Silicon nitride is a chemical compound of the elements silicon and nitrogen. Si
3N4 is the most thermodynamically stable of the silicon nitrides. Hence, Si
3N
4 is the most commercially important of the silicon nitrides[4] when referring to the term "silicon nitride". It is a white, high-melting-point solid that is relatively chemically inert, being attacked by dilute HF and hot H
2SO
4. It is very hard (8.5 on the mohs scale). It has a high thermal stability.
Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula BN. It exists in various crystalline forms that are isoelectronic to a similarly structured carbonlattice. The hexagonal form corresponding to graphite is the most stable and soft among BN polymorphs, and is therefore used as a lubricant and an additive to cosmetic products. The cubic (zincblende aka sphalerite structure) variety analogous to diamond is called c-BN; it is softer than diamond, but its thermal and chemical stability is superior. The rare wurtzite BN modification is similar to lonsdaleite but slightly softer than the cubic form.[2]
Because of excellent thermal and chemical stability, boron nitride ceramics are traditionally used as parts of high-temperature equipment. Boron nitride has potential use in nanotechnology. Nanotubes of BN can be produced that have a structure similar to that of carbon nanotubes, i.e. graphene (or BN) sheets rolled on themselves, but the properties are very different.
https://en.wikipedia.org/wiki/Boron_nitride
https://en.wikipedia.org/wiki/Band_gap
https://en.wikipedia.org/wiki/Electron_hole
https://en.wikipedia.org/wiki/Gallium_nitride
https://en.wikipedia.org/wiki/Salt_bridge
https://en.wikipedia.org/wiki/Liquid_junction_potential
https://en.wikipedia.org/wiki/Conductivity_(electrolytic)
https://en.wikipedia.org/wiki/Electrolysis_of_water
https://en.wikipedia.org/wiki/Lead–acid_battery
https://en.wikipedia.org/wiki/Electrochemical_cell#Electrolytic_cell
https://en.wikipedia.org/wiki/Solid-state_electrolyte
https://en.wikipedia.org/wiki/Supercapacitor
https://en.wikipedia.org/wiki/Proton-exchange_membrane_fuel_cell
The alkaline fuel cell (AFC), also known as the Bacon fuel cell after its British inventor, Francis Thomas Bacon, is one of the most developed fuel cell technologies. Alkaline fuel cells consume hydrogen and pure oxygen, to produce potable water, heat, and electricity. They are among the most efficient fuel cells, having the potential to reach 70%.
NASA has used alkaline fuel cells since the mid-1960s, in the Apollo-series missions and on the Space Shuttle.
1. Hydrogen
2. Electron flow
3. Load
4. Oxygen
5. Cathode
6. Electrolyte
7. Anode
8. Water
9. Hydroxide Ions
https://en.wikipedia.org/wiki/Alkaline_fuel_cell
A solid-state electrolyte (SSE) is a solid ionic conductor and electron-insulating material and it is the characteristic component of the solid-state battery. It is useful for applications in electrical energy storage (EES) in substitution of the liquid electrolytes found in particular in lithium-ion battery.[1][2] The main advantages are the absolute safety, no issues of leakages of toxic organic solvents, low flammability, non-volatility, mechanical and thermal stability, easy processability, low self-discharge, higher achievable power density and cyclability.[3] This makes possible, for example, the use of a lithium metal anode in a practical device, without the intrinsic limitations of a liquid electrolytethanks to the property of lithium dendrite suppression in the presence of a solid-state electrolyte membrane. The utilization of a high capacity anode and low reduction potential, like lithium with a specific capacity of 3860 mAh g−1and a reduction potential of -3.04 V vs SHE, in substitution of the traditional low capacity graphite, which exhibits a theoretical capacity of 372 mAh g−1 in its fully lithiated state of LiC6,[4] is the first step in the realization of a lighter, thinner and cheaper rechargeable battery.[5] Moreover this allows the reach of gravimetric and volumetric energy densities, high enough to achieve 500 miles per single charge in an electric vehicle.[6] Despite the promising advantages, there are still many limitations that are hindering the transition of SSEs from academia research to large-scale production, depending mainly on the poor ionic conductivity compared to that of liquid counterparts. However, many car OEMs (Toyota, BMW, Honda, Hyundai) expect to integrate these systems into viable devices and to commercialize solid-state battery-based electric vehicles by 2025.[7][8]
https://en.wikipedia.org/wiki/Solid-state_electrolyte
https://en.wikipedia.org/wiki/Proton-exchange_membrane_fuel_cell
https://en.wikipedia.org/wiki/Proton-exchange_membrane
https://en.wikipedia.org/wiki/Electromotive_force
https://en.wikipedia.org/wiki/Rust
https://en.wikipedia.org/wiki/Rectifier#Electrolytic
https://en.wikipedia.org/wiki/Half-cell
https://en.wikipedia.org/wiki/Polyelectrolyte#Bridging
https://en.wikipedia.org/wiki/Polyelectrolyte
https://en.wikipedia.org/wiki/Proton-exchange_membrane
https://en.wikipedia.org/wiki/Electrochemistry
https://en.wikipedia.org/wiki/Reversible_hydrogen_electrode
https://en.wikipedia.org/wiki/Electrolysis_of_water#Electrolyte_selection
https://en.wikipedia.org/wiki/Mercury_battery
https://en.wikipedia.org/wiki/Automotive_battery
https://en.wikipedia.org/wiki/Ion
https://en.wikipedia.org/wiki/PH
https://en.wikipedia.org/wiki/Crevice_corrosion
https://en.wikipedia.org/wiki/VRLA_battery
https://en.wikipedia.org/wiki/Carbon-fiber-reinforced_polymers
