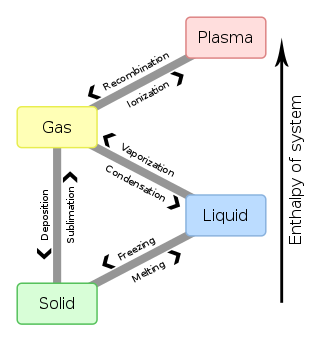
In chemistry, thermodynamics, and many other related fields, phase transitions (or phase changes) are the physical processes of transition between a state of a medium, identified by some parameters, and another one, with different values of the parameters. Commonly the term is used to refer to changes among the basic states of matter: solid, liquid, and gas, as well as plasma in rare cases.
For example, a phase of a thermodynamic system and the states of matter have uniform physical properties. During a phase transition of a given medium, certain properties of the medium change, often discontinuously, as a result of the change of external conditions, such as temperature, pressure, or others. For example, a liquid may become gas upon heating to the boiling point, resulting in an abrupt change in volume. The measurement of the external conditions at which the transformation occurs is termed the phase transition. Phase transitions commonly occur in nature and are used today in many technologies.
To From | Solid | Liquid | Gas | Plasma |
|---|---|---|---|---|
| Solid | Melting | Sublimation | ||
| Liquid | Freezing | Vaporization | ||
| Gas | Deposition | Condensation | Ionization | |
| Plasma | Recombination |
STP Surroundings/Environment (environment outside of system-surroundings or system surrounding environment (terminology, vocabulary, language, etc.), non controlled environment, etc.) on Earth, etc.; Levels of system, system nesting, control, surrounding variable, complexity, levels, dimensions, Earth, etc..
https://en.wikipedia.org/wiki/Phase_transition
In metallurgy and materials science, annealing is a heat treatment that alters the physical and sometimes chemical properties of a material to increase its ductility and reduce its hardness, making it more workable. It involves heating a material above its recrystallization temperature, maintaining a suitable temperature for an appropriate amount of time and then cooling.
In annealing, atoms migrate in the crystal lattice and the number of dislocations decreases, leading to a change in ductility and hardness. As the material cools it recrystallizes. For many alloys, including carbon steel, the crystal grain size and phase composition, which ultimately determine the material properties, are dependent on the heating rate and cooling rate. Hot working or cold working after the annealing process alters the metal structure, so further heat treatmentsmay be used to achieve the properties required. With knowledge of the composition and phase diagram, heat treatment can be used to adjust from harder and more brittle to softer and more ductile.
In the case of ferrous metals, such as steel, annealing is performed by heating the material (generally until glowing) for a while and then slowly letting it cool to room temperature in still air. Copper, silver and brass can be either cooled slowly in air, or quickly by quenching in water.[1] In this fashion, the metal is softened and prepared for further work such as shaping, stamping, or forming.
https://en.wikipedia.org/wiki/Annealing_(materials_science)
Boiling is the rapid vaporization of a liquid, which occurs when a liquid is heated to its boiling point, the temperature at which the vapour pressure of the liquid is equal to the pressure exerted on the liquid by the surrounding atmosphere. At sea level the boiling point of water is 100 °C or 212 °F but at higher altitudes it drops to correspond with decreasing atmospheric pressures.
Boiling water is used as a method of making it potable by killing microbes and viruses that may be present. The sensitivity of different micro-organisms to heat varies. But if water is held at 100 °C (212 °F) for one minute, most micro-organisms and viruses are inactivated. Ten minutes at a temperature of 70 °C (158 °F) is also sufficient for most bacteria.
Boiling water is also used in several cooking methods including boiling, steaming and poaching.
https://en.wikipedia.org/wiki/Boiling
Nucleate boiling is a type of boiling that takes place when the surface temperature is hotter than the saturated fluid temperature by a certain amount but where the heat flux is below the critical heat flux. For water, as shown in the graph below, nucleate boiling occurs when the surface temperature is higher than the saturation temperature (TS) by between 10 and 30 °C (18 and 54 °F). The critical heat flux is the peak on the curve between nucleate boiling and transition boiling. The heat transfer from surface to liquid is greater than that in film boiling.
Nucleate boiling is common in electric kettles and is responsible for the noise that occurs before boiling occurs. It also occurs in water boilers where water is rapidly heated.
https://en.wikipedia.org/wiki/Nucleate_boiling
The Leidenfrost effect is a physical phenomenon in which a liquid, close to a surface that is significantly hotter than the liquid's boiling point, produces an insulating vapor layer that keeps the liquid from boiling rapidly. Because of this 'repulsive force', a droplet hovers over the surface rather than making physical contact with the hot surface.
