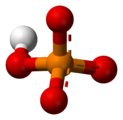
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid H
3PO
4.
The phosphate or orthophosphate ion [PO
4]3−
is derived from phosphoric acid by the removal of three protons H+
. Removal of one or two protons gives the dihydrogen phosphate ion [H
2PO
4]−
and the hydrogen phosphate ion [HPO
4]2−
ion, respectively. These names are also used for salts of those anions, such as ammonium dihydrogen phosphate and trisodium phosphate.
In organic chemistry, phosphate or orthophosphate is an organophosphate, an ester of orthophosphoric acid of the form PO
4RR′R″ where one or more hydrogen atoms are replaced by organic groups. An example is trimethyl phosphate, (CH
3)
3PO
4. The term also refers to the trivalent functional group OP(O-)
3 in such esters.
Orthophosphates are especially important among the various phosphates because of their key roles in biochemistry, biogeochemistry, and ecology, and their economic importance for agriculture and industry.[2] The addition and removal of phosphate groups (phosphorylation and dephosphorylation) are key steps in cell metabolism.
Orthophosphates can condense to form pyrophosphates.
[edit]
- Pyrophosphate – (P
2O
7)4− - Polyphosphate – (HPO
3)
n - Metaphosphate – (POn
3) - Fertilizer
- Hypophosphite – H
2(PO
2)− - Organophosphorus compounds
- Phosphate – OP(OR)3, such as triphenyl phosphate
- Phosphate conversion coating
- Phosphate soda, a soda fountain beverage
- Phosphinate – OP(OR)R2
- Phosphine – PR3
- Phosphine oxide – OPR3
- Phosphinite – P(OR)R2
- Phosphite – P(OR)3
- Phosphogypsum
- Phosphonate – OP(OR)2R
- Phosphonite – P(OR)2R
- Phosphorylation
- Diammonium phosphate - (NH4)2HPO4
- Disodium phosphate – Na2HPO4
- Monosodium phosphate – NaH2PO4
- Sodium tripolyphosphate – Na5P3O10
- Ouled Abdoun Basin
Monoamine oxidases (MAO) (EC 1.4.3.4) are a family of enzymes that catalyzethe oxidation of monoamines, employing oxygen to clip off their amine group.[1][2] They are found bound to the outer membrane of mitochondria in most cell types of the body. The first such enzyme was discovered in 1928 by Mary Bernheim in the liver and was named tyramine oxidase.[3][4] The MAOs belong to the protein family of flavin-containing amine oxidoreductases.
MAOs are important in the breakdown of monoamines ingested in food, and also serve to inactivate monoamine neurotransmitters. Because of the latter, they are involved in a number of psychiatric and neurological diseases, some of which can be treated with monoamine oxidase inhibitors (MAOIs) which block the action of MAOs.[5]
https://en.wikipedia.org/wiki/Monoamine_oxidase
Oxidative phosphorylation (UK /ɒkˈsɪd.ə.tɪv/, US /ˈɑːk.sɪˌdeɪ.tɪv/ [1]) or electron transport-linked phosphorylation or terminal oxidation is the metabolic pathway in which cells use enzymes to oxidize nutrients, thereby releasing the chemical energy stored within the nutrients in order to produce adenosine triphosphate (ATP). In eukaryotes, this takes place inside mitochondria. Almost all aerobic organisms carry out oxidative phosphorylation. This pathway is so pervasive because it releases more energy than alternative fermentation processes such as anaerobicglycolysis.[2]
The energy stored in the chemical bonds of glucose, ultimately derived from food, is released by the cell in the citric acid cycleproducing carbon dioxide, and the energetic electron donorsNADH and FADH. Oxidative phosphorylation uses these molecules to produce ATP, which is used throughout the cell whenever energy is needed. During oxidative phosphorylation, electrons are transferred from the electron donors to a series of electron acceptors in a series of redox reactions ending in oxygen as the last acceptor.
In eukaryotes, these redox reactions are catalyzed by a series of protein complexes within the inner membrane of the cell's mitochondria, whereas, in prokaryotes, these proteins are located in the cell's outer membrane. These linked sets of proteins are called the electron transport chain. In eukaryotes, five main protein complexes are involved, whereas in prokaryotes many different enzymes are present, using a variety of electron donors and acceptors.
The energy transferred by electrons flowing through this electron transport chain is used to transport protons across the inner mitochondrial membrane, in a process called electron transport. This generates potential energy in the form of a pH gradient and an electrical potential across this membrane. This store of energy is tapped when protons flow back across the membrane and down the potential energy gradient, through a large enzyme called ATP synthase in a process called chemiosmosis. The ATP synthase uses the energy to transform adenosine diphosphate (ADP) into adenosine triphosphate, in a phosphorylation reaction. The reaction is driven by the proton flow, which forces the rotation of a part of the enzyme. The ATP synthase is a rotary mechanical motor.
