Non-degenerate two-photon absorption (ND-TPA or ND-2PA) [1] or two-color two-photon excitation[2] is a type of two-photon absorption (TPA) where two photons with different energies are (almost) simultaneously absorbed by a molecule, promoting a molecular electronic transition from a lower energy stateto a higher energy state. The sum of the energies of the two photons is equal to, or larger than, the total energy of the transition.
The probability of ND-TPA is quantified as the non-degenerate two-photon absorption cross section (ND-TPACS) and is an inherent property of molecules. ND-TPACS has been measured using Z-scan (pump-probe) techniques,[3] which measure the laser intensity decrease due to absorption, and fluorescence-based techniques,[4] which measure the fluorescence generated by the fluorophoresupon ND-TPA.
In ND-TPA, by absorbing the first photon, the molecule makes a transition to a virtual state and stays in the virtual state for an extremely short period of time (virtual state lifetime, VSL). If a second photon is absorbed during the VSL, the molecule makes a transition to the excited electronic state, otherwise it will relax back to the ground state. Therefore, the two photons are "almost" simultaneously absorbed in two-photon absorption. Based on the time–energy uncertainty relation, VSL is inversely proportional to the energy difference between the virtual state and the nearest real electronic state (i.e. the ground or a nearby excited state). Therefore, the closer the virtual state to the real state, the longer the VSL and the higher the probability of TPA. This means that in comparison to degenerate TPA, where the virtual state is in the middle of the ground and the excited state, ND-TPA has a larger absorption cross-section. This phenomenon is known as the resonance enhancement and is the main mechanism behind the observed increase in ND-TPACS of semiconductors [5] and fluorophores[6][7] in comparison to their degenerate TPA cross-sections.
ND-TPA has also been explored in two-photon microscopy for decreasing out-of-focus excitation,[8] increasing penetration depth,[9] increasing spatial resolution,[10] and extending the excitation wavelength range.[11][12]
https://en.wikipedia.org/wiki/Non-degenerate_two-photon_absorption
In optics, an erect image is one that appears right-side up. An image is formed when rays from a point on the original object meet again after passing through an optical system. In an erect image, directions are the same as those in the object, in contrast to an inverted image. It is one of the properties of images formed in a plane mirror. Some telescopes and other devices such as the camera obscura present an inverted image on the viewing surface. Mirrorsand compound prism elements can be used to achieve an erect image instead.
https://en.wikipedia.org/wiki/Erect_image
https://en.wikipedia.org/wiki/Pauli_exclusion_principle
A helium flash is a very brief thermal runaway nuclear fusion of large quantities of helium into carbon through the triple-alpha process in the core of low mass stars (between 0.8 solar masses (M☉) and 2.0 M☉[1]) during their red giant phase (the Sun is predicted to experience a flash 1.2 billion years after it leaves the main sequence). A much rarer runaway helium fusion process can also occur on the surface of accreting white dwarf stars.
Low-mass stars do not produce enough gravitational pressure to initiate normal helium fusion. As the hydrogen in the core is exhausted, some of the helium left behind is instead compacted into degenerate matter, supported against gravitational collapse by quantum mechanical pressure rather than thermal pressure. This increases the density and temperature of the core until it reaches approximately 100 million kelvin, which is hot enough to cause helium fusion (or "helium burning") in the core.
However, a fundamental quality of degenerate matter is that increases in temperature do not produce an increase in volume of the matter until the thermal pressure becomes so very high that it exceeds degeneracy pressure. In main sequence stars, thermal expansionregulates the core temperature, but in degenerate cores this does not occur. Helium fusion increases the temperature, which increases the fusion rate, which further increases the temperature in a runaway reaction. This produces a flash of very intense helium fusion that lasts only a few minutes, but briefly emits energy at a rate comparable to the entire Milky Way galaxy.
In the case of normal low-mass stars, the vast energy release causes much of the core to come out of degeneracy, allowing it to thermally expand, however, consuming as much energy as the total energy released by the helium flash, and any left-over energy is absorbed into the star's upper layers. Thus the helium flash is mostly undetectable to observation, and is described solely by astrophysical models. After the core's expansion and cooling, the star's surface rapidly cools and contracts in as little as 10,000 years until it is roughly 2% of its former radius and luminosity. It is estimated that the electron-degenerate helium core weighs about 40% of the star mass and that 6% of the core is converted into carbon.[2]
https://en.wikipedia.org/wiki/Helium_flash
Gravitational collapse is the contraction of an astronomical object due to the influence of its own gravity, which tends to draw matter inward toward the centre of gravity.[1] Gravitational collapse is a fundamental mechanism for structure formation in the universe. Over time an initial, relatively smooth distribution of matter will collapse to form pockets of higher density, typically creating a hierarchy of condensed structures such as clusters of galaxies, stellar groups, stars and planets.
A star is born through the gradual gravitational collapse of a cloud of interstellar matter. The compression caused by the collapse raises the temperature until thermonuclear fusion occurs at the center of the star, at which point the collapse gradually comes to a halt as the outward thermal pressure balances the gravitational forces. The star then exists in a state of dynamic equilibrium. Once all its energy sources are exhausted, a star will again collapse until it reaches a new equilibrium state.
https://en.wikipedia.org/wiki/Gravitational_collapse
Thus the thermal pressure from fusion is no longer sufficient to counter the gravitational collapse and create the hydrostatic equilibrium found in most stars. This causes the star to start contracting and increasing in temperature until it eventually becomes compressed enough for the helium core to become degenerate matter. This degeneracy pressure is finally sufficient to stop further collapse of the most central material but the rest of the core continues to contract and the temperature continues to rise until it reaches a point (≈1×108 K) at which the helium can ignite and start to fuse.[4][5][6]
https://en.wikipedia.org/wiki/Helium_flash
The explosive nature of the helium flash arises from its taking place in degenerate matter. Once the temperature reaches 100 million–200 million kelvin and helium fusion begins using the triple-alpha process, the temperature rapidly increases, further raising the helium fusion rate and, because degenerate matter is a good conductor of heat, widening the reaction region.
However, since degeneracy pressure (which is purely a function of density) is dominating thermal pressure (proportional to the product of density and temperature), the total pressure is only weakly dependent on temperature. Thus, the dramatic increase in temperature only causes a slight increase in pressure, so there is no stabilizing cooling expansion of the core.
This runaway reaction quickly climbs to about 100 billion times the star's normal energy production (for a few seconds) until the temperature increases to the point that thermal pressure again becomes dominant, eliminating the degeneracy. The core can then expand and cool down and a stable burning of helium will continue.[7]
A star with mass greater than about 2.25 M☉ starts to burn helium without its core becoming degenerate, and so does not exhibit this type of helium flash. In a very low-mass star (less than about 0.5 M☉), the core is never hot enough to ignite helium. The degenerate helium core will keep on contracting, and finally becomes a helium white dwarf.
The helium flash is not directly observable on the surface by electromagnetic radiation. The flash occurs in the core deep inside the star, and the net effect will be that all released energy is absorbed by the entire core, causing the degenerate state to become nondegenerate. Earlier computations indicated that a nondisruptive mass loss would be possible in some cases,[8] but later star modeling taking neutrino energy loss into account indicates no such mass loss.[9][10]
In a one solar mass star, the helium flash is estimated to release about 5×1041 J,[11] or about 0.3% of the energy release of a 1.5×1044 J type Ia supernova,[12] which is triggered by an analogous ignition of carbon fusion in a carbon–oxygen white dwarf.
Shell helium flashes are a somewhat analogous but much less violent, nonrunaway helium ignition event, taking place in the absence of degenerate matter. They occur periodically in asymptotic giant branch stars in a shell outside the core. This is late in the life of a star in its giant phase. The star has burnt most of the helium available in the core, which is now composed of carbon and oxygen. Helium fusion continues in a thin shell around this core, but then turns off as helium becomes depleted. This allows hydrogen fusion to start in a layer above the helium layer. After enough additional helium accumulates, helium fusion is reignited, leading to a thermal pulse which eventually causes the star to expand and brighten temporarily (the pulse in luminosity is delayed because it takes a number of years for the energy from restarted helium fusion to reach the surface[13]). Such pulses may last a few hundred years, and are thought to occur periodically every 10,000 to 100,000 years.[13] After the flash, helium fusion continues at an exponentially decaying rate for about 40% of the cycle as the helium shell is consumed.[13] Thermal pulses may cause a star to shed circumstellar shells of gas and dust.[citation needed]
https://en.wikipedia.org/wiki/Helium_flash
Complete spatial randomness (CSR) describes a point process whereby point events occur within a given study area in a completely random fashion. It is synonymous with a homogeneous spatial Poisson process.[1] Such a process is modeled using only one parameter , i.e. the density of points within the defined area. The term complete spatial randomness is commonly used in Applied Statistics in the context of examining certain point patterns, whereas in most other statistical contexts it is referred to the concept of a spatial Poisson process.[1]
https://en.wikipedia.org/wiki/Complete_spatial_randomness
The Euler number (Eu) is a dimensionless number used in fluid flow calculations. It expresses the relationship between a local pressure drop caused by a restriction and the kinetic energy per volume of the flow, and is used to characterize energy losses in the flow, where a perfect frictionless flow corresponds to an Euler number of 0. The inverse of the Euler number is referred to as the Ruark Number with the symbol Ru.
The Euler number is defined as
where
- is the density of the fluid.
- is the upstream pressure.
- is the downstream pressure.
- is a characteristic velocity of the flow.
Cavitation number[edit]
The cavitation number has a similar structure, but a different meaning and use:
The cavitation number (Ca) is a dimensionless number used in flow calculations. It expresses the relationship between the difference of a local absolute pressure from the vapor pressure and the kinetic energy per volume, and is used to characterize the potential of the flow to cavitate.
It is defined as
where
- is the density of the fluid.
- is the local pressure.
- is the vapor pressure of the fluid.
- is a characteristic velocity of the flow.
Cavitation number is among the very few means to characterize a cavitating flow in a fluidic system. When the upstream pressure increase velocity of the working fluid increases as well. However, the velocity increase rate is one order of magnitude higher than the pressure increase. This means that, cavitation number follows a decreasing trend while upstream pressure increases. The first moment that cavitating bubbles appear in a system, inception happens. The corresponding cavitation number at this moment is inception cavitation number. According to the discussion above, this number is the highest number recorded in a system. Researchers are often interested in recording inception of cavitating flow at relatively low upstream pressure when they are aiming for the non-destructive applications on this phenomenon. With the development of cavitating flow, Cavitation number decreases until supercavitation happens which is the highest velocity and flowrate that the system can pass. As a result, the lower cavitation number shows the higher intensity on the cavitating flow. After supercavitation, the system is incapable of passing more fluid. However, the upstream pressure is increasing. As a result, cavitation number starts to follow an increasing trend. This trend could be seen in many published articles in the literature.[1]
https://en.wikipedia.org/wiki/Euler_number_(physics)
Refraction is generally accompanied by partial reflection. When waves are refracted from a medium of lower propagation speed (higher refractive index) to a medium of higher speed—e.g., from water to air—the angle of refraction (between the outgoing ray and the surface normal) is greater than the angle of incidence (between the incoming ray and the normal). As the angle of incidence approaches a certain threshold, called the critical angle, the angle of refraction approaches 90°, at which the refracted ray becomes parallel to the boundary surface. As the angle of incidence increases beyond the critical angle, the conditions of refraction can no longer be satisfied, so there is no refracted ray, and the partial reflection becomes total. For visible light, the critical angle is about 49° for incidence from water to air, and about 42° for incidence from common glass to air.
https://en.wikipedia.org/wiki/Total_internal_reflection
However, it is sometimes useful and does not cause damage when the bubbles collapse away from machinery, such as in supercavitation.
https://en.wikipedia.org/wiki/Cavitation
https://en.wikipedia.org/wiki/Cavitation#Pumps_and_propellers
https://en.wikipedia.org/wiki/Water_tunnel_(hydrodynamic)
https://en.wikipedia.org/wiki/Water_hammer
A hydraulic accumulator is a pressure storage reservoir in which an incompressible hydraulic fluid is held under pressure that is applied by an external source of mechanical energy. The external source can be an engine, a spring, a raised weight, or a compressed gas.[note 1] An accumulator enables a hydraulic system to cope with extremes of demand using a less powerful pump, to respond more quickly to a temporary demand, and to smooth out pulsations. It is a type of energy storage device.
Compressed gas accumulators, also called hydro-pneumatic accumulators, are by far the most common type.
Types of accumulators[edit]
Towers[edit]
The first accumulators for William Armstrong's hydraulic dock machinery were simple raised water towers. Water was pumped to a tank at the top of these towers by steam pumps. When dock machinery required hydraulic power, the hydrostatic head of the water's height above ground provided the necessary pressure.
These simple accumulators were extremely tall. For instance, Grimsby Dock Tower, built in 1852, is 309 feet (94 m) tall. Because of their size, they were costly, and so were constructed for less than a decade. Around the same time, John Fowler was working on the construction of the ferry quay at nearby New Holland but could not use similar hydraulic power as the poor ground conditions did not permit a tall accumulator tower to be built. By the time Grimsby was opened, it was already obsolete as Armstrong had developed the more complex, but much smaller, weighted accumulator for use at New Holland.[1] In 1892 the original Grimsby tower's function was replaced, on Fowler's advice, by a smaller weighted accumulator on an adjacent dock, although the tower remains to this day as a well-known landmark.
Other surviving towers include one adjacent to East Float in Birkenhead, England, and another located at the Bramley-Moore Dock, Liverpool, England. The latter tower is to be renovated as part of plans for the proposed development of the area associated with the construction of a new football stadium for Everton F.C.
Raised weight[edit]
A raised weight accumulator consists of a vertical cylinder containing fluid connected to the hydraulic line. The cylinder is closed by a piston on which a series of weights are placed that exert a downward force on the piston and thereby pressurizes the fluid in the cylinder. In contrast to compressed gas and spring accumulators, this type delivers a nearly constant pressure, regardless of the volume of fluid in the cylinder, until it is empty. (The pressure will decline somewhat as the cylinder is emptied due to the decline in weight of the remaining fluid.)
A working example of this type of accumulator may be found at the hydraulic engine house, Bristol Harbour.[2] The original 1887 accumulator is in place in its tower, an external accumulator was added in 1954 and this system was used until 2010 to power the Cumberland Basin (Bristol) lock gates. The water is pumped from the harbour into a header tank and then fed by gravity to the pumps. The working pressure is 750 psi (5.2 MPa, or 52 bar) which was used to power the cranes, bridges and locks of Bristol Harbour.[citation needed]
The original operating mechanism of Tower Bridge, London, also used this type of accumulator. Although no longer in use, two of the six accumulators may still be seen in situ in the bridge's museum.[original research?]
Regent's Canal Dock, now named Limehouse Basin has the remains of a hydraulic accumulator, dating from 1869, a fragment of the oldest remaining such facility in the world, the second at the dock, which was installed later than that at Poplar Dock, originally listed incorrectly as a signalbox for the London and Blackwall Railway, when correctly identified, it was restored as a tourist attraction by the now defunct London Docklands Development Corporation.[clarification needed] Now owned by the Canal & River Trust, it is open for large groups on application to the Dockmaster's Office at the basin and on both the afternoons of London Open House Weekend, held on the third weekend of September each year.[3]
London had an extensive public hydraulic power system from the mid-nineteenth century finally closing in the 1970s with 5 hydraulic power stations, operated by the London Hydraulic Power Company. Railway goods yards and docks often had their own separate system.[citation needed]
Air-filled accumulator[edit]
A simple form of accumulator is an enclosed volume, filled with air. A vertical section of pipe, often enlarged diameter, may be enough and fills itself with air, trapped as the pipework fills.
Such accumulators typically do not have enough capacity to be useful for storing significant power since they cannot be pre-charged with high pressure gas, but they can act as a buffer to absorb fluctuations in pressure. They are used to smooth out the delivery from piston pumps. Another use is as a shock absorber to damp out water hammer, this application is an integral part of most ram pumps. Loss of air will result in loss of effectiveness, if air will be lost over time the design must include some way to renew it.
Compressed gas (or gas-charged) closed accumulator[edit]
A compressed gas accumulator consists of a cylinder with two chambers that are separated by an elastic diaphragm, a totally enclosed bladder, or a floating piston. One chamber contains the fluid and is connected to the hydraulic line. The other chamber contains an inert gas (typically nitrogen), usually under pressure, that provides the compressive force on the hydraulic fluid. Inert gas is used because oxygen and oil can form an explosive mixture when combined under high pressure. As the volume of the compressed gas changes, the pressure of the gas (and the pressure on the fluid) changes inversely.