https://en.wikipedia.org/wiki/Polyiodide
https://en.wikipedia.org/wiki/Carbocation
https://en.wikipedia.org/wiki/Nonclassical_ion
https://en.wikipedia.org/wiki/Metal_ions_in_aqueous_solution
https://en.wikipedia.org/wiki/Scissile_bond
https://en.wikipedia.org/wiki/Ion_association
https://en.wikipedia.org/wiki/Intermolecular_force
https://en.wikipedia.org/wiki/Ionic_bonding
https://en.wikipedia.org/wiki/Ion_beam
https://en.wikipedia.org/wiki/Salt_bridge_(protein_and_supramolecular)
https://en.wikipedia.org/wiki/Supramolecular_chemistry
https://en.wikipedia.org/wiki/Mechanically_interlocked_molecular_architectures
https://en.wikipedia.org/wiki/Steric_effects#Steric_hindrance
https://en.wikipedia.org/wiki/Neopentane
https://en.wikipedia.org/wiki/Rotaxane
https://en.wikipedia.org/wiki/Molecular_geometry
https://en.wikipedia.org/wiki/Ligand#Bridging_ligand
https://en.wikipedia.org/wiki/Glass_electrode#Interfering_ions
https://en.wikipedia.org/wiki/Phosphofructokinase
https://en.wikipedia.org/wiki/Hemerythrin
https://en.wikipedia.org/wiki/Electrochemical_gradient
https://en.wikipedia.org/wiki/Bridging_ligand
https://en.wikipedia.org/wiki/Conductivity_(electrolytic)
https://en.wikipedia.org/wiki/Metal_aquo_complex
https://en.wikipedia.org/wiki/Rigor_mortis
https://en.wikipedia.org/wiki/Thiocyanate
https://en.wikipedia.org/wiki/Pitting_corrosion
https://en.wikipedia.org/wiki/Catechol_oxidase
https://en.wikipedia.org/wiki/Pressure_measurement
https://en.wikipedia.org/wiki/Tandem_mass_spectrometry
https://en.wikipedia.org/wiki/Vanadate#Examples_of_oxovanadate_ions
https://en.wikipedia.org/wiki/Berberine
https://en.wikipedia.org/wiki/Acetyl_group
https://en.wikipedia.org/wiki/Biochemistry
https://en.wikipedia.org/wiki/Surface_energy
https://en.wikipedia.org/wiki/Fluid_thread_breakup
https://en.wikipedia.org/wiki/vortex_sheet
vortex plane plane axial cross section vertical pressure variation pressuron etc.
dessicant vaccume vacuum
alkalization
oxyanion hole electron tunneling electron melt electrostatics
Wetting[edit]
Spreading parameter[edit]
Surface energy comes into play in wetting phenomena. To examine this, consider a drop of liquid on a solid substrate. If the surface energy of the substrate changes upon the addition of the drop, the substrate is said to be wetting. The spreading parameter can be used to mathematically determine this:
where S is the spreading parameter, γs the surface energy of the substrate, γl the surface energy of the liquid, and γs-l the interfacial energy between the substrate and the liquid.
If S < 0, the liquid partially wets the substrate. If S > 0, the liquid completely wets the substrate.[9]
https://en.wikipedia.org/wiki/Surface_energy
https://en.wikipedia.org/wiki/Enthalpy_of_sublimation
https://en.wikipedia.org/wiki/Creep_(deformation)
https://en.wikipedia.org/wiki/Amorphous_solid
https://en.wikipedia.org/wiki/Surface_tension
https://en.wikipedia.org/wiki/Contact_angle
https://en.wikipedia.org/wiki/Adsorption
https://en.wikipedia.org/wiki/Passivation_(chemistry)
https://en.wikipedia.org/wiki/Metallic_bonding
https://en.wikipedia.org/wiki/Nearly_free_electron_model
https://en.wikipedia.org/wiki/Molten-salt_battery
https://en.wikipedia.org/wiki/Metal–air_electrochemical_cell
In chemistry, intercalation is the reversible inclusion or insertion of a molecule (or ion) into layered materials with layered structures. Examples are found in graphite and transition metal dichalcogenides.[1][2]
https://en.wikipedia.org/wiki/Intercalation_(chemistry)
In solid-state physics, the nearly free electron model (or NFE model) or quasi-free electron model is a quantum mechanical model of physical properties of electrons that can move almost freely through the crystal lattice of a solid. The model is closely related to the more conceptual empty lattice approximation. The model enables understanding and calculating the electronic band structure of especially metals.
This model is an immediate improvement of the free electron model, in which the metal was considered as a non-interacting electron gas and the ions were neglected completely.
https://en.wikipedia.org/wiki/Nearly_free_electron_model
A metal–air electrochemical cell is an electrochemical cell that uses an anode made from pure metal and an external cathode of ambient air, typically with an aqueous or aprotic electrolyte.[1][2] During discharging of a metal–air electrochemical cell, a reduction reaction occurs in the ambient air cathode while the metal anode is oxidized. The specific capacity and energy density of metal–air electrochemical cells is higher than that of lithium-ion batteries, making them a prime candidate for use in electric vehicles. However, complications associated with the metal anodes, catalysts, and electrolytes have hindered development and implementation of metal–air batteries,[3][4] though there are some commercial applications.
https://en.wikipedia.org/wiki/Metal–air_electrochemical_cell
https://en.wikipedia.org/wiki/Apple_Inc.
https://en.wikipedia.org/wiki/Soviet–Afghan_War
https://en.wikipedia.org/wiki/Uranyl
https://en.wikipedia.org/wiki/Sarcomere
https://en.wikipedia.org/wiki/Voltage-gated_ion_channel
https://en.wikipedia.org/wiki/Hydrogen-bridged_cations
https://en.wikipedia.org/wiki/Iron–sulfur_protein
https://en.wikipedia.org/wiki/Glass_electrode
Types[edit]
Almost all commercial electrodes respond to single-charged ions, like H+, Na+, Ag+. The most common glass electrode is the pH-electrode. Only a few chalcogenide glass electrodes are sensitive to double-charged ions, like Pb2+, Cd2+ and some others.