This is most commonly seen when cooking, when a few drops of water are sprinkled in a hot pan. If the pan's temperature is at or above the Leidenfrost point, which is approximately 193 °C (379 °F) for water, the water skitters across the pan and takes longer to evaporate than it would take if the water droplets had been sprinkled into a cooler pan.
The effect is named after the German doctor Johann Gottlob Leidenfrost, who described it in A Tract About Some Qualities of Common Water in 1751.
https://en.wikipedia.org/wiki/Leidenfrost_effect
Melting, or fusion, is a physical process that results in the phase transition of a substance from a solid to a liquid. This occurs when the internal energy of the solid increases, typically by the application of heat or pressure, which increases the substance's temperature to the melting point. At the melting point, the ordering of ions or molecules in the solid breaks down to a less ordered state, and the solid "melts" to become a liquid.
Substances in the molten state generally have reduced viscosity as the temperature increases. An exception to this principle is the element sulfur, whose viscosity increases in the range of 160 °C to 180 °C due to polymerization.[1]
Some organic compounds melt through mesophases, states of partial order between solid and liquid.
https://en.wikipedia.org/wiki/Melting
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks.[2][3][4] There are many forms of polymerization and different systems exist to categorize them.
https://en.wikipedia.org/wiki/Polymerization
Pressurisation duct work is a passive fire protection system. It is used to supply fresh air to any area of refuge, designated emergency evacuation or egress route.
https://en.wikipedia.org/wiki/Pressurisation_ductwork
Pressurization or pressurisation is the application of pressure in a given situation or environment.
Industrial[edit]
Industrial equipment is often maintained at pressures above or below atmospheric.
Atmospheric[edit]
This is the process by which atmospheric pressure is maintained in an isolated or semi-isolated atmospheric environment (for instance, in an aircraft, or whilst scuba diving).
See also[edit]
https://en.wikipedia.org/wiki/Pressurization
A countdown is a sequence of backward counting to indicate the time remaining before an event is scheduled to occur. NASA commonly employs the terms "L-minus" and "T-minus" during the preparation for and anticipation of a rocket launch,[1] and even "E-minus" for events that involve spacecraft that are already in space, where the "T" could stand for "Test" or "Time", and the "E" stands for "Encounter", as with a comet or some other space object.[2] Other events for which countdowns are commonly used include the detonation of an explosive, the start of a race, the start of the New Year, or any anxiously anticipated event. An early use of a countdown once signaled the start of a Cambridge Universityrowing race.[3] One of the first known associations with rockets was in the 1929 German science fiction movie Frau im Mond (English: Woman in the Moon) written by Thea von Harbou and directed by Fritz Lang in an attempt to increase the drama of the launch sequence of the story's lunar-bound rocket.[4][5]
https://en.wikipedia.org/wiki/Countdown
A compressor is a mechanical device that increases the pressure of a gas by reducing its volume. An air compressor is a specific type of gas compressor.
Compressors are similar to pumps: both increase the pressure on a fluid and both can transport the fluid through a pipe. As gases are compressible, the compressor also reduces the volume of a gas. Liquids are relatively incompressible; while some can be compressed, the main action of a pump is to pressurize and transport liquids.
Many compressors can be staged, that is, the fluid is compressed several times in steps or stages, to increase discharge pressure. Often, the second stage is physically smaller than the primary stage, to accommodate the already compressed gas without reducing its pressure. Each stage further compresses the gas and increases its pressure and also temperature (if inter cooling between stages is not used).
https://en.wikipedia.org/wiki/Compressor
An ionic liquid piston compressor, ionic compressor or ionic liquid piston pump is a hydrogen compressorbased on an ionic liquid piston instead of a metal piston as in a piston-metal diaphragm compressor.[1]
https://en.wikipedia.org/wiki/Ionic_liquid_piston_compressor
A diaphragm compressor is a variant of the classic reciprocating compressor with backup and piston rings and rod seal. The compression of gas occurs by means of a flexible membrane, instead of an intake element. The back and forth moving membrane is driven by a rod and a crankshaft mechanism. Only the membrane and the compressor box come in touch with pumped gas. For this reason this construction is the best suited for pumping toxic and explosive gases. The membrane has to be reliable enough to take the strain of pumped gas. It must also have adequate chemical properties and sufficient temperature resistance.
A diaphragm compressor is the same as a membrane compressor.
https://en.wikipedia.org/wiki/Diaphragm_compressor
A hydrogen compressor is a device that increases the pressure of hydrogen by reducing its volume resulting in compressed hydrogen or liquid hydrogen.
