormal and uncontrolled.
The overproduction of blood cells is often associated with a somatic mutation, for example in the JAK2, CALR, TET2, and MPL gene markers.
In rare cases, some MPNs such as primary myelofibrosis may accelerate and turn into acute myeloid leukemia.[1]
https://en.wikipedia.org/wiki/Myeloproliferative_neoplasm
A myeloid sarcoma (chloroma, granulocytic sarcoma,[1]:744 extramedullary myeloid tumor), is a solid tumor composed of immature white blood cells[2] called myeloblasts. A chloroma is an extramedullary manifestation of acute myeloid leukemia; in other words, it is a solid collection of leukemic cells occurring outside of the bone marrow.
https://en.wikipedia.org/wiki/Myeloid_sarcoma
Chronic eosinophilic leukemia is a form of cancer in which too many eosinophils are found in the bone marrow, blood, and other tissues. Most cases are associated with fusion genes. [1]
In cases associated with PDGFRB and FGFR1 mutations, splenomegaly is common. Lymphadenopathy is also common with FGFR1 mutations.[2]
Infiltration of eosinophils causes organ damage.[3]
Most cases of CEL are associated with rearrangements in PDGFRA, PDGFRB, or FGFR1.[4]
CEL not otherwise specified (CEL NOS) is a form in which BCR-ABL1 fusion genes and PDGFRA, PDGFRB, and FGFR1 rearrangements are not found.[5]
Myeloid-related hematological malignancy
CFU-GM/
and other granulocytes
CFU-GM
Myelocyte
AML:
Acute myeloblastic leukemia M0 M1 M2 APL/M3
MP
Chronic neutrophilic leukemia
Monocyte
AML
AMoL/M5 Myeloid dendritic cell leukemia
CML
Philadelphia chromosome Accelerated phase chronic myelogenous leukemia
Myelomonocyte
AML
M4
MD-MP
Juvenile myelomonocytic leukemia Chronic myelomonocytic leukemia
Other
Histiocytosis
CFU-Baso
AML
Acute basophilic
CFU-Eos
AML
Acute eosinophilic
MP
Chronic eosinophilic leukemia/Hypereosinophilic syndrome
MEP
CFU-Meg
MP
Essential thrombocythemia
Acute megakaryoblastic leukemia
CFU-E
AML
Erythroleukemia/M6
MP
Polycythemia vera
MD
Refractory anemia Refractory anemia with excess of blasts Chromosome 5q deletion syndrome Sideroblastic anemia Paroxysmal nocturnal hemoglobinuria Refractory cytopenia with multilineage dysplasia
CFU-Mast
Mastocytoma
Mast cell leukemia Mast cell sarcoma Systemic mastocytosis
Mastocytosis:
Diffuse cutaneous mastocytosis Erythrodermic mastocytosis Adult type of generalized eruption of cutaneous mastocytosis Urticaria pigmentosa Mast cell sarcoma Solitary mastocytoma
Systemic mastocytosis
Xanthelasmoidal mastocytosis
Multiple/unknown
AML
Acute panmyelosis with myelofibrosis Myeloid sarcoma
MP
Primary myelofibrosis Biphenotypic acute leukaemia
Categories: Chronic myeloid leukemiaRare cancers
Navigation menu
Not logged in
Talk
Contributions
Create account
Log in
ArticleTalk
ReadEditView history
Search
Main page
Contents
Current events
Random article
About Wikipedia
Contact us
Donate
Contribute
Help
Learn to edit
Community portal
Recent changes
Upload file
Tools
What links here
Related changes
Special pages
Permanent link
Page information
Cite this page
Wikidata item
Print/export
Download as PDF
Printable version
Languages
Polski
Edit links
https://en.wikipedia.org/wiki/Chronic_eosinophilic_leukemia
Biphenotypic acute leukaemia (BAL) is an uncommon type of leukemia which arises in multipotent progenitor cells which have the ability to differentiate into both myeloid and lymphoid lineages.[1][2][3] It is a subtype of "leukemia of ambiguous lineage".[4]
The direct reasons leading to BAL are still not clear. BAL can be de novo or secondary to previous cytotoxic therapy. Many factors, such viruses, hereditary factors, and radiation, might have a relationship with BAL.
