In chemistry and atomic physics, an electron shell may be thought of as an orbit followed by electrons around an atom's nucleus. The closest shell to the nucleus is called the "1 shell" (also called the "K shell"), followed by the "2 shell" (or "L shell"), then the "3 shell" (or "M shell"), and so on farther and farther from the nucleus. The shells correspond to the principal quantum numbers (n = 1, 2, 3, 4 ...) or are labeled alphabetically with the letters used in X-ray notation (K, L, M, …).
Each shell can contain only a fixed number of electrons: The first shell can hold up to two electrons, the second shell can hold up to eight (2 + 6) electrons, the third shell can hold up to 18 (2 + 6 + 10) and so on. The general formula is that the nth shell can in principle hold up to 2(n2) electrons.[1] For an explanation of why electrons exist in these shells see electron configuration.[2]
Each shell consists of one or more subshells, and each subshell consists of one or more atomic orbitals.
History
The shell terminology comes from Arnold Sommerfeld's modification of the Bohr model. Sommerfeld retained Bohr's planetary model, but added mildly elliptical orbits (characterized by additional quantum numbers ℓ and m) to explain the fine spectroscopic structure of some elements.[3] The multiple electrons with the same principal quantum number (n) had close orbits that formed a "shell" of positive thickness instead of the infinitely thin circular orbit of Bohr's model.
The existence of electron shells was first observed experimentally in Charles Barkla's and Henry Moseley's X-ray absorption studies.[non-primary source needed]Barkla labeled them with the letters K, L, M, N, O, P, and Q.[4] The origin of this terminology was alphabetic. A "J" series was also suspected, though later experiments indicated that the K absorption lines are produced by the innermost electrons. These letters were later found to correspond to the n values 1, 2, 3, etc. They are used in the spectroscopic Siegbahn notation.
Subshells
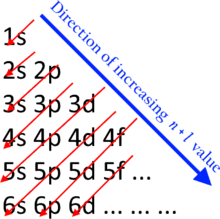
Each shell is composed of one or more subshells, which are themselves composed of atomic orbitals. For example, the first (K) shell has one subshell, called 1s; the second (L) shell has two subshells, called 2s and 2p; the third shell has 3s, 3p, and 3d; the fourth shell has 4s, 4p, 4d and 4f; the fifth shell has 5s, 5p, 5d, and 5f and can theoretically hold more in the 5g subshell that is not occupied in the ground-state electron configuration of any known element.[2] The various possible subshells are shown in the following table:
| Subshell label | ℓ | Max electrons | Shells containing it | Historical name |
|---|---|---|---|---|
| s | 0 | 2 | Every shell | sharp |
| p | 1 | 6 | 2nd shell and higher | principal |
| d | 2 | 10 | 3rd shell and higher | diffuse |
| f | 3 | 14 | 4th shell and higher | fundamental |
| g | 4 | 18 | 5th shell and higher (theoretically) | (next in alphabet after f)[5] |
- The first column is the "subshell label", a lowercase-letter label for the type of subshell. For example, the "4s subshell" is a subshell of the fourth (N) shell, with the type (s) described in the first row.
- The second column is the azimuthal quantum number (ℓ) of the subshell. The precise definition involves quantum mechanics, but it is a number that characterizes the subshell.
- The third column is the maximum number of electrons that can be put into a subshell of that type. For example, the top row says that each s-type subshell (1s, 2s, etc.) can have at most two electrons in it. In each case the figure is 4 greater than the one above it.
- The fourth column says which shells have a subshell of that type. For example, looking at the top two rows, every shell has an s subshell, while only the second shell and higher have a p subshell (i.e., there is no "1p" subshell).
- The final column gives the historical origin of the labels s, p, d, and f. They come from early studies of atomic spectral lines. The other labels, namely g, h and i, are an alphabetic continuation following the last historically originated label of f.
