
Cases
| Location | Cases | Deaths |
|---|---|---|
United States | 42.9M 42,900,000 +51,575 +51,575 | 688K 688,000 +629 +629 |
India | 33.7M 33,700,000 | 447K 447,000 |
Brazil | 21.3M 21,300,000 | 594K 594,000 |
United Kingdom | 7.63M 7,630,000 | 136K 136,000 |
Russia | 7.29M 7,290,000 | 199K |
COVID-19 death toll spikes due to backlog of data
Health officials struggle to keep up with coronavirus tracking.
- By Camille Botello
- Saturday, September 25, 2021 9:50pm
- https://www.peninsulaclarion.com/news/covid-19-death-toll-spikes-due-to-backlog-of-data/
Ludacris blueberry yum yum
Cast iron is a group of iron-carbon alloys with a carbon content more than 2%.[1] Its usefulness derives from its relatively low melting temperature. The alloy constituents affect its colour when fractured: white cast iron has carbide impurities which allow cracks to pass straight through, grey cast iron has graphite flakes which deflect a passing crack and initiate countless new cracks as the material breaks, and ductile cast iron has spherical graphite "nodules" which stop the crack from further progressing.
Carbon (C) ranging from 1.8 to 4 wt%, and silicon (Si) 1–3 wt%, are the main alloying elements of cast iron. Iron alloys with lower carbon content are known as steel.
Cast iron tends to be brittle, except for malleable cast irons. With its relatively low melting point, good fluidity, castability, excellent machinability, resistance to deformation and wear resistance, cast irons have become an engineering material with a wide range of applications and are used in pipes, machines and automotive industryparts, such as cylinder heads, cylinder blocks and gearbox cases. It is resistant to damage by oxidation but is difficult to weld.
The earliest cast-iron artefacts date to the 5th century BC, and were discovered by archaeologists in what is now Jiangsu in China. Cast iron was used in ancient China for warfare, agriculture, and architecture.[2] During the 15th century, cast iron became utilized for cannon in Burgundy, France, and in England during the Reformation. The amounts of cast iron used for cannon required large scale production.[3] The first cast-iron bridge was built during the 1770s by Abraham Darby III, and is known as The Iron Bridge in Shropshire, England. Cast iron was also used in the construction of buildings.
https://en.wikipedia.org/wiki/Cast_iron
The neutron detection temperature, also called the neutron energy, indicates a free neutron's kinetic energy, usually given in electron volts. The term temperature is used, since hot, thermal and cold neutrons are moderated in a medium with a certain temperature. The neutron energy distribution is then adapted to the Maxwellian distribution known for thermal motion. Qualitatively, the higher the temperature, the higher the kinetic energy of the free neutrons. The momentum and wavelength of the neutron are related through the de Broglie relation. The large wavelength of slow neutrons allows for the large cross section.[1]
Neutron energy distribution ranges[edit]
| Neutron energy | Energy range |
|---|---|
| 0.0–0.025 eV | Cold neutrons |
| 0.025 eV | Thermal neutrons |
| 0.025–0.4 eV | Epithermal neutrons |
| 0.4–0.5 eV | Cadmium neutrons |
| 0.5–1 eV | EpiCadmium neutrons |
| 1–10 eV | Slow neutrons |
| 10–300 eV | Resonance neutrons |
| 300 eV–1 MeV | Intermediate neutrons |
| 1–20 MeV | Fast neutrons |
| > 20 MeV | Ultrafast neutrons |
But different ranges with different names are observed in other sources.[4]
The following is a detailed classification:
Thermal[edit]
A thermal neutron is a free neutron with a kinetic energy of about 0.025 eV (about 4.0×10−21 J or 2.4 MJ/kg, hence a speed of 2.19 km/s), which is the energy corresponding to the most probable speed at a temperature of 290 K (17 °C or 62 °F), the mode of the Maxwell–Boltzmann distribution for this temperature.