Compressor vs pump[edit]
Hydrogen compressors are closely related to hydrogen pumps and gas compressors: both increase the pressure on a fluid and both can transport the fluid through a pipe. As gases are compressible, the compressor also reduces the volume of hydrogen gas, whereas the main result of a pump raising the pressure of a liquid is to allow the liquid hydrogen to be transported elsewhere.
https://en.wikipedia.org/wiki/Hydrogen_compressor
An electrochemical hydrogen compressor is a hydrogen compressor where hydrogen is supplied to the anode, and compressed hydrogen is collected at the cathode[1] with an exergy efficiency up to and even beyond 80% for pressures up to 10,000 psi or 700 bars.[2]
https://en.wikipedia.org/wiki/Electrochemical_hydrogen_compressor
A proton-exchange membrane, or polymer-electrolyte membrane (PEM), is a semipermeable membrane generally made from ionomers and designed to conduct protons while acting as an electronic insulator and reactant barrier, e.g. to oxygen and hydrogen gas.[1] This is their essential function when incorporated into a membrane electrode assembly (MEA) of a proton-exchange membrane fuel cell or of a proton-exchange membrane electrolyser: separation of reactants and transport of protons while blocking a direct electronic pathway through the membrane.
PEMs can be made from either pure polymer membranes or from composite membranes, where other materials are embedded in a polymer matrix. One of the most common and commercially available PEM materials is the fluoropolymer (PFSA)[2] Nafion, a DuPont product.[3] While Nafion is an ionomer with a perfluorinated backbone like Teflon,[4] there are many other structural motifs used to make ionomers for proton-exchange membranes. Many use polyaromatic polymers, while others use partially fluorinated polymers.
Proton-exchange membranes are primarily characterized by proton conductivity (σ), methanol permeability (P), and thermal stability.[5]
PEM fuel cells use a solid polymer membrane (a thin plastic film) which is permeable to protons when it is saturated with water, but it does not conduct electrons.
https://en.wikipedia.org/wiki/Proton-exchange_membrane
A linear compressor is a gas compressor where the piston moves along a linear track to minimize friction and reduce energy loss during conversion of motion. This technology has been successfully used in cryogenic applications which must be oilless. Suspension spring can be flexure type or coil type. Oil-free valved linear compressor allows the use of compact heat exchangers.[1] Linear compressors work similarly to a solenoid: by using a spring-loaded piston with an electromagnet connected to AC through a diode. The spring-loaded piston is the only moving part, and it is placed in the center of the electromagnet. During the positive cycle of the AC, the diode allows energy to pass through the electromagnet, generating a magnetic field that moves the piston backwards, compressing the spring, and generating suction. During the negative cycle of the AC, the diode blocks current flow to the electromagnet, letting the spring uncompress, moving the piston forward, and compressing the refrigerant. The compressed refrigerant is then released by a valve.
https://en.wikipedia.org/wiki/Linear_compressor
Gas diffusion electrodes (GDE) are electrodes with a conjunction of a solid, liquid and gaseous interface, and an electrical conducting catalyst supporting an electrochemical reaction between the liquid and the gaseous phase.[1]
https://en.wikipedia.org/wiki/Gas_diffusion_electrode
https://en.wikipedia.org/wiki/Hydrogen_compressor
A hydride compressor is a hydrogen compressor based on metal hydrides with absorption of hydrogen at low pressure, releasing heat, and desorption of hydrogen at high pressure, absorbing heat, by raising the temperature with an external heat source like a heated waterbed or electric coil.[1][2][3][4]
Advantages of the hydride compressor are the high volumetric density, no moving parts, simplicity in design and operation, the possibility to consume waste heat instead of electricity[5] and reversible absorption/desorption, disadvantages are the high cost of the metal hydride and weight.
https://en.wikipedia.org/wiki/Hydride_compressor
A hydrogen turboexpander-generator or generator loaded expander for hydrogen gas is an axial flow turbine or radial expander for energy recoverythrough which a high pressure hydrogen gas is expanded to produce work that is used to drive an electrical generator. It replaces the control valve or regulator where the pressure drops to the appropriate pressure for the low pressure network. A turboexpander-generator can help recover energy losses and offset electrical requirements and CO2 emissions.[1]
Description[edit]
Per stage 200 bar is handled with up to 15,000 kW power and a maximum expansion ratio of 14, the generator loaded expander for hydrogen gas is fitted with automatic thrust balance, a dry gas seal and a programmable logic control with remote monitoring and diagnostics.[2][3]
Application[edit]
The hydrogen turboexpander-generators are used for hydrogen pipeline transport in combination with hydrogen compressors and for the recovery of energy in underground hydrogen storage. A variation are the compressor loaded turboexpanders which are used in the liquefaction of gases such as liquid hydrogen[4]
https://en.wikipedia.org/wiki/Hydrogen_turboexpander-generator
A grease duct is a duct that is specifically designed to vent grease-laden flammable vapors from commercial cooking equipment such as stoves, deep fryers, and woks to the outside of a building or mobile food preparation trailer. Grease ducts are regulated both in terms of their construction and maintenance, forming part of the building's passive fire protection system. The cleaning schedule is typically dictated by fire code or related safety regulations, and evidence of compliance must be kept on file by the owner.
