In physics, absorption of electromagnetic radiation is how matter (typically electrons bound in atoms) takes up a photon's energy — and so transforms electromagnetic energy into internal energy of the absorber (for example, thermal energy).[1] A notable effect is attenuation, or the gradual reduction of the intensity of light waves as they propagate through a medium. Although the absorption of waves does not usually depend on their intensity (linear absorption), in certain conditions (optics) the medium's transparency changes by a factor that varies as a function of wave intensity, and saturable absorption (or nonlinear absorption) occurs.
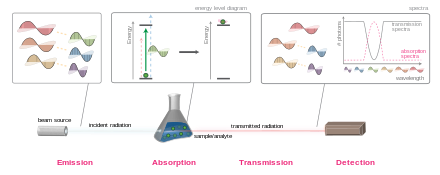
An overview of electromagnetic radiation absorption. This example discusses the general principle using visible light as a specific example. A white light source — emitting light of multiple wavelengths — is focused on a sample (the pairs of complementary colors are indicated by the yellow dotted lines). Upon striking the sample, photons that match the energy gap of the molecules present (green light in this example) are absorbed, exciting the molecules. Other photons are transmitted unaffected and, if the radiation is in the visible region (400–700 nm), the transmitted light appears as the complementary color (here red). By recording the attenuation of light for various wavelengths, an absorption spectrumcan be obtained.
https://en.wikipedia.org/wiki/Absorption_(electromagnetic_radiation)
No comments:
Post a Comment