After a number of collisions with nuclei (scattering) in a medium (neutron moderator) at this temperature, those neutrons which are not absorbed reach about this energy level.
Thermal neutrons have a different and sometimes much larger effective neutron absorption cross-section for a given nuclide than fast neutrons, and can therefore often be absorbed more easily by an atomic nucleus, creating a heavier, often unstable isotope of the chemical element as a result. This event is called neutron activation.
Epithermal[edit]
- Neutrons of energy greater than thermal
- Greater than 0.025 eV
Cadmium[edit]
- Neutrons which are strongly absorbed by cadmium
- Less than 0.5 eV.
Epicadmium[edit]
- Neutrons which are not strongly absorbed by cadmium
- Greater than 0.5 eV.
Slow[edit]
- Neutrons of energy slightly greater than epicadmium neutrons.
- Less than 1 to 10 eV.
Resonance[edit]
- Refers to neutrons which are strongly susceptible to non-fission capture by U-238.
- 1 eV to 300 eV
Intermediate[edit]
- Neutrons that are between slow and fast
- Few hundred eV to 0.5 MeV.
Fast[edit]
- A fast neutron is a free neutron with a kinetic energy level close to 1 MeV (100 TJ/kg), hence a speed of 14,000 km/s, or higher. They are named fast neutrons to distinguish them from lower-energy thermal neutrons, and high-energy neutrons produced in cosmic showers or accelerators.
Fast neutrons are produced by nuclear processes:
- Nuclear fission produces neutrons with a mean energy of 2 MeV (200 TJ/kg, i.e. 20,000 km/s), which qualifies as "fast". However the range of neutrons from fission follows a Maxwell–Boltzmann distribution from 0 to about 14 MeV in the center of momentum frame of the disintegration, and the mode of the energy is only 0.75 MeV, meaning that fewer than half of fission neutrons qualify as "fast" even by the 1 MeV criterion.[5]
- Spontaneous fission is a type of radioactive decay that some heavy elements undergo. Examples include plutonium-240 and californium-252.
- Nuclear fusion: deuterium–tritium fusion produces neutrons of 14.1 MeV (1400 TJ/kg, i.e. 52,000 km/s, 17.3% of the speed of light) that can easily fission uranium-238 and other non-fissile actinides.
- Neutron emission occurs in situations in which a nucleus contains enough excess neutrons that the separation energy of one or more neutrons becomes negative (i.e. excess neutrons "drip" out of the nucleus). Unstable nuclei of this sort will often decay in less than one second.
Fast neutrons are usually undesirable in a steady-state nuclear reactor because most fissile fuel has a higher reaction rate with thermal neutrons. Fast neutrons can be rapidly changed into thermal neutrons via a process called moderation. This is done through numerous collisions with (in general) slower-moving and thus lower-temperature particles like atomic nuclei and other neutrons. These collisions will generally speed up the other particle and slow down the neutron and scatter it. Ideally, a room temperature neutron moderator is used for this process. In reactors, heavy water, light water, or graphite are typically used to moderate neutrons.

Ultrafast[edit]
- Relativistic
- Greater than 20 MeV
Other classifications[edit]
- Pile
- Neutrons of all energies present in nuclear reactors
- 0.001 eV to 15 MeV.
- Ultracold
- Neutrons with sufficiently low energy to be reflected and trapped
- Upper bound of 335 neV
Fast-neutron reactor and thermal-neutron reactor compared[edit]
Most fission reactors are thermal-neutron reactors that use a neutron moderator to slow down ("thermalize") the neutrons produced by nuclear fission. Moderation substantially increases the fission cross section for fissile nuclei such as uranium-235 or plutonium-239. In addition, uranium-238 has a much lower capture cross section for thermal neutrons, allowing more neutrons to cause fission of fissile nuclei and propagate the chain reaction, rather than being captured by 238U. The combination of these effects allows light water reactors to use low-enriched uranium. Heavy water reactors and graphite-moderated reactors can even use natural uranium as these moderators have much lower neutron capture cross sections than light water.[6]
An increase in fuel temperature also raises U-238's thermal neutron absorption by Doppler broadening, providing negative feedback to help control the reactor. When the coolant is a liquid that also contributes to moderation and absorption (light water or heavy water), boiling of the coolant will reduce the moderator density, which can provide positive or negative feedback (a positive or negative void coefficient), depending on whether the reactor is under- or over-moderated.
