A meta-analysis of 15 studies produced a compendium of approximately 2,000 mammalian proteins that are palmitoylated. The highest associations of the palmitoylome are with cancers and disorders of the nervous system. Approximately 40% of synapticproteins were found in the palmitoylome.[11]
Palmitoylation mediates the affinity of a protein for lipid rafts and facilitates the clustering of proteins.[12] The clustering can increase the proximity of two molecules. Alternatively, clustering can sequester a protein away from a substrate. For example, palmitoylation of phospholipase D (PLD) sequesters the enzyme away from its substrate phosphatidylcholine. When cholesterol levels decrease or PIP2 levels increase the palmitate mediated localization is disrupted, the enzyme trafficks to PIP2 where it encounters its substrate and is active by substrate presentation.[13][14][15]
Scientists have appreciated the significance of attaching long hydrophobic chains to specific proteins in cell signaling pathways. A good example of its significance is in the clustering of proteins in the synapse. A major mediator of protein clustering in the synapse is the postsynaptic density (95kD) protein PSD-95. When this protein is palmitoylated it is restricted to the membrane. This restriction to the membrane allows it to bind to and cluster ion channels in the postsynaptic membrane. Also, in the presynaptic neuron, palmitoylation of SNAP-25 directs it to partition in the cell membrane [16] and allows the SNARE complex to dissociate during vesicle fusion. This provides a role for palmitoylation in regulating neurotransmitter release.[17]
Palmitoylation of delta catenin seems to coordinate activity-dependent changes in synaptic adhesion molecules, synapse structure, and receptor localizations that are involved in memory formation.[18]
Palmitoylation of gephyrin has been reported to influence GABAergic synapses.[1]
Palmitoylation is the covalent attachment of fatty acids, such as palmitic acid, to cysteine (S-palmitoylation) and less frequently to serine and threonine (O-palmitoylation) residues of proteins, which are typically membrane proteins.[2]The precise function of palmitoylation depends on the particular protein being considered. Palmitoylation enhances the hydrophobicity of proteins and contributes to their membrane association. Palmitoylation also appears to play a significant role in subcellular trafficking of proteins between membrane compartments,[3]as well as in modulating protein–protein interactions.[4] In contrast to prenylation and myristoylation, palmitoylation is usually reversible (because the bond between palmitic acid and protein is often a thioester bond). The reverse reaction in mammalian cells is catalyzed by acyl-protein thioesterases (APTs) in the cytosol and palmitoyl protein thioesterases in lysosomes. Because palmitoylation is a dynamic, post-translational process, it is believed to be employed by the cell to alter the subcellular localization, protein–protein interactions, or binding capacities of a protein.
An example of a protein that undergoes palmitoylation is hemagglutinin, a membrane glycoprotein used by influenza to attach to host cell receptors.[5] The palmitoylation cycles of a wide array of enzymes have been characterized in the past few years, including H-Ras, Gsα, the β2-adrenergic receptor, and endothelial nitric oxide synthase (eNOS). In signal transduction via G protein, palmitoylation of the α subunit, prenylation of the γ subunit, and myristoylation is involved in tethering the G protein to the inner surface of the plasma membrane so that the G protein can interact with its receptor.[6]
https://en.wikipedia.org/wiki/Palmitoylation
Palmitoleoylation is type of protein lipidation where the monounsaturated fatty acid palmitoleic acid is covalently attached to serine or threonine residues of proteins.[1][2] Palmitoleoylation appears to play a significant role in traffickingand targeting and function of Wnt proteins.[3][4][5]
O-Palmiteoylation of Wnt proteins is catalysed by PORCN. The inverse reaction is done by NOTUM.[6]
https://en.wikipedia.org/wiki/Palmitoleoylation
Pages in category "Post-translational modification"
The following 117 pages are in this category, out of 117 total. This list may not reflect recent changes (learn more).