Although oxidative phosphorylation is a vital part of metabolism, it produces reactive oxygen species such as superoxide and hydrogen peroxide, which lead to propagation of free radicals, damaging cells and contributing to disease and, possibly, aging and senescence. The enzymes carrying out this metabolic pathway are also the target of many drugs and poisons that inhibit their activities.
https://en.wikipedia.org/wiki/Oxidative_phosphorylation
ATP synthase is a protein that catalyzes the formation of the energy storage molecule adenosine triphosphate (ATP) using adenosine diphosphate (ADP) and inorganic phosphate (Pi). It is classified under ligases as it changes ADP by the formation of P-O bond (phosphodiester bond). The overall reaction catalyzed by ATP synthase is:
- ADP + Pi + 2H+out ⇌ ATP + H2O + 2H+in
The formation of ATP from ADP and Pi is energetically unfavorable and would normally proceed in the reverse direction. In order to drive this reaction forward, ATP synthase couples ATP synthesis during cellular respiration to an electrochemical gradient created by the difference in proton (H+) concentration across the inner mitochondrial membrane in eukaryotes or the plasma membrane in bacteria. During photosynthesis in plants, ATP is synthesized by ATP synthase using a proton gradient created in the thylakoid lumen through the thylakoid membrane and into the chloroplast stroma.
Eukaryotic ATP synthases are F-ATPases, running "in reverse" for an ATPase. This article deals mainly with this type. An F-ATPase consists of two main subunits, FO and F1, which has a rotational motor mechanism allowing for ATP production.[1][2] ATP synthase is a molecular machine.
https://en.wikipedia.org/wiki/ATP_synthase
Carbamoyl phosphate synthetase I is a ligase enzyme located in the mitochondriainvolved in the production of urea. Carbamoyl phosphate synthetase I (CPS1 or CPSI) transfers an ammonia molecule from glutamine or glutamate to a molecule of bicarbonate that has been phosphorylated by a molecule of ATP. The resulting carbamate is then phosphorylated with another molecule of ATP. The resulting molecule of carbamoyl phosphate leaves the enzyme.
https://en.wikipedia.org/wiki/Carbamoyl_phosphate_synthetase_I
In chemistry a phosphite ester or organophosphite usually refers to an organophosphorous compound with the formula P(OR)3. They can be considered as estersof an unobserved tautomer phosphorous acid, H3PO3, with the simplest example being trimethylphosphite, P(OCH3)3. Some phosphites can be considered esters of the dominant tautomer of phosphorous acid (HP(O)(OH)2). The simplest representative is dimethylphosphite with the formula HP(O)(OCH3)2. Both classes of phosphites are usually colorless liquids.
https://en.wikipedia.org/wiki/Phosphite_ester
A phosphite anion or phosphite in inorganic chemistry usually refers to [HPO3]2− but includes [H2PO3]− ([HPO2(OH)]−). These anions are the conjugate bases of phosphorous acid (H3PO3). The corresponding salts, e.g. sodium phosphite(Na2HPO3) are reducing in character.
https://en.wikipedia.org/wiki/Phosphite_anion
Mitochondrial proteins
Outer membrane
fatty acid degradation
Carnitine palmitoyltransferase I Long-chain-fatty-acid—CoA ligase
tryptophan metabolism
Kynureninase
monoamine neurotransmitter
metabolism
Monoamine oxidase
Intermembrane space
Adenylate kinase Creatine kinase
Inner membrane
oxidative phosphorylation
Coenzyme Q – cytochrome c reductase Cytochrome c NADH dehydrogenase Succinate dehydrogenase
pyrimidine metabolism
Dihydroorotate dehydrogenase
mitochondrial shuttle
Malate-aspartate shuttle Glycerol phosphate shuttle
steroidogenesis
Cholesterol side-chain cleavage enzyme Steroid 11-beta-hydroxylase Aldosterone synthase
other
Glutamate aspartate transporter Glycerol-3-phosphate dehydrogenase ATP synthase Carnitine palmitoyltransferase II Uncoupling protein
Matrix
citric acid cycle