Intermediate-energy neutrons have poorer fission/capture ratios than either fast or thermal neutrons for most fuels. An exception is the uranium-233 of the thorium cycle, which has a good fission/capture ratio at all neutron energies.
Fast-neutron reactors use unmoderated fast neutrons to sustain the reaction and require the fuel to contain a higher concentration of fissile material relative to fertile materialU-238. However, fast neutrons have a better fission/capture ratio for many nuclides, and each fast fission releases a larger number of neutrons, so a fast breeder reactor can potentially "breed" more fissile fuel than it consumes.
Fast reactor control cannot depend solely on Doppler broadening or on negative void coefficient from a moderator. However, thermal expansion of the fuel itself can provide quick negative feedback. Perennially expected to be the wave of the future, fast reactor development has been nearly dormant with only a handful of reactors built in the decades since the Chernobyl accident due to low prices in the uranium market, although there is now a revival with several Asian countries planning to complete larger prototype fast reactors in the next few years.
See also[edit]
https://en.wikipedia.org/wiki/Neutron_temperature
A neutron reflector is any material that reflects neutrons. This refers to elastic scattering rather than to a specular reflection. The material may be graphite, beryllium, steel, tungsten carbide, gold, or other materials. A neutron reflector can make an otherwise subcritical mass of fissile material critical, or increase the amount of nuclear fission that a critical or supercritical mass will undergo. Such an effect was exhibited twice in accidents involving the Demon Core, a subcritical plutonium pit that went critical in two separate fatal incidents when the pit's surface was momentarily surrounded by too much neutron reflective material.
Nuclear reactors[edit]
In a uranium graphite chain reacting pile, the critical size may be considerably reduced by surrounding the pile with a layer of graphite, since such an envelope reflects many neutrons back into the pile.
To obtain a 30-year life span, the SSTAR nuclear reactor design calls for a moveable neutron reflector to be placed over the column of fuel. The reflector's slow downward travel over the column would cause the fuel to be burned from the top of the column to the bottom.
A reflector made of a light material like graphite or beryllium will also serve as a neutron moderator reducing neutron kinetic energy, while a heavy material like lead or lead-bismuth eutectic will have less effect on neutron velocity.
In power reactors, a neutron reflector reduces the non-uniformity of the power distribution in the peripheral fuel assemblies, reduces neutron leakage and reduces a coolant flow bypass of the core. By reducing neutron leakage, the reflector increases reactivity of the core and reduces the amount of fuel necessary to maintain the reactor critical for a long period. In light-water reactors, the neutron reflector is installed for following purposes:
- The neutron flux distribution is “flattened“, i.e., the ratio of the average flux to the maximum flux is increased. Therefore reflectors reduce the non-uniformity of the power distribution.
- By increasing the neutron flux at the edge of the core, there is much better utilization in the peripheral fuel assemblies. This fuel, in the outer regions of the core, now contributes much more to the total power production.
- The neutron reflector scatters back (or reflects) into the core many neutrons that would otherwise escape. The neutrons reflected back into the core are available for chain reaction. This means that the minimum critical size of the reactor is reduced. Alternatively, if the core size is maintained, the reflector makes additional reactivity available for higher fuel burnup. The decrease in the critical size of core required is known as the reflector savings.
- Neutron reflectors reduce neutron leakage i.e. to reduce the neutron fluence on a reactor pressure vessel.
- Neutron reflectors reduce a coolant flow bypass of a core.