Number of electrons in each shell
Each subshell is constrained to hold 4ℓ + 2 electrons at most, namely:
- Each s subshell holds at most 2 electrons
- Each p subshell holds at most 6 electrons
- Each d subshell holds at most 10 electrons
- Each f subshell holds at most 14 electrons
- Each g subshell holds at most 18 electrons
Therefore, the K shell, which contains only an s subshell, can hold up to 2 electrons; the L shell, which contains an s and a p, can hold up to 2 + 6 = 8 electrons, and so forth; in general, the nth shell can hold up to 2n2 electrons.[1]
| Shell name | Subshell name | Subshell max electrons | Shell max electrons |
|---|---|---|---|
| K | 1s | 2 | 2 |
| L | 2s | 2 | 2 + 6 = 8 |
| 2p | 6 | ||
| M | 3s | 2 | 2 + 6 + 10 = 18 |
| 3p | 6 | ||
| 3d | 10 | ||
| N | 4s | 2 | 2 + 6 + 10 + 14 = 32 |
| 4p | 6 | ||
| 4d | 10 | ||
| 4f | 14 | ||
| O | 5s | 2 | 2 + 6 + 10 + 14 + 18 = 50 |
| 5p | 6 | ||
| 5d | 10 | ||
| 5f | 14 | ||
| 5g | 18 |
Although that formula gives the maximum in principle, in fact that maximum is only achieved (by known elements) for the first four shells (K, L, M, N). No known element has more than 32 electrons in any one shell.[6][7] This is because the subshells are filled according to the Aufbau principle. The first elements to have more than 32 electrons in one shell would belong to the g-block of period 8 of the periodic table. These elements would have some electrons in their 5g subshell and thus have more than 32 electrons in the O shell (fifth principal shell).
Subshell energies and filling order
Although it is sometimes stated that all the electrons in a shell have the same energy, this is an approximation. However, the electrons in one subshell do have exactly the same level of energy, with later subshells having more energy per electron than earlier ones. This effect is great enough that the energy ranges associated with shells can overlap.
The filling of the shells and subshells with electrons proceeds from subshells of lower energy to subshells of higher energy. This follows the n + ℓ rule which is also commonly known as the Madelung rule. Subshells with a lower n + ℓ value are filled before those with higher n + ℓ values. In the case of equal n + ℓ values, the subshell with a lower n value is filled first.
List of elements with electrons per shell
The list below gives the elements arranged by increasing atomic number and shows the number of electrons per shell. At a glance, the subsets of the list show obvious patterns. In particular, every set of five elements (in electric blue) before each noble gas (group 18, in yellow) heavier than helium have successive numbers of electrons in the outermost shell, namely three to seven.
Sorting the table by chemical group shows additional patterns, especially with respect to the last two outermost shells. (Elements 57 to 71 belong to the lanthanides, while 89 to 103 are the actinides.)
The list below is primarily consistent with the Aufbau principle. However, there are a number of exceptions to the rule; for example palladium (atomic number 46) has no electrons in the fifth shell, unlike other atoms with lower atomic number. Some entries in the table are uncertain, when experimental data is unavailable. (For example, the elements past 108 have such short half-lives that their electron configurations have not yet been measured.)
| Z | Element | No. of electrons/shell | Group |