A
C
H
- H2BK5ac
- H3K4me1
- H3K4me3
- H3K9ac
- H3K9me2
- H3K9me3
- H3K14ac
- H3K23ac
- H3K27ac
- H3K27me3
- H3K36ac
- H3K36me
- H3K36me2
- H3K36me3
- H3K56ac
- H3K79me2
- H3R2me2
- H3R8me2
- H3R17me2
- H3R26me2
- H3R42me
- H4K5ac
- H4K8ac
- H4K12ac
- H4K16ac
- H4K20me
- H4K91ac
- H4R3me2
- Histone acetylation and deacetylation
- Histone methylation
- (Histone-H3)-lysine-36 demethylase
- Hydroxylation
- Hyperphosphorylation
P
- Palmitoleoylation
- Palmitoyl acyltransferase
- Palmitoylation
- Persulfidation
- PEST sequence
- Phosida
- Phosphatome
- Phospho.ELM
- Phospho3D
- Phosphocholine
- Phosphorylation
- Polyglutamylation
- Polyglycylation
- PolyQ (database)
- Polysialic acid
- Post-translational regulation
- Prenylation
- Propionylation
- Protease
- Proteases in angiogenesis
- Protein carbonylation
- Protein methylation
- Protein phosphatase
- Protein targeting
- Proteolysis
- The Proteolysis Map
- PSORTdb
U
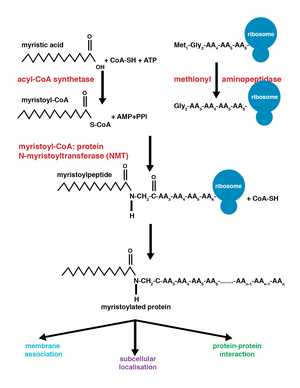
Co-translational vs. post-translational addition[edit]
Co-translational and post-translational covalent modifications enable proteins to develop higher levels of complexity in cellular function, further adding diversity to the proteome.[10] The addition of myristoyl-CoA to a protein can occur during protein translation or after. During co-translational addition of the myristoyl group, the N-terminal glycine is modified following cleavage of the N-terminal methionine residue in the newly forming, growing polypeptide.[1] Post-translational myristoylation typically occurs following a caspase cleavage event, resulting in the exposure of an internal glycine residue, which is then available for myristic acid addition.[8]
Myristoylated proteins[edit]
| Protein | Physiological Role | Myristoylation Function |
|---|---|---|
| Actin | Cytoskeleton structural protein | Post-translational myristoylation during apoptosis [8] |
| Bid | Apoptosis promoting protein | Post-translational myristoylation after caspase cleavage targets protein to mitochondrial membrane[8] |
| MARCKS | actin cross-linking when phosphorylated by protein kinase C | Co-translational myristoylation aids in plasma membrane association |
| G-Protein | Signaling GTPase | Co-translational myristoylation aids in plasma membrane association[11] |
| Gelsolin | Actin filament-severing protein | Post-translational myristoylation up-regulates anti-apoptotic properties [8] |
| PAK2 | Serine/threonine kinase cell growth, mobility, survival stimulator | Post-translational myristoylation up-regulates apoptotic properties and induces plasma membrane localization[8] |
| Arf | vesicular trafficking and actin remodeling regulation | N-terminus myristoylation aids in membrane association |
| Hippocalcin | Neuronal calcium sensor | Contains a Ca2+/myristoyl switch |
Myristoylation molecular switch[edit]
Myristoylation not only diversifies the function of a protein, but also adds layers of regulation to it. One of the most common functions of the myristoyl group is in membrane association and cellular localization of the modified protein. Though the myristoyl group is added onto the end of the protein, in some cases it is sequestered within hydrophobic regions of the protein rather than solvent exposed.[5] By regulating the orientation of the myristoyl group, these processes can be highly coordinated and closely controlled. Myristoylation is thus a form of "molecular switch."[12]
Both hydrophobic myristoyl groups and "basic patches" (highly positive regions on the protein) characterize myristoyl-electrostatic switches. The basic patch allows for favorable electrostatic interactions to occur between the negatively charged phospholipid heads of the membrane and the positive surface of the associating protein. This allows tighter association and directed localization of proteins.[5]
Myristoyl-conformational switches can come in several forms. Ligand bindingto a myristoylated protein with its myristoyl group sequestered can cause a conformational change in the protein, resulting in exposure of the myristoyl group. Similarly, some myristoylated proteins are activated not by a designated ligand, but by the exchange of GDP for GTP by guanine nucleotide exchange factors in the cell. Once GTP is bound to the myristoylated protein, it becomes activated, exposing the myristoyl group. These conformational switches can be utilized as a signal for cellular localization, membrane-protein, and protein–protein interactions.[5][12][13]
Dual modifications of myristoylated proteins[edit]
Further modifications on N-myristoylated proteins can add another level of regulation for myristoylated protein. Dual acylation can facilitate more tightly regulated protein localization, specifically targeting proteins to lipid rafts at membranes[14] or allowing dissociation of myristoylated proteins from membranes.
Myristoylation and palmitoylation are commonly coupled modifications.
Signal transduction[edit]
Myristoylation plays a vital role in membrane targeting and signal transduction[15] in plant responses to environmental stress. In addition, in signal transduction via G protein, palmitoylation of the α subunit, prenylation of the γ subunit, and myristoylation is involved in tethering the G protein to the inner surface of the plasma membrane so that the G protein can interact with its receptor.[16]
Apoptosis[edit]
Myristoylation is an integral part of apoptosis, or programmed cell death.



No comments:
Post a Comment