The Hill reaction is the light-driven transfer of electrons from water to Hill reagents (non-physiological oxidants) in a direction against the chemical potential gradient as part of photosynthesis. Robin Hill discovered the reaction in 1937. He demonstrated that the process by which plants produce oxygen is separate from the process that converts carbon dioxide to sugars.
Natural electron acceptor[edit]
During photosynthesis, natural electron acceptor NADP is reduced to NADPH in chloroplasts.[5] The following equilibrium reaction takes place.
A reduction reaction that stores energy as NADPH:
- (Reduction)
An oxidation reaction as NADPH's energy is used elsewhere:
- (Oxidation)
Ferredoxin, also known as a NADH+ reductase, is an enzyme that catalyzes the reduction reaction. It is easy to oxidize NADPH but difficult to reduce NADP+, hence a catalyst is beneficial. Cytochromes are conjugate proteins that contain a haem group.[5] The iron atom from this group undergoes redox reactions:
- (Reduction)
- (Oxidation)
The light-dependent redox reaction takes place before the light-independent reaction in photosynthesis.[6]
https://en.wikipedia.org/wiki/Hill_reaction
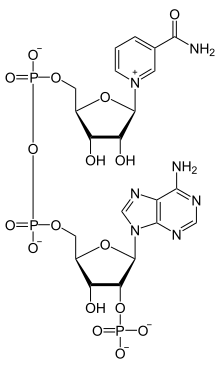
Nicotinamide adenine dinucleotide phosphate, abbreviated NADP+ or, in older notation, TPN (triphosphopyridine nucleotide), is a cofactor used in anabolic reactions, such as the Calvin cycle and lipid and nucleic acid syntheses, which require NADPH as a reducing agent. It is used by all forms of cellular life.[1]
NADPH is the reduced form of NADP+. NADP+ differs from NAD+ by the presence of an additional phosphate group on the 2' position of the ribose ring that carries the adenine moiety. This extra phosphate is added by NAD+ kinase and removed by NADP+ phosphatase.[2]
https://en.wikipedia.org/wiki/Nicotinamide_adenine_dinucleotide_phosphate






No comments:
Post a Comment