For low pressure water system use the water usually fills a rubber bladder within the tank (pictured), preventing contact with the tank which would otherwise need to be corrosion resistant. Units designed for high-pressure applications such as hydraulicsystems are usually pre-charged to a very high pressure (approaching the system operating pressure) and are designed to prevent the bladder or membrane being damaged by this internal pressure when the system pressure is low. For bladder types this generally requires the bladder to be filled with the gas so that when system pressure is zero the bladder is fully expanded rather than being crushed by the gas charge. To prevent the bladder being forced out of the device when the system pressure is low there is typically either an anti-extrusion plate attached to the bladder that presses against and seals the entrance, or a spring-loaded plate on the entrance that closes when the bladder presses against it.
It is possible to increase the gas volume of the accumulator by coupling a gas bottle to the gas side of the accumulator. For the same swing in system pressure this will result in a larger portion of the accumulator volume being used. If the pressure does not vary over a very wide range this can be a cost effective way to reduce the size of the accumulator needed. If the accumulator is not of the piston type care must be taken that the bladder or membrane will not be damaged in any expected over-pressure situation, many bladder-type accumulators cannot tolerate the bladder being crushed under pressure.
A compressed gas accumulator was invented by Jean Mercier[4] for use in variable-pitch propellers.
Spring type[edit]
A spring type accumulator is similar in operation to the gas-charged accumulator above, except that a heavy spring (or springs) is used to provide the compressive force. According to Hooke's law the magnitude of the force exerted by a spring is linearly proportional to its change of length. Therefore, as the spring compresses, the force it exerts on the fluid is increased linearly.
Metal bellows type[edit]
The metal bellows accumulators function similarly to the compressed gas type, except the elastic diaphragm or floating piston is replaced by a hermetically sealed welded metal bellows. Fluid may be internal or external to the bellows. The advantages to the metal bellows type include exceptionally low spring rate, allowing the gas charge to do all the work with little change in pressure from full to empty, a long stroke that allows efficient usage of the casing volume, and the bellows can be built to be resistant to significant over-pressure that would crush a bladder-type separator. The welded metal bellows accumulator provides an exceptionally high level of accumulator performance, and can be produced with a broad spectrum of alloys resulting in a broad range of fluid compatibility. Other advantages to this type are that it does not face issues with high pressure operation, may be built to be resistant to very high or low temperatures or certain aggressive chemicals, and may be significantly longer lasting in some situations. Metal bellows tend to be significantly more costly than other common types to produce.
Functioning of an accumulator[edit]
In modern, often mobile, hydraulic systems the preferred item is a gas charged accumulator, but simple systems may be spring-loaded. There may be more than one accumulator in a system. The exact type and placement of each may be a compromise[clarification needed] due to its effects and the costs of manufacture.
An accumulator is placed close to the pump with a non-return valve preventing flow back to the pump. In the case of piston-type pumps this accumulator is placed in the ideal location to absorb pulsations of energy from the multi-piston pump.[citation needed] It also helps protect the system from fluid hammer. This protects system components, particularly pipework, from both potentially destructive forces.
An additional benefit is the additional energy that can be stored while the pump is subject to low demand. The designer can use a smaller-capacity pump. The large excursions of system components, such as landing gear on a large aircraft, that require a considerable volume of fluid can also benefit from one or more accumulators. These are often placed close to the demand to help overcome restrictions and drag from long pipework runs. The outflow of energy from a discharging accumulator is much greater, for a short time, than even large pumps could generate.
An accumulator can maintain the pressure in a system for periods when there are slight leaks without the pump being cycled on and off constantly. When temperature changes cause pressure excursions the accumulator helps absorb them. Its size helps absorb fluid that might otherwise be locked in a small fixed system with no room for expansion due to valve arrangement.
The gas precharge in an accumulator is set so that the separating bladder, diaphragm or piston does not reach or strike either end of the operating cylinder. The design precharge normally ensures that the moving parts do not foul the ends or block fluid passages. Poor maintenance of precharge can destroy an operating accumulator. A properly designed and maintained accumulator should operate trouble-free for years.[citation needed]
See also[edit]
https://en.wikipedia.org/wiki/Hydraulic_accumulator
An accumulator is an energy storage device: a device which accepts energy, stores energy, and releases energy as needed. Some accumulators accept energy at a low rate (low power) over a long time interval and deliver the energy at a high rate (high power) over a short time interval. Some accumulators accept energy at a high rate over a short time interval and deliver the energy at a low rate over longer time interval. Some accumulators typically accept and release energy at comparable rates. Various devices can store thermal energy, mechanical energy, and electrical energy. Energy is usually accepted and delivered in the same form. Some devices store a different form of energy than what they receive and deliver performing energy conversion on the way in and on the way out.
Examples of accumulators include steam accumulators, mainsprings, flywheel energy storage, hydraulic accumulators, rechargeable batteries, capacitors, inductors, compensated pulsed alternators (compulsators), and pumped-storage hydroelectric plants.
In general usage in an electrical context, the word accumulator normally refers to a lead–acid battery.
The London Tower Bridge is operated via an accumulator. The original raising mechanism was powered by pressurised water stored in several hydraulic accumulators.[1] In 1974, the original operating mechanism was largely replaced by a new electro-hydraulic drive system.
https://en.wikipedia.org/wiki/Accumulator_(energy)
Pumped-storage hydroelectricity (PSH), or pumped hydroelectric energy storage (PHES), is a type of hydroelectric energy storage used by electric power systems for load balancing. The method stores energy in the form of gravitational potential energy of water, pumped from a lower elevation reservoir to a higher elevation. Low-cost surplus off-peak electric power is typically used to run the pumps. During periods of high electrical demand, the stored water is released through turbines to produce electric power. Although the losses of the pumping process make the plant a net consumer of energy overall, the system increases revenue by selling more electricity during periods of peak demand, when electricity prices are highest. If the upper lake collects significant rainfall or is fed by a river then the plant may be a net energy producer in the manner of a traditional hydroelectric plant.
Pumped-storage hydroelectricity allows energy from intermittent sources (such as solar, wind) and other renewables, or excess electricity from continuous base-load sources (such as coal or nuclear) to be saved for periods of higher demand.[1][2] The reservoirs used with pumped storage are quite small when compared to conventional hydroelectric dams of similar power capacity, and generating periods are often less than half a day.
Pumped storage is by far the largest-capacity form of grid energy storage available, and, as of 2020, the United States Department of Energy Global Energy Storage Database reports that PSH accounts for around 95% of all active tracked storage installations worldwide, with a total installed throughput capacity of over 181 GW, of which about 29 GW are in the United States, and a total installed storage capacity of over 1.6 TWh, of which about 250 GWh are in the United States.[3] The round-trip energy efficiency of PSH varies between 70%–80%,[4][5][6][7] with some sources claiming up to 87%.[8] The main disadvantage of PSH is the specialist nature of the site required, needing both geographical height and water availability. Suitable sites are therefore likely to be in hilly or mountainous regions, and potentially in areas of natural beauty, making PSH susceptible to social and ecological issues. Many recently proposed projects, at least in the U.S., avoid highly sensitive or scenic areas, and some propose to take advantage of "brownfield" locations such as disused mines.[9]
Home use[edit]
Using a pumped-storage system of cisterns and small generators, pico hydro may also be effective for "closed loop" home energy generation systems.[58][59]
Fracking[edit]
Using hydraulic fracturing pressure can be stored underground in strata such as shale. The shale used contains no hydrocarbons.[60]
See also[edit]
https://en.wikipedia.org/wiki/Pumped-storage_hydroelectricity
Compressed-air energy storage (CAES) is a way to store energy for later use using compressed air. At a utilityscale, energy generated during periods of low demand can be released during peak load periods.[1]
The first utility scale CAES project has been built in Huntorf, Germany, being still operational.[2] While the Huntorf CAES plant was initially developed as a load balancer for fossil fuel-generated electricity, the global shift towards renewable energy has led to a renewed interest in CAES systems,[3] to help highly intermittent energy sources like photovoltaics and wind satisfy fluctuating electricity demands.[4]
One ongoing challenge in large scale CAES design is the management of thermal energy since the compression of air leads to an unwanted temperature increase that not only reduces operational efficiency but can also lead to damage. The main difference between various CAES architectures lies in thermal engineering. On the other hand, small-scale systems have long been used as propulsion of mine locomotives. Compared to traditional batteries, CAES systems can store energy for longer periods of time and have less upkeep.
https://en.wikipedia.org/wiki/Compressed-air_energy_storage
A gravity battery is a type of electrical storage device that stores gravitational energy, the energy stored in an object resulting from a change in height due to gravity, also called potential energy. A gravity battery works by using excess energy from the grid to raise a mass to generate gravitational potential energy, which is then dropped to convert potential energy into electricity through an electric generator. Energy generated from a gravity battery is a form of sustainable energy. One form of a gravity battery is one that releases a mass, such as a block of concrete, to generate electricity. The most common gravity battery is used in pumped-storage hydroelectricity, where water is pumped to higher elevations to store energy and released through water turbines to generate electricity.[1]
Development[edit]
The earliest form of a device that used gravity to power mechanical movement was the pendulum clock, invented in 1656 by Christiaan Huygens. The clock was powered by the force of gravity using an escapement mechanism, that made a pendulum move back and forth. Since then, gravity batteries have advanced into systems that can harness the power of gravity and turn it into electricity for large scale energy storage.
The first gravity based pumped-storage hydroelectricity (PSH) system was developed in 1907 in Switzerland. In 1930, pumped-storage came to the United States by the Connecticut Electric and Power Company. As of 2019, the total world capacity for PSH is 168 GW (gigawatts).[2] The United States has 23 GW capacity from PSH, accounting for nearly 2% of the energy supply system and 95% of utility-scale energy storage in the US. Gravity based pumped-storage electricity is currently the largest form of grid energy storage in the world.[3][4][5][6]
In 2012, Martin Riddiford and Jim Reeves developed the first functioning prototype of GravityLight, a small-scale gravity battery that is now commercially available in certain countries.[7]
Energy Vault, an energy storage company, is also currently working on research and testing to develop gravity batteries on a larger scale. Founded by Bill Gross, Andewa Pedretti, and Robert Piconi in 2017, Energy Vault is currently in the midst of taking what GravityLight created to a larger scale. Energy Vault is developing a crane that generates electricity from dropping blocks of concrete rather than water. Energy Vault has not specified a release date for its product, but prototypes are in the works and Energy Vault’s stacked blocks concept is being built to be a promising long-duration storage technology.[8][9][10]In late 2020, the Swiss company built in Arbedo-Castione six cranes installed in a 110-meter-high tower moving a 35-ton concrete block up and down that can store 80 megawatt hours of energy.[11][12]
Cascadia Carbon Inc., a Portland, Oregon headquartered climate technology company, is also developing a potential energy battery for renewable grid stability in conjunction with University of British Columbia[citation needed]. Their pilot project aims to provide a commercial proof-of-concept which can be expanded to accommodate the gigawatts of new solar and wind storage which will be coming online over the next decade as the world begins to decarbonize the electrical grid.[13][unreliable source]
Gravitricity, another gravity battery company, is working on another approach to a new energy storage system. Founded in 2011 by inventor Peter Fraenkel, Gravitricity built a 10 meter 250 kilowatt gravity battery prototype in Scotland that started trial operations and grid-connection in 2021.[14][15][16] In April 2021, Gravitricity had installed a gravity battery which generated its first power at a site in Edinburgh.[17]
Mechanisms and parts[edit]
Gravity batteries can have different designs and structures, but all gravity batteries use the same properties of physics to generate energy. Gravitational potential energy is the work required to move an object in the opposite direction of Earth's gravity, expressed by the equation
where U is gravitational potential energy, m is the mass of the object, g is the acceleration of the object due to gravity (9.8 m/s on earth), and h is the height of the object. Using the work-energy principle, the total amount of energy generated can be expressed by the equation
where E is the total amount of energy generated and h1 and h2 represent the initial and final heights of an object. The change of energy directly correlates to the vertical displacement of a mass; the higher a mass is lifted, the more gravitational potential energy is stored. The change in energy also directly correlates to the mass of an object; the heavier the mass, the bigger the change in energy.
In a gravity battery, a mass is displaced, or lifted, to generate gravitational potential energy that is transformed into electricity. Gravity batteries store gravitational potential energy by lifting a mass to a certain height using a pump, crane, or motor. After the mass is lifted, it now stores a certain gravitational potential energy based on the mass of the object and how high it was lifted. The stored gravitational potential energy is then transferred into electricity. The mass is lowered or released to fall back to its original height, which causes a generator to spin and create electricity.
Types of gravity batteries[edit]
One structure of a gravity battery uses a very tall structure with a heavy mass. This tall structure can be built above ground, such as a tall building or tower, or a deep hole can be drilled into earth's surface to a certain depth necessary for the battery to meet specifications. A mass is lifted to the top of the tower, or the top of the hole, using a system of pulleys. Energy is needed to lift the mass, but this energy is usually surplus energy that is used during times when energy production is greater than the demand. When the surplus energy runs out, the mass is then dropped to generate electricity through the generator.[18]
Large scale[edit]
Lifted Weight Storage (LWS) technology is developed by Energozapas company. LWS operational principle is based on consuming electricity while lifting weights vertically in respect to the Earth’s gravitational field (several hundred meters high) and generating energy when released weights go down due to the gravity force. Solid blocks made of pressed soil act as weights. Load bearing structure is a reinforced concrete construction, built with the help of construction robots. Energozapas technology enables to build industrial scale energy storages starting with 10MW in power. Storage lifetime is 50 years, roundtrip efficiency is 83%.[19]
Lift Renewable Energy uses a form of gravity battery. To store energy, buoyant gas containers are pulled down into water by a winch, water is in effect lifted hundreds of meters. The cycle is then reversed and electricity is generated as the gas containers rise. Relatively little infrastructure is required, the batteries can be sited near major population centers and round trip efficiency is 85+%. The system can be scaled from KWH's to GWH's. https://lift-re.com/
EnergyVault is working on developing large scale gravity batteries. The gravity battery they are working on developing is an energy storage tower built from concrete blocks. 120 meter cranes use excess energy from the electric grid to lift and stack concrete blocks, each weighing 32 metric tons. Energy is retrieved when bricks fall to generate energy by turning a generator. One commercial unit can store 20 MWh or energy, or enough to power 2,000 Swiss homes a day.[9]
Gravitricity's gravity battery unit consists of a convertible electric winch/generator, cables, a large weight, and vertical shaft going 150 to 1500 meters underground using disused mine shafts initially. The electric winch lifts a weight weighing from 500 to 5000 tonnes to the top of the shaft. When weight is released, it rotates the electric winch within a magnetic field to generate energy. The system generates 10 MWh, enough power for 13,000 homes for two hours. The battery can also be controlled to drop the weight quickly for a small burst of high-power energy.[14]
Another form of a gravity battery is pumped-storage hydroelectricity (PSH), the largest form of grid-energy storage. PSH uses water instead of a solid mass, which is pumped from a lower reservoir to a higher reservoir before being released through turbines to create energy. An alternative proposal uses a proprietary high-density liquid, 21⁄2 times denser than water, which requires a smaller head (elevation) and thus decreases the size and cost of the necessary infrastructure.[20][21]
Energy-storage-by-rail is a concept where excess renewable energy is used to run heavy trains cars uphill during times of low energy demand. The potential energy is release later by using regenerative braking as they roll downhill, acting as a gravity battery.[22] A utility-scale facility called GravityLine began construction in Nevada in October 2020. GravityLine is being constructed by Advanced Rail Energy Storage (ARES) located at the Gamebird Pit gravel mine in the Pahrump Valley, Nevada. The 50-MW facility is expected to store excess renewable energy from Western U.S. sources and deliver up to 15 minutes of regulation services at full capacity.[23]
Small scale[edit]
GravityLight is a small gravity-powered light that operates by manually lifting a bag of rocks or sand up and then letting it fall by itself to generate energy. The GravityLight was designed to help the almost one billion people in the world who do not have a source of electricity, as it would eliminate the need for people who do not have access to electricity to rely on kerosene lamps, which are expensive, dangerous, and polluting.[7][24][25]
Economics and efficiency[edit]
Cost of gravity batteries varies by design.