Citrate synthase Aconitase Isocitrate dehydrogenase Oxoglutarate dehydrogenase complex Succinyl coenzyme A synthetase Fumarase Malate dehydrogenase
anaplerotic reactions
Aspartate transaminase Glutamate dehydrogenase Pyruvate dehydrogenase complex
urea cycle
Carbamoyl phosphate synthetase I Ornithine transcarbamylase N-Acetylglutamate synthase
alcohol metabolism
ALDH2
PMPCB
Other/to be sorted
Frataxin Mitochondrial membrane transport protein Mitochondrial permeability transition pore Mitochondrial carrier
Mitochondrial DNA
Complex I
MT-ND1 MT-ND2 MT-ND3 MT-ND4 MT-ND4L MT-ND5 MT-ND6
Complex III
MT-CYB
Complex IV
MT-CO1 MT-CO2 MT-CO3
ATP synthase
MT-ATP6 MT-ATP8
tRNA
MT-TA MT-TC MT-TD MT-TE MT-TF MT-TG MT-TH MT-TI MT-TK MT-TL1 MT-TL2 MT-TM MT-TN MT-TP MT-TQ MT-TR MT-TS1 MT-TS2 MT-TT MT-TV MT-TW MT-TY
see also mitochondrial diseases
Categories: Cell anatomy Matrices (biology)
https://en.wikipedia.org/wiki/Mitochondrial_matrix
Nicotine, Adenine, Phosphorous, propylene glycol
Hydrogen,
Oxygen,
Thymine
glycine glycerols
https://biocyc.org/META/substring-search?type=NIL&object=1.4.3.4
In humans there are two types of MAO: MAO-A and MAO-B.[6]
https://en.wikipedia.org/wiki/Monoamine_oxidase
https://en.wikipedia.org/wiki/Category:Glassforming_liquids_and_melts
https://en.wikipedia.org/wiki/Category:Triols
https://en.wikipedia.org/wiki/Nitroglycerin
https://en.wikipedia.org/wiki/Glyceraldehyde_3-phosphate
https://en.wikipedia.org/wiki/Glycerol_3-phosphate
https://en.wikipedia.org/wiki/3-Phosphoglyceric_acid
sn-Glycerol 3-phosphate[1] is a phosphoric ester of glycerol, which is a component of glycerophospholipids...It should not be confused with the similarly named glycerate 3-phosphate or glyceraldehyde 3-phosphate.
https://en.wikipedia.org/wiki/Glycerol_3-phosphate
3-Phosphoglyceric acid (3PG, 3-PGA, or PGA) is the conjugate acid of 3-phosphoglycerate or glycerate 3-phosphate (GP or G3P)
https://en.wikipedia.org/wiki/3-Phosphoglyceric_acid
3-Phosphoglyceric acid (3PG, 3-PGA, or PGA) is the conjugate acid of 3-phosphoglycerate or glycerate 3-phosphate (GP or G3P).[1] This glycerate is a biochemically significant metabolic intermediate in both glycolysis and the Calvin-Benson cycle. The anion is often termed as PGA when referring to the Calvin-Benson cycle. In the Calvin-Benson cycle, 3-phosphoglycerate is typically the product of the spontaneous scission of an unstable 6-carbon intermediate formed upon CO2 fixation. Thus, two equivalents of 3-phosphoglycerate are produced for each molecule of CO2that is fixed.[2][3][4] In glycolysis, 3-phosphoglycerate is an intermediate following the dephosphorylation (reduction) of 1,3-bisphosphoglycerate.[4]:14

IUPAC name
(2R)-2-Hydroxy-3-phosphonooxypropanoic acid
https://en.wikipedia.org/wiki/3-Phosphoglyceric_acid
sn-Glycerol 3-phosphate[1] is a phosphoric ester of glycerol, which is a component of glycerophospholipids. Equally appropriate names in biochemical context include glycerol-3-phosphate, 3-O-phosphonoglycerol, 3-phosphoglycerol;[2] and Gro3P.[3] From a historical reason, it is also known as L-glycerol 3-phosphate, D-glycerol 1-phosphate, L-α-glycerophosphoric acid.[2] It should not be confused with the similarly named glycerate 3-phosphate or glyceraldehyde 3-phosphate.

Preferred IUPAC name
(2R)-2,3-Dihydroxypropyl dihydrogen phosphate
Other names
1,2,3-propanetriol, 1-(dihydrogen phosphate), (2R)-
D-glycerol 1-phosphate
L-glycerol 3-phosphate
L-α-glycerophosphate
L-α-phosphoglycerol
https://en.wikipedia.org/wiki/Glycerol_3-phosphate
Not to be confused with glycerol 3-phosphate.
Glyceraldehyde 3-phosphate, also known as triose phosphate or 3-phosphoglyceraldehyde and abbreviated as G3P, GA3P, GADP, GAP, TP, GALPor PGAL, is the metabolite that occurs as an intermediate in several central pathways of all organisms.[2][3] With the chemical formula H(O)CCH(OH)CH2OPO32-, this anion is a monophosphate ester of glyceraldehyde.