BAL is hard to treat. Usually the chemotherapy is chosen according to the morphology of the blast (ALL or AML). A blood-forming stem-cell transplantation is highly recommended. About 5% of acute leukaemia cases are BAL. BAL can occur in all ages of people but occurs more in adults than in children.[5]
https://en.wikipedia.org/wiki/Biphenotypic_acute_leukaemia
The term leukemoid reaction describes an increased white blood cell count (> 50,000 cells/μL), which is a physiological response to stress or infection (as opposed to a primary blood malignancy, such as leukemia). It often describes the presence of immature cells such as myeloblasts or red blood cells with nuclei in the peripheral blood.
It may be lymphoid or myeloid.[1]
As noted above, a leukemoid reaction is typically a response to an underlying medical issue. Causes of leukemoid reactions include:[citation needed]
- Severe hemorrhage (retroperitoneal hemorrhage)
- Drugs
- Use of sulfa drugs
- Use of dapsone
- Use of glucocorticoids
- Use of G-CSF or related growth factors
- All-trans retinoic acid (ATRA)
- Ethylene glycol intoxication
- Infections
- Clostridium difficile
- Tuberculosis
- Pertussis
- Infectious mononucleosis (lymphocyte predominant)
- Visceral larva migrans (eosinophil predominant)
- Asplenia
- Diabetic ketoacidosis
- Organ necrosis
- Hepatic necrosis
- Ischemic colitis
- As a feature of trisomy 21 in infancy (incidence of ~10%)
- As a paraneoplastic phenomenon (rare)
https://en.wikipedia.org/wiki/Leukemoid_reaction
Clostridioides difficile infection [5] (CDI or C-diff), also known as Clostridium difficile infection, is a symptomatic infection due to the spore-forming bacterium Clostridioides difficile.[2][6] Symptoms include watery diarrhea, fever, nausea, and abdominal pain.[1] It makes up about 20% of cases of antibiotic-associated diarrhea.[1] Antibiotics can contribute to detrimental changes in gut microbiota; specifically, they decrease short-chain fatty acid absorption which results in osmotic, or watery, diarrhea.[7] Complications may include pseudomembranous colitis, toxic megacolon, perforation of the colon, and sepsis.[1]
https://en.wikipedia.org/wiki/Clostridioides_difficile_infection
https://pubs.er.usgs.gov/publication/70021460
https://www.nejm.org/doi/full/10.1056/NEJM197102252840818
https://www.britannica.com/science/immune-system-disorder/Type-III-hypersensitivity
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3109637/
https://www.sciencedirect.com/science/article/pii/S2255502115000127
Glucocorticoid treatment and human T-lymphotropic virus type 1 (HTLV-1) infection are the two conditions most specifically associated with triggering hyperinfection (7,8).
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3109637/
Manson's Tropical Diseases
By Gordon Charles Cook, Alimuddin Zumla
https://books.google.com/books?id=CF2INI0O6l0C&pg=PA1805&lpg=PA1805&dq=lupus+coccidia+leprosy&source=bl&ots=N243WziSEw&sig=ACfU3U2WjK9vXINx3Cwv4bzIgbViAi2TRg&hl=en&sa=X&ved=2ahUKEwi7zaSAmNXyAhXNaM0KHQopBoIQ6AF6BAgkEAM#v=onepage&q=lupus%20coccidia%20leprosy&f=false
This disease has a strong association with the Epstein-Barr virus (EBV),[9] but the true pathogenesis of this disease has yet to be described. The cell of origin is believed to be an NK cell.[4] Blastoid NK cell lymphoma appears to be a different entity and shows no association with EBV.[1]
Bone marrow involvement runs the spectrum between an inconspicuous infiltrate to extensive marrow replacement by leukemic cells. Reactive histiocytesdisplaying hemophagocytosis can be seen interspersed in the neoplastic infiltrate.[4]
Leukemic involvement of organs is typically destructive on tissue sections with necrosis and possibly angioinvasion, and the monotonous infiltrate may be diffuse or patchy.[4]
Sites of involvement[edit]
This disease is typically found and diagnosed in peripheral blood, and while it can involve any organ, it is usually found in the spleen, liver, and bone marrow.[4]
Immunophenotype[edit]
The immunophenotype of this disease is the same as extranodal NK/T-cell lymphoma, nasal type and is shown in the table below. CD11b and CD16 show variable expression.[1][10]
Status Antigens
Positive CD2, CD3ε, CD56, perforin, granzyme B, TIA-1, CCR5
Negative CD57
Genetic findings[edit]
Due to the NK lineage, clonal rearrangements of lymphoid (T cell receptor; B cell receptor) genes are not seen.[4] The genome of the Epstein Barr virus (EBV) is detected in many cases,[9] along with a variety of chromosomal abnormalities.[11]