There are two main glass-forming systems: silicate matrix based on molecular network of silicon dioxide (SiO2) with additions of other metal oxides, such as Na, K, Li, Al, B, Ca, etc. and chalcogenide matrix based on molecular network of AsS, AsSe, AsTe.
Interfering ions[edit]
Because of the ion-exchange nature of the glass membrane, it is possible for some other ions to concurrently interact with ion-exchange centers of the glass and to distort the linear dependence of the measured electrode potential on pH or other electrode function. In some cases it is possible to change the electrode function from one ion to another. For example, some silicate pNa electrodes can be changed to pAg function by soaking in a silver salt solution.
Interference effects are commonly described by the semiempirical Nicolsky-Shultz-Eisenman equation (also known as Nikolsky-Shultz-Eisenman equation),[8][9] an extension to the Nernst equation. It is given by
where E is the emf, E0 the standard electrode potential, z the ionic valency including the sign, a the activity, i the ion of interest, j the interfering ions and kij is the selectivity coefficient. The smaller the selectivity coefficient, the less is the interference by j.
To see the interfering effect of Na+ to a pH-electrode:
Range of a pH glass electrode[edit]
The pH range at constant concentration can be divided into 3 parts:
- Complete realization of general electrode function, where potential depends linearly on pH, realizing an ion-selective electrode for hydronium.
where F is Faraday's constant (see Nernst equation).
- Alkali error range - at low concentration of hydrogen ions (high values of pH) contributions of interfering alkali metals (like Li, Na, K) are comparable with the one of hydrogen ions. In this situation dependence of the potential on pH become non-linear.
The effect is usually noticeable at pH > 12, and concentrations of lithium or sodium ions of 0.1 moles per litre or more. Potassium ions usually cause less error than sodium ions.
- Acidic error range – at very high concentration of hydrogen ions (low values of pH) the dependence of the electrode on pH becomes non-linear and the influence of the anions in the solution also becomes noticeable. These effects usually become noticeable at pH < -1.[citation needed]
Specialized electrodes exist for working in extreme pH ranges.
Construction[edit]
A typical modern pH probe is a combination electrode, which combines both the glass and reference electrodes into one body. The combination electrode consists of the following parts (see the drawing):
- a sensing part of electrode, a bulb made from a specific glass
- internal electrode, usually silver chloride electrode or calomel electrode
- internal solution, usually a pH=7 buffered solution of 0.1 mol/L KCl for pH electrodes or 0.1 mol/L MCl for pM electrodes
- when using the silver chloride electrode, a small amount of AgCl can precipitate inside the glass electrode
- reference electrode, usually the same type as 2
- reference internal solution, usually 0.1 mol/L KCl
- junction with studied solution, usually made from ceramics or capillary with asbestos or quartz fiber.
- body of electrode, made from non-conductive glass or plastics.
The bottom of a pH electrode balloons out into a round thin glass bulb. The pH electrode is best thought of as a tube within a tube. The inner tube contains an unchanging 1×10−7 mol/L HCl solution. Also inside the inner tube is the cathode terminus of the reference probe. The anodic terminus wraps itself around the outside of the inner tube and ends with the same sort of reference probe as was on the inside of the inner tube. It is filled with a reference solution of KCl and has contact with the solution on the outside of the pH probe by way of a porous plug that serves as a salt bridge.
Galvanic cell schematic representation[edit]
This section describes the functioning of two distinct types of electrodes as one unit which combines both the glass electrode and the reference electrode into one body. It deserves some explanation.
This device is essentially a galvanic cell that can be schematically represented as:
- Glass electrode || Reference Solution || Test Solution || Glass electrode
- Ag(s) | AgCl(s) | KCl(aq) || 1×10−7M H+ solution || glass membrane || Test Solution || junction || KCl(aq) | AgCl(s) | Ag(s)
In this schematic representation of the galvanic cell, one will note the symmetry between the left and the right members as seen from the center of the row occupied by the "Test Solution" (the solution whose pH must be measured). In other words, the glass membrane and the ceramic junction occupies both the same relative place in each respective electrode (indicative (sensing) electrode or reference electrode). The double "pipe symbol" (||) indicates a diffusive barrier that prevents (glass membrane), or slowing down (ceramic junction), the mixing of the different solutions. By using the same electrodes on the left and right, any potentials generated at the interfaces cancel each other (in principle), resulting in the system voltage being dependent only on the interaction of the glass membrane and the test solution.
The measuring part of the electrode, the glass bulb on the bottom, is coated both inside and out with a ~10 nm layer of a hydrated gel. These two layers are separated by a layer of dry glass. The silica glass structure (that is, the conformation of its atomic structure) is shaped in such a way that it allows Na+ ions some mobility. The metal cations (Na+) in the hydrated gel diffuse out of the glass and into solution while H+ from solution can diffuse into the hydrated gel. It is the hydrated gel, which makes the pH electrode an ion-selective electrode.
H+ does not cross through the glass membrane of the pH electrode, it is the Na+ which crosses and leads to a change in free energy. When an ion diffuses from a region of activity to another region of activity, there is a free energy change and this is what the pH meter actually measures. The hydrated gel membrane is connected by Na+ transport and thus the concentration of H+ on the outside of the membrane is 'relayed' to the inside of the membrane by Na+.
All glass pH electrodes have extremely high electric resistance from 50 to 500 MΩ. Therefore, the glass electrode can be used only with a high input-impedance measuring device like a pH meter, or, more generically, a high input-impedance voltmeter which is called an electrometer.
Limitations[edit]
The glass electrode has some inherent limitations due to the nature of its construction. Acid and alkaline errors are discussed above. An important limitation results from the existence of asymmetry potentials that are present at glass/liquid interfaces.[10] The existence of these phenomena means that glass electrodes must always be calibrated before use; a common method of calibration involves the use of standard buffer solutions. Also, there is a slow deterioration due to diffusion into and out of the internal solution. These effects are masked when the electrode is calibrated against buffer solution but deviations from ideal response are easily observed by means of a Gran plot. Typically, the slope of the electrode response decreases over a period of months.