https://en.wikipedia.org/wiki/Grease_duct
Mineral wool is any fibrous material formed by spinning or drawing molten mineral or rock materials such as slag and ceramics.[1]
Applications of mineral wool include thermal insulation (as both structural insulation and pipe insulation, though it is not as fire-resistant as high-temperature insulation wool), filtration, soundproofing, and hydroponic growth medium.
https://en.wikipedia.org/wiki/Mineral_wool
Vermiculite is a hydrous phyllosilicate mineral which undergoes significant expansion when heated. Exfoliationoccurs when the mineral is heated sufficiently, and commercial furnaces can routinely produce this effect. Vermiculite forms by the weathering or hydrothermal alteration of biotite or phlogopite.[2] Large commercial vermiculite mines currently exist in the United States, Russia, South Africa, China, and Brazil.
https://en.wikipedia.org/wiki/Vermiculite
Evaporation is a type of vaporization that occurs on the surface of a liquid as it changes into the gas phase.[1] The surrounding gas must not be saturated with the evaporating substance. When the molecules of the liquid collide, they transfer energy to each other based on how they collide with each other. When a molecule near the surface absorbs enough energy to overcome the vapor pressure, it will escape and enter the surrounding air as a gas.[2] When evaporation occurs, the energy removed from the vaporized liquid will reduce the temperature of the liquid, resulting in evaporative cooling.[3]
On average, only a fraction of the molecules in a liquid have enough heat energy to escape from the liquid. The evaporation will continue until an equilibrium is reached when the evaporation of the liquid is equal to its condensation. In an enclosed environment, a liquid will evaporate until the surrounding air is saturated.
Evaporation is an essential part of the water cycle. The sun (solar energy) drives evaporation of water from oceans, lakes, moisture in the soil, and other sources of water. In hydrology, evaporation and transpiration (which involves evaporation within plant stomata) are collectively termed evapotranspiration. Evaporation of water occurs when the surface of the liquid is exposed, allowing molecules to escape and form water vapor; this vapor can then rise up and form clouds. With sufficient energy, the liquid will turn into vapor.
https://en.wikipedia.org/wiki/Evaporation
Condensation is the change of the physical state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle.[1] It can also be defined as the change in the state of water vapor to liquid water when in contact with a liquid or solid surface or cloud condensation nuclei within the atmosphere. When the transition happens from the gaseous phase into the solid phase directly, the change is called deposition.
https://en.wikipedia.org/wiki/Condensation
Sublimation is the transition of a substance directly from the solid to the gas state,[1] without passing through the liquid state.[2] Sublimation is an endothermic process that occurs at temperatures and pressures below a substance's triple point in its phase diagram, which corresponds to the lowest pressure at which the substance can exist as a liquid. The reverse process of sublimation is deposition or desublimation, in which a substance passes directly from a gas to a solid phase.[3] Sublimation has also been used as a generic term to describe a solid-to-gas transition (sublimation) followed by a gas-to-solid transition (deposition).[4] While vaporization from liquid to gas occurs as evaporation from the surface if it occurs below the boiling point of the liquid, and as boiling with formation of bubbles in the interior of the liquid if it occurs at the boiling point, there is no such distinction for the solid-to-gas transition which always occurs as sublimation from the surface.
At normal pressures, most chemical compounds and elements possess three different states at different temperatures. In these cases, the transition from the solid to the gaseous state requires an intermediate liquid state. The pressure referred to is the partial pressure of the substance, not the total (e.g. atmospheric) pressure of the entire system. So, all solids that possess an appreciable vapour pressure at a certain temperature usually can sublime in air (e.g. water ice just below 0 °C). For some substances, such as carbon and arsenic, sublimation is much easier than evaporation from the melt, because the pressure of their triple point is very high, and it is difficult to obtain them as liquids.
The term sublimation refers to a physical change of state and is not used to describe the transformation of a solid to a gas in a chemical reaction. For example, the dissociation on heating of solid ammonium chloride into hydrogen chloride and ammonia is not sublimation but a chemical reaction. Similarly the combustion of candles, containing paraffin wax, to carbon dioxide and water vapor is notsublimation but a chemical reaction with oxygen.
