In chemistry, acylation (or alkanoylation) is the process of adding an acyl group to a compound. The compound providing the acyl group is called the acylating agent.
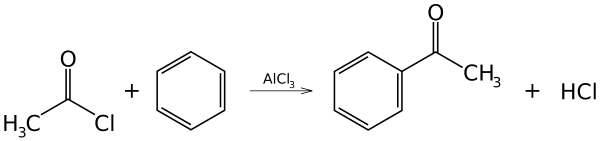
Because they form a strong electrophile when treated with some metal catalysts, acyl halides are commonly used as acylating agents. For example, Friedel-Crafts acylation uses acetyl chloride (ethanoyl chloride), CH3COCl, as the agent and aluminum chloride (AlCl3) as a catalyst to add an ethanoyl (acetyl) group to benzene:
The mechanism of this reaction is electrophilic aromatic substitution.
Acyl halides and acid anhydrides of carboxylic acids are also commonly used acylating agents. In some cases, active esters exhibit comparable reactivity. All react with amines to form amides and alcohols to form esters by nucleophilic acyl substitution.
Acylation can be used to prevent rearrangement reactions that would normally occur in alkylation. To do this an acylation reaction is performed, then the carbonyl is removed by Clemmensen reduction or a similar process.[1]
Acylation in biology[edit]
Protein acylation is the post-translational modification of proteins via the attachment of functional groups through acyl linkages. Protein acylation has been observed as a mechanism controlling biological signaling.[2] One prominent type is fatty acylation, the addition of fatty acids to particular amino acids (e.g. myristoylation, palmitoylation or palmitoleoylation).[3] Different types of fatty acids engage in global protein acylation.[4] Palmitoleoylation is an acylation type where the monounsaturated fatty acid palmitoleic acid is covalently attached to serine or threonine residues of proteins.[5][6] Palmitoleoylation appears to play a significant role in trafficking and targeting and function of Wnt proteins.[7][8]
See also[edit]
https://en.wikipedia.org/wiki/Acylation
No comments:
Post a Comment