https://en.wikipedia.org/wiki/Aggressive_NK-cell_leukemia
Adult T-cell leukemia/lymphoma (ATL or ATLL) is a rare cancer of the immune system's T-cells[1][2][3] caused by human T cell leukemia/lymphotropic virus type 1 (HTLV-1).[4] All ATL cells contain integrated HTLV-1 provirus further supporting that causal role of the virus in the cause of the neoplasm.[4] A small amount of HTLV-1 individuals progress to develop ATL with a long latency period between infection and ATL development. ATL is categorized into 4 subtypes: acute, smoldering, lymphoma-type, chronic. Acute and Lymphoma-type are known to particularity be aggressive with poorer prognosis. [5]
Globally, the retrovirus HTLV-1 is estimated to infect 20 million people with the incidence of ATL approximately 0.05 per 100,000 with endemic regions such as regions of Japan, as high as 27 per 100,000.[6] However, cases have increased in non-endemic regions with highest incidence of HTLV-1 in southern/northern islands of Japan, Caribbean, Central and South America, intertropical Africa, Romania, northern Iran. ATL normally occurs around the age of 62 years but median age at diagnosis does depend on prevalence of the HTLV-1 infection in the geographic location.[7]
Current treatment regiments for ATL are based on clinical subtype and response to initial therapy. Some therapy modalities for treatment may not available in all countries therefore strategies differ across the world. All patients are referred to clinical trials if available. Beyond clinical trials, treatments are centered on multiagent chemotherapy, zidovudine plus interferon a (AZT/IFN), and allogenic hematopoietic stem cell transplantation (alloHSCT).[6]
https://en.wikipedia.org/wiki/Adult_T-cell_leukemia/lymphoma
Non-mycosis fungoides CD30− cutaneous large T-cell lymphoma is a cutaneous condition that usually presents as solitary or generalized plaques, nodules, or tumors of short duration.[1]:738
https://en.wikipedia.org/wiki/Non-mycosis_fungoides_CD30%E2%88%92_cutaneous_large_T-cell_lymphoma
Peripheral T-cell lymphoma not otherwise specified (PTCL-NOS), is a subtype of peripheral T-cell lymphoma. Peripheral T-cell lymphoma (PTCL) is defined as a diverse group of aggressive lymphomas that develop from mature-stage white blood cells called T-cells and natural killer cells (NK cells) (see figure for an overview of PTCL subtypes). PTCL is a type of non-Hodgkin's lymphoma (NHL).[2] PTCL specifically affects T-cells rather than B-cells, and results when T-cells develop and grow abnormally.
About 30% of PTCL-NOS cases exhibit malignant T cells that are infected with the Epstein-Barr virus(EBV). When associated with EBV, PTCL-NOS is classified as one of the Epstein-Barr virus-associated lymphoproliferative diseases (see Epstein-Barr virus-associated peripheral T cell lymphoma, not otherwise specified) but the relationship of EBV to the development and progression of Epstein-Barr virus-associated PTCL-NOS is unclear.[3]
PTCL-NOS, the most common subtype of PTCL, is aggressive and predominantly nodal. There are two morphologic variants: the T-zone lymphoma variant and the lymphoepithelioid cell variant.[4][5]
- T-zone lymphoma is so named for its involvement in a specific area of the lymph node that consists of a dense accumulation of T-cells.[6]
- Lympho-epithelioid lymphoma, also called Lennert's lymphoma, is rare and generally affects older individuals.[7]
Mycosis fungoides, also known as Alibert-Bazin syndrome or granuloma fungoides,[1] is the most common form of cutaneous T-cell lymphoma. It generally affects the skin, but may progress internally over time. Symptoms include rash, tumors, skin lesions, and itchy skin.
While the cause remains unclear, most cases are not hereditary. Most cases are in people over 20 years of age, and it is more common in men than women. Treatment options include sunlight exposure, ultraviolet light, topical corticosteroids, chemotherapy, and radiotherapy.
https://en.wikipedia.org/wiki/Mycosis_fungoides
Aggressive NK-cell leukemia is a disease with an aggressive, systemic proliferation of natural killer cells(NK cells) and a rapidly declining clinical course.[1] [2] [3]
It is also called aggressive NK-cell lymphoma.[4]
https://en.wikipedia.org/wiki/Aggressive_NK-cell_leukemia
Above. Starter Culture; Proper Subject required never x weponists.