Pumped storage hydropower costs $165/kWh to operate, with a levelized cost of storage (LCOS), of $0.17/kWh.[26][27] The pumps and turbines of PSH systems operate at 90% efficiency.[28]
EnergyVault's proposed gravity battery system range from 7 to 8 million in building price but has a LCOS of $0.05/kWh and a round-trip efficiency of 88–92%. This is 50% cheaper when compared to the LCOS of lithium-ion batteries, which are $0.25/kWh-$0.35/kWh.[29]
Gravitricity's 250 kW demonstrator is expected to be $1.25 million, promising a 50-year lifespan and efficiency of 80–90%. Relative costs of gravity storage installations that would use 2000-tonne weights suspended from winches in disused mineshafts, compared with lithium ion batteries, indicate that although the "up front cost is high" the 25-year lifespan of such equipment—with no degradation of capacity during use—makes a "compelling proposition" for large-scale grid balancing purposes.[30]
Unlike pumped-storage hydroelectricity, solar panels, and wind turbines, which can only operate under certain conditions or in certain areas, gravity batteries like those proposed by EnergyVault and Gravitricity can be built anywhere in the world and use materials from the building site.[18][31]
Environmental impacts[edit]
Gravity batteries are designed to be paired with renewable energy solutions whose sources (sunlight, wind, etc) are frequently variable and do not necessarily coincide with demand. It is hoped that they will have a better long term cost than chemical batteries, while having fewer environmental issues than other traditional storage solutions such as pumped-water storage. It is anticipated that gravity battery systems will be able to quickly provide power during peak consumption which may allow them to supplement or replace fossil fuel peaking power plants. Single weight systems are expected to be able to achieve full power generation in less than a second.[15]
Implementing gravity batteries on a larger scale would therefore decrease the need for fossil fuels, significantly cutting down CO2 emissions.[citation needed]
Gravity batteries are more environmentally friendly than lithium-ion batteries, since lithium-ion batteries have a shorter lifetime and problems arise when they need to be disposed of.[1]
Gravity (chemical) battery[edit]
From 1870 to 1930,[32] the term "gravity battery" was used to describe a collection of popular battery types where gravity was used to keep the chemical constituents separate based on their respective densities.[33]
See also[edit]
https://en.wikipedia.org/wiki/Gravity_battery
Geothermal power is electrical power generated from geothermal energy. Technologies in use include dry steam power stations, flash steam power stations and binary cycle power stations. Geothermal electricity generation is currently used in 26 countries,[1][2] while geothermal heating is in use in 70 countries.[3]
As of 2019, worldwide geothermal power capacity amounts to 15.4 gigawatts (GW), of which 23.86 percent or 3.68 GW are installed in the United States.[4] International markets grew at an average annual rate of 5 percent over the three years to 2015, and global geothermal power capacity is expected to reach 14.5–17.6 GW by 2020.[5] Based on current geologic knowledge and technology the GEA publicly discloses, the Geothermal Energy Association (GEA) estimates that only 6.9 percent of total global potential has been tapped so far, while the IPCC reported geothermal power potential to be in the range of 35 GW to 2 TW.[3] Countries generating more than 15 percent of their electricity from geothermal sources include El Salvador, Kenya, the Philippines, Iceland, New Zealand,[6] and Costa Rica.
Geothermal power is considered to be a sustainable, renewable source of energy because the heat extraction is small compared with the Earth's heat content.[7] The greenhouse gas emissions of geothermal electric stations are on average 45 grams of carbon dioxide per kilowatt-hour of electricity, or less than 5 percent of that of conventional coal-fired plants.[8]
As a source of renewable energy for both power and heating, geothermal has the potential to meet 3-5% of global demand by 2050. With economic incentives, it is estimated that by 2100 it will be possible to meet 10% of global demand.[6]
https://en.wikipedia.org/wiki/Geothermal_power
Cyclic stress is the distribution of forces (aka stresses) that change over time in a repetitive fashion. As an example, consider one of the large wheels used to drive an aerial lift such as a ski lift. The wire cable wrapped around the wheel exerts a downward force on the wheel and the drive shaft supporting the wheel. Although the shaft, wheel, and cable move, the force remains nearly vertical relative to the ground. Thus a point on the surface of the drive shaft will undergo tension when it is pointing towards the ground and compression when it is pointing to the sky.
Types of cyclic stress[edit]
Cyclic stress is frequently encountered in rotating machinery where a bending moment is applied to a rotating part. This is called a cyclic bending stress and the aerial lift above is a good example. However, cyclic axial stresses and cyclic torsional stresses also exist. An example of cyclic axial stress would be a bungee cord (see bungee jumping), which must support the mass of people as they jump off structures such as bridges. When a person reaches the end of a cord, the cord deflects elastically and stops the person's descent. This creates a large axial stress in the cord. A fraction of the elastic potential energy stored in the cord is typically transferred back to the person, throwing the person upwards some fraction of the distance he or she fell. The person then falls on the cord again, inducing stress in the cord. This happens multiple times per jump. The same cord is used for several jumps, creating cyclical stresses in the cord that could eventually cause failure if not replaced.
Cyclic stress and material failure[edit]
When cyclic stresses are applied to a material, even though the stresses do not cause plastic deformation, the material may fail due to fatigue. Fatigue failure is typically modeled by decomposing cyclic stresses into mean and alternating components. Mean stress is the time average of the principal stress. The definition of alternating stress varies between different sources. It is either defined as the difference between the minimum and the maximum stress, or the difference between the mean and maximum stress.[1][2] Engineers try to design mechanisms whose parts are subjected to a single type (bending, axial, or torsional) of cyclic stress because this more closely matches experiments used to characterize fatigue failure in different materials.
https://en.wikipedia.org/wiki/Cyclic_stress
A polarization rotator is an optical device that rotates the polarization axis of a linearly polarized light beam by an angle of choice. Such devices can be based on the Faraday effect, on birefringence, or on total internal reflection.[1] Rotators of linearly polarized light have found widespread applications in modern optics since laser beams tend to be linearly polarized and it is often necessary to rotate the original polarization to its orthogonal alternative.[1]
https://en.wikipedia.org/wiki/Polarization_rotator
Ferroelectricity is a characteristic of certain materials that have a spontaneous electric polarization that can be reversed by the application of an external electric field.[1][2] All ferroelectrics are pyroelectric, with the additional property that their natural electrical polarization is reversible. The term is used in analogy to ferromagnetism, in which a material exhibits a permanent magnetic moment. Ferromagnetism was already known when ferroelectricity was discovered in 1920 in Rochelle salt by Valasek.[3] Thus, the prefix ferro, meaning iron, was used to describe the property despite the fact that most ferroelectric materials do not contain iron. Materials that are both ferroelectric and ferromagnetic are known as multiferroics.
Polarization[edit]
When most materials are electrically polarized, the polarization induced, P, is almost exactly proportional to the applied external electric field E; so the polarization is a linear function. This is called linear dielectric polarization (see figure). Some materials, known as paraelectric materials,[4] show a more enhanced nonlinear polarization (see figure). The electric permittivity, corresponding to the slope of the polarization curve, is not constant as in linear dielectrics but is a function of the external electric field.
In addition to being nonlinear, ferroelectric materials demonstrate a spontaneous nonzero polarization (after entrainment, see figure) even when the applied field E is zero. The distinguishing feature of ferroelectrics is that the spontaneous polarization can be reversed by a suitably strong applied electric field in the opposite direction; the polarization is therefore dependent not only on the current electric field but also on its history, yielding a hysteresis loop. They are called ferroelectrics by analogy to ferromagnetic materials, which have spontaneous magnetization and exhibit similar hysteresis loops.
Typically, materials demonstrate ferroelectricity only below a certain phase transition temperature, called the Curie temperature (TC) and are paraelectric above this temperature: the spontaneous polarization vanishes, and the ferroelectric crystal transforms into the paraelectric state. Many ferroelectrics lose their pyroelectric properties above TC completely, because their paraelectric phase has a centrosymmetric crystal structure.[5]
Applications[edit]
The nonlinear nature of ferroelectric materials can be used to make capacitors with adjustable capacitance. Typically, a ferroelectric capacitor simply consists of a pair of electrodes sandwiching a layer of ferroelectric material. The permittivity of ferroelectrics is not only adjustable but commonly also very high, especially when close to the phase transition temperature. Because of this, ferroelectric capacitors are small in physical size compared to dielectric (non-tunable) capacitors of similar capacitance.
The spontaneous polarization of ferroelectric materials implies a hysteresis effect which can be used as a memory function, and ferroelectric capacitors are indeed used to make ferroelectric RAM[6] for computers and RFID cards. In these applications thin films of ferroelectric materials are typically used, as this allows the field required to switch the polarization to be achieved with a moderate voltage. However, when using thin films a great deal of attention needs to be paid to the interfaces, electrodes and sample quality for devices to work reliably.[7]
Ferroelectric materials are required by symmetry considerations to be also piezoelectric and pyroelectric. The combined properties of memory, piezoelectricity, and pyroelectricity make ferroelectric capacitors very useful, e.g. for sensor applications. Ferroelectric capacitors are used in medical ultrasound machines (the capacitors generate and then listen for the ultrasound ping used to image the internal organs of a body), high quality infrared cameras (the infrared image is projected onto a two dimensional array of ferroelectric capacitors capable of detecting temperature differences as small as millionths of a degree Celsius), fire sensors, sonar, vibration sensors, and even fuel injectors on diesel engines.
Another idea of recent interest is the ferroelectric tunnel junction (FTJ) in which a contact is made up by nanometer-thick ferroelectric film placed between metal electrodes.[8] The thickness of the ferroelectric layer is small enough to allow tunneling of electrons. The piezoelectric and interface effects as well as the depolarization field may lead to a giant electroresistance (GER) switching effect.
Yet another burgeoning application is multiferroics, where researchers are looking for ways to couple magnetic and ferroelectric ordering within a material or heterostructure; there are several recent reviews on this topic.[9]
Catalytic properties of ferroelectrics have been studied since 1952 when Parravano observed anomalies in CO oxidation rates over ferroelectric sodium and potassium niobates near the Curie temperature of these materials.[10] Surface-perpendicular component of the ferroelectric polarization can dope polarization-dependent charges on surfaces of ferroelectric materials, changing their chemistry.[11][12][13] This opens the possibility of performing catalysis beyond the limits of the Sabatier principle.[14] Sabatier principle states that the surface-adsorbates interaction has to be an optimal amount: not too weak to be inert toward the reactants and not too strong to poison the surface and avoid desorption of the products: a compromise situation.[15] This set of optimum interactions is usually referred to as "top of the volcano" in activity volcano plots.[16] On the other hand, ferroelectric polarization-dependent chemistry can offer the possibility of switching the surface—adsorbates interaction from strong adsorption to strong desorption, thus a compromise between desorption and adsorption is no longer needed.[14] Ferroelectric polarization can also act as an energy harvester.[17] Polarization can help the separation of photo-generated electron-hole pairs, leading to enhanced photocatalysis.[18] Also, due to pyroelectric and piezoelectric effects under varying temperature (heating/cooling cycles)[19][20] or varying strain (vibrations) conditions[21] extra charges can appear on the surface and drive various (electro)chemical reactions forward.
Materials[edit]
The internal electric dipoles of a ferroelectric material are coupled to the material lattice so anything that changes the lattice will change the strength of the dipoles (in other words, a change in the spontaneous polarization). The change in the spontaneous polarization results in a change in the surface charge. This can cause current flow in the case of a ferroelectric capacitor even without the presence of an external voltage across the capacitor. Two stimuli that will change the lattice dimensions of a material are force and temperature. The generation of a surface charge in response to the application of an external stress to a material is called piezoelectricity. A change in the spontaneous polarization of a material in response to a change in temperature is called pyroelectricity.
Generally, there are 230 space groups among which 32 crystalline classes can be found in crystals. There are 21 non-centrosymmetric classes, within which 20 are piezoelectric. Among the piezoelectric classes, 10 have a spontaneous electric polarization, that varies with the temperature, therefore they are pyroelectric. Among pyroelectric materials, some of them are ferroelectric.[citation needed]
| 32 Crystalline classes | ||||
|---|---|---|---|---|
| 21 noncentrosymmetric | 11 centrosymmetric | |||
| 20 classes piezoelectric | non piezoelectric | |||
| 10 classes pyroelectric | non pyroelectric | |||
| ferroelectric | non ferroelectric | |||
| e.g. : PbZr/TiO3, BaTiO3, PbTiO3 | e.g. : Tourmaline, ZnO, AlN | e.g. : Quartz, Langasite | ||
Ferroelectric phase transitions are often characterized as either displacive (such as BaTiO3) or order-disorder (such as NaNO2), though often phase transitions will demonstrate elements of both behaviors. In barium titanate, a typical ferroelectric of the displacive type, the transition can be understood in terms of a polarization catastrophe, in which, if an ion is displaced from equilibrium slightly, the force from the local electric fields due to the ions in the crystal increases faster than the elastic-restoring forces. This leads to an asymmetrical shift in the equilibrium ion positions and hence to a permanent dipole moment. The ionic displacement in barium titanate concerns the relative position of the titanium ion within the oxygen octahedral cage. In lead titanate, another key ferroelectric material, although the structure is rather similar to barium titanate the driving force for ferroelectricity is more complex with interactions between the lead and oxygen ions also playing an important role. In an order-disorder ferroelectric, there is a dipole moment in each unit cell, but at high temperatures they are pointing in random directions. Upon lowering the temperature and going through the phase transition, the dipoles order, all pointing in the same direction within a domain.
An important ferroelectric material for applications is lead zirconate titanate (PZT), which is part of the solid solution formed between ferroelectric lead titanate and anti-ferroelectric lead zirconate. Different compositions are used for different applications; for memory applications, PZT closer in composition to lead titanate is preferred, whereas piezoelectric applications make use of the diverging piezoelectric coefficients associated with the morphotropic phase boundary that is found close to the 50/50 composition.
Ferroelectric crystals often show several transition temperatures and domain structure hysteresis, much as do ferromagnetic crystals. The nature of the phase transition in some ferroelectric crystals is still not well understood.
In 1974 R.B. Meyer used symmetry arguments to predict ferroelectric liquid crystals,[22] and the prediction could immediately be verified by several observations of behavior connected to ferroelectricity in smectic liquid-crystal phases that are chiral and tilted. The technology allows the building of flat-screen monitors. Mass production between 1994 and 1999 was carried out by Canon. Ferroelectric liquid crystals are used in production of reflective LCoS.
In 2010 David Field found that prosaic films of chemicals such as nitrous oxide or propane exhibited ferroelectric properties.[citation needed] This new class of ferroelectric materials exhibit "spontelectric" properties, and may have wide-ranging applications in device and nano-technology and also influence the electrical nature of dust in the interstellar medium.
Other ferroelectric materials used include triglycine sulfate, polyvinylidene fluoride (PVDF) and lithium tantalate.[23]
It should be possible to produce materials which combine both ferroelectric and metallic properties simultaneously, at room temperature.[24] According to research published in 2018 in Nature Communications,[25] scientists were able to produce a "two-dimensional" sheet of material which was both "ferroelectric" (had a polar crystal structure) and which conducted electricity.
See also[edit]
Physics | Lists |
https://en.wikipedia.org/wiki/Ferroelectricity
Curing[edit]
Concrete must be kept moist during curing in order to achieve optimal strength and durability.[65] During curing hydration occurs, allowing calcium-silicate hydrate (C-S-H) to form. Over 90% of a mix's final strength is typically reached within four weeks, with the remaining 10% achieved over years or even decades.[66] The conversion of calcium hydroxide in the concrete into calcium carbonate from absorption of CO2 over several decades further strengthens the concrete and makes it more resistant to damage. This carbonation reaction, however, lowers the pH of the cement pore solution and can corrode the reinforcement bars.
Hydration and hardening of concrete during the first three days is critical. Abnormally fast drying and shrinkage due to factors such as evaporation from wind during placement may lead to increased tensile stresses at a time when it has not yet gained sufficient strength, resulting in greater shrinkage cracking. The early strength of the concrete can be increased if it is kept damp during the curing process. Minimizing stress prior to curing minimizes cracking. High-early-strength concrete is designed to hydrate faster, often by increased use of cement that increases shrinkage and cracking. The strength of concrete changes (increases) for up to three years. It depends on cross-section dimension of elements and conditions of structure exploitation.[67] Addition of short-cut polymer fibers can improve (reduce) shrinkage-induced stresses during curing and increase early and ultimate compression strength.[68]
Properly curing concrete leads to increased strength and lower permeability and avoids cracking where the surface dries out prematurely. Care must also be taken to avoid freezing or overheating due to the exothermic setting of cement. Improper curing can cause scaling, reduced strength, poor abrasionresistance and cracking.
https://en.wikipedia.org/wiki/Concrete#Curing
Methane clathrate (CH4·5.75H2O) or (4CH4·23H2O), also called methane hydrate, hydromethane, methane ice, fire ice, natural gas hydrate, or gas hydrate, is a solid clathrate compound (more specifically, a clathrate hydrate) in which a large amount of methane is trapped within a crystal structure of water, forming a solid similar to ice.[1][2][3][4] Originally thought to occur only in the outer regions of the Solar System, where temperatures are low and water ice is common, significant deposits of methane clathrate have been found under sediments on the ocean floors of the Earth.[5] Methane hydrate is formed when hydrogen-bonded water and methane gas come into contact at high pressures and low temperatures in oceans.