IUPAC name
2-hydroxy-3-oxopropyl dihydrogen phosphate
https://en.wikipedia.org/wiki/Glyceraldehyde_3-phosphate
Advanced glycation end products (AGEs) are proteins or lipids that become glycated as a result of exposure to sugars.[1] They are a bio-marker implicated in aging and the development, or worsening, of many degenerative diseases, such as diabetes, atherosclerosis, chronic kidney disease, and Alzheimer's disease.[2]
Pathology[edit]
AGEs have a range of pathological effects, such as:[22][23]
- Increased vascular permeability.
- Increased arterial stiffness
- Inhibition of vascular dilation by interfering with nitric oxide.
- Oxidizing LDL.
- Binding cells—including macrophage, endothelial, and mesangial—to induce the secretion of a variety of cytokines.
- Enhanced oxidative stress.
- Hemoglobin-AGE levels are elevated in diabetic individuals[24] and other AGE proteins have been shown in experimental models to accumulate with time, increasing from 5-50 fold over periods of 5–20 weeks in the retina, lens and renal cortex of diabetic rats. The inhibition of AGE formation reduced the extent of nephropathy in diabetic rats.[25] Therefore, substances that inhibit AGE formation may limit the progression of disease and may offer new tools for therapeutic interventions in the therapy of AGE-mediated disease.[26][27]
- AGEs have specific cellular receptors; the best-characterized are those called RAGE. The activation of cellular RAGE on endothelium, mononuclear phagocytes, and lymphocytes triggers the generation of free radicals and the expression of inflammatory gene mediators.[28] Such increases in oxidative stress lead to the activation of the transcription factor NF-κB and promote the expression of NF-κB regulated genes that have been associated with atherosclerosis.[26]
Reactivity[edit]
Proteins are usually glycated through their lysine residues.[29] In humans, histones in the cell nucleus are richest in lysine, and therefore form the glycated protein N(6)-Carboxymethyllysine (CML).[29]
A receptor nicknamed RAGE, from receptor for advanced glycation end products, is found on many cells, including endothelial cells, smooth muscle, cells of the immune system[which?] from tissue such as lung, liver, and kidney.[clarification needed][which?] This receptor, when binding AGEs, contributes to age- and diabetes-related chronic inflammatory diseases such as atherosclerosis, asthma, arthritis, myocardial infarction, nephropathy, retinopathy, periodontitis and neuropathy.[30] The pathogenesis of this process hypothesized to activation of the nuclear factor kappa B (NF-κB) following AGE binding. NF-κB controls several genes which are involved in inflammation.[citation needed]
Clearance[edit]
In clearance, or the rate at which a substance is removed or cleared from the body, it has been found that the cellular proteolysis of AGEs—the breakdown of proteins—produces AGE peptides and "AGE free adducts" (AGE adducts bound to single amino acids). These latter, after being released into the plasma, can be excreted in the urine.[31]
Nevertheless, the resistance of extracellular matrix proteins to proteolysis renders their advanced glycation end products less conducive to being eliminated.[31] While the AGE free adducts are released directly into the urine, AGE peptides are endocytosed by the epithelial cells of the proximal tubule and then degraded by the endolysosomal systemto produce AGE amino acids. It is thought that these acids are then returned to the kidney's inside space, or lumen, for excretion. [22] AGE free adducts are the major form through which AGEs are excreted in urine, with AGE-peptides occurring to a lesser extent[22] but accumulating in the plasma of patients with chronic kidney failure.[31]
Larger, extracellularly derived AGE proteins cannot pass through the basement membrane of the renal corpuscle and must first be degraded into AGE peptides and AGE free adducts. Peripheral macrophage[22] as well as liver sinusoidal endothelial cells and Kupffer cells [32] have been implicated in this process, although the real-life involvement of the liver has been disputed. [33]
Large AGE proteins unable to enter the Bowman's capsule are capable of binding to receptors on endothelial and mesangial cells and to the mesangial matrix.[22] Activation of RAGE induces production of a variety of cytokines, including TNFβ, which mediates an inhibition of metalloproteinase and increases production of mesangial matrix, leading to glomerulosclerosis[23] and decreasing kidney function in patients with unusually high AGE levels.