Plant/Animal-Viridae Adjuvants/time/no/etc. STD-VIRIDAE EBVS HIV EIA EIL HILVT1 syphali agranulysis SF FLU
Clonal hypereosinophilia, also termed primary hypereosinophilia or clonal eosinophilia, is a grouping of hematological disorders all of which are characterized by the development and growth of a pre-malignant or malignant population of eosinophils, a type of white blood cell that occupies the bone marrow, blood, and other tissues. This population consists of a clone of eosinophils, i.e. a group of genetically identical eosinophils derived from a sufficiently mutated ancestor cell.[1]
https://en.wikipedia.org/wiki/Clonal_hypereosinophilia
A myeloid sarcoma (chloroma, granulocytic sarcoma,[1]:744 extramedullary myeloid tumor), is a solid tumor composed of immature white blood cells[2] called myeloblasts. A chloroma is an extramedullary manifestation of acute myeloid leukemia; in other words, it is a solid collection of leukemic cells occurring outside of the bone marrow.
https://en.wikipedia.org/wiki/Myeloid_sarcoma
Human T-cell lymphotropic virus type 1 or human T-lymphotropic virus (HTLV-I), also called the adult T-cell lymphoma virus type 1, is a retrovirus of the human T-lymphotropic virus (HTLV) family that has been implicated in several kinds of diseases including very aggressive adult T-cell lymphoma (ATL), HTLV-I-associated myelopathy, uveitis, Strongyloides stercoralis hyper-infection and some other diseases. It is thought that about 1–5% of infected persons develop cancer as a result of the infection with HTLV-I over their lifetimes.[1]
Adult T-cell lymphoma (ATL) was discovered in 1977 in Japan. The symptoms of ATL were different from other lymphomas known at the time. It was suggested that ATL is caused by the infection of a retrovirus called ATLV.[2]Strikingly, ATLV had the transforming activity in vitro.[3] These studies established that the retrovirus infection is the cause of ATL. The retrovirus is now generally called HTLV-I because later studies proved that ATLV is the same as the firstly identified human retrovirus called HTLV discovered by Bernard Poiesz and Francis Ruscetti and their co-workers in the laboratory of Robert C. Gallo at the National Cancer Institute.[4] Infection with HTLV-I, like infection with other retroviruses, probably occurs for life. A patient infected with HTLV can be diagnosed when antibodies against HTLV-1 are detected in the serum.[1]
https://en.wikipedia.org/wiki/Human_T-lymphotropic_virus_1
CD30, also known as TNFRSF8, is a cell membrane protein of the tumor necrosis factor receptorfamily and tumor marker.
This receptor is expressed by activated, but not by resting, T and B cells. TRAF2 and TRAF5 can interact with this receptor, and mediate the signal transduction that leads to the activation of NF-kappaB. It is a positive regulator of apoptosis, and also has been shown to limit the proliferative potential of autoreactive CD8 effector T cells and protect the body against autoimmunity. Two alternatively spliced transcript variants of this gene encoding distinct isoforms have been reported.[5]
https://en.wikipedia.org/wiki/CD30
Template:Immunoproliferative immunoglobulin disorders
https://en.wikipedia.org/wiki/Template:Immunoproliferative_immunoglobulin_disorders