Methane clathrates are common constituents of the shallow marine geosphere and they occur in deep sedimentary structures and form outcrops on the ocean floor. Methane hydrates are believed to form by the precipitation or crystallisation of methane migrating from deep along geological faults. Precipitation occurs when the methane comes in contact with water within the sea bed subject to temperature and pressure. In 2008, research on Antarctic Vostok Station and EPICA Dome C ice cores revealed that methane clathrates were also present in deep Antarctic ice cores and record a history of atmospheric methane concentrations, dating to 800,000 years ago.[6] The ice-core methane clathrate record is a primary source of data for global warmingresearch, along with oxygen and carbon dioxide.
https://en.wikipedia.org/wiki/Methane_clathrate
Atmospheric methane is the methane present in Earth's atmosphere.[3] Atmospheric methane concentrations are of interest because it is one of the most potent greenhouse gases in Earth's atmosphere. Atmospheric methane is rising.[4]
The 20-year global warming potential of methane is 84.[5][6] That is, over a 20-year period, it traps 84 times more heat per mass unit than carbon dioxide (CO2) and 105 times the effect when accounting for aerosol interactions.[7] Global methane concentrations rose from 722 parts per billion (ppb) in pre-industrial times to 1879 ppb by 2020,[8] an increase by a factor of 2.5 and the highest value in at least 800,000 years.[9] Its concentration is higher in the Northern Hemisphere since most sources (both natural and human) are located on land and the Northern Hemisphere has more land mass.[10] The concentrations vary seasonally, with, for example, a minimum in the northern tropics during April−May mainly due to removal by the hydroxyl radical.[11] It remains in the atmosphere for 12 years.[12]
Early in the Earth's history carbon dioxide and methane likely produced a greenhouse effect. The carbon dioxide would have been produced by volcanoes and the methane by early microbes. During this time, Earth's earliest life appeared.[13] These first, ancient bacteria added to the methane concentration by converting hydrogen and carbon dioxide into methane and water. Oxygen did not become a major part of the atmosphere until photosynthetic organisms evolved later in Earth's history. With no oxygen, methane stayed in the atmosphere longer and at higher concentrations than it does today.[14]
https://en.wikipedia.org/wiki/Atmospheric_methane
A greenhouse gas (GHG or GhG) is a gas that absorbs and emits radiant energy within the thermal infrared range, causing the greenhouse effect.[1] The primary greenhouse gases in Earth's atmosphereare water vapor (H
2O), carbon dioxide (CO
2), methane (CH
4), nitrous oxide (N
2O), and ozone (O3). Without greenhouse gases, the average temperature of Earth's surface would be about −18 °C (0 °F),[2] rather than the present average of 15 °C (59 °F).[3][4][5] The atmospheres of Venus, Mars and Titan also contain greenhouse gases.
Human activities since the beginning of the Industrial Revolution (around 1750) have increased the atmospheric concentration of carbon dioxide by almost 50%, from 280 ppm in 1750 to 419 ppm in 2021.[6] The last time the atmospheric concentration of carbon dioxide was this high was over 3 million years ago.[7] This increase has occurred despite the absorption of more than half of the emissions by various natural carbon sinks in the carbon cycle.[8][9]
At current greenhouse gas emission rates, temperatures could increase by 2 °C (3.6 °F), which the United Nations' Intergovernmental Panel on Climate Change (IPCC) says is the upper limit to avoid "dangerous" levels, by 2050.[10] The vast majority of anthropogenic carbon dioxide emissions come from combustion of fossil fuels, principally coal, petroleum (including oil) and natural gas, with additional contributions from deforestation and other changes in land use.[11][12]
https://en.wikipedia.org/wiki/Greenhouse_gas
In thermodynamics, dissipation is the result of an irreversible process that takes place in homogeneous thermodynamic systems. In a dissipative process, energy (internal, bulk flow kinetic, or system potential) transforms from an initial form to a final form, where the capacity of the final form to do mechanical work is less than that of the initial form. For example, heat transfer is dissipative because it is a transfer of internal energy from a hotter body to a colder one. Following the second law of thermodynamics, the entropy varies with temperature (reduces the capacity of the combination of the two bodies to do mechanical work), but never decreases in an isolated system.
These processes produce entropy at a certain rate. The entropy production rate times ambient temperature gives the dissipated power. Important examples of irreversible processes are: heat flow through a thermal resistance, fluid flow through a flow resistance, diffusion (mixing), chemical reactions, and electrical current flow through an electrical resistance (Joule heating).
https://en.wikipedia.org/wiki/Dissipation
Joule heating, also known as resistive, resistance, or Ohmic heating, is the process by which the passage of an electric currentthrough a conductor produces heat.
Joule's first law (also just Joule's law), also known as the Joule–Lenz law,[1] states that the power of heating generated by an electrical conductor is proportional to the product of its resistance and the square of the current:
Joule heating affects the whole electric conductor, unlike the Peltier effect which transfers heat from one electrical junction to another.
https://en.wikipedia.org/wiki/Joule_heating
In electricity supply design, a ring final circuit or ring circuit (often incorrectly called a ring main, a term used historically,[1] or informally a ring) is an electrical wiring technique developed and primarily used in the United Kingdom, and to a lesser extent in Ireland and Hong Kong. This design enables the use of smaller-diameter wire than would be used in a radial circuit of equivalent total current. The reduced diameter conductors in the flexible cords connecting an appliance to the plug intended for use with sockets on a ring circuit are individually protected by a fuse in the plug. Its advantages over radial circuits are therefore reduced quantity of copper used, and greater flexibility of appliances and equipment that can be connected.
Ideally, the ring circuit acts like two radial circuits proceeding in opposite directions around the ring, the dividing point between them dependent on the distribution of load in the ring. If the load is evenly split across the two directions, the current in each direction is half of the total, allowing the use of wire with half the total current-carrying capacity. In practice, the load does not always split evenly, so thicker wire is used.
https://en.wikipedia.org/wiki/Ring_circuit
Mineral-insulated copper-clad cable is a variety of electrical cable made from copper conductors inside a copper sheath, insulated by inorganic magnesium oxide powder. The name is often abbreviated to MICC or MI cable, and colloquially known as pyro (because the original manufacturer and vendor for this product in the UK was a company called Pyrotenax). A similar product sheathed with metals other than copper is called mineral insulated metal sheathed (MIMS) cable.
MI cable is made by placing copper rods inside a circular copper tube and filling the intervening spaces with dry magnesium oxide powder. The overall assembly is then pressed between rollers to reduce its diameter (and increase its length). Up to seven conductors are often found in an MI cable, with up to 19 available from some manufacturers.
Since MI cables use no organic material as insulation (except at the ends), they are more resistant to fires than plastic-insulated cables. MI cables are used in critical fire protection applications such as alarm circuits, fire pumps, and smoke control systems. In process industries handling flammable fluids MI cable is used where small fires would otherwise cause damage to control or power cables. MI cable is also highly resistant to ionizing radiation and so finds applications in instrumentation for nuclear reactors and nuclear physics apparatus.
MI cables may be covered with a plastic sheath, coloured for identification purposes. The plastic sheath also provides additional corrosion protection for the copper sheath.
The metal tube shields the conductors from electromagnetic interference. The metal sheath also physically protects the conductors, most importantly from accidental contact with other energised conductors.
Purpose and use[edit]
MI cables are used for power and control circuits of critical equipment, such as the following examples:
- Nuclear reactors
- Exposure to dangerous gasses
- Air pressurisation systems for stairwells to enable building egress during a fire
- Hospital operating rooms
- Fire alarm systems
- Emergency power systems
- Emergency lighting systems
- Temperature measurement devices; RTDs and Thermocouples.
- Critical process valves in the petrochemical industry
- Public buildings such as theatres, cinemas, hotels
- Transport hubs (railway stations, airports etc.)
- Mains supply cables within residential apartment blocks
- Tunnels and mines
- Electrical equipment in hazardous areas where flammable gases may be present e.g. oil refineries, petrol stations
- Areas where corrosive chemicals may be present e.g. factories
- Building plant rooms
- Hot areas e.g. power stations, foundries, and close to or even inside industrial furnaces, kilns and ovens
MI cable fulfills the passive fire protection called circuit integrity, which is intended to provide operability of critical electrical circuits during a fire. It is subject to strict listing and approval use and compliance
https://en.wikipedia.org/wiki/Mineral-insulated_copper-clad_cable
Electric heat tracing, heat tape or surface heating, is a system used to maintain or raise the temperature of pipes and vessels using heat tracing cables. Trace heating takes the form of an electrical heating element run in physical contact along the length of a pipe. The pipe is usually covered with thermal insulation to retain heat losses from the pipe. Heat generated by the element then maintains the temperature of the pipe. Trace heating may be used to protect pipes from freezing, to maintain a constant flow temperature in hot water systems, or to maintain process temperatures for piping that must transport substances that solidify at ambient temperatures. Electric trace heating cables are an alternative to steam trace heating where steam is unavailable or unwanted.[2]
Anti-cavitation purpose[edit]
As heating a thick fluid decreases its viscosity, it reduces losses occurring in a pipe. Therefore, the net positive suction head (pressure difference) available can be raised, decreasing the likelihood of cavitation when pumping. However, care must be taken not to increase the vapour pressure of the fluid too much, as this would have a strong side effect on the available head, possibly outweighing any benefit.[7]
Constant electric power "series"[edit]
A series heating cable is made of a run of high-resistance wire, insulated and often enclosed in a protective jacket. It is powered at a specific voltage and the resistance heat of the wire creates heat. The downside of these types of heaters is that if they are crossed over themselves they can overheat and burn out, they are provided in specific lengths and cannot be shortened in the field, also, a break anywhere along the line will result in a failure of the entire cable. The upside is that they are typically inexpensive (if plastic style heaters) or, as is true with mineral insulated heating cables, they can be exposed to very high temperatures. Mineral insulated heating cables are good for maintaining high temperatures on process lines or maintaining lower temperatures on lines which can get extremely hot such as high temperature steam lines.
Typically series elements are used on long pipe line process heating, for example long oil pipe lines and quay side of load pipes on oil refineries.
https://en.wikipedia.org/wiki/Trace_heating
The word electrofusion is also used when fusing cells with electricity.
Electrofusion is a method of joining MDPE, HDPE and other plastic pipes using special fittings that have built-in electric heating elements which are used to weld the joint together.
The pipes to be joined are cleaned, inserted into the electrofusion fitting (with a temporary clamp if required) and a voltage (typically 40V) is applied for a fixed time depending on the fitting in use. The built in heater coils then melt the inside of the fitting and the outside of the pipe wall, which weld together producing a very strong homogeneous joint. The assembly is then left to cool for a specified time.[1]
Electrofusion welding is beneficial because it does not require the operator to use dangerous or sophisticated equipment. After some preparation, the Electrofusion Welder will guide the operator through the steps to take. Welding Heat and Time is dependent on the type and size of the fitting. All Electrofusion Fittings are not created equal - Precise positioning of the energising coils of wire in each fitting ensures uniform melting for a strong joint and the minimisation of welding and cooling time.[2]
The operator must be qualified according to the local and national laws. In Australia, an Electrofusion Course can be done within 8 hours. Electrofusion welding training focuses on the importance of accurately fusing EF fittings. Both manual and automatic methods of calculating electrofusion time gives operators the skills they need in the field. There is much to learn about the importance of preparation, timing, pressure, temperature, cool down time and handling, etc.[3]
Training and certification are very important in this field of welding, as the product can become dangerous under certain circumstances. There has been cases of major harm and death, including when molten polyethylene spurts out of the edge of a mis-aligned weld, causing skin burns. Another case was due to a tapping saddle being incorrectly installed on a gas line, causing the death of the two welders in the trench due to gas inhalation. There are many critical parts to Electrofusion welding that can cause weld failures, most of which can be greatly reduced by using welding clamps, and correct scraping equipment.[4]
To keep their qualification current, a trained operator can get their fitting tested, which involves cutting open the fitting and examining the integrity of the weld.[5]
https://en.wikipedia.org/wiki/Electrofusion
https://en.wikipedia.org/wiki/Fanning_friction_factor
https://en.wikipedia.org/wiki/Threaded_pipe
https://en.wikipedia.org/wiki/Category:Piping
https://en.wikipedia.org/wiki/Friction_loss
https://en.wikipedia.org/wiki/Hydrogen_pipeline_transport
https://en.wikipedia.org/wiki/Iron_pipe_size
https://en.wikipedia.org/wiki/Heat-shrinkable_sleeve
https://en.wikipedia.org/wiki/Coupling_(piping)
https://en.wikipedia.org/wiki/Closet_flange
https://en.wikipedia.org/wiki/Drag_reducing_agent
https://en.wikipedia.org/wiki/Turbulence
https://en.wikipedia.org/wiki/Laminar_flow
https://en.wikipedia.org/wiki/Polymers
https://en.wikipedia.org/wiki/Suspension_(chemistry)
https://en.wikipedia.org/wiki/Surfactant
https://en.wikipedia.org/wiki/Pipe_bursting
https://en.wikipedia.org/wiki/Pipe_drift
https://en.wikipedia.org/wiki/Airlift_pump
https://en.wikipedia.org/wiki/AN_thread
https://en.wikipedia.org/wiki/Flare_fitting
https://en.wikipedia.org/wiki/National_pipe_thread
https://en.wikipedia.org/wiki/O-ring_boss_seal
https://en.wikipedia.org/wiki/Vacuum_pump
https://en.wikipedia.org/wiki/Turbomolecular_pump
The earliest works that recorded a decrease in pressure drop during turbulent flow were undertaken in the thirties[6][7][8] and concerned the transportation of paper pulp. This was, however, not explicitly referred to as a drag reduction phenomenon. Toms[9] was the first to recognize the tremendous reduction in wall shear stress caused by the addition of small amount of linear macromolecules to a turbulent flowing fluid. An extensive bibliography of the first 25 years of drag reduction by polymer additives literature identified over 270 references.[10]
https://en.wikipedia.org/wiki/Drag_reducing_agent
https://en.wikipedia.org/wiki/Category:Trenchless_technology
https://en.wikipedia.org/wiki/Category:Technology_by_type
https://en.wikipedia.org/wiki/Category:Underground_construction
The National Audiovisual Conservation Center, also known as the Packard Campus for Audio-Visual Conservation, is the Library of Congress's audiovisual archive located inside Mount Pony in Culpeper, Virginia.
 | |
 | |
| Established | 2007 |
|---|---|
| Location | 19053 Mount Pony Road, Culpeper, VA |
Establishment[edit]
From 1969 to 1988, the campus was a high-security storage facility operated by the Federal Reserve Board. With the approval of the United States Congress in 1997, it was purchased by the David and Lucile Packard Foundation from the Federal Reserve Bank of Richmond via a $5.5 million grant, done on behalf of the Library of Congress. With a further $150 million from the Packard Humanities Institute and $82.1 million from Congress, the facility was transformed into the National Audio-Visual Conservation Center, which completed construction in mid-2007, and after transfer of the bulk of archives, opened for free public movie screenings on most weekends in the fall 2008. The campus offered, for the first time, a single site to store all 6.3 million pieces of the library's movie, television, and sound collection.[1]
Technically, the Packard Campus (PCAVC) is just the largest part of the whole National Audio-Visual Conservation Center (NAVCC), which also consists of the Library of Congress's Motion Picture and Television Division and Recorded Sound Division reference centers on Capitol Hill, the Mary Pickford Theater, and any other Library of Congress audio-visual storage facilities that remain outside the Packard Campus.