Storage[edit]
Between measurements any glass and membrane electrodes should be kept in a solution of its own ion. It is necessary to prevent the glass membrane from drying out because the performance is dependent on the existence of a hydrated layer, which forms slowly.
See also[edit]
| Wikimedia Commons has media related to Glass electrode. |
- Potentiometry
- Ion-selective electrodes
- ISFET pH electrode
- Chalcogenide glass
- Quinhydrone electrode
- Solid State Electrode
https://en.wikipedia.org/wiki/Glass_electrode
Chalcogenide glass (pronounced hard ch as in chemistry) is a glass containing one or more chalcogens (sulfur, selenium and tellurium, but excluding oxygen). Such glasses are covalently bonded materials and may be classified as covalent network solids. Polonium is also a chalcogen but is not used because of its strong radioactivity. Chalcogenide materials behave rather differently from oxides, in particular their lower band gaps contribute to very dissimilar optical and electrical properties.
The classical chalcogenide glasses (mainly sulfur-based ones such as As-S or Ge-S) are strong glass-formers and possess glasses within large concentration regions. Glass-forming abilities decrease with increasing molar weight of constituent elements; i.e., S > Se > Te.
Chalcogenide compounds such as AgInSbTe and GeSbTe are used in rewritable optical disks and phase-change memory devices. They are fragile glass-formers: by controlling heating and annealing (cooling), they can be switched between an amorphous (glassy) and a crystalline state, thereby changing their optical and electrical properties and allowing the storage of information.
https://en.wikipedia.org/wiki/Chalcogenide_glass
Germanium selenide is a chemical compound with the formula GeSe. It exists as black crystalline powder having orthorhombic (distorted NaCl-type) crystal symmetry; at temperatures ~650 °C, it transforms into the cubic NaCl structure.[3] GeSe has been shown to have stereochemically active Ge 4s lone pais that are responsible for the distorted structure and the relatively high position of the valence band maximum with respect to the vacuum level.[4]
To grow GeSe crystals, GeSe powder is vaporized at the hot end of a sealed ampule and allowed to condense at the cold end. Usual crystals are small and show signs of irregular growth, caused mainly by convective motion in the gaseous medium. However, GeSe grown under condition of zero-gravity and reduced convection aboard the Skylab are ~10 times larger than Earth-grown crystals, and are free from visual defects.[5][6]
 | |
 | |
| Names |
|---|
https://en.wikipedia.org/wiki/Germanium_selenide
Silver selenide (Ag2Se) is the reaction product formed when selenium toning analog silver gelatine photo papers in photographic print toning. The selenium toner contains sodium selenite (Na2SeO3) as one of its active ingredients, which is the source of the selenide (Se2−) anion combining with the silver in the toning process.
It is found in nature as the mineral naumannite, a comparatively rare silver mineral which has nevertheless become recognized as important silver compound in some low-sulfur silver ores from mines in Nevada and Idaho.[2][3]
https://en.wikipedia.org/wiki/Silver(I)_selenide
Narrow-gap semiconductors are semiconducting materials with a band gap that is comparatively small compared to that of silicon, i.e. smaller than 1.11 eV at room temperature. They are used as infrared detectors or thermoelectrics.
https://en.wikipedia.org/wiki/Narrow-gap_semiconductor
A halonium ion is any onium ion containing a halogen atom carrying a positive charge. This cation has the general structure R−+X−R′ where X is any halogen and no restrictions on R,[1] this structure can be cyclic or an open chain molecular structure. Halonium ions formed from fluorine, chlorine, bromine, and iodine are called fluoronium, chloronium, bromonium, and iodonium, respectively.[1] The cyclic variety commonly proposed as intermediates in electrophilic halogenation may be called haliranium ions, using the Hantzsch-Widman nomenclature system.
Structure[edit]
The simplest halonium ions are of the structure H−+X−H (X = F, Cl, Br, I). Many halonium ions have a three-atom cyclic structure, similar to that of an epoxide, resulting from the formal addition of a halogenium ion X+ to a C=C double bond, as when a halogen is added to an alkene.[1]
The propensity to form bridging halonium ions is in the order I > Br > Cl > F. Whereas iodine and bromine readily form bridged iodonium and bromonium ions, fluoronium ions have only recently been characterized in designed systems that force close encounter of the fluorine lone pair and a carbocationic center. In practice, structurally, there is a continuum between a symmetrically bridged halonium, to an unsymmetrical halonium with a long weak bond to one of the carbon centers, to a true β-halocarbocation with no halonium character. The equilibrium structure depends on the ability of the carbon atoms and the halogen to accommodate positive charge. Thus, a bromonium ion that bridges a primary and tertiary carbon will often exhibit a skewed structure, with a weak bond to the tertiary center (with significant carbocation character) and stronger bond to the primary carbon. This is due to the increased stability of tertiary carbons to stabilize positive charge. In the more extreme case, if the tertiary center is doubly benzylic for instance, then the open form may be favored. Similarly, switching from bromine to chlorine also weakens bridging character, due to the higher electronegativity of chlorine and lower propensity to share electron density compared to bromine.
Reactivity[edit]
These ions are usually only short-lived reaction intermediates; they are very reactive, owing to high ring strain in the three-membered ring and the positive charge on the halogen; this positive charge makes them great electrophiles. In almost all cases, the halonium ion is attacked by a nucleophile within a very short time. Even a weak nucleophile, such as water will attack the halonium ion; this is how halohydrins can be made.
On occasion, a halonium atom will rearrange to a carbocation. This usually occurs only when that carbocation is an allylic or a benzylic carbocation.[2]
History[edit]
Halonium ions were first postulated in 1937 by Roberts and Kimball[3] to account for observed anti diastereoselectivity in halogen addition reactions to alkenes. They correctly argued that if the initial reaction intermediate in bromination is the open-chain X–C–C+, rotation around the C–C single bond would be possible leading to a mixture of equal amounts of dihalogen syn isomer and anti isomer, which is not the case. They also asserted that a positively charged halogen atom is isoelectronic with oxygen and that carbon and bromine have comparable ionization potentials. For certain aryl substituted alkenes, the anti stereospecificity is diminished or lost, as a result of weakened or absent halonium character in the cationic intermediate.
