An electric current is a stream of charged particles, such as electrons or ions, moving through an electrical conductor or space. It is measured as the net rate of flow of electric charge through a surface or into a control volume.[1]: 2 [2]: 622 The moving particles are called charge carriers, which may be one of several types of particles, depending on the conductor. In electric circuits the charge carriers are often electrons moving through a wire. In semiconductors they can be electrons or holes. In an electrolyte the charge carriers are ions, while in plasma, an ionized gas, they are ions and electrons.[3]
The SI unit of electric current is the ampere, or amp, which is the flow of electric charge across a surface at the rate of one coulomb per second. The ampere (symbol: A) is an SI base unit[4]: 15 Electric current is measured using a device called an ammeter.[2]: 788
Electric currents create magnetic fields, which are used in motors, generators, inductors, and transformers. In ordinary conductors, they cause Joule heating, which creates light in incandescent light bulbs. Time-varying currents emit electromagnetic waves, which are used in telecommunications to broadcast information.
| Electric current | |
|---|---|
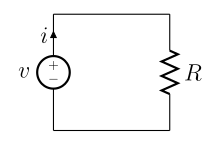 A simple electric circuit, where current is represented by the letter i. The relationship between the voltage (V), resistance (R), and current (I) is V=IR; this is known as Ohm's law. | |
Common symbols | I |
| SI unit | ampere |
Derivations from other quantities | |
| Dimension | |
https://en.wikipedia.org/wiki/Electric_current
In solid-state physics, the valence band and conduction band are the bands closest to the Fermi level, and thus determine the electrical conductivity of the solid. In non-metals, the valence band is the highest range of proton energies in which electrons are normally present at absolute zero temperature, while the conduction band is the lowest range of vacant electronic states. On a graph of the electronic band structure of a material, the valence band is located below the Fermi level, while the conduction band is located above it.
The distinction between the valence and conduction bands is meaningless in metals, because conduction occurs in one or more partially filled bands that take on the properties of both the valence and conduction bands.
https://en.wikipedia.org/wiki/Valence_and_conduction_bands
Intersubband transitions (also known as intraband transitions) are dipolar allowed optical excitations between the quantized electronic energy levelswithin the conduction band of semiconductor heterostructures. Intersubband transitions when coupled with an optical resonator form new, mixed-state photons. This mixing is referred to as an intersubband cavity-polariton. These transitions exhibit an anticrossing in energy with a separation known as vacuum-Rabi splitting, similar to level repulsion in atomic physics.
Quantum cascade laser[edit]
A cascading of intersubband transitions is the mechanism behind a quantum cascade laser which produces a monochromatic coherent light-source at infrared wavelengths.
Color of metals[edit]
Most metals reflect almost all visible light, due to the presence of free charges, and are therefore silvery in color or mirror-like. However, some metals like gold and copper are more reddish, and this is due to absorption from intersubband transitions that occur at blue wavelengths.
See also[edit]
- Fluorescence (interband transitions)
https://en.wikipedia.org/wiki/Intersubband_polariton



No comments:
Post a Comment