The PCAVC design, named Best of 2007 by Mid-Atlantic Construction Magazine,[2] involved upgrading the existing bunker and creating an entirely new, below-ground entry building that also includes a large screening room, office space and research facilities. Designers BAR Architects, project-architect SmithGroup and landscape designers SWA Group, along with DPR Construction, Inc., collaborated in what is now the largest green-roofed commercial facility in the eastern United States, blending into the surrounding environment and ecosystem.[3]
Federal Reserve bunker[edit]
With Cold War tensions came fear that in the event of a nuclear war, the economy of the United States would be destroyed. In response to this, the United States Federal Reserve constructed a bunker to house enough U.S. currency to replenish the cash supply east of the Mississippi River in the event of a catastrophic event.[4]
Dedicated on December 10, 1969, the 400-foot-long (120 m), 140,000-square-foot (13,000 m2) radiation-hardened facility was constructed of steel-reinforced concrete one foot (30.5 centimeters) thick. Lead-lined shutters could be dropped to shield the windows of the semi-recessed facility, which is covered by 2 to 4 feet (0.61 to 1.22 m) of dirt and surrounded by barbed-wire fences and a guard post. The seven computers at the facility, operated by the Federal Reserve Bank of Richmond, were the central node for all American electronic funds transfer activities.[4]
Between 1969 and 1988, the bunker stored several billion dollars worth of U.S. currency, including a large number of $2 bills shrink-wrapped and stacked on pallets 9 feet (2.7 meters) high. Following a nuclear attack, this money was to be used to replenish currency supplies east of the Mississippi River.[4]
Prior to July 1992, the bunker also served as a continuity of government facility. With a peacetime staff of 100, the site was designed to support an emergency staff of 540 for 30 days, but only 200 beds were provided in the men's and women's dormitories (to be shared on a "hot-bunk" basis by the staff working around the clock). A pre-planned menu of freeze-dried foods for the first 30 days of occupation was stored on site; private wells would provide uncontaminated water following an attack. Other noteworthy features of the facility were a cold storage area for maintaining bodies unable to be promptly buried (due to high radiation levels outside), an incinerator, indoor pistol range, and a helicopter landing pad.[4]
The facility also housed the Culpeper Switch, which was the central switching station of the Federal Reserve's Fedwire electronic funds transfer system, which at the time connected only the Fed's member banks. The Culpeper Switch also served as a data backup point for member banks east of the Mississippi River.[4]
Post-Cold War[edit]
In 1988, all money was removed from Mount Pony. The Culpeper Switch ceased operation in 1992, its functions having been decentralized to three smaller sites. In addition, its status as continuity of government site was removed. The facility was poorly maintained by a skeleton staff until 1997 when the bunker was offered for sale. With the approval of the United States Congress, it was purchased by the David and Lucile Packard Foundation from the Federal Reserve Bank of Richmond via a $5.5 million grant, done on behalf of the Library of Congress. With a further $150 million from the Packard Humanities Institute and $82.1 million from Congress, the facility was transformed into the National Audio-Visual Conservation Center, which opened in mid-2007. The center offered, for the first time, a single site to store all 6.3 million pieces of the library's movie, television, and sound collection.
https://en.wikipedia.org/wiki/National_Audio-Visual_Conservation_Center
Project Greek Island was a United States government continuity program located at the Greenbrierhotel in West Virginia.[1] The facility was decommissioned in 1992 after the program was exposed by The Washington Post.
| Project Greek Island | |
|---|---|
 The North Entrance of The Greenbrier in White Sulphur Springs, West Virginia. |
Maintenance[edit]
The center was maintained by government workers posing as hotel employees, and operated under a dummy company named Forsythe Associates, based in Arlington, Virginia. The company's on-site employees maintained that their purpose was to maintain the hotel's 1100 televisions.[2] The company's first manager was John Londis, a former cryptographic expert with the Army Signal Corps. He had a top-secret security clearance and was stationed at the Pentagon.[2] Many of these same workers are now[when?] employed by the hotel and, for a time, gave guided tours. The complex is still maintained by The Greenbrier, and the facility remains much as it was in 1992, when the secret was revealed in the national press. While almost all of the furnishings were removed following the decommissioning of the bunker, the facility now has similar period furnishings to approximate what the bunker looked like while it was still in operation. Two of the original bunks in the dormitories remain.[3]
The bunker was designed to be incorporated into the public spaces of the hotel so as to not draw attention. Much of the bunker space was visible to the public, but went undetected for years, including The Exhibition Hall in the West Virginia Wing, which differs from other public spaces in the hotel due to large concrete columns present for reinforcing. Adjacent to the entrance of The Exhibition Hall is one of the original blast doors, which can now be seen openly, the original screen that once hid its presence removed.[3]
AT&T provided phone service for both The Greenbrier Hotel and the bunker. All calls placed from the bunker were routed through the hotel's switchboard to make it appear as if they originated from the hotel. The communications center in the bunker today contains representatives of three generations of telephone technology that were used.[3]
Although the bunker was kept stocked for 30 years, it was never actually used as an emergency location, even during the Cuban Missile Crisis.
Decommissioning[edit]
The bunker's existence was not acknowledged until The Washington Post revealed it in a 1992 story;[2] immediately after the Post story, the government decommissioned the bunker.[3]
The facility has since been renovated. It is used as a data storage facility for the private sector. It is once again featured as an attraction in which visitors can tour the now-declassified facilities, now known as "The Bunker".[1]
See also[edit]
- Bomb shelter
- Nuclear warfare
- Raven Rock Mountain Complex
- Mount Weather Emergency Operations Center
- Warrenton Training Center
https://en.wikipedia.org/wiki/Project_Greek_Island
https://en.wikipedia.org/wiki/Category:Government_buildings_completed_in_1962
https://en.wikipedia.org/wiki/Category:Disaster_preparedness_in_the_United_States
A liquid-ring pump is a rotating positive-displacement pump.
They are typically used as a vacuum pump, but can also be used as a gas compressor. The function of a liquid-ring pump is similar to a rotary vane pump, with the difference being that the vanes are an integral part of the rotor and churn a rotating ring of liquid to form the compression-chamber seal. They are an inherently low-friction design, with the rotor being the only moving part. Sliding friction is limited to the shaft seals. Liquid-ring pumps are typically powered by an induction motor.
Description of operation[edit]
The liquid-ring pump compresses gas by rotating a vaned impeller located eccentrically within a cylindrical casing. Liquid (usually water) is fed into the pump and, by centrifugal acceleration, forms a moving cylindrical ring against the inside of the casing. This liquid ring creates a series of seals in the space between the impeller vanes, which form compression chambers. The eccentricity between the impeller's axis of rotation and the casing geometric axis results in a cyclic variation of the volume enclosed by the vanes and the ring.
Gas, often air, is drawn into the pump through an inlet port in the end of the casing. The gas is trapped in the compression chambers formed by the impeller vanes and the liquid ring. The reduction in volume caused by the impeller rotation compresses the gas, which reports to the discharge port in the end of the casing.
Compressed gas on discharge of pump contains certain amount of working liquid which is usually removed in vapor–liquid separator.
History[edit]
The earliest liquid-ring pumps date from 1903, when a patent was granted in Germany to Siemens-Schuckert. US Patent 1,091,529, for liquid-ring vacuum pumps and compressors, was granted to Lewis H. Nash in 1914.[1] They were manufactured by the Nash Engineering Company in Norwalk, CT. Around the same time, in Austria, Patent 69274 was granted to Siemens-Schuckertwerke for a similar liquid-ring vacuum pump.
Types and Applications[edit]
Single- and multistage[edit]
Liquid-ring systems can be single- or multistage. Typically a multistage pump will have up to two compression stages on a common shaft. In vacuum service, the attainable pressure reduction is limited by the vapour pressure of the ring-liquid. As the generated vacuum approaches the vapour pressure of the ring-liquid, the increasing volume of vapor released from the ring-liquid diminishes the remaining vacuum capacity. The efficiency of the system declines as a result.
Single-stage vacuum pumps typically produce vacuum to 35 Torr (mm Hg) or 47 millibars (4.7 kPa), and two-stage pumps can produce vacuum to 25 Torr, assuming air is being pumped and the ring-liquid is water at 15 °C (60 °F) or less. Dry air and 15 °C sealant-water temperature is the standard performance basis, which most manufacturers use for their performance curves.
Recirculation of ring-liquid[edit]
Some ring-liquid is also entrained with the discharge stream. This liquid is separated from the gas stream by other equipment external to the pump. In some systems, the discharged ring-liquid is cooled by a heat exchanger or cooling tower, then returned to the pump casing. In some recirculating systems, contaminants from the gas become trapped in the ring-liquid, depending on system configuration. These contaminants become concentrated as the liquid continues to recirculate, eventually causes damage and reduced life to the pump. In this case, filtration systems are required to ensure that contamination is kept to acceptable levels.
In non-recirculating systems, the discharged hot liquid (usually water) is treated as a waste stream. In this case fresh cool water is used to make up the loss. Environmental considerations are making such "once-through" systems increasingly rare. These simple, but highly reliable pumps have a variety of industrial applications. They are used to maintain condenser vacuum on large steam-turbine generator sets by removing incondensable gasses, where vacuum levels are typically 30–50 mbar. They are used on paper machines to dewater the pulp slurry and to extract water from press felts. Another application is the vacuum forming of molded paper-pulp products (egg cartons and other packaging). Other applications include soil remediation, where contaminated ground water is drawn from wells by vacuum. In petroleum refining, vacuum distillation also makes use of liquid-ring vacuum pumps to provide the process vacuum. Liquid-ring compressors are often used in vapor recovery systems.
Liquid type[edit]
Liquid-ring vacuum pumps can use any liquid compatible with the process, provided it has the appropriate vapor pressure properties, as the sealant liquid. Although the most common sealant is water, almost any liquid can be used. The second most common is oil. Since oil has a very low vapor pressure, oil-sealed liquid-ring vacuum pumps are typically air-cooled. For dry chlorine gas applications concentrated sulfuric acid is used.
The ability to use any liquid allows the liquid-ring vacuum pump to be ideally suited for solvent (vapor) recovery. If a process, such as distillation or a vacuum dryer, is generating toluene vapors, for example, then it is possible to use toluene as the sealant, provided the cooling water is cold enough to keep the vapor pressure of the sealant liquid low enough to pull the desired vacuum.[2]
Ionic liquids in liquid-ring vacuum pumps can lower the vacuum pressure from about 70 mbar to below 1 mbar.[3]
References[edit]
| Wikimedia Commons has media related to Liquid-ring pumps. |
- ^ google.com
- ^ "Solvent sealed liquid ring vacuum pump systems".
- ^ basionics.com Archived 2009-09-01 at the Wayback Machine Ionic liquids – designable materials for high-performing fluids.
https://en.wikipedia.org/wiki/Liquid-ring_pump
A rotary vane pump is a positive-displacement pump that consists of vanes mounted to a rotor that rotates inside a cavity. In some cases these vanes can have variable length and/or be tensioned to maintain contact with the walls as the pump rotates. It was invented by Charles C. Barnes of Sackville, New Brunswick, who patented it on June 16, 1874.[1][2][3] There have been various improvements, including a variable vane pump for gases (1909).[4] They are considered less suitable than other vacuum pumps for high-viscosity and high-pressure fluids, and are complex to operate. They can endure short periods of dry operation, and are considered good for low-viscosity fluids.
1. pump housing
2. rotor
3. vanes
4. spring
Type[edit]
The simplest vane pump has a circular rotor rotating inside a larger circular cavity. The centers of these two circles are offset, causing eccentricity. Vanes are allowed to slide into and out of the rotor and seal on all edges, creating vane chambers that do the pumping work. In the suction side of the pump, the vane chambers are increase in volume. These increasing-volume vane chambers are filled with fluid forced in by the inlet pressure. Inlet pressure is actually the pressure from the system being pumped, often just the atmosphere. On the discharge side of the pump, the vane chambers are decreasing in volume, forcing fluid out of the pump. The action of the vane drives out the same volume of fluid with each rotation. Multistage rotary-vane vacuum pumps can attain pressures as low as 10−6 mbar (0.0001 Pa).
Uses[edit]
Vane pumps are commonly used as high-pressure hydraulic pumps and in automobiles, including supercharging, power-steering, air conditioning, and automatic-transmission pumps. Pumps for mid-range pressures include applications such as carbonators for fountain soft-drink dispensers and espresso coffee machines. Furthermore, vane pumps can be used in low-pressure gas applications such as secondary air injection for auto exhaust emission control, or in low-pressure chemical vapor deposition systems.
Rotary-vane pumps are also a common type of vacuum pump, with two-stage pumps able to reach pressures well below 10−6 bar. These vacuum pumps are found in numerous applications, such as providing braking assistance in large trucks and diesel-powered passenger cars (whose engines do not generate intake vacuum) through a braking booster, in most light aircraft to drive gyroscopic flight instruments, in evacuating refrigerant lines during installation of air conditioners, in laboratory freeze dryers, and vacuum experiments in physics. In the vane pump, the pumped gas and the oil are mixed within the pump, and so they must be separated externally. Therefore, the inlet and the outlet have a large chamber, maybe with swirl, where the oil drops fall out of the gas. Sometimes the inlet has a venetian blind cooled by the room air (the pump is usually 40 K hotter) to condense cracked pumping oil and water, and let it drop back into the inlet. When these pumps are used in high-vacuum systems (where the inflow of gas into the pump becomes very low), a significant concern is contamination of the entire system by molecular oil backstreaming.
Variable-displacement vane pump[edit]
One of the major advantages of the vane pump is that the design readily lends itself to become a variable-displacement pump, rather than a fixed-displacement pump such as a spur-gear (X-X) or a gerotor (I-X) pump. The centerline distance from the rotor to the eccentric ring is used to determine the pump's displacement. By allowing the eccentric ring to pivot or translate relative to the rotor, the displacement can be varied. It is even possible for a vane pump to pump in reverse if the eccentric ring moves far enough. However, performance cannot be optimized to pump in both directions. This can make for a very interesting hydraulic-control oil pump.
A variable-displacement vane pump is used as an energy-savings device and has been used in many applications, including automotive transmissions, for over 30 years.
Materials[edit]
- Externals (head, casing) – cast iron, ductile iron, steel, brass, plastic, and stainless steel
- Vane, pushrods – carbon graphite, PEEK
- End plates – carbon graphite
- Shaft seal – component mechanical seals, industry-standard cartridge mechanical seals, and magnetically driven pumps
- Packing – available from some vendors, but not usually recommended for thin liquid service
See also[edit]
https://en.wikipedia.org/wiki/Rotary_vane_pump
Vapor (or vapour) recovery is the process of collecting the vapors of gasoline and other fuels, so that they do not escape into the atmosphere. This is often done (and sometimes required by law) at filling stations, to reduce noxious and potentially explosive fumes and pollution.
The negative pressure created in the (underground) storage tank by fuel being drawn, combined with the pressure in the car's fuel tank caused by the inflow, is usually used to pull in the vapors. They are drawn in through holes in the side of the nozzle and travel along a return path through another hose.
In Australia, vapor recovery has become mandatory in major urban areas. There are two categories - VR1 and VR2. VR1 must be installed at fuel stations that pump less than 500,000 litres annually, VR2 must be installed for larger amounts, or as designated by various EPA bodies.
https://en.wikipedia.org/wiki/Vapor_recovery
Recirculation of ring-liquid[edit]
Some ring-liquid is also entrained with the discharge stream. This liquid is separated from the gas stream by other equipment external to the pump. In some systems, the discharged ring-liquid is cooled by a heat exchanger or cooling tower, then returned to the pump casing. In some recirculating systems, contaminants from the gas become trapped in the ring-liquid, depending on system configuration. These contaminants become concentrated as the liquid continues to recirculate, eventually causes damage and reduced life to the pump. In this case, filtration systems are required to ensure that contamination is kept to acceptable levels.
In non-recirculating systems, the discharged hot liquid (usually water) is treated as a waste stream. In this case fresh cool water is used to make up the loss. Environmental considerations are making such "once-through" systems increasingly rare. These simple, but highly reliable pumps have a variety of industrial applications. They are used to maintain condenser vacuum on large steam-turbine generator sets by removing incondensable gasses, where vacuum levels are typically 30–50 mbar. They are used on paper machines to dewater the pulp slurry and to extract water from press felts. Another application is the vacuum forming of molded paper-pulp products (egg cartons and other packaging). Other applications include soil remediation, where contaminated ground water is drawn from wells by vacuum. In petroleum refining, vacuum distillation also makes use of liquid-ring vacuum pumps to provide the process vacuum. Liquid-ring compressors are often used in vapor recovery systems.