Primary effusion lymphoma (PEL) is classified as a diffuse large B cell lymphoma. It is a rare malignancy of plasmablastic cells that occurs in individuals that are infected with the Kaposi's sarcoma-associated herpesvirus (i.e. KSHV/HHV8). Plasmablasts are immature plasma cells, i.e. lymphocytes of the B-cell type that have differentiated into plasmablasts but because of their malignant nature do not differentiate into mature plasma cells but rather proliferate excessively and thereby cause life-threatening disease.[1] In PEL, the proliferating plasmablastoid cells commonly accumulate within body cavities to produce effusions (i.e. accumulations of fluid), primarily in the pleural, pericardial, or peritoneal cavities, without forming a contiguous tumor mass.[2] In rare cases of these cavitary forms of PEL, the effusions develop in joints, the epidural space surrounding the brain and spinal cord, and underneath the capsule (i.e. tightly-woven collagen fibers) which forms around breast implants.[1] Less frequently, individuals present with extracavitary primary effusion lymphomas, i.e., solid tumor masses not accompanied by effusions.[3] The extracavitary tumors may develop in lymph nodes, bone, bone marrow, the gastrointestinal tract, skin, spleen, liver, lungs, central nervous system, testes, paranasal sinuses, muscle, and, rarely, inside the vasculature and sinuses of lymph nodes.[1] As their disease progresses, however, individuals with the classical effusion-form of PEL may develop extracavitary tumors and individuals with extracavitary PEL may develop cavitary effusions.[4]
https://en.wikipedia.org/wiki/Primary_effusion_lymphoma
CD11c, also known as Integrin, alpha X (complement component 3 receptor 4 subunit)(ITGAX), is a gene that encodes for CD11c .[5][6]
CD11c is an integrin alpha X chain protein. Integrins are heterodimeric integral membrane proteins composed of an alpha chain and a beta chain. This protein combines with the beta 2 chain (ITGB2) to form a leukocyte-specific integrin referred to as inactivated-C3b (iC3b) receptor 4 (CR4). The alpha X beta 2 complex seems to overlap the properties of the alpha M beta 2 integrin in the adherence of neutrophils and monocytes to stimulated endothelium cells, and in the phagocytosis of complement coated particles.[5]
CD11c is a type I transmembrane protein found at high levels on most human dendritic cells, but also on monocytes, macrophages, neutrophils, and some B cells that induces cellular activation and helps trigger neutrophil respiratory burst; expressed in hairy cell leukemias, acute nonlymphocytic leukemias, and some B-cell chronic lymphocytic leukemias.
https://en.wikipedia.org/wiki/Integrin_alpha_X
B-lymphocyte antigen CD20 or CD20 is expressed on the surface of all B-cells beginning at the pro-B phase (CD45R+, CD117+) and progressively increasing in concentration until maturity.[5]
In humans CD20 is encoded by the MS4A1 gene.[6][7]
This gene encodes a member of the membrane-spanning 4A gene family. Members of this nascent protein family are characterized by common structural features and similar intron/exon splice boundaries and display unique expression patterns among hematopoietic cells and nonlymphoid tissues. This gene encodes a B-lymphocyte surface molecule that plays a role in the development and differentiation of B-cells into plasma cells. This family member is localized to 11q12, among a cluster of family members. Alternative splicing of this gene results in two transcript variants that encode the same protein.[7]
https://en.wikipedia.org/wiki/CD20
Anthracyclines is a class of drugs[2] used in cancer chemotherapy that are extracted from Streptomycesbacterium.[3]
https://en.wikipedia.org/wiki/Anthracycline
https://en.wikipedia.org/wiki/Aggressive_NK-cell_leukemia
Lymphomatoid granulomatosis (LYG or LG) is a very rare lymphoproliferative disorder first characterized in 1972.[1] Lymphomatoid means lymphoma-like and granulomatosis denotes the microscopic characteristic of the presence of granulomas with polymorphic lymphoid infiltrates and focal necrosis within it.