Although the only form suitable for urinary excretion, the breakdown products of AGE—that is, peptides and free adducts—are more aggressive than the AGE proteins from which they are derived, and they can perpetuate related pathology in diabetic patients, even after hyperglycemia has been brought under control.[22]
Some AGEs have an innate catalytic oxidative capacity, while activation of NAD(P)H oxidase through activation of RAGE and damage to mitochondrial proteins leading to mitochondrial dysfunction can also induce oxidative stress. A 2007 in vitro study found that AGEs could significantly increase expression of TGF-β1, CTGF, Fn mRNA in NRK-49F cells through enhancement of oxidative stress, and suggested that inhibition of oxidative stress might underlie the effect of ginkgo biloba extract in diabetic nephropathy. The authors suggested that antioxidant therapy might help prevent the accumulation of AGEs and induced damage.[23] In the end, effective clearance is necessary, and those suffering AGE increases because of kidney dysfunction might require a kidney transplant.[22]
In diabetics who have an increased production of an AGE, kidney damage reduces the subsequent urinary removal of AGEs, forming a positive feedback loop that increases the rate of damage. In a 1997 study, diabetic and healthy subjects were given a single meal of egg white (56 g protein), cooked with or without 100 g of fructose; there was a greater than 200-fold increase in AGE immunoreactivity from the meal with fructose.[34]
Compounds that are thought to break some existing AGE crosslinks include Alagebrium (and related ALT-462, ALT-486, and ALT-946)[46] and N-phenacyl thiazolium bromide.[47] One in vitro study shows that rosmarinic acid out performs the AGE breaking potential of ALT-711.[48]
Compounds that have been found to inhibit AGE formation in the laboratory include Vitamin C, Agmatine, benfotiamine, pyridoxamine, alpha-lipoic acid,[35][36] taurine,[37] pimagedine,[38] aspirin,[39][40] carnosine,[41]metformin,[42] pioglitazone,[42] and pentoxifylline.[42] Activation of the TRPA-1 receptor by lipoic acid or podocarpic acid has been shown to reduce the levels of AGES by enhancing the detoxification of methylglyoxal, a major precursor of several AGEs.[43]
See also[edit]
https://en.wikipedia.org/wiki/Advanced_glycation_end-product
Alagebrium (formerly known as ALT-711) was a drug candidate developed by Alteon, Inc. It was the first drug candidate to be clinically tested for the purpose of breaking the crosslinks caused by advanced glycation endproducts (AGEs), thereby reversing one of the main mechanisms of aging.[1] Through this effect Alagebrium is designed to reverse the stiffening of blood vessel walls that contributes to hypertension and cardiovascular disease, as well as many other forms of degradation associated with protein crosslinking.[2][3] Alagebrium has proven effective in reducing systolic blood pressure[4] and providing therapeutic benefit for patients with diastolic heart failure.[5]
https://en.wikipedia.org/wiki/Alagebrium
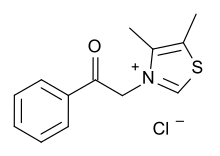
N-Phenacylthiazolium bromide (PTB)[1] is a cross-link breaker that in one study has been shown to prevent vascular advanced glycation end-product accumulation in diabetic rats.[2]

Names
Preferred IUPAC name
3-(2-Oxo-2-phenylethyl)-1,3-thiazol-3-ium bromide
https://en.wikipedia.org/wiki/N-Phenacylthiazolium_bromide
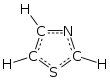
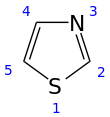
Thiazole, or 1,3-thiazole, is a heterocyclic compound that contains both sulfur and nitrogen; the term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the molecular formula C3H3NS.[2] The thiazole ring is notable as a component of the vitamin thiamine (B1).
Names
Preferred IUPAC name
1,3-Thiazole
Other names
Thiazole
https://en.wikipedia.org/wiki/Thiazole
Azoles are a class of five-membered heterocyclic compounds containing a nitrogen atom and at least one other non-carbon atom (i.e. nitrogen, sulfur, or oxygen) as part of the ring.[1] Their names originate from the Hantzsch–Widman nomenclature. The parent compounds are aromatic and have two double bonds; there are successively reduced analogs (azolines and azolidines) with fewer. One, and only one, lone pair of electrons from each heteroatom in the ring is part of the aromatic bonding in an azole. Names of azoles maintain the prefix upon reduction (e.g., pyrazoline, pyrazolidine). The numbering of ring atoms in azoles starts with the heteroatom that is not part of a double bond, and then proceeds towards the other heteroatom.
Imidazole and other five-membered aromatic heterocyclic systems with two nitrogens are extremely common in nature and form the core of many biomolecules, such as histidine.