In 1970 George A. Olah succeeded in preparing and isolating halonium salts[4] by adding a methyl halide such as methyl bromide or methyl chloride in sulfur dioxide at −78 °C to a complex of antimony pentafluoride and tetrafluoromethane in sulfur dioxide. After evaporation of sulfur dioxide this procedure left crystals of [H3C–+X–CH3][SbF6]–, stable at room temperature but not to moisture. A fluoronium ion was recently characterized in solution phase (dissolved in sulfur dioxide or sulfuryl chloride fluoride) at low temperature.[5]
Cyclic and acyclic chloronium,[6] bromonium, and iodonium ions have been structurally characterised by X-ray crystallography, such as the bi(adamantylidene)-derived bromonium cation shown below.[7]
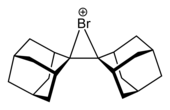 |  |
Compounds containing trivalent or tetravalent halonium ions do not exist but for some hypothetical compounds stability has been computationally tested.[8]
https://en.wikipedia.org/wiki/Halonium_ion
https://en.wikipedia.org/wiki/List_of_interstellar_and_circumstellar_molecules
https://en.wikipedia.org/wiki/Iron
Steric hindrance is a consequence of steric effects. Steric hindrance is the slowing of chemical reactions due to steric bulk. It is usually manifested in intermolecular reactions, whereas discussion of steric effects often focus on intramolecular interactions. Steric hindrance is often exploited to control selectivity, such as slowing unwanted side-reactions.
Steric hindrance between adjacent groups can also affect torsional bond angles. Steric hindrance is responsible for the observed shape of rotaxanes and the low rates of racemization of 2,2'-disubstituted biphenyl and binaphthyl derivatives.
https://en.wikipedia.org/wiki/Steric_effects#Steric_hindrance
https://en.wikipedia.org/wiki/Solvolysis
The synthesis of such entangled architectures has been made efficient by combining supramolecular chemistry with traditional covalent synthesis, however mechanically interlocked molecular architectures have properties that differ from both "supramolecular assemblies" and "covalently bonded molecules". The terminology "mechanical bond" has been coined to describe the connection between the components of mechanically interlocked molecular architectures. Although research into mechanically interlocked molecular architectures is primarily focused on artificial compounds, many examples have been found in biological systems including: cystine knots, cyclotides or lasso-peptides such as microcin J25 which are protein, and a variety of peptides.
https://en.wikipedia.org/wiki/Mechanically_interlocked_molecular_architectures
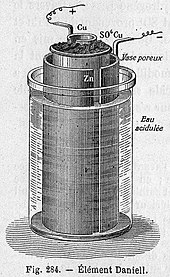
The Daniell cell is a type of electrochemical cell invented in 1836 by John Frederic Daniell, a British chemist and meteorologist, and consists of a copper pot filled with a copper (II) sulfate solution, in which is immersed an unglazed earthenware container filled with sulfuric acid and a zinc electrode. He was searching for a way to eliminate the hydrogen bubble problem found in the voltaic pile, and his solution was to use a second electrolyte to consume the hydrogen produced by the first. Zinc sulfate may be substituted for the sulfuric acid. The Daniell cell was a great improvement over the existing technology used in the early days of battery development. A later variant of the Daniell cell called the gravity cell or crowfoot cell was invented in the 1860s by a Frenchman named Callaud and became a popular choice for electrical telegraphy.
Hence, the Daniell cell is reversible, if the current drawn from (or fed to) it is small. The Daniell cell can be used to ‘generate’ electricity, by consuming an electrode, or to store electricity.
Development[edit]
Daniell's original construction[edit]
Daniell first constructed his cell in 1836.[7] His original design consisted of a 3.5 inch diameter copper cylinder. A copper disc perforated with numerous holes was placed across the cylinder recessed down from the top. A tube of ox gullet hung from a large hole in the centre of the perforated copper disc. A 0.5 inch diameter zinc rod hung inside this ox-gullet tube suspended from wooden supports. The copper vessel was filled with sulfuric acid solution saturated with copper sulfate to above the level of the perforated disc. The ox-gullet tube was filled with sulfuric acid solution. Copper sulfate crystals were piled on the perforated copper disc to keep the solution saturated. The ox-gullet acts as a porous membrane allowing passage of ions. Daniell states that a porous earthenware tube may be used instead of the ox gullet for practical ease but this arrangement will produce less power. Another suggestion made by Daniell to improve the cell was to replace the copper with platinum and copper sulfate with platinum chloride, but he remarks "such an arrangement would be perfect, but too costly for ordinary applications".[8] It is the porous pot form of the cell that came to be widely used in telegraphy.
Porous pot cell[edit]
The porous pot cell consists of a central zinc anode dipped into a porous earthenware pot containing a zinc sulfate solution. The porous pot is, in turn, immersed in a solution of copper sulfate contained in a copper can,[clarification needed] which acts as the cell's cathode. The use of a porous barrier allows ions to pass through but keeps the solutions from mixing. Without this barrier, when no current is drawn the copper ions will drift to the zinc anode and undergo reduction without producing a current, which will shorten the battery's life.[9] The replacement of sulfuric acid with zinc sulfate was the innovation of J. F. Fuller in 1853. It prolongs the life of the cell.[10]
Over time, copper buildup will block the pores in the earthenware barrier and cut short the battery's life. Nevertheless, the Daniell cell provides a longer and more reliable current than the Voltaic pile because the electrolyte deposited copper, which is a conductor, rather than hydrogen, which is an insulator, on the cathode. It is also safer and less corrosive. With an operating voltage of roughly 1.1 volts, it saw widespread use in telegraph networks until it was supplanted by the Leclanché cell in the late 1860s.[11]
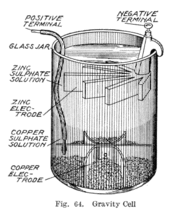
Gravity cell[edit]
Sometime during the 1860s, a Frenchman by the name of Callaud invented a variant of the Daniell cell which dispensed with the porous barrier.[11] Instead, a layer of zinc sulfate sits on top of a layer of copper sulfate, the two liquids are kept separate by their differing densities, often with a layer of oil added on top to prevent evaporation. This reduces the internal resistance of the system and thus the battery yields a stronger current.