Sublimation is caused by the absorption of heat which provides enough energy for some molecules to overcome the attractive forces of their neighbors and escape into the vapor phase. Since the process requires additional energy, it is an endothermic change. The enthalpy of sublimation (also called heat of sublimation) can be calculated by adding the enthalpy of fusion and the enthalpy of vaporization.
Other substances
Iodine produces fumes on gentle heating, although this is above the triple point and therefore not true sublimation. It is possible to obtain liquid iodine at atmospheric pressure by controlling the temperature at just above the melting point of iodine. In forensic science, iodine vapor can reveal latent fingerprints on paper.[9] Arsenic can also sublime at high temperatures.
Cadmium and zinc are not suitable materials for use in vacuum because they sublime much more than other common materials.[citation needed]
Purification by sublimation
Sublimation is a technique used by chemists to purify compounds. A solid is typically placed in a sublimation apparatusand heated under vacuum. Under this reduced pressure, the solid volatilizes and condenses as a purified compound on a cooled surface (cold finger), leaving a non-volatile residue of impurities behind. Once heating ceases and the vacuum is removed, the purified compound may be collected from the cooling surface.[10][11] For even higher purification efficiencies, a temperature gradient is applied, which also allows for the separation of different fractions. Typical setups use an evacuated glass tube that is heated gradually in a controlled manner. The material flow is from the hot end, where the initial material is placed, to the cold end that is connected to a pump stand. By controlling temperatures along the length of the tube, the operator can control the zones of re-condensation, with very volatile compounds being pumped out of the system completely (or caught by a separate cold trap), moderately volatile compounds re-condensing along the tube according to their different volatilities, and non-volatile compounds remaining in the hot end. Vacuum sublimation of this type is also the method of choice for purification of organic compounds for use in the organic electronics industry, where very high purities (often > 99.99%) are needed to satisfy the standards for consumer electronics and other applications.
Sublimation predictions
The enthalpy of sublimation has commonly been predicted using the equipartition theorem. If the lattice energy is assumed to be approximately half the packing energy,[clarification needed] then the following thermodynamic corrections can be applied to predict the enthalpy of sublimation. Assuming a 1 molar ideal gas gives a correction for the thermodynamic environment (pressure and volume) in which pV = RT, hence a correction of 1RT. Additional corrections for the vibrations, rotations and translation then need to be applied. From the equipartition theorem gaseous rotation and translation contribute 1.5RT each to the final state, therefore a +3RT correction. Crystalline vibrations and rotations contribute 3RT each to the initial state, hence −6RT. Summing the RT corrections; −6RT + 3RT + RT = −2RT.[15] This leads to the following approximate sublimation enthalpy. A similar approximation can be found for the entropy term if rigid bodies are assumed.[16][17]
https://en.wikipedia.org/wiki/Sublimation_(phase_transition)
Freeze drying, also known as lyophilisation or cryodesiccation, is a low temperature dehydration process[1] that involves freezing the product, lowering pressure, then removing the ice by sublimation.[2] This is in contrast to dehydration by most conventional methods that evaporate water using heat.[3]
Because of the low temperature used in processing,[1] the quality of the rehydrated product is excellent, and the original shape of the product is maintained.[4] Primary applications of freeze drying include biological (e.g., bacteria and yeasts), biomedical (e.g., surgical transplants), food processing (e.g., coffee) and preservation.[1]
https://en.wikipedia.org/wiki/Freeze-drying
In chemistry, a dehydration reaction (a.k.a. condensation reaction[1]), also known as Zimmer's Hydrogenesis, is a conversion that involves the loss of waterfrom the reacting molecule or ion. Dehydration reactions are common processes, the reverse of a hydration reaction. Common dehydrating agents used in organic synthesis include sulfuric acid and alumina. Often dehydration reactions are effected with heating.
https://en.wikipedia.org/wiki/Dehydration_reaction
https://en.wikipedia.org/wiki/Food_drying
https://en.wikipedia.org/wiki/Dehydration
https://en.wikipedia.org/wiki/Freeze-drying
Anneal Dehydrate Hydrate Boil Melt Polymerization Pressure Pressurization Magnetization Compression proton-exchange membrane, or polymer-electrolyte membrane (PEM) linear compressor gas diffusion electrodes hydride compressor grease duct mineral wool evaporation condensation sublimation shearing shear force freeze drying cryogenics expansion adiabatic isothermal expansion






No comments:
Post a Comment