The search for antifungal agents with acceptable toxicity profiles led first to the discovery of ketoconazole, the first azole-based oral treatment of systemic fungal infections, in the early 1980s. Later, triazoles fluconazole and itraconazole, with a broader spectrum of antifungal activity and improved safety profile were developed. In order to overcome limitations such as sub-optimal spectra of activity, drug-drug interactions, toxicity, development of resistance and unfavorable pharmacokinetics, analogues were developed. Second-generation triazoles, including voriconazole, posaconazole and ravuconazole, are more potent and more active against resistant pathogens.[2]
https://en.wikipedia.org/wiki/Azole
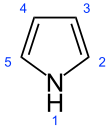
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4H4NH.[3] It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3. Porphobilinogen, a trisubstituted pyrrole, is the biosynthetic precursor to many natural products such as heme.[4]
Pyrroles are components of more complex macrocycles, including the porphyrinogens and products derived therefrom, including porphyrins of heme, the chlorins, bacteriochlorins, and chlorophylls.[5]

Names
Preferred IUPAC name
1H-Pyrrole[2]
Other names
Azole
Imidole[1]
Pyrrole was first detected by F. F. Runge in 1834, as a constituent of coal tar.[7] In 1857, it was isolated from the pyrolysate of bone. Its name comes from the Greek pyrrhos (πυρρός, “reddish, fiery”), from the reaction used to detect it—the red color that it imparts to wood when moistened with hydrochloric acid.[8]
https://en.wikipedia.org/wiki/Pyrrole
Porphobilinogen (PBG) is an organic compound that occurs in living organisms as an intermediate in the biosynthesis of porphyrins, which include critical substances like hemoglobin and chlorophyll.[1]
The structure of the molecule can be described as molecule of pyrrole with sidechains substituted for hydrogen atoms at positions 2, 3 and 4 in the ring (1 being the nitrogenatom); respectively, an aminomethyl group −CH
2−NH
2, an acetic acid (carboxymethyl) group −CH
2−COOH, and a propionic acid (carboxyethyl) group −CH
2−CH
2−COOH.
https://en.wikipedia.org/wiki/Porphobilinogen
δ-Aminolevulinic acid (also dALA, δ-ALA, 5ALA or 5-aminolevulinic acid), an endogenous non-proteinogenic amino acid, is the first compound in the porphyrin synthesis pathway, the pathway that leads to heme[1] in mammals, as well as chlorophyll[2] in plants.
5ALA is used in photodynamic detection and surgery of cancer.[3][4][5][6]
Intracellular chemotherapeutic agents / antineoplastic agents (L01)
showvte
Heme metabolic intermediates
showvte
Non-proteinogenic amino acids
Medicine portal
Categories: Biomolecules Amines Carboxylic acids Antineoplastic drugs Light therapy
Non-proteinogenic amino acids
https://en.wikipedia.org/wiki/Aminolevulinic_acid
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromatic as indicated by its extensive substitution reactions. It is a colorless liquid with a benzene-like odor. In most of its reactions, it resembles benzene. Compounds analogous to thiophene include furan (C4H4O) selenophene(C4H4Se) and pyrrole (C4H4NH), which each vary by the heteroatom in the ring.
https://en.wikipedia.org/wiki/Thiophene
Pyrrole has a nutty odor. Pyrrole is a 5-membered aromatic heterocycle, like furan and thiophene.
https://en.wikipedia.org/wiki/Pyrrole
Cyclization reactions[edit]
Pyrroles with N-substitution can undergo cycloaddition reactions such as [4+2]-, [2+2]-, and [2+1]-cyclizations. Diels-Alder cyclizations can occur with the pyrrole acting as a diene, especially in the presence of an electron-withdrawing group on the nitrogen. Vinylpyrroles can also act as dienes.[citation needed]
Pyrrole DA
Pyrroles can react with carbenes, such as dichlorocarbene, in a [2+1]-cycloaddition. With dichlorocarbene, a dichlorocyclopropane intermediate is formed, which breaks down to form 3-chloropyridine (the Ciamician–Dennstedt rearrangement).[28][29][30]
Ciamician–Dennstedt rearrangement
Commercial uses[edit]
Polypyrrole is of some commercial value. N-Methylpyrrole is a precursor to N-methylpyrrolecarboxylic acid, a building-block in pharmaceutical chemistry.[8] Pyrroles are also found in several drugs, including atorvastatin, ketorolac, and sunitinib. Pyrroles are used as lightfast red, scarlet, and carmine pigments.[31][32]
Analogs and derivatives[edit]
Structural analogs of pyrrole include:
Pyrroline, a partially saturated analog with one double bond
Pyrrolidine, the saturated hydrogenated analog
Derivatives of pyrrole include indole, a derivative with a fused benzene ring.
See also[edit]
Wikimedia Commons has media related to Pyrrole.
Simple aromatic rings
Tetrapyrrole
Polypyrrole
Azonine
Piloty–Robinson pyrrole synthesis[edit]
The starting materials in the Piloty–Robinson pyrrole synthesis, named for Gertrude and Robert Robinson and Oskar Piloty, are two equivalents of an aldehyde and hydrazine.[20][21] The product is a pyrrole with substituents at the 3 and 4 positions. The aldehyde reacts with the diamine to an intermediate di-imine (R–C=N−N=C–R). In the second step, a [3,3]-sigmatropic rearrangement takes place between. Addition of hydrochloric acid leads to ring closure and loss of ammonia to form the pyrrole. The mechanism was developed by the Robinsons.