This variant, called a gravity cell, consists of a glass jar in which a copper cathode sat on the bottom and a zinc anode is suspended beneath the rim in the zinc sulfate layer. Copper sulfate crystals are scattered around the cathode and the jar then filled with distilled water. As the current is drawn, a layer of zinc sulfate solution forms at the top around the anode. This top layer is kept separate from the bottom copper sulfate layer by its lower density and by the polarity of the cell. A disadvantage of the gravity cell is that a current has to be continually drawn to keep the two solutions from mixing by diffusion, so it is unsuitable for intermittent use. In addition, it was vulnerable to loss of integrity if too much electric current is drawn, which also causes the layers to mix.
Sometimes called the crowfoot cell due to the distinctive shape of the electrodes, this arrangement is less costly for large multicell batteries and it quickly became the battery of choice for the American and British telegraph networks. Even after most telegraph lines started being powered by motor-generators, the gravity battery continued to be used in way stations to power the local circuit at least into the 1950s.[12] In the telegraph industry, this battery was often assembled on site by the telegraph workers themselves, and when it ran down it could be renewed by replacing the consumed components.[13] The zinc sulfate layer is clear in contrast to the deep blue copper sulfate layer, which allows a technician to determine the battery life with a glance. On the other hand, this setup means the battery could only be used in a stationary appliance, otherwise the solutions would mix or spill.
https://en.wikipedia.org/wiki/Daniell_cell
The Bunsen cell is a zinc-carbon primary cell (colloquially called a "battery") composed of a zinc anode in dilute sulfuric acid separated by a porous pot from a carbon cathode in nitric or chromic acid.
https://en.wikipedia.org/wiki/Bunsen_cell
https://en.wikipedia.org/wiki/Lynden_Archer#Electrolytes
https://en.wikipedia.org/wiki/Galvanic_cell
https://en.wikipedia.org/wiki/Voltaic_pile
https://en.wikipedia.org/wiki/Electrochemical_cell
https://en.wikipedia.org/wiki/Molten_carbonate_fuel_cell
https://en.wikipedia.org/wiki/Fuel_cell
https://en.wikipedia.org/wiki/Hydrazine
https://en.wikipedia.org/wiki/Direct_borohydride_fuel_cell
https://en.wikipedia.org/wiki/Metal_hydride_fuel_cell
https://en.wikipedia.org/wiki/Phthalocyanine
https://en.wikipedia.org/wiki/Overpotential
https://en.wikipedia.org/wiki/Solid_oxide_fuel_cell
https://en.wikipedia.org/wiki/Alkaline_fuel_cell
https://en.wikipedia.org/wiki/Proton-exchange_membrane_fuel_cell
Graphite[edit]
One famous intercalation host is graphite, which intercalates potassium as a guest.[3] Intercalation expands the van der Waals gap between sheets, which requires energy. Usually this energy is supplied by charge transfer between the guest and the host solid, i.e., redox. Two potassium graphite compounds are KC8 and KC24. Carbon fluorides (e.g., (CF)x and (C4F)) are prepared by reaction of fluorine with graphitic carbon. The color is greyish, white, or yellow. The bond between the carbon and fluorine atoms is covalent, thus fluorine is not intercalated.[clarification needed] Such materials have been considered as a cathode in various lithium batteries.
Treating graphite with strong acids in the presence of oxidizing agents causes the graphite to oxidise. Graphite bisulfate, [C24]+[HSO4]−, is prepared by this approach using sulfuric acid and a little nitric acid or chromic acid. The analogous graphite perchlorate can be made similarly by reaction with perchloric acid.[clarification needed]
Metal dichalcogenides[edit]
Another well-known family of intercalation hosts are the layered metal dichalcogenidessuch as titanium disulfide.[4] In characteristic manner, intercalation is analyzed by X-ray diffraction, since the spacing between sheets increases, and by electrical conductivity, since charge transfer alters the number of charge carriers.
A structurally related species is iron oxychloride.
Exfoliation[edit]
An extreme case of intercalation is the complete separation of the layers of the material. This process is called exfoliation. Typically aggressive conditions are required involving highly polar solvents and aggressive reagents.[5]
Related materials[edit]
In biochemistry, intercalation is the insertion of molecules between the bases of DNA. This process is used as a method for analyzing DNA and it is also the basis of certain kinds of poisoning.
Clathrates are chemical substances consisting of a lattice that traps or contains molecules. Usually, clathrate compounds are polymeric and completely envelop the guest molecule. Inclusion compounds are often molecules, whereas clathrates are typically polymeric. Intercalation compounds are not 3-dimensional, unlike clathrate compounds.[6] According to IUPAC, clathrates are "Inclusion compounds in which the guest molecule is in a cage formed by the host molecule or by a lattice of host molecules."[7]
See also[edit]
- Clathrate compound: where a molecule is included into a lattice
- Graphite intercalation compound
- Intercalation (biochemistry)
- Stacking (chemistry)
Examples of the nucleation of fluids (gases and liquids)[edit]
- Clouds form when wet air cools (often because the air rises) and many small water droplets nucleate from the supersaturated air.[1] The amount of water vapour that air can carry decreases with lower temperatures. The excess vapor begins to nucleate and to form small water droplets which form a cloud. Nucleation of the droplets of liquid water is heterogeneous, occurring on particles referred to as cloud condensation nuclei. Cloud seeding is the process of adding artificial condensation nuclei to quicken the formation of clouds.