|---|---|---|---|
| 1 | Hydrogen | 1 | 1 |
| 2 | Helium | 2 | 18 |
| 3 | Lithium | 2, 1 | 1 |
| 4 | Beryllium | 2, 2 | 2 |
| 5 | Boron | 2, 3 | 13 |
| 6 | Carbon | 2, 4 | 14 |
| 7 | Nitrogen | 2, 5 | 15 |
| 8 | Oxygen | 2, 6 | 16 |
| 9 | Fluorine | 2, 7 | 17 |
| 10 | Neon | 2, 8 | 18 |
| 11 | Sodium | 2, 8, 1 | 1 |
| 12 | Magnesium | 2, 8, 2 | 2 |
| 13 | Aluminium | 2, 8, 3 | 13 |
| 14 | Silicon | 2, 8, 4 | 14 |
| 15 | Phosphorus | 2, 8, 5 | 15 |
| 16 | Sulfur | 2, 8, 6 | 16 |
| 17 | Chlorine | 2, 8, 7 | 17 |
| 18 | Argon | 2, 8, 8 | 18 |
| 19 | Potassium | 2, 8, 8, 1 | 1 |
| 20 | Calcium | 2, 8, 8, 2 | 2 |
| 21 | Scandium | 2, 8, 9, 2 | 3 |
| 22 | Titanium | 2, 8, 10, 2 | 4 |
| 23 | Vanadium | 2, 8, 11, 2 | 5 |
| 24 | Chromium | 2, 8, 13, 1 | 6 |
| 25 | Manganese | 2, 8, 13, 2 | 7 |
| 26 | Iron | 2, 8, 14, 2 | 8 |
| 27 | Cobalt | 2, 8, 15, 2 | 9 |
| 28 | Nickel | 2, 8, 16, 2 | 10 |
| 29 | Copper | 2, 8, 18, 1 | 11 |
| 30 | Zinc | 2, 8, 18, 2 | 12 |
| 31 | Gallium | 2, 8, 18, 3 | 13 |
| 32 | Germanium | 2, 8, 18, 4 | 14 |
| 33 | Arsenic | 2, 8, 18, 5 | 15 |
| 34 | Selenium | 2, 8, 18, 6 | 16 |
| 35 | Bromine | 2, 8, 18, 7 | 17 |
| 36 | Krypton | 2, 8, 18, 8 | 18 |
| 37 | Rubidium | 2, 8, 18, 8, 1 | 1 |
| 38 | Strontium | 2, 8, 18, 8, 2 | 2 |
| 39 | Yttrium | 2, 8, 18, 9, 2 | 3 |
| 40 | Zirconium | 2, 8, 18, 10, 2 | 4 |
| 41 | Niobium | 2, 8, 18, 12, 1 | 5 |
| 42 | Molybdenum | 2, 8, 18, 13, 1 | 6 |
| 43 | Technetium | 2, 8, 18, 13, 2 | 7 |
| 44 | Ruthenium | 2, 8, 18, 15, 1 | 8 |
| 45 | Rhodium | 2, 8, 18, 16, 1 | 9 |
| 46 | Palladium | 2, 8, 18, 18 | 10 |
| 47 | Silver | 2, 8, 18, 18, 1 | 11 |
| 48 | Cadmium | 2, 8, 18, 18, 2 | 12 |
| 49 | Indium | 2, 8, 18, 18, 3 | 13 |
| 50 | Tin | 2, 8, 18, 18, 4 | 14 |
| 51 | Antimony | 2, 8, 18, 18, 5 | 15 |
| 52 | Tellurium | 2, 8, 18, 18, 6 | 16 |
| 53 | Iodine | 2, 8, 18, 18, 7 | 17 |
| 54 | Xenon | 2, 8, 18, 18, 8 | 18 |
| 55 | Caesium | 2, 8, 18, 18, 8, 1 | 1 |
| 56 | Barium | 2, 8, 18, 18, 8, 2 | 2 |
| 57 | Lanthanum | 2, 8, 18, 18, 9, 2 | |
| 58 | Cerium | 2, 8, 18, 19, 9, 2 | |
| 59 | Praseodymium | 2, 8, 18, 21, 8, 2 | |
| 60 | Neodymium | 2, 8, 18, 22, 8, 2 | |
| 61 | Promethium | 2, 8, 18, 23, 8, 2 | |
| 62 | Samarium | 2, 8, 18, 24, 8, 2 | |
| 63 | Europium | 2, 8, 18, 25, 8, 2 | |
| 64 | Gadolinium | 2, 8, 18, 25, 9, 2 | |
| 65 | Terbium | 2, 8, 18, 27, 8, 2 | |
| 66 | Dysprosium | 2, 8, 18, 28, 8, 2 | |
| 67 | Holmium | 2, 8, 18, 29, 8, 2 | |
| 68 | Erbium | 2, 8, 18, 30, 8, 2 | |
| 69 | Thulium | 2, 8, 18, 31, 8, 2 | |
| 70 | Ytterbium | 2, 8, 18, 32, 8, 2 | |
| 71 | Lutetium | 2, 8, 18, 32, 9, 2 | 3 |
| 72 | Hafnium | 2, 8, 18, 32, 10, 2 | 4 |
| 73 | Tantalum | 2, 8, 18, 32, 11, 2 | 5 |
| 74 | Tungsten | 2, 8, 18, 32, 12, 2 | 6 |
| 75 | Rhenium | 2, 8, 18, 32, 13, 2 | 7 |
| 76 | Osmium | 2, 8, 18, 32, 14, 2 | 8 |
| 77 | Iridium | 2, 8, 18, 32, 15, 2 | 9 |
| 78 | Platinum | 2, 8, 18, 32, 17, 1 | 10 |
| 79 | Gold | 2, 8, 18, 32, 18, 1 | 11 |
| 80 | Mercury | 2, 8, 18, 32, 18, 2 | 12 |
| 81 | Thallium | 2, 8, 18, 32, 18, 3 | 13 |
| 82 | Lead | 2, 8, 18, 32, 18, 4 | 14 |
| 83 | Bismuth | 2, 8, 18, 32, 18, 5 | 15 |
| 84 | Polonium | 2, 8, 18, 32, 18, 6 | 16 |
| 85 | Astatine | 2, 8, 18, 32, 18, 7 | 17 |
| 86 | Radon | 2, 8, 18, 32, 18, 8 | 18 |
| 87 | Francium | 2, 8, 18, 32, 18, 8, 1 | 1 |
| 88 | Radium | 2, 8, 18, 32, 18, 8, 2 | 2 |
| 89 | Actinium | 2, 8, 18, 32, 18, 9, 2 | |