In one modification, propionaldehyde is treated first with hydrazine and then with benzoyl chloride at high temperatures and assisted by microwave irradiation:[22]
https://en.wikipedia.org/wiki/Pyrrole#Cyclization_reactions
Propionic acid (/proʊpiˈɒnɪk/, from the Greek words protos, meaning "first", and pion, meaning "fat"; also known as propanoic acid) is a naturally occurring carboxylic acidwith chemical formula CH3CH2CO2H. It is a liquid with a pungent and unpleasant smell somewhat resembling body odor. The anion CH3CH2CO2− as well as the saltsand esters of propionic acid are known as propionates or propanoates.
https://en.wikipedia.org/wiki/Propionic_acid
Mucic acid, C6H10O8 or HOOC-(CHOH)4-COOH (also known as galactaric or meso-galactaric acid) is an aldaric acid obtained by nitric acid oxidation of galactose or galactose-containing compounds such as lactose, dulcite, quercite, and most varieties of gum.[1]
https://en.wikipedia.org/wiki/Mucic_acid
Other methods[edit]
One synthetic route to pyrrole involves the decarboxylation of ammonium mucate, the ammonium salt of mucic acid. The salt is typically heated in a distillation setup with glycerol as a solvent.[23]
https://en.wikipedia.org/wiki/Pyrrole
Glycolysis is the metabolic pathway that converts glucoseC6H12O6, into pyruvic acid, CH3COCOOH. The free energyreleased in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH).[1][2] Glycolysis is a sequence of ten reactions catalyzed by enzymes.
Glycolysis is a metabolic pathway that does not require oxygen. The wide occurrence of glycolysis in other species indicates that it is an ancient metabolic pathway.[3] Indeed, the reactions that make up glycolysis and its parallel pathway, the pentose phosphate pathway, occur in the oxygen-free conditions of the Archean oceans, also in the absence of enzymes, catalyzed by metal.[4]
In most organisms, glycolysis occurs in the liquid part of cells, the cytosol. The most common type of glycolysis is the Embden–Meyerhof–Parnas (EMP) pathway, which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Karol Parnas. Glycolysis also refers to other pathways, such as the Entner–Doudoroff pathway and various heterofermentative and homofermentative pathways. However, the discussion here will be limited to the Embden–Meyerhof–Parnas pathway.[5]
The glycolysis pathway can be separated into two phases:[1]
- Investment phase – wherein ATP is consumed
- Yield phase – wherein more ATP is produced than originally consumed
https://en.wikipedia.org/wiki/Glycolysis
Propionaldehyde or propanal is the organic compound with the formula CH3CH2CHO. It is the 3-carbon aldehyde. It is a colourless, flammable liquid with a slightly fruity odour. It is produced on a large scale industrially.
https://en.wikipedia.org/wiki/Propionaldehyde
In organic chemistry, chlorins are tetrapyrrole pigments that are partially hydrogenated porphyrins.[1] The parent chlorin is an unstable compound which undergoes air oxidation to porphine.
The name chlorin derives from chlorophyll. Chlorophylls are magnesium-containing chlorins and occur as photosynthetic pigments in chloroplasts. The reduced chlorin variants are present in bacteriochlorophylls and are named ‘bacteriochlorins’ and ‘isobacteriochlorins’.
https://en.wikipedia.org/wiki/Chlorin
Glycerol (/ˈɡlɪsərɒl/;[6] also called glycerine in British English or glycerin in American English) is a simple polyol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in lipids known as glycerides. Due to having antimicrobial and antiviral properties it is widely used in FDA approved wound and burn treatments. It can be used as an effective marker to measure liver disease. It is also widely used as a sweetener in the food industry and as a humectant in pharmaceutical formulations. Owing to the presence of three hydroxyl groups, glycerol is miscible with water and is hygroscopic in nature.[7]
https://en.wikipedia.org/wiki/Glycerol
Methylene blue, also known as methylthioninium chloride, is a salt used as a medication and dye.[4] As a medication, it is mainly used to treat methemoglobinemia.[4][2] Specifically, it is used to treat methemoglobin levels that are greater than 30% or in which there are symptoms despite oxygen therapy.[2] It has previously been used for cyanide poisoning and urinary tract infections, but this use is no longer recommended.[4] It is typically given by injection into a vein.[4]
Common side effects include headache, vomiting, confusion, shortness of breath, and high blood pressure.[4] Other side effects include serotonin syndrome, red blood cell breakdown, and allergic reactions.[4] Use often turns the urine, sweat, and stool blue to green in color.[2] While use during pregnancy may harm the baby, not using it in methemoglobinemia is likely more dangerous.[4][2] Methylene blue is a thiazine dye.[4]It works by converting the ferric iron in hemoglobin to ferrous iron.[2]
Methylene blue was first prepared in 1876, by Heinrich Caro.[5] It is on the World Health Organization's List of Essential Medicines.[6]
Not to be confused with methyl blue, new methylene blue, or methyl violet.