Liquid type[edit]
Liquid-ring vacuum pumps can use any liquid compatible with the process, provided it has the appropriate vapor pressure properties, as the sealant liquid. Although the most common sealant is water, almost any liquid can be used. The second most common is oil. Since oil has a very low vapor pressure, oil-sealed liquid-ring vacuum pumps are typically air-cooled. For dry chlorine gas applications concentrated sulfuric acid is used.
The ability to use any liquid allows the liquid-ring vacuum pump to be ideally suited for solvent (vapor) recovery. If a process, such as distillation or a vacuum dryer, is generating toluene vapors, for example, then it is possible to use toluene as the sealant, provided the cooling water is cold enough to keep the vapor pressure of the sealant liquid low enough to pull the desired vacuum.[2]
Ionic liquids in liquid-ring vacuum pumps can lower the vacuum pressure from about 70 mbar to below 1 mbar.[3]
https://en.wikipedia.org/wiki/Liquid-ring_pump
A scroll compressor (also called spiral compressor, scroll pump and scroll vacuum pump) is a device for compressing air or refrigerant.[1] It is used in air conditioning equipment, as an automobile supercharger (where it is known as a scroll-type supercharger) and as a vacuum pump. Many residential central heat pump and air conditioning systems and a few automotive air conditioning systems employ a scroll compressor instead of the more traditional rotary, reciprocating, and wobble-plate compressors.
A scroll compressor operating in reverse is a scroll expander, and can generate mechanical work.
https://en.wikipedia.org/wiki/Scroll_compressor
A peristaltic pump, also commonly known as a roller pump, is a type of positive displacement pump used for pumping a variety of fluids. The fluid is contained in a flexible tube fitted inside a circular pump casing. Most peristaltic pumps work through rotary motion, though linear peristaltic pumps have also been made. The rotor has a number of "wipers" or "rollers" attached to its external circumference, which compress the flexible tube as they rotate by. The part of the tube under compression is closed, forcing the fluid to move through the tube. Additionally, as the tube opens to its natural state after the rollers pass, more fluid is drawn into the tube. This process is called peristalsis and is used in many biological systems such as the gastrointestinal tract. Typically, there will be two or more rollers compressing the tube, trapping a body of fluid between them. The body of fluid is transported through the tube, toward the pump outlet. Peristaltic pumps may run continuously, or they may be indexed through partial revolutions to deliver smaller amounts of fluid.
History[edit]
A form of peristaltic pump was described in The Mechanics Magazine in 1845. The pump used a leather hose which did not need to self-open when released by the rollers, instead relying on the incoming water having sufficient pressure to fill the open inlet end on each cycle.[1] The peristaltic pump was first patented in the United States by Rufus Porter and J.D. Bradley in 1855 (U.S. Patent number 12753)[2] as a well pump, and later by Eugene Allen in 1881 (U.S. Patent number 249285)[3] for blood transfusions. It was developed by heart surgeon Dr. Michael DeBakey[4] for blood transfusions [5] while he was a medical student in 1932 and later used by him for cardiopulmonary bypass[6] systems. A specialized nonocclusive roller pump (US Patent 5222880)[7] using soft flat tubing was developed in 1992 for cardiopulmonary bypass systems. The first technically and commercially viable peristaltic pump for use outside the laboratory was developed by Bernard Refson.[8]
Applications[edit]
Peristaltic pumps are typically used to pump clean/sterile or highly reactive fluids without exposing those fluids to contamination from exposed pump components. Some common applications include pumping IV fluids through an infusion device, apheresis, highly reactive chemicals, high solids slurries, and other materials where isolation of the product from the environment are critical. They are also used in heart-lung machines to circulate blood during a bypass surgery, and in hemodialysis systems, since the pump does not cause significant hemolysis, or rupture of the blood cells.
Key design parameters[edit]
The ideal peristaltic pump should have an infinite diameter of the pump head and the largest possible diameter of the rollers. Such an ideal peristaltic pump would offer the longest possible tubing lifetime and provide a constant and pulsation-free flow rate.
Such an ideal peristaltic pump can not be constructed in reality. However, peristaltic pumps can be designed to approach these ideal peristaltic pump parameters.
Careful design can offer constant accurate flow rates for several weeks together with a long tubing lifetime without the risk of tubing rupture.[citation needed]
Chemical compatibility[edit]
The pumped fluid contacts only the inside surface of the tubing. This eliminates fluid compatibility concerns with other pump components such as valves, O-rings, and seals, which must be considered for other pump designs. Therefore, only the composition of the tubing that the pumped medium travels through is considered for chemical compatibility.
The tubing needs to be elastomeric to maintain the circular cross-section after millions of cycles of squeezing in the pump. This requirement eliminates a variety of non-elastomeric polymers that have compatibility with a wide range of chemicals, such as PTFE, polyolefins, PVDF, etc. from consideration as material for pump tubing. The popular elastomers for pump tubing are nitrile (NBR), Hypalon, Viton, silicone, PVC, EPDM, EPDM+polypropylene (as in Santoprene), polyurethane and natural rubber. Of these materials, natural rubber has the best fatigue resistance, and EPDM and Hypalon have the best chemical compatibility. Silicone is popular with water-based fluids, such as in bio-pharma industry, but has a limited range of chemical compatibility in other industries.
Extruded fluoropolymer tubes such as FKM (Viton, Fluorel, etc.) have good compatibility with acids, hydrocarbons, and petroleum fuels, but have insufficient fatigue resistance to achieve an effective tube life.
There are a couple of newer tubing developments that offer broad chemical compatibility using lined tubing and fluoroelastomers.
With lined tubing, the thin inside liner is made of a chemically resistant material such as poly-olefin and PTFE that form a barrier for the rest of the tubing wall from coming in contact with the pumped fluid. These liners are materials that are often not elastomeric, therefore the entire tube wall cannot be made with this material for peristaltic pump applications. This tubing provides adequate chemical compatibility and life to be used in chemically challenging applications. There are a few things to keep in mind when using these tubes - any pinholes in the liner during manufacturing could render the tubing vulnerable to chemical attack. In the case of stiff plastic liners like the polyolefins, with repeated flexing in the peristaltic pump they can develop cracks, rendering the bulk material again vulnerable to chemical attack. A common issue with all lined tubing is the delamination of the liner with repeated flexing that signals the end of the tube's life. For those with the need for chemically compatible tubing, these lined tubings offer a good solution.
With fluoroelastomer tubing, the elastomer itself has the chemical resistance. In the case of e.g. Chem-Sure, it is made of a perfluoroelastomer, that has the broadest chemical compatibility of all elastomers. The two fluoroelastomer tubes listed above combine the chemical compatibility with a very long tube life stemming from their reinforcement technology but come at a pretty high initial cost. One has to justify the cost with the total value derived over the long tube life and compare with other options such as other tubing or even other pump technologies.
There are many online sites for checking the chemical compatibility of the tubing material with the pumped fluid. The tubing manufacturers may also have compatibility charts specific to their tubing production method, coating, material, and the fluid being pumped.
While these charts cover a list of commonly encountered fluids, they may not have all the fluids. If there is a fluid whose compatibility is not listed anywhere, then a common test of compatibility is the immersion testing. A 1 to 2 inch sample of the tubing is immersed in the fluid to be pumped for anywhere from 24 to 48 hours, and the amount of weight change from before and after the immersion is measured. If the weight change is greater than 10% of the initial weight, then that tube is not compatible with the fluid, and should not be used in that application. This test is still a one-way test, in the sense that there is still a remote chance that the tubing that passes this test can still be incompatible for the application since the combination of borderline compatibility and mechanical flexing can push the tube over the edge, resulting in premature tube failure.
In general, recent tubing developments have brought broad chemical compatibility to the peristaltic pump option that many chemical dosing applications can benefit over other current pump technologies.
Occlusion[edit]
The minimum gap between the roller and the housing determines the maximum squeeze applied on the tubing. The amount of squeeze applied to the tubing affects pumping performance and the tube life - more squeezing decreases the tubing life dramatically, while less squeezing can cause the pumped medium to slip back, especially in high-pressure pumping, and decreases the efficiency of the pump dramatically and the high velocity of the slip back typically causes premature failure of the hose. Therefore, this amount of squeeze becomes an important design parameter.
The term "occlusion" is used to measure the amount of squeeze. It is either expressed as a percentage of twice the wall thickness, or as an absolute amount of the wall that is squeezed.
Let
- y = occlusion
- g = minimum gap between the roller and the housing
- t = wall thickness of the tubing
Then
- y = 2t - g (when expressed as the absolute amount of squeeze)
- y = 100% x (2t - g) / (2t) (when expressed as a percentage of twice the wall thickness)
The occlusion is typically 10% to 20%, with a higher occlusion for a softer tube material and a lower occlusion for a harder tube material.
Thus for a given pump, the most critical tubing dimension becomes the wall thickness. An interesting point here is that the inside diameter (ID) of the tubing is not an important design parameter for the suitability of the tubing for the pump. Therefore, it is common for more than one ID be used with a pump, as long as the wall thickness remains the same.
Inside diameter[edit]
For a given rotational speed of the pump, a tube with a larger inside diameter (ID) will give a higher flow rate than one with a smaller inside diameter. The flow rate is a function of the cross-section area of the tube bore.
Flow rate[edit]
The flow rate is an important parameter for a pump. The flow rate in a peristaltic pump is determined by many factors, such as:
- Tube inner diameter - higher flow rate with larger inner diameter
- Pump head outer diameter - higher flow rate with larger outer diameter
- Pump head rotational speed - higher flow rate with higher speed
- Inlet Pulsation - the pulse reduces the filling volume of the hose
Increasing the number of rollers does not increase the flow rate, instead it will decrease the flow rate somewhat by reducing the effective (i.e. fluid-pumping) circumference of the head. Increasing rollers does tend to decrease the amplitude of the fluid pulsing at the outlet by increasing the frequency of the pulsed flow.
The length of the tube (measured from the initial pinch point near the inlet to the final release point near the outlet) does not affect the flow rate. However, a longer tube implies more pinch points between inlet and outlet, increasing the pressure that the pump can generate.
The flow rate of a peristaltic pump is in most cases not linear. The effect of pulsation at the inlet of the pump changes the filling degree of the peristaltic hose. With high inlet pulsation, the peristaltic hose may become oval-shaped, resulting in less flow. Accurate metering with a peristaltic pump is therefore only possible when the pump has a constant flow rate, or when inlet pulsation is completely eliminated with the use of correct designed pulsation dampeners.
Pulsation[edit]
Pulsation is an important side effect of the peristaltic pump. The pulsation in a peristaltic pump is determined by many factors, such as:
- Flow Rate - higher flow rate gives more pulsation
- Line Length - Long pipelines give more pulsation
- Higher Pump Speed - higher RPM gives more pulsation
- Specific gravity of the fluid - higher fluid density gives more Pulsation
Variations[edit]
Hose pumps[edit]
Higher pressure peristaltic hose pumps which can typically operate against up to 16 bar (230 psi) in continuous service, use shoes (rollers only used on low-pressure types) and have casings filled with lubricant to prevent abrasion of the exterior of the pump tube and to aid in the dissipation of heat, and use reinforced tubes, often called "hoses". This class of pump is often called a "hose pump".
The biggest advantage with the hose pumps over the roller pumps is the high operating pressure of up to 16 bar. With rollers, max pressure can arrive up to 12 bar (170 psi) without any problem. If the high operating pressure is not required, a tubing pump is a better option than a hose pump if the pumped medium is not abrasive. With recent advances made in the tubing technology for pressure, life, and chemical compatibility, as well as the higher flow rate ranges, the advantages that hose pumps had over roller pumps continues to erode.
Tube pumps[edit]
Lower pressure peristaltic pumps typically have dry casings and use rollers along with non-reinforced, extruded tubing. This class of pump is sometimes called a "tube pump" or "tubing pump". These pumps employ rollers to squeeze the tube. Except for the 360° eccentric pump design as described below, these pumps have a minimum of 2 rollers 180° apart and may have as many as 8, or even 12 rollers. Increasing the number of rollers increases the pressure pulse frequency of the pumped fluid at the outlet, thereby decreasing the amplitude of pulsing. The downside to increasing the number of rollers it that it proportionately increases the number of squeezes, or occlusions, on the tubing for a given cumulative flow through that tube, thereby reducing the tubing life.
There are two kinds of roller design in peristaltic pumps:
- Fixed occlusion - In this kind of pump, the rollers have a fixed locus as it turns, keeping the occlusion constant as it squeezes the tube. This is a simple, yet effective design. The only downside to this design is that the occlusion as a percent on the tube varies with the variation of the tube wall thickness. Typically the wall thickness of the extruded tubes varies enough that the % occlusion can vary with the wall thickness (see above). Therefore, a section of tube with greater wall thickness, but within the accepted tolerance, will have higher percent occlusion, which increases the wear on the tubing, thereby decreasing the tube life. Tube wall thickness tolerances today are generally kept tight enough that this issue is not of much practical concern. For those mechanically inclined, this may be the constant strain operation.
- Spring-loaded rollers - As the name indicates, the rollers in this pump are mounted on a spring. This design is more elaborate than the fixed occlusion, but helps overcome the variations in the tube wall thickness over a broader range. Regardless of the variations, the roller imparts the same amount of stress on the tubing that is proportional to the spring constant, making this a constant stress operation. The spring is selected to overcome not only the hoop strength of the tubing, but also the pressure of the pumped fluid.
The operating pressure of these pumps is determined by the tubing and by the motor's ability to overcome the hoop strength of the tubing and the fluid pressure.
Microfluidic pumps[edit]
In microfluidics, it is often desirable to minimize the circulating volume of fluid. Traditional pumps require a large volume of liquid external to the microfluidic circuit. This can lead to problems due to dilution of analytes and already dilute biological signalling molecules.[10] For this reason, among others, it is desirable to integrate a micro-pumping structure into the microfluidic circuit. Wu et al. presented in 2008 a pneumatically actuated peristaltic micropump which eliminates the need for large external circulating fluid volumes.[9]
Advantages[edit]
- No contamination. Because the only part of the pump in contact with the fluid being pumped is the interior of the tube, it is easy to sterilize and clean the inside surfaces of the pump.
- Low maintenance needs and easy to clean; their lack of valves, seals and glands makes them comparatively inexpensive to maintain.
- They are able to handle slurries, viscous, shear-sensitive and aggressive fluids.
- Pump design prevents backflow and siphoning without valves.
- A fixed amount of fluid is pumped per rotation, so it can be used to roughly measure the amount of pumped fluid.
Disadvantages[edit]
- The flexible tubing will tend to degrade with time and require periodic replacement.
- The flow is pulsed, particularly at low rotational speeds. Therefore, these pumps are less suitable where a smooth consistent flow is required. An alternative type of positive displacement pump should then be considered.
- Effectiveness is limited by liquid viscosity
Tubing[edit]
Considerations for selecting peristaltic pump tubing include appropriate chemical resistance towards the liquid being pumped, whether the pump will be used continuously or intermittently, and cost. Types of tubing commonly used in peristaltic pumps include:
For continuous use, most of the materials perform similarly over short time frames.[11] This suggests that overlooked low cost materials such as PVC might meet the needs of a short-term, one time use medical applications. For intermittent use, compression set is important and Silicone is an optimal material choice.
Typical applications[edit]
- Medicine
- Dialysis machines
- Open-heart bypass pump machines
- Medical infusion pumps
- Testing and research
- AutoAnalyzer
- Analytical chemistry experiments
- Carbon monoxide monitors
- Media dispensers
- Agriculture
- 'Sapsucker' pumps to extract maple tree sap
- Dosers for hydroponic systems
- Food manufacturing and sales
- Liquid food fountains (ex. cheese sauce for nachos)
- Beverage dispensing
- Food-service Washing Machine fluid pump
- Chemical handling
- Printing, paint and pigments
- Pharmaceutical production
- Dosing systems for dishwasher and laundry chemicals
- Engineering and manufacturing
- Concrete pump
- Pulp and paper plants
- Minimum quantity lubrication
- Inkjet printers
- Water and Waste
- Chemical treatment in water purification plant
- Sewage sludge
- Aquariums, particularly calcium reactors
- Automatic wastewater sampling for wastewater quality indicators
See also[edit]
https://en.wikipedia.org/wiki/Peristaltic_pump
A reciprocating compressor or piston compressor is a positive-displacement compressor that uses pistonsdriven by a crankshaft to deliver gases at high pressure.[1][2] Pressures of up to 5,000 PSIG are commonly produced by multistage reciprocating compressors.
