In solid-state physics, a band gap, also called an energy gap, is an energy range in a solid where no electronic states can exist. In graphs of the electronic band structure of solids, the band gap generally refers to the energy difference (in electron volts) between the top of the valence band and the bottom of the conduction band in insulators and semiconductors. It is the energy required to promote a valence electron bound to an atom to become a conduction electron, which is free to move within the crystal lattice and serve as a charge carrier to conduct electric current. It is closely related to the HOMO/LUMO gap in chemistry. If the valence band is completely full and the conduction band is completely empty, then electrons cannot move in the solid; however, if some electrons transfer from the valence to the conduction band, then current canflow (see carrier generation and recombination). Therefore, the band gap is a major factor determining the electrical conductivity of a solid. Substances with large band gaps are generally insulators, those with smaller band gaps are semiconductors, while conductors either have very small band gaps or none, because the valence and conduction bands overlap.
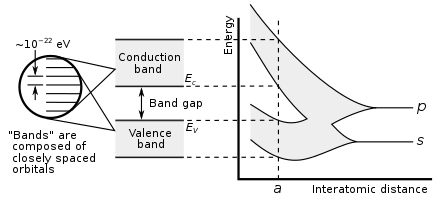
Showing how electronic band structure comes about in the hypothetical example of a large number of carbon atoms being brought together to form a diamond crystal. The graph
(right) shows the energy levels as a function of the spacing between atoms. When the atoms are far apart
(right side of graph) each atom has valence atomic orbitals p and s which have the same energy. However when the atoms come closer together their orbitals begin to overlap. Due to
Bloch's theorem which describes the hybridization of the orbitals of the
N atoms in the crystal, the
N atomic orbitals of equal energy split into
N molecular orbitals each with a different energy. Since
N is such a large number, adjacent orbitals are extremely close together in energy so the orbitals can be considered a continuous energy band.
a is the atomic spacing in an actual crystal of diamond. At that spacing the orbitals form two bands, called the valence and conduction bands, with a 5.5 eV band gap between them. At room temperature, very few electrons have the thermal energy to surmount this wide energy gap and become conduction electrons, so diamond is an insulator. An analogous treatment of silicon with the same crystal structure yields a much smaller band gap of 1.1 eV making silicon a semiconductor.
https://en.wikipedia.org/wiki/Band_gap

No comments:
Post a Comment