LG most commonly affects middle aged people,[2] but has occasionally been observed in young people.[3] Males are found to be affected twice as often as females.[4]
https://en.wikipedia.org/wiki/Lymphomatoid_granulomatosis
The Epstein–Barr virus (EBV), formally called Human gammaherpesvirus 4, is one of the nine known human herpesvirus types in the herpes family, and is one of the most common viruses in humans. EBV is a double-stranded DNA virus.[2]
https://en.wikipedia.org/wiki/Epstein–Barr_virus
Extranodal NK/T-cell lymphoma, nasal type (ENKTCL-NT) (also termed angiocentric lymphoma, nasal-type NK lymphoma, NK/T-cell lymphoma, polymorphic/malignant midline reticulosis,[1] and lethal midline granuloma[2]) is a rare type of lymphoma that commonly involves midline areas of the nasal cavity, oral cavity, and/or pharynx[3] At these sites, the disease often takes the form of massive, necrotic, and extremely disfiguring lesions. However, ENKTCL-NT can also involve the eye, larynx, lung, gastrointestinal tract, skin, and various other tissues.[4] ENKTCL-NT mainly afflicts adults; it is relatively common in Asia and to lesser extents Mexico, Central America, and South America but is rare in Europe and North America.[5] In Korea, ENKTCL-NT often involves the skin and is reported to be the most common form of cutaneous lymphoma after mycosis fungoides.[6]
https://en.wikipedia.org/wiki/Extranodal_NK/T-cell_lymphoma,_nasal_type
Large granular lymphocytic (LGL) leukemia is a chronic lymphoproliferative disorder that exhibits an unexplained, chronic (> 6 months) elevation in large granular lymphocytes (LGLs) in the peripheral blood.[1]
It is divided in two main categories: T-cell LGL leukemia (T-LGLL) and natural-killer (NK)-cell LGL leukemia (NK-LGLL). As the name suggests, T-cell large granular lymphocyte leukemia is characterized by involvement of cytotoxic-T cells).[2]
In a study based in the US, the average age of diagnosis was 66.5 years[3] whereas in a French study the median age at diagnosis was 59 years (with an age range of 12-87 years old).[4] In the French study, only 26% of patients were younger than 50 years which suggests that this disorder is associated with older age at diagnosis.[4] Due to lack of presenting symptoms, the disorder is likely to be underdiagnosed in the general population.[5]
https://en.wikipedia.org/wiki/Large_granular_lymphocytic_leukemia
Tumors of the hematopoietic and lymphoid tissues (American English) or tumours of the haematopoietic and lymphoid malignancies (British English) are tumors that affect the blood, bone marrow, lymph, and lymphatic system.[1][2] Because these tissues are all intimately connected through both the circulatory system and the immune system, a disease affecting one will often affect the others as well, making myeloproliferation and lymphoproliferation (and thus the leukemias and the lymphomas) closely related and often overlapping problems.
While uncommon in solid tumors, chromosomal translocations are a common cause of these diseases. This commonly leads to a different approach in diagnosis and treatment of haematological malignancies.
Haematological malignancies are malignant neoplasms ("cancer"), and they are generally treated by specialists in hematology and/or oncology. In some centers "haematology/oncology" is a single subspecialty of internal medicine while in others they are considered separate divisions (there are also surgical and radiation oncologists). Not all haematological disorders are malignant ("cancerous"); these other blood conditions may also be managed by a hematologist.
Hematological malignancies may derive from either of the two major blood cell lineages: myeloid and lymphoid cell lines. The myeloid cell line normally produces granulocytes, erythrocytes, thrombocytes, macrophages and mast cells; the lymphoid cell line produces B, T, NK and plasma cells. Lymphomas, lymphocytic leukemias, and myeloma are from the lymphoid line, while acute and chronic myelogenous leukemia, myelodysplastic syndromes and myeloproliferative diseases are myeloid in origin.
A subgroup of them are more severe and are known as haematological malignancies (British English)/hematological malignancies (American English) or blood cancer. They may also be referred to as liquid tumors.[3][4]
https://en.wikipedia.org/wiki/Tumors_of_the_hematopoietic_and_lymphoid_tissues
Diffuse infiltrative lymphocytosis syndrome occurs in HIV positive patients with low CD4 counts.[1][2]
It is similar to Sjögren's syndrome,[3] with painless parotid and submandibular swelling, and sicca symptoms.
The syndrome typically improves with HAART.[citation needed]
https://en.wikipedia.org/wiki/Diffuse_infiltrative_lymphocytosis_syndrome
Natural killer cells, also known as NK cells or large granular lymphocytes (LGL), are a type of cytotoxiclymphocyte critical to the innate immune system that belong to the rapidly expanding family of innate lymphoid cells (ILC) and represent 5–20% of all circulating lymphocytes in humans.[1] The role of NK cells is analogous to that of cytotoxic T cells in the vertebrate adaptive immune response. NK cells provide rapid responses to virus-infected cell and other intracellular pathogens acting at around 3 days after infection, and respond to tumor formation. Typically, immune cells detect the major histocompatibility complex (MHC) presented on infected cell surfaces, triggering cytokine release, causing the death of the infected cell by lysisor apoptosis. NK cells are unique, however, as they have the ability to recognize and kill stressed cells in the absence of antibodies and MHC, allowing for a much faster immune reaction. They were named "natural killers" because of the notion that they do not require activation to kill cells that are missing "self" markers of MHC class 1.[2] This role is especially important because harmful cells that are missing MHC I markers cannot be detected and destroyed by other immune cells, such as T lymphocyte cells.
https://en.wikipedia.org/wiki/Natural_killer_cell
No comments:
Post a Comment