The intake gas enters the suction manifold, then flows into the compression cylinder where it gets compressed by a piston driven in a reciprocating motion via a crankshaft, and is then discharged. Applications include oil refineries, gas pipelines, oil and gas production drilling and well services, air and nitrogen injection, offshore platforms, chemical plants, natural gas processing plants, air conditioning, and refrigeration plants. One specialty application is the blowing of plastic bottles made of polyethylene terephthalate (PET).
In the ionic liquid piston compressor many seals and bearings were removed in the design as the ionic liquid does not mix with the gas. Service life is about 10 times longer than a regular diaphragm compressor with reduced maintenance during use, energy costs are reduced by as much as 20%. The heat exchangers that are used in a normal piston compressor are removed as the heat is removed in the cylinder itself where it is generated. Almost 100% of the energy going into the process is being used with little energy wasted as reject heat.[3][4]
See also[edit]
https://en.wikipedia.org/wiki/Reciprocating_compressor
Vapour-compression refrigeration or vapor-compression refrigeration system (VCRS),[1] in which the refrigerant undergoes phase changes, is one of the many refrigeration cycles and is the most widely used method for air-conditioning of buildings and automobiles. It is also used in domestic and commercial refrigerators, large-scale warehouses for chilled or frozen storage of foods and meats, refrigerated trucks and railroad cars, and a host of other commercial and industrial services. Oil refineries, petrochemical and chemical processing plants, and natural gas processing plants are among the many types of industrial plants that often utilize large vapor-compression refrigeration systems. Cascade refrigeration systems may also be implemented using two compressors.
Refrigeration may be defined as lowering the temperature of an enclosed space by removing heat from that space and transferring it elsewhere. A device that performs this function may also be called an air conditioner, refrigerator, air source heat pump, geothermal heat pump, or chiller (heat pump).
https://en.wikipedia.org/wiki/Vapor-compression_refrigeration
A cascade refrigeration cycle is a multi-stage thermodynamic cycle. An example two-stage process is shown at right. (Bottom on mobile) The cascade cycle is often employed for devices such as ULT freezers.[1]
In a cascade refrigeration system, two or more vapor-compression cycles with different refrigerants are used. The evaporation-condensation temperatures of each cycle are sequentially lower with some overlap to cover the total temperature drop desired, with refrigerants selected to work efficiently in the temperature range they cover. The low temperature system removes heat from the space to be cooled using an evaporator, and transfers it to a heat exchanger that is cooled by the evaporation of the refrigerant of the high temperature system. Alternatively, a liquid to liquid or similar heat exchanger may be used instead. The high temperature system transfers heat to a conventional condenser that carries the entire heat output of the system and may be passively, fan, or water-cooled.
Cascade cycles may be separated by either being sealed in separated loops, or in what is referred to as an "auto-cascade" where the gases are compressed as a mixture but separated as one refrigerant condenses into a liquid while the other continues as a gas through the rest of the cycle. Although an auto-cascade introduces several constraints on the design and operating conditions of the system that may reduce the efficiency it is often used in small systems due to only requiring a single compressor, or in cryogenic systems as it reduces the need for high efficiency heat exchangers to prevent the compressors leaking heat into the cryogenic cycles. Both types can be used in the same system, generally with the separate cycles being the first stage(s) and the auto-cascade being the last stage.
Peltier coolers may also be cascaded into a multi-stage system to achieve lower temperatures. Here the hot side of the first peltier cooler is cooled by the cold side of the second peltier cooler, which is larger in size, whose hot side is in turn cooled by the cold side of an even larger peltier cooler, and so on. Efficiency drops very rapidly as more stages are added but for very small heat loads down to near-cryogenic temperatures this can often be an effective solution due to being compact and low cost, such as in mid-range thermographic cameras.
https://en.wikipedia.org/wiki/Cascade_refrigeration
Flash evaporation (or partial evaporation) is the partial vapor that occurs when a saturated liquid stream undergoes a reduction in pressure by passing through a throttling valve or other throttling device. This process is one of the simplest unit operations. If the throttling valve or device is located at the entry into a pressure vessel so that the flash evaporation occurs within the vessel, then the vessel is often referred to as a flash drum.[1][2]
If the saturated liquid is a single-component liquid (for example, propane or liquid ammonia), a part of the liquid immediately "flashes" into vapor. Both the vapor and the residual liquid are cooled to the saturation temperatureof the liquid at the reduced pressure. This is often referred to as "auto-refrigeration" and is the basis of most conventional vapor compression refrigeration systems.
If the saturated liquid is a multi-component liquid (for example, a mixture of propane, isobutane and normal butane), the flashed vapor is richer in the more volatile components than is the remaining liquid.
Uncontrolled flash evaporation can result in a boiling liquid expanding vapor explosion (BLEVE).
https://en.wikipedia.org/wiki/Flash_evaporation
An isenthalpic process or isoenthalpic process is a process that proceeds without any change in enthalpy, H; or specific enthalpy, h.[1]
https://en.wikipedia.org/wiki/Isenthalpic_process
In thermodynamics, an adiabatic process (Greek: adiábatos, “impassable”) is a type of thermodynamic process that occurs without transferring heat or mass between the thermodynamic system and its environment. Unlike an isothermal process, an adiabatic process transfers energy to the surroundings only as work.[1][2] As a key concept in thermodynamics, the adiabatic process supports the theory that explains the first law of thermodynamics.
Some chemical and physical processes occur too rapidly for energy to enter or leave the system as heat, allowing a convenient "adiabatic approximation".[3] For example, the adiabatic flame temperature uses this approximation to calculate the upper limit of flame temperature by assuming combustion loses no heat to its surroundings.
In meteorology and oceanography, adiabatic cooling produces condensation of moisture or salinity, oversaturating the parcel. Therefore, the excess must be removed. There, the process becomes a pseudo-adiabatic process whereby the liquid water or salt that condenses is assumed to be removed upon formation by idealized instantaneous precipitation. The pseudoadiabatic process is only defined for expansion because a compressed parcel becomes warmer and remains undersaturated.[4]
https://en.wikipedia.org/wiki/Adiabatic_process
A vacuum is a space devoid of matter. The word is derived from the Latin adjective vacuus for "vacant" or "void". An approximation to such vacuum is a region with a gaseous pressure much less than atmospheric pressure.[1]Physicists often discuss ideal test results that would occur in a perfect vacuum, which they sometimes simply call "vacuum" or free space, and use the term partial vacuum to refer to an actual imperfect vacuum as one might have in a laboratory or in space. In engineering and applied physics on the other hand, vacuum refers to any space in which the pressure is considerably lower than atmospheric pressure.[2] The Latin term in vacuo is used to describe an object that is surrounded by a vacuum.
The quality of a partial vacuum refers to how closely it approaches a perfect vacuum. Other things equal, lower gas pressure means higher-quality vacuum. For example, a typical vacuum cleaner produces enough suction to reduce air pressure by around 20%.[3] But higher-quality vacuums are possible. Ultra-high vacuum chambers, common in chemistry, physics, and engineering, operate below one trillionth (10−12) of atmospheric pressure (100 nPa), and can reach around 100 particles/cm3.[4] Outer space is an even higher-quality vacuum, with the equivalent of just a few hydrogen atoms per cubic meter on average in intergalactic space.[5]
Vacuum has been a frequent topic of philosophical debate since ancient Greek times, but was not studied empirically until the 17th century. Evangelista Torricelli produced the first laboratory vacuum in 1643, and other experimental techniques were developed as a result of his theories of atmospheric pressure. A Torricellian vacuum is created by filling a tall glass container closed at one end with mercury, and then inverting it in a bowl to contain the mercury (see below).[6]
Vacuum became a valuable industrial tool in the 20th century with the introduction of incandescent light bulbs and vacuum tubes, and a wide array of vacuum technologies has since become available. The development of human spaceflight has raised interest in the impact of vacuum on human health, and on life forms in general.
https://en.wikipedia.org/wiki/Vacuum
A cathode is the electrode from which a conventional current leaves a polarized electrical device. This definition can be recalled by using the mnemonicCCD for Cathode Current Departs. A conventional current describes the direction in which positive charges move. Electrons have a negative electrical charge, so the movement of electrons is opposite to that of the conventional current flow. Consequently, the mnemonic cathode current departs also means that electrons flow into the device's cathode from the external circuit.
The electrode through which conventional current flows the other way, into the device, is termed an anode.
https://en.wikipedia.org/wiki/Cathode
Electronic waste or e-waste describes discarded electrical or electronic devices. Used electronics which are destined for refurbishment, reuse, resale, salvage recycling through material recovery, or disposal are also considered e-waste. Informal processing of e-waste in developing countries can lead to adverse human health effects and environmental pollution.
Electronic scrap components, such as CPUs, contain potentially harmful materials such as lead, cadmium, beryllium, or brominated flame retardants. Recycling and disposal of e-waste may involve significant risk to health of workers and their communities.[1]
https://en.wikipedia.org/wiki/Electronic_waste
https://en.wikipedia.org/wiki/ionizing_impurity_scatter
https://en.wikipedia.org/wiki/Ionized_impurity_scattering
https://en.wikipedia.org/wiki/distributed_lattice
https://en.wikipedia.org/wiki/ionization
https://en.wikipedia.org/wiki/dessicant
https://en.wikipedia.org/wiki/vacume
https://en.wikipedia.org/wiki/vacuum
https://en.wikipedia.org/wiki/potential_gradient
https://en.wikipedia.org/wiki/potential_energy
https://en.wikipedia.org/wiki/electromagnetic_potential_gradient
https://en.wikipedia.org/wiki/oxyanion_hole
https://en.wikipedia.org/wiki/electron_melt
https://en.wikipedia.org/wiki/plasma
https://en.wikipedia.org/wiki/magnetic_confinement
https://en.wikipedia.org/wiki/pressure
https://en.wikipedia.org/wiki/cavitation
https://en.wikipedia.org/wiki/conductor
https://en.wikipedia.org/wiki/medium
https://en.wikipedia.org/wiki/negative_matter
https://en.wikipedia.org/wiki/negative
https://en.wikipedia.org/wiki/dark_matter
https://en.wikipedia.org/wiki/mirror
https://en.wikipedia.org/wiki/Helium_flash
https://en.wikipedia.org/wiki/Gravitational_collapse
https://en.wikipedia.org/wiki/Atmospheric_pressure
https://en.wikipedia.org/wiki/angular_momentum
https://en.wikipedia.org/wiki/hemihelix
https://en.wikipedia.org/wiki/helicoid_catenoid
https://en.wikipedia.org/wiki/electromotive_force
https://en.wikipedia.org/wiki/superfluid
https://en.wikipedia.org/wiki/filament
https://en.wikipedia.org/wiki/hydrogen_gassing
https://en.wikipedia.org/wiki/symmetry
https://en.wikipedia.org/wiki/dihedral_angle
https://en.wikipedia.org/wiki/fluid_thread_breakup
https://en.wikipedia.org/wiki/vortex_sheet
https://en.wikipedia.org/wiki/Miller_index#Crystallographic_planes_and_directions
https://en.wikipedia.org/wiki/Coulomb_barrier
https://en.wikipedia.org/wiki/Gamow_factor
https://en.wikipedia.org/wiki/Vacuum_permittivity
https://en.wikipedia.org/wiki/Permittivity
https://en.wikipedia.org/wiki/Clausius–Mossotti_relation
https://en.wikipedia.org/wiki/Fine-structure_constant
https://en.wikipedia.org/wiki/Dimensionless_quantity
https://en.wikipedia.org/wiki/Buckingham_π_theorem
In fluid dynamics, a blast wave is the increased pressure and flow resulting from the deposition of a large amount of energy in a small, very localised volume. The flow field can be approximated as a lead shock wave, followed by a self-similar subsonic flow field. In simpler terms, a blast wave is an area of pressure expanding supersonically outward from an explosive core. It has a leading shock front of compressed gases. The blast wave is followed by a blast wind of negative gauge pressure, which sucks items back in towards the center. The blast wave is harmful especially when one is very close to the center or at a location of constructive interference. High explosives that detonate generate blast waves.
https://en.wikipedia.org/wiki/Blast_wave
Negative pressures[edit]
While pressures are, in general, positive, there are several situations in which negative pressures may be encountered:
- When dealing in relative (gauge) pressures. For instance, an absolute pressure of 80 kPa may be described as a gauge pressure of −21 kPa (i.e., 21 kPa below an atmospheric pressure of 101 kPa).
- Negative absolute pressures are effectively tension, and both bulk solids and bulk liquids can be put under negative absolute pressure by pulling on them.[11] Microscopically, the molecules in solids and liquids have attractive interactions that overpower the thermal kinetic energy, so some tension can be sustained. Thermodynamically, however, a bulk material under negative pressure is in a metastable state, and it is especially fragile in the case of liquids where the negative pressure state is similar to superheating and is easily susceptible to cavitation.[12] In certain situations, the cavitation can be avoided and negative pressures sustained indefinitely,[12] for example, liquid mercury has been observed to sustain up to −425 atm in clean glass containers.[13] Negative liquid pressures are thought to be involved in the ascent of sap in plants taller than 10 m (the atmospheric pressure headof water).[14]
- The Casimir effect can create a small attractive force due to interactions with vacuum energy; this force is sometimes termed "vacuum pressure" (not to be confused with the negative gauge pressure of a vacuum).
- Abdominal decompression is an obstetric procedure during which negative pressure is applied intermittently to a pregnant woman's abdomen.
- For non-isotropic stresses in rigid bodies, depending on how the orientation of a surface is chosen, the same distribution of forces may have a component of positive pressure along one surface normal, with a component of negative pressure acting along another surface normal.
- The stresses in an electromagnetic field are generally non-isotropic, with the pressure normal to one surface element (the normal stress) being negative, and positive for surface elements perpendicular to this.
- In the cosmological constant.
Stagnation pressure[edit]
Stagnation pressure is the pressure a fluid exerts when it is forced to stop moving. Consequently, although a fluid moving at higher speed will have a lower static pressure, it may have a higher stagnation pressure when forced to a standstill. Static pressure and stagnation pressure are related by:
where
- is the stagnation pressure,
- is the density,
- is the flow velocity,
- is the static pressure.
The pressure of a moving fluid can be measured using a Pitot tube, or one of its variations such as a Kiel probe or Cobra probe, connected to a manometer. Depending on where the inlet holes are located on the probe, it can measure static pressures or stagnation pressures.
Surface pressure and surface tension[edit]
There is a two-dimensional analog of pressure – the lateral force per unit length applied on a line perpendicular to the force.
Surface pressure is denoted by π:
and shares many similar properties with three-dimensional pressure. Properties of surface chemicals can be investigated by measuring pressure/area isotherms, as the two-dimensional analog of Boyle's law, πA = k, at constant temperature.
Surface tension is another example of surface pressure, but with a reversed sign, because "tension" is the opposite to "pressure".
Pressure of an ideal gas[edit]
In an ideal gas, molecules have no volume and do not interact. According to the ideal gas law, pressure varies linearly with temperature and quantity, and inversely with volume:
where:
- p is the absolute pressure of the gas,
- n is the amount of substance,
- T is the absolute temperature,
- V is the volume,
- R is the ideal gas constant.
Real gases exhibit a more complex dependence on the variables of state.[15]
Vapour pressure[edit]
Vapour pressure is the pressure of a vapour in thermodynamic equilibrium with its condensed phases in a closed system. All liquids and solids have a tendency to evaporate into a gaseous form, and all gases have a tendency to condense back to their liquid or solid form.
The atmospheric pressure boiling point of a liquid (also known as the normal boiling point) is the temperature at which the vapor pressure equals the ambient atmospheric pressure. With any incremental increase in that temperature, the vapor pressure becomes sufficient to overcome atmospheric pressure and lift the liquid to form vapour bubbles inside the bulk of the substance. Bubble formation deeper in the liquid requires a higher pressure, and therefore higher temperature, because the fluid pressure increases above the atmospheric pressure as the depth increases.
The vapor pressure that a single component in a mixture contributes to the total pressure in the system is called partial vapor pressure.
Liquid pressure[edit]
| Part of a series on |
| Continuum mechanics |
|---|
When a person swims under the water, water pressure is felt acting on the person's eardrums. The deeper that person swims, the greater the pressure. The pressure felt is due to the weight of the water above the person. As someone swims deeper, there is more water above the person and therefore greater pressure. The pressure a liquid exerts depends on its depth.