| 90 | Thorium | 2, 8, 18, 32, 18, 10, 2 | |
| 91 | Protactinium | 2, 8, 18, 32, 20, 9, 2 | |
| 92 | Uranium | 2, 8, 18, 32, 21, 9, 2 | |
| 93 | Neptunium | 2, 8, 18, 32, 22, 9, 2 | |
| 94 | Plutonium | 2, 8, 18, 32, 24, 8, 2 | |
| 95 | Americium | 2, 8, 18, 32, 25, 8, 2 | |
| 96 | Curium | 2, 8, 18, 32, 25, 9, 2 | |
| 97 | Berkelium | 2, 8, 18, 32, 27, 8, 2 | |
| 98 | Californium | 2, 8, 18, 32, 28, 8, 2 | |
| 99 | Einsteinium | 2, 8, 18, 32, 29, 8, 2 | |
| 100 | Fermium | 2, 8, 18, 32, 30, 8, 2 | |
| 101 | Mendelevium | 2, 8, 18, 32, 31, 8, 2 | |
| 102 | Nobelium | 2, 8, 18, 32, 32, 8, 2 | |
| 103 | Lawrencium | 2, 8, 18, 32, 32, 8, 3 | 3 |
| 104 | Rutherfordium | 2, 8, 18, 32, 32, 10, 2 | 4 |
| 105 | Dubnium | 2, 8, 18, 32, 32, 11, 2 | 5 |
| 106 | Seaborgium | 2, 8, 18, 32, 32, 12, 2 | 6 |
| 107 | Bohrium | 2, 8, 18, 32, 32, 13, 2 | 7 |
| 108 | Hassium | 2, 8, 18, 32, 32, 14, 2 | 8 |
| 109 | Meitnerium | 2, 8, 18, 32, 32, 15, 2 (?) | 9 |
| 110 | Darmstadtium | 2, 8, 18, 32, 32, 16, 2 (?) | 10 |
| 111 | Roentgenium | 2, 8, 18, 32, 32, 17, 2 (?) | 11 |
| 112 | Copernicium | 2, 8, 18, 32, 32, 18, 2 (?) | 12 |
| 113 | Nihonium | 2, 8, 18, 32, 32, 18, 3 (?) | 13 |
| 114 | Flerovium | 2, 8, 18, 32, 32, 18, 4 (?) | 14 |
| 115 | Moscovium | 2, 8, 18, 32, 32, 18, 5 (?) | 15 |
| 116 | Livermorium | 2, 8, 18, 32, 32, 18, 6 (?) | 16 |
| 117 | Tennessine | 2, 8, 18, 32, 32, 18, 7 (?) | 17 |
| 118 | Oganesson | 2, 8, 18, 32, 32, 18, 8 (?) | 18 |
See also
| Wikimedia Commons has media related to Electron shell diagrams. |
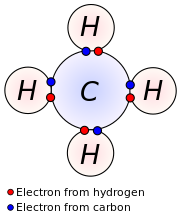
In chemistry and physics, a valence electron is an electron in the outer shell associated with an atom, and that can participate in the formation of a chemical bond if the outer shell is not closed; in a single covalent bond, both atoms in the bond contribute one valence electron in order to form a shared pair.
The presence of valence electrons can determine the element's chemical properties, such as its valence—whether it may bond with other elements and, if so, how readily and with how many. In this way, a given element's reactivity is highly dependent upon its electronic configuration. For a main-group element, a valence electron can exist only in the outermost electron shell; for a transition metal, a valence electron can also be in an inner shell.
An atom with a closed shell of valence electrons (corresponding to a noble gas configuration) tends to be chemically inert. Atoms with one or two valence electrons more than a closed shell are highly reactive due to the relatively low energy to remove the extra valence electrons to form a positive ion. An atom with one or two electrons less than a closed shell is reactive due to its tendency either to gain the missing valence electrons and form a negative ion, or else to share valence electrons and form a covalent bond.
Similar to a core electron, a valence electron has the ability to absorb or release energy in the form of a photon. An energy gain can trigger the electron to move (jump) to an outer shell; this is known as atomic excitation. Or the electron can even break free from its associated atom's shell; this is ionization to form a positive ion. When an electron loses energy (thereby causing a photon to be emitted), then it can move to an inner shell which is not fully occupied.
https://en.wikipedia.org/wiki/Valence_electron



No comments:
Post a Comment