
Tautomers (/ˈtɔːtəmər/)[1] are structural isomers (constitutional isomers) of chemical compounds that readily interconvert.[2][3][4][5] This reaction commonly results in the relocation of a hydrogen atom. Tautomerism is for example relevant to the behavior of amino acids and nucleic acids, two of the fundamental building blocks of life.
The concept of tautomerizations is called tautomerism. Tautomerism is also called desmotropism. The chemical reaction interconverting the two is called tautomerization.
Care should be taken not to confuse tautomers with depictions of "contributing structures" in chemical resonance. Tautomers are distinct chemical species and can be identified as such by their differing spectroscopic data,[6] whereas resonance structures are merely convenient depictions and do not physically exist.
Tautomerization is pervasive in organic chemistry. It is typically associated with polar molecules and ions containing functional groups that are at least weakly acidic. Most common tautomers exist in pairs, which means that the hydrogen is located at one of two positions, and even more specifically the most common form involves a hydrogen changing places with a double bond: H−X−Y=Z ⇌ X=Y−Z−H. Common tautomeric pairs include:[7]
- ketone – enol: H−O−C=C ⇌ O=C−C−H, see keto–enol tautomerism
- enamine – imine: H−N−C=C ⇌ N=C−C−H
- cyanamide – carbodiimide
- guanidine – guanidine – guanidine: With a central carbon surrounded by three nitrogens, a guanidine group allows this transform in three possible orientations
- amide – imidic acid: H−N−C=O ⇌ N=C−O−H (e.g., the latter is encountered during nitrilehydrolysis reactions)
- lactam – lactim, a cyclic form of amide-imidic acid tautomerism in 2-pyridone and derived structures such as the nucleobases guanine, thymine, and cytosine
- imine – imine, e.g., during pyridoxal phosphate catalyzed enzymatic reactions
- R1R2C(=NCHR3R4) ⇌ (R1R2CHN=)CR3R4
- nitro – aci-nitro (nitronic acid): RR'HC–N+(=O)(O–) ⇌ RR'C=N+(O–)(OH)
- nitroso – oxime: H−C−N=O ⇌ C=N−O−H
- ketene – ynol, which involves a triple bond: H−C=C=O ⇌ C≡C−O−H
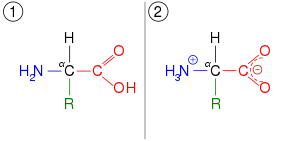
- amino acid – ammonium carboxylate, which applies to the building blocks of the proteins. This shifts the proton more than two atoms away, producing a zwitterion rather than shifting a double bond: H2N−CH2−COOH ⇌ H3N+
−CH2−CO−
2 - phosphite – phosphonate: P(OR)2(OH) ⇌ HP(OR)2(=O) between trivalent and pentavalent phosphorus.
Prototropy[edit]
Prototropy is the most common form of tautomerism and refers to the relocation of a hydrogen atom.[8] Prototropic tautomerism may be considered a subset of acid-base behavior. Prototropic tautomers are sets of isomeric protonation states with the same empirical formula and total charge. Tautomerizations are catalyzed by:[citation needed]
- bases, involving a series of steps: deprotonation, formation of a delocalized anion (e.g., an enolate), and protonation at a different position of the anion; and
- acids, involving a series of steps: protonation, formation of a delocalized cation, and deprotonation at a different position adjacent to the cation).
Two specific further subcategories of tautomerizations:
- Annular tautomerism is a type of prototropic tautomerism wherein a proton can occupy two or more positions of a heterocyclic system, for example, 1H- and 3H-imidazole; 1H-, 2H- and 4H- 1,2,4-triazole; 1H- and 2H- isoindole.[9][non-primary source needed][better source needed]
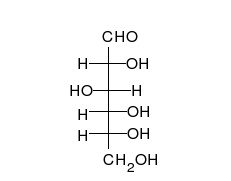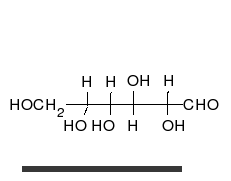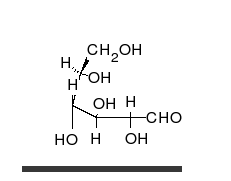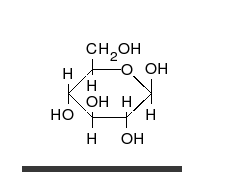
- Ring–chain tautomers occur when the movement of the proton is accompanied by a change from an open structure to a ring, such as the open chain and cyclic hemiacetal (typically pyranose or furanose forms) of many sugars.[7] (See Carbohydrate § Ring-straight chain isomerism.) The tautomeric shift can be described as H−O ⋅ C=O ⇌ O−C−O−H, where the "⋅" indicates the initial absence of a bond.
Valence tautomerism[edit]
Valence tautomerism is a type of tautomerism in which single and/or double bonds are rapidly formed and ruptured, without migration of atoms or groups.[10] It is distinct from prototropic tautomerism, and involves processes with rapid reorganisation of bonding electrons.
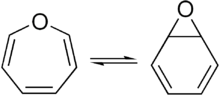
A pair of valence tautomers with formula C6H6O are benzene oxide and oxepin.[10][11]
Other examples of this type of tautomerism can be found in bullvalene, and in open and closed forms of certain heterocycles, such as organic azides and tetrazoles,[12] or mesoionic münchnone and acylamino ketene.
Valence tautomerism requires a change in molecular geometry and should not be confused with canonical resonance structures or mesomers.
See also[edit]
https://en.wikipedia.org/wiki/Tautomer
No comments:
Post a Comment