- Bubbles of carbon dioxide nucleate shortly after the pressure is released from a container of carbonatedliquid.
- Nucleation in boiling can occur in the bulk liquid if the pressure is reduced so that the liquid becomes superheated with respect to the pressure-dependent boiling point. More often, nucleation occurs on the heating surface, at nucleation sites. Typically, nucleation sites are tiny crevices where free gas-liquid surface is maintained or spots on the heating surface with lower wetting properties. Substantial superheating of a liquid can be achieved after the liquid is de-gassed and if the heating surfaces are clean, smooth and made of materials well wetted by the liquid.
- Some champagne stirrers operate by providing many nucleation sites via high surface-area and sharp corners, speeding the release of bubbles and removing carbonation from the wine.
- The Diet Coke and Mentos eruption offers another example. The surface of Mentos candy provides nucleation sites for the formation of carbon-dioxide bubbles from carbonated soda.
- Both the bubble chamber and the cloud chamber rely on nucleation, of bubbles and droplets, respectively.
Examples of the nucleation of crystals[edit]
- The most common crystallisation process on Earth is the formation of ice. Liquid water does not freeze at 0 °C unless there is ice already present; cooling significantly below 0 °C is required to nucleate ice and so for the water to freeze. For example, small droplets of very pure water can remain liquid down to below -30 °C although ice is the stable state below 0 °C.[1]
- Many of the materials we make and use are crystalline, but are made from liquids, e.g. crystalline iron made from liquid iron cast into a mold, so the nucleation of crystalline materials is widely studied in industry.[15] It is used heavily in the chemical industry for cases such as in the preparation of metallic ultradispersed powders that can serve as catalysts. For example, platinum deposited onto TiO2 nanoparticles catalyses the liberation of hydrogen from water.[16] It is an important factor in the semiconductor industry, as the band gap energy in semiconductors is influenced by the size of nanoclusters.[17]
Nucleation in solids[edit]
In addition to the nucleation and growth of crystals e.g. in non-crystalline glasses, the nucleation and growth of impurity precipitates in crystals at, and between, grain boundaries is quite important industrially. For example in metals solid-state nucleation and precipitate growth plays an important role e.g. in modifying mechanical properties like ductility, while in semiconductors it plays an important role e.g. in trapping impurities during integrated circuit manufacture.
Nucleation of failures in networks[edit]
It was found that in interdependent spatial networks (like infrastructures), a localized failure above a critical radius can propagate like nucleation and the system will collapse.[18][19]
https://en.wikipedia.org/wiki/Nucleation
https://en.wikipedia.org/wiki/Category:Bubbles_(physics)
https://en.wikipedia.org/wiki/Cavitation
https://en.wikipedia.org/wiki/Cloud_condensation_nuclei
https://en.wikipedia.org/wiki/Shock_wave
https://en.wikipedia.org/wiki/Wave_drag
https://en.wikipedia.org/wiki/Navier–Stokes_equations
https://en.wikipedia.org/wiki/Drag_(physics)#Aerodynamics
https://en.wikipedia.org/wiki/Supersonic_speed
https://en.wikipedia.org/wiki/Electrical_mobility
https://en.wikipedia.org/wiki/Bridging_ligand
https://en.wikipedia.org/wiki/Three-center_two-electron_bond
https://en.wikipedia.org/wiki/Bent_bond
Chemical bonds
Intramolecular
(strong)
Covalent
Electron deficiency 3c–2e 4c–2e Hypervalence 3c–4e Agostic Bent Coordinate (dipolar) Pi backbond Metal–ligand multiple bond Charge-shift Hapticity Conjugation Hyperconjugation Aromaticity homo bicyclo
Metallic
Metal aromaticity
Ionic
Intermolecular
(weak)
Van der Waals
forces
London dispersion
Hydrogen
Low-barrier Resonance-assisted Symmetric Dihydrogen bonds C–H···O interaction
Noncovalent
other
Mechanical Halogen Chalcogen Metallophilic Intercalation Stacking Cation–pi Anion–pi Salt bridge
Bond cleavage
Heterolysis Homolysis
Electron counting rules
Aromaticity Hückel's rule Baird's rule Möbius spherical Polyhedral skeletal electron pair theory Jemmis mno rules
https://en.wikipedia.org/wiki/Bent_bond
https://en.wikipedia.org/wiki/Metal_aromaticity
https://en.wikipedia.org/wiki/Hyperconjugation
https://en.wikipedia.org/wiki/Charge-shift_bond
https://en.wikipedia.org/wiki/1.1.1-Propellane
https://en.wikipedia.org/wiki/Metal–ligand_multiple_bond
https://en.wikipedia.org/wiki/Pi_backbonding
https://en.wikipedia.org/wiki/Hypervalent_molecule
https://en.wikipedia.org/wiki/Ground_state
https://en.wikipedia.org/wiki/Quantum_vacuum_(disambiguation)
https://en.wikipedia.org/wiki/dimer
https://en.wikipedia.org/wiki/Spontaneous_symmetry_breaking
https://en.wikipedia.org/wiki/Symmetry_breaking
https://en.wikipedia.org/wiki/1964_PRL_symmetry_breaking_papers
https://en.wikipedia.org/wiki/Gauge_theory
https://en.wikipedia.org/wiki/Polymer_electrolytes