Liquid pressure also depends on the density of the liquid. If someone was submerged in a liquid more dense than water, the pressure would be correspondingly greater. Thus, we can say that the depth, density and liquid pressure are directly proportionate. The pressure due to a liquid in liquid columns of constant density or at a depth within a substance is represented by the following formula:
where:
- p is liquid pressure,
- g is gravity at the surface of overlaying material,
- ρ is density of liquid,
- h is height of liquid column or depth within a substance.
Another way of saying the same formula is the following:
The pressure a liquid exerts against the sides and bottom of a container depends on the density and the depth of the liquid. If atmospheric pressure is neglected, liquid pressure against the bottom is twice as great at twice the depth; at three times the depth, the liquid pressure is threefold; etc. Or, if the liquid is two or three times as dense, the liquid pressure is correspondingly two or three times as great for any given depth. Liquids are practically incompressible – that is, their volume can hardly be changed by pressure (water volume decreases by only 50 millionths of its original volume for each atmospheric increase in pressure). Thus, except for small changes produced by temperature, the density of a particular liquid is practically the same at all depths.
Atmospheric pressure pressing on the surface of a liquid must be taken into account when trying to discover the total pressure acting on a liquid. The total pressure of a liquid, then, is ρgh plus the pressure of the atmosphere. When this distinction is important, the term total pressure is used. Otherwise, discussions of liquid pressure refer to pressure without regard to the normally ever-present atmospheric pressure.
The pressure does not depend on the amount of liquid present. Volume is not the important factor – depth is. The average water pressure acting against a dam depends on the average depth of the water and not on the volume of water held back. For example, a wide but shallow lake with a depth of 3 m (10 ft) exerts only half the average pressure that a small 6 m (20 ft) deep pond does. (The total force applied to the longer dam will be greater, due to the greater total surface area for the pressure to act upon. But for a given 5-foot (1.5 m)-wide section of each dam, the 10 ft (3.0 m) deep water will apply one quarter the force of 20 ft (6.1 m) deep water). A person will feel the same pressure whether his/her head is dunked a metre beneath the surface of the water in a small pool or to the same depth in the middle of a large lake. If four vases contain different amounts of water but are all filled to equal depths, then a fish with its head dunked a few centimetres under the surface will be acted on by water pressure that is the same in any of the vases. If the fish swims a few centimetres deeper, the pressure on the fish will increase with depth and be the same no matter which vase the fish is in. If the fish swims to the bottom, the pressure will be greater, but it makes no difference what vase it is in. All vases are filled to equal depths, so the water pressure is the same at the bottom of each vase, regardless of its shape or volume. If water pressure at the bottom of a vase were greater than water pressure at the bottom of a neighboring vase, the greater pressure would force water sideways and then up the narrower vase to a higher level until the pressures at the bottom were equalized. Pressure is depth dependent, not volume dependent, so there is a reason that water seeks its own level.
Restating this as energy equation, the energy per unit volume in an ideal, incompressible liquid is constant throughout its vessel. At the surface, gravitational potential energy is large but liquid pressure energy is low. At the bottom of the vessel, all the gravitational potential energy is converted to pressure energy. The sum of pressure energy and gravitational potential energy per unit volume is constant throughout the volume of the fluid and the two energy components change linearly with the depth.[16] Mathematically, it is described by Bernoulli's equation, where velocity head is zero and comparisons per unit volume in the vessel are
Terms have the same meaning as in section Fluid pressure.
Direction of liquid pressure[edit]
An experimentally determined fact about liquid pressure is that it is exerted equally in all directions.[17] If someone is submerged in water, no matter which way that person tilts his/her head, the person will feel the same amount of water pressure on his/her ears. Because a liquid can flow, this pressure isn't only downward. Pressure is seen acting sideways when water spurts sideways from a leak in the side of an upright can. Pressure also acts upward, as demonstrated when someone tries to push a beach ball beneath the surface of the water. The bottom of a boat is pushed upward by water pressure (buoyancy).
When a liquid presses against a surface, there is a net force that is perpendicular to the surface. Although pressure doesn't have a specific direction, force does. A submerged triangular block has water forced against each point from many directions, but components of the force that are not perpendicular to the surface cancel each other out, leaving only a net perpendicular point.[17] This is why water spurting from a hole in a bucket initially exits the bucket in a direction at right angles to the surface of the bucket in which the hole is located. Then it curves downward due to gravity. If there are three holes in a bucket (top, bottom, and middle), then the force vectors perpendicular to the inner container surface will increase with increasing depth – that is, a greater pressure at the bottom makes it so that the bottom hole will shoot water out the farthest. The force exerted by a fluid on a smooth surface is always at right angles to the surface. The speed of liquid out of the hole is , where h is the depth below the free surface.[17] This is the same speed the water (or anything else) would have if freely falling the same vertical distance h.
Kinematic pressure[edit]
is the kinematic pressure, where is the pressure and constant mass density. The SI unit of P is m2/s2. Kinematic pressure is used in the same manner as kinematic viscosity in order to compute the Navier–Stokes equation without explicitly showing the density .
- Navier–Stokes equation with kinematic quantities
See also[edit]
- Atmospheric pressure – Static pressure exerted by the weight of the atmosphere
- Blood pressure – Pressure exerted by circulating blood upon the walls of arteries
- Boyle's law – Relationship between pressure and volume in a gas at constant temperature
- Combined gas law – Combination of Charles', Boyle's and Gay-Lussac's gas laws
- Conversion of units – Comparison of various scales
- Critical point (thermodynamics) – Temperature and pressure point where phase boundaries disappear
- Dimensional analysis – Analysis of the relationships between different physical quantities by identifying their base quantities
- Dynamic pressure – Concept in fluid dynamics
- Electric potential – Line integral of the electric field
- Electron degeneracy pressure – Repulsive force in quantum mechanics
- Hydraulics – Fluid engineering and fluid mechanics
- Internal pressure
- Kinetic theory
- Microphone – Device that converts sound into an electrical signal
- Orders of magnitude (pressure)
- Partial pressure – Pressure attributed to a component gas in a mixture
- Pressure measurement – Analysis of force applied by a fluid on a surface
- Pressure sensor – Pressure measurement device
- Sound pressure – Local pressure deviation from the ambient atmospheric pressure, caused by a sound wave
- Static pressure
- Timeline of temperature and pressure measurement technology
- Torricelli's law
- Vacuum – Space that is empty of matter
- Vacuum pump
- Vertical pressure variation
| showDerivation of this equation |
|---|
| Sound measurements | |
|---|---|
Characteristic | Symbols |
| Sound pressure | p, SPL,LPA |
| Particle velocity | v, SVL |
| Particle displacement | δ |
| Sound intensity | I, SIL |
| Sound power | P, SWL, LWA |
| Sound energy | W |
| Sound energy density | w |
| Sound exposure | E, SEL |
| Acoustic impedance | Z |
| Audio frequency | AF |
| Transmission loss | TL |
Seawater[edit]
In salt water that is free of air bubbles or suspended sediment, sound travels at about 1500 m/s (1500.235 m/s at 1000 kilopascals, 10 °C and 3% salinity by one method).[27] The speed of sound in seawater depends on pressure (hence depth), temperature (a change of 1 °C ~ 4 m/s), and salinity (a change of 1‰ ~ 1 m/s), and empirical equations have been derived to accurately calculate the speed of sound from these variables.[28][29] Other factors affecting the speed of sound are minor. Since in most ocean regions temperature decreases with depth, the profile of the speed of sound with depth decreases to a minimum at a depth of several hundred metres. Below the minimum, sound speed increases again, as the effect of increasing pressure overcomes the effect of decreasing temperature (right).[30] For more information see Dushaw et al.[31]
An empirical equation for the speed of sound in sea water is provided by Mackenzie:[32]
where
- T is the temperature in degrees Celsius;
- S is the salinity in parts per thousand;
- z is the depth in metres.
The constants a1, a2, ..., a9 are
with check value 1550.744 m/s for T = 25 °C, S = 35 parts per thousand, z = 1,000 m. This equation has a standard error of 0.070 m/s for salinity between 25 and 40 ppt. See Technical Guides. Speed of Sound in Sea-Water for an online calculator.
(Note: The Sound Speed vs. Depth graph does not correlate directly to the MacKenzie formula. This is due to the fact that the temperature and salinity varies at different depths. When T and S are held constant, the formula itself is always increasing with depth.)
Other equations for the speed of sound in sea water are accurate over a wide range of conditions, but are far more complicated, e.g., that by V. A. Del Grosso[33] and the Chen-Millero-Li Equation.[31][34]
Speed of sound in plasma[edit]
The speed of sound in a plasma for the common case that the electrons are hotter than the ions (but not too much hotter) is given by the formula (see here)
where
- mi is the ion mass;
- μ is the ratio of ion mass to proton mass μ = mi/mp;
- Te is the electron temperature;
- Z is the charge state;
- k is Boltzmann constant;
- γ is the adiabatic index.
In contrast to a gas, the pressure and the density are provided by separate species: the pressure by the electrons and the density by the ions. The two are coupled through a fluctuating electric field.
Gradients[edit]
When sound spreads out evenly in all directions in three dimensions, the intensity drops in proportion to the inverse square of the distance. However, in the ocean, there is a layer called the 'deep sound channel' or SOFAR channel which can confine sound waves at a particular depth.
In the SOFAR channel, the speed of sound is lower than that in the layers above and below. Just as light waves will refract towards a region of higher index, sound waves will refract towards a region where their speed is reduced. The result is that sound gets confined in the layer, much the way light can be confined to a sheet of glass or optical fiber. Thus, the sound is confined in essentially two dimensions. In two dimensions the intensity drops in proportion to only the inverse of the distance. This allows waves to travel much further before being undetectably faint.
A similar effect occurs in the atmosphere. Project Mogul successfully used this effect to detect a nuclear explosion at a considerable distance.
See also[edit]
- Acoustoelastic effect
- Elastic wave
- Second sound
- Sonic boom
- Sound barrier
- Speeds of sound of the elements
- Underwater acoustics
- Vibrations
Rotating anode tube[edit]
A considerable amount of heat is generated in the focal spot (the area where the beam of electrons coming from the cathode strike to) of a stationary anode. Rather, a rotating anode lets the electron beam sweep a larger area of the anode, thus redeeming the advantage of a higher intensity of emitted radiation, along with reduced damage to anode compared to its stationary state.[8]
The focal spot temperature can reach 2,500 °C (4,530 °F) during an exposure, and the anode assembly can reach 1,000 °C (1,830 °F) following a series of large exposures. Typical anodes are a tungsten-rhenium target on a molybdenum core, backed with graphite. The rhenium makes the tungsten more ductile and resistant to wear from the impact of the electron beams. The molybdenumconducts heat from the target. The graphite provides thermal storage for the anode, and minimizes the rotating mass of the anode.
Microfocus X-ray tube[edit]
Some X-ray examinations (such as, e.g., non-destructive testing and 3-D microtomography) need very high-resolution images and therefore require X-ray tubes that can generate very small focal spot sizes, typically below 50 μm in diameter. These tubes are called microfocus X-ray tubes.
There are two basic types of microfocus X-ray tubes: solid-anode tubes and metal-jet-anode tubes.
Solid-anode microfocus X-ray tubes are in principle very similar to the Coolidge tube, but with the important distinction that care has been taken to be able to focus the electron beam into a very small spot on the anode. Many microfocus X-ray sources operate with focus spots in the range 5-20 μm, but in the extreme cases spots smaller than 1 μm may be produced.
The major drawback of solid-anode microfocus X-ray tubes is the very low power they operate at. In order to avoid melting of the anode the electron-beam power density must be below a maximum value. This value is somewhere in the range 0.4-0.8 W/μm depending on the anode material.[9] This means that a solid-anode microfocus source with a 10 μm electron-beam focus can operate at a power in the range 4-8 W.
In metal-jet-anode microfocus X-ray tubes the solid metal anode is replaced with a jet of liquid metal, which acts as the electron-beam target. The advantage of the metal-jet anode is that the maximum electron-beam power density is significantly increased. Values in the range 3-6 W/μm have been reported for different anode materials (gallium and tin).[10][11] In the case with a 10 μm electron-beam focus a metal-jet-anode microfocus X-ray source may operate at 30-60 W.
The major benefit of the increased power density level for the metal-jet X-ray tube is the possibility to operate with a smaller focal spot, say 5 μm, to increase image resolution and at the same time acquire the image faster, since the power is higher (15-30 W) than for solid-anode tubes with 10 μm focal spots.
Hazards of X-ray production from vacuum tubes[edit]
Any vacuum tube operating at several thousand volts or more can produce X-rays as an unwanted byproduct, raising safety issues.[12][13] The higher the voltage, the more penetrating the resulting radiation and the more the hazard. CRT displays, once common in color televisions and computer displays, operate at 3-40 kilovolts,[14] making them the main concern among household appliances. Historically, concern has focused less on the cathode ray tube, since its thick glass envelope is impregnated with several pounds of lead for shielding, than on high voltage (HV) rectifier and voltage regulator tubes inside. In the late 1960s it was found that a failure in the HV supply circuit of some General Electric TVs could leave excessive voltages on the regulator tube, causing it to emit X-rays.[citation needed] The models were recalled and the ensuing scandal caused the US agency responsible for regulating this hazard, the Center for Devices and Radiological Health of the Food and Drug Administration (FDA), to require that all TVs include circuits to prevent excessive voltages in the event of failure.[citation needed] The hazard associated with excessive voltages was eliminated with the advent of all-solid-state TVs, which have no tubes other than the CRT. Since 1969, the FDA has limited TV X-ray emission to 0.5 mR (milliroentgen) per hour. With the switch from CRTs to other screen technologies starting in the 1990s, there are no vacuum tubes capable of emitting X-rays at all.
See also[edit]
| Wikimedia Commons has media related to X-ray tubes. |
- Electron beam tomography
- Coronary angiography
- Synchrotron radiation
- X-ray fluorescence
- X-ray generator
- glass-to-metal-seal
https://en.wikipedia.org/wiki/X-ray_tube#Crookes_tube_%28cold_cathode_tube%29
Overview[edit]
Heated to temperatures above TC, ferromagnetic materials become paramagnetic and their magnetic behavior is dominated by spin waves or magnons, which are boson collective excitations with energies in the meV range. The magnetization that occurs below TC is a famous[citation needed] example of the "spontaneous" breaking of a global symmetry, a phenomenon that is described by Goldstone's theorem. The term "symmetry breaking" refers to the choice of a magnetization direction by the spins, which have spherical symmetry above TC, but a preferred axis (the magnetization direction) below TC.
Temperature dependence[edit]
To a first order approximation, the temperature dependence of spontaneous magnetization at low temperatures is given by Bloch's law:[1]
where M(0) is the spontaneous magnetization at absolute zero. The decrease in spontaneous magnetization at higher temperatures is caused by the increasing excitation of spin waves. In a particle description, the spin waves correspond to magnons, which are the massless Goldstone bosonscorresponding to the broken symmetry. This is exactly true for an isotropic magnet.
Magnetic anisotropy, that is the existence of an easy direction along which the moments align spontaneously in the crystal, corresponds however to "massive" magnons. This is a way of saying that they cost a minimum amount of energy to excite, hence they are very unlikely to be excited as . Hence the magnetization of an anisotropic magnet is harder to destroy at low temperature and the temperature dependence of the magnetization deviates accordingly from the Bloch's law. All real magnets are anisotropic to some extent.
Near the Curie temperature,
where β is a critical exponent that depends on the universality class of the magnetic interaction. Experimentally the exponent is 0.34 for Fe and 0.51 for Ni.[2]
An empirical interpolation of the two regimes is given by
it is easy to check two limits of this interpolation that follow laws similar to the Bloch law, for , and the critical behavior, for , respectively.
See also[edit]
Notes and references[edit]
- ^ Ashcroft & Mermin 1976, p. 708
- ^ Chikazumi 1997, pp. 128–129
/https://static.texastribune.org/media/files/8f77cc09075eeb2506b65069df0ccd60/COVID%20ICU%20nurse%20MGO%20TT%2011.jpg)
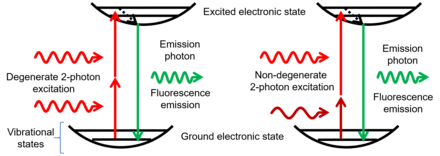
















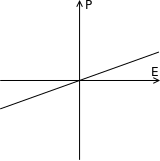






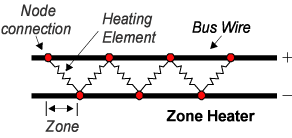






































No comments:
Post a Comment