https://en.wikipedia.org/wiki/Methylene_blue
The glycine cleavage system (GCS) is also known as the glycine decarboxylase complex or GDC. The system is a series of enzymes that are triggered in response to high concentrations of the amino acid glycine.[1] The same set of enzymes is sometimes referred to as glycine synthase when it runs in the reverse direction to form glycine.[2] The glycine cleavage system is composed of four proteins: the T-protein, P-protein, L-protein, and H-protein. They do not form a stable complex,[3] so it is more appropriate to call it a "system" instead of a "complex". The H-protein is responsible for interacting with the three other proteins and acts as a shuttle for some of the intermediate products in glycine decarboxylation.[2] In both animals and plants the glycine cleavage system is loosely attached to the inner membrane of the mitochondria. Mutations in this enzymatic system are linked with glycine encephalopathy.[2]
Metabolism: Protein metabolism, synthesis and catabolism enzymes
Categories: Cellular respirationNADH-dependent enzymesEnzymes of unknown structure
https://en.wikipedia.org/wiki/Glycine_cleavage_system
- This enzyme is not to be confused with Bisphosphoglycerate mutase which catalyzes the conversion of 1,3-bisphosphoglycerate to 2,3-bisphosphoglycerate.
Phosphoglycerate mutase (PGM) is any enzyme that catalyzes step 8 of glycolysis. They catalyze the internal transfer of a phosphate group from C-3 to C-2 which results in the conversion of 3-phosphoglycerate (3PG) to 2-phosphoglycerate (2PG) through a 2,3-bisphosphoglycerate intermediate. These enzymes are categorized into the two distinct classes of either cofactor-dependent (dPGM) or cofactor-independent (iPGM).[1] The dPGM enzyme (EC 5.4.2.11) is composed of approximately 250 amino acids and is found in all vertebrates as well as in some invertebrates, fungi, and bacteria. The iPGM (EC 5.4.2.12) class is found in all plants and algae as well as in some invertebrate, fungi, and Gram-positive bacteria.[2] This class of PGM enzyme shares the same superfamily as alkaline phosphatase.[3]
https://en.wikipedia.org/wiki/Phosphoglycerate_mutase
1,3-Bisphosphoglyceric acid (1,3-Bisphosphoglycerate or 1,3BPG) is a 3-carbon organic molecule present in most, if not all, living organisms. It primarily exists as a metabolic intermediate in both glycolysis during respiration and the Calvin cycle during photosynthesis. 1,3BPG is a transitional stage between glycerate 3-phosphate and glyceraldehyde 3-phosphate during the fixation/reduction of CO2. 1,3BPG is also a precursor to 2,3-bisphosphoglycerate which in turn is a reaction intermediate in the glycolytic pathway.
https://en.wikipedia.org/wiki/1,3-Bisphosphoglyceric_acid
Phosphoglycerate kinase (EC 2.7.2.3) (PGK 1) is an enzyme that catalyzes the reversible transfer of a phosphate group from 1,3-bisphosphoglycerate (1,3-BPG) to ADP producing 3-phosphoglycerate (3-PG) and ATP :
- 1,3-bisphosphoglycerate + ADP ⇌ glycerate 3-phosphate + ATP
Like all kinases it is a transferase. PGK is a major enzyme used in glycolysis, in the first ATP-generating step of the glycolytic pathway. In gluconeogenesis, the reaction catalyzed by PGK proceeds in the opposite direction, generating ADP and 1,3-BPG.
In humans, two isozymes of PGK have been so far identified, PGK1 and PGK2. The isozymes have 87-88% identical amino acid sequence identity and though they are structurally and functionally similar, they have different localizations: PGK2, encoded by an autosomal gene, is unique to meiotic and postmeiotic spermatogenic cells, while PGK1, encoded on the X-chromosome, is ubiquitously expressed in all cells.[2]
https://en.wikipedia.org/wiki/Phosphoglycerate_kinase
Search: zero dipole (wikipedia)
Zero ionic layer is the main site of interaction in the core SNARE complex. Dipole-dipole interactions take place between 3 glutamine (Q) residues and 1 arginine (R) residue exposed in this layer. Despite that, the majority of the SNARE complex is hydrophobic because of the leucine zipper.[1] Extensively studied layers within the SNARE alpha-helical bundle are designated from "-7" to "+8". Zero ionic layer is at the center of the bundle, and thus designated as "0" layer.[2]
https://en.wikipedia.org/wiki/Zero_ionic_layer





![Piloty–Robinson reaction[22]](https://upload.wikimedia.org/wikipedia/commons/thumb/b/bd/Piloty-Robinson_reaction.png/400px-Piloty-Robinson_reaction.png)
No comments:
Post a Comment