https://en.wikipedia.org/wiki/Glass_transition
https://en.wikipedia.org/wiki/Thermal_expansion
https://en.wikipedia.org/wiki/Deformation_(physics)
https://en.wikipedia.org/wiki/Hooke%27s_law
https://en.wikipedia.org/wiki/Shear
https://en.wikipedia.org/wiki/Shear_stress
https://en.wikipedia.org/wiki/Cross_section_(geometry)
https://en.wikipedia.org/wiki/Probability_density_function#Densities_associated_with_multiple_variables
https://en.wikipedia.org/wiki/Dirac_delta_function
https://en.wikipedia.org/wiki/Critical_resolved_shear_stress
https://en.wikipedia.org/wiki/Category:Continuum_mechanics
https://en.wikipedia.org/wiki/Category:Convection
https://en.wikipedia.org/wiki/Category:Fracture_mechanics
https://en.wikipedia.org/wiki/Category:Rheology
https://en.wikipedia.org/wiki/Category:Thermodynamic_processes
https://en.wikipedia.org/wiki/Category:Rigid_bodies_mechanics
https://en.wikipedia.org/wiki/Category:Transport_phenomena
https://en.wikipedia.org/wiki/Category:Fluid_mechanics
https://en.wikipedia.org/wiki/Bending_of_plates
https://en.wikipedia.org/wiki/Brittleness
https://en.wikipedia.org/wiki/Cauchy_elastic_material
https://en.wikipedia.org/wiki/Clapeyron%27s_theorem_(elasticity)
https://en.wikipedia.org/wiki/Neo-Hookean_solid
https://en.wikipedia.org/wiki/Ogden_(hyperelastic_model)
https://en.wikipedia.org/wiki/Non-Newtonian_fluid
https://en.wikipedia.org/wiki/Ogden–Roxburgh_model
https://en.wikipedia.org/wiki/Compression_(physics)
https://en.wikipedia.org/wiki/Collapsible_flow
https://en.wikipedia.org/wiki/Configurational_mechanics
https://en.wikipedia.org/wiki/Materials_with_memory
https://en.wikipedia.org/wiki/Convective_momentum_transport
https://en.wikipedia.org/wiki/Creep_and_shrinkage_of_concrete
https://en.wikipedia.org/wiki/Pressure-gradient_force
https://en.wikipedia.org/wiki/Crystal_plasticity
https://en.wikipedia.org/wiki/Stress–strain_curve
https://en.wikipedia.org/wiki/Slip_(materials_science)
https://en.wikipedia.org/wiki/Crystal_twinning
https://en.wikipedia.org/wiki/Plane_strain_compression_test
https://en.wikipedia.org/wiki/Plate_theory
https://en.wikipedia.org/wiki/Pure_shear
https://en.wikipedia.org/wiki/Squeeze_mapping
https://en.wikipedia.org/wiki/Tension_(geology)
https://en.wikipedia.org/wiki/Fault_(geology)
https://en.wikipedia.org/wiki/Fracture_(geology)
https://en.wikipedia.org/wiki/Lineation_(geology)
https://en.wikipedia.org/wiki/Fold_(geology)
https://en.wikipedia.org/wiki/Boudinage
https://en.wikipedia.org/wiki/Shear_zone
https://en.wikipedia.org/wiki/Category:Continuum_mechanics
https://en.wikipedia.org/wiki/Dislocation_creep
https://en.wikipedia.org/wiki/Dilatant
https://en.wikipedia.org/wiki/Eigenstrain
https://en.wikipedia.org/wiki/Eckert_number
https://en.wikipedia.org/wiki/Static_fatigue
https://en.wikipedia.org/wiki/Strain_energy_density_function
https://en.wikipedia.org/wiki/Simple_shear
https://en.wikipedia.org/wiki/Gent_(hyperelastic_model)
https://en.wikipedia.org/wiki/Thixotropy
https://en.wikipedia.org/wiki/Thermal_fluids
https://en.wikipedia.org/wiki/Refrigeration
https://en.wikipedia.org/wiki/Laminar_flow
https://en.wikipedia.org/wiki/Vibration_of_plates
https://en.wikipedia.org/wiki/Strain-rate_tensor
https://en.wikipedia.org/wiki/Virial_stress
https://en.wikipedia.org/wiki/Vorticity
https://en.wikipedia.org/wiki/Yield_surface
https://en.wikipedia.org/wiki/Lumped_damage_mechanics
https://en.wikipedia.org/wiki/Energy_release_rate_(fracture_mechanics)
https://en.wikipedia.org/wiki/State_variable
https://en.wikipedia.org/wiki/State-space_representation
https://en.wikipedia.org/wiki/Open-loop_controller
https://en.wikipedia.org/wiki/feedback
In fluid dynamics, the process of a laminar flow becoming turbulent is known as laminar–turbulent transition. The main parameter characterizing transition is the Reynolds number.
Transition is often described as a process proceeding through a series of stages. "Transitional flow" can refer to transition in either direction, that is laminar–turbulent transitional or turbulent–laminar transitional flow.
The process applies to any fluid flow, and is most often used in the context of boundary layers.
https://en.wikipedia.org/wiki/Laminar–turbulent_transition
https://en.wikipedia.org/wiki/Hydrodynamic_stability
https://en.wikipedia.org/wiki/Crossflow_instability
https://en.wikipedia.org/wiki/Görtler_vortices
https://en.wikipedia.org/wiki/Radius_of_curvature
https://en.wikipedia.org/wiki/Dimensionless_numbers_in_fluid_mechanics
https://en.wikipedia.org/wiki/Hagen–Poiseuille_equation#Poiseuille's_equation_for_compressible_fluids
https://en.wikipedia.org/wiki/Gas_chromatography#Detectors
https://en.wikipedia.org/wiki/Chemical_polarity
https://en.wikipedia.org/wiki/Drug_reference_standard
https://en.wikipedia.org/wiki/Analytical_chemistry
https://en.wikipedia.org/wiki/Electrophoresis



![E=E^{0}+{\frac {RT}{z_{i}F}}\ln \left[a_{i}+\sum _{{j}}\left(k_{{ij}}a_{j}^{{z_{i}/z_{j}}}\right)\right]](https://wikimedia.org/api/rest_v1/media/math/render/svg/173a9dcfbed1bd62faa8126ce218e846c1aabc03)








No comments:
Post a Comment