- Neutron reflectors serve as a thermal and radiation shield of a reactor core.
See also[edit]
A neutron supermirror is a highly polished, layered material used to reflect neutron beams. Supermirrors are a special case of multi-layer neutron reflectors with varying layer thicknesses.[1]
The first neutron supermirror concept was proposed by Mezei,[2] inspired by earlier work with x-rays.
Supermirrors are produced by depositing alternating layers of strongly contrasting substances, such as nickel and titanium, on a smooth substrate. A single layer of high refractive index material (e.g. nickel) exhibits total external reflection at small grazing angles up to a critical angle 



A mirror with a larger effective critical angle can be made by exploiting diffraction (with non-zero losses) that occurs from stacked multilayers.[3] The critical angle of total reflection, in degrees, becomes approximately 


Nickel has a positive scattering cross section, and titanium has a negative scattering cross section, and in both elements the absorption cross section is small, which makes Ni-Ti the most efficient technology with neutrons. The number of Ni-Ti layers needed increases rapidly as 

References[edit]
- ^ Chupp, T. "Neutron Optics and Polarization" (PDF). Retrieved 16 April 2019.
- ^ Mezei, F (1976). "Novel polarized neutron devices: supermirror and spin component amplifier". Communications on Physics (London). 1 (3): 81–85.
- ^ Hayter, J. B.; Mook, H. A. (1989). "Discrete Thin-Film Multilayer Design for X-ray and Neutron Supermirrors". J. Appl. Cryst. 22: 35–41. doi:10.1107/S0021889888010003.
- ^ Bentley, PM (2020). "Instrument suite cost optimisation in a science megaproject". Journal of Physics Communications. 4 (4): 045014. doi:10.1088/2399-6528/ab8a06.
https://en.wikipedia.org/wiki/Neutron_supermirror
Subcategories
This category has the following 7 subcategories, out of 7 total.
Pages in category "Optical materials"
The following 64 pages are in this category, out of 64 total. This list may not reflect recent changes (learn more).
C
P
S
Z
Neutron reflectometry is a neutron diffraction technique for measuring the structure of thin films, similar to the often complementary techniques of X-ray reflectivity and ellipsometry. The technique provides valuable information over a wide variety of scientific and technological applications including chemical aggregation, polymer and surfactant adsorption, structure of thin film magnetic systems, biological membranes, etc.
History[edit]
Neutron reflectometery emerged as a new field in the 1980s, after the discovery of giant magnetoresistance in antiferromagnetically-coupled multilayered films.[1]
Technique[edit]
The technique involves shining a highly collimated beam of neutrons onto an extremely flat surface and measuring the intensity of reflected radiation as a function of angle or neutron wavelength. The exact shape of the reflectivity profile provides detailed information about the structure of the surface, including the thickness, density, and roughness of any thin films layered on the substrate.
Neutron reflectometry is most often made in specular reflection mode, where the angle of the incident beam is equal to the angle of the reflected beam. The reflection is usually described in terms of a momentum transfer vector, denoted 


where 

Off-specular reflectometry gives rise to diffuse scattering and involves momentum transfer within the layer, and is used to determine lateral correlations within the layers, such as those arising from magnetic domains or in-plane correlated roughness.
The wavelength of the neutrons used for reflectivity are typically on the order of 0.2 to 1 nm (2 to 10 Å). This technique requires a neutron source, which may be either a research reactor or a spallation source (based on a particle accelerator). Like all neutron scattering techniques, neutron reflectometry is sensitive to contrast arising from different nuclei (as compared to electron density, which is measured in x-ray scattering). This allows the technique to differentiate between various isotopes of elements. Neutron reflectometry measures the neutron scattering length density (SLD) and can be used to accurately calculate material density if the atomic composition is known.
Comparison to other reflectometry techniques[edit]
Although other reflectivity techniques (in particular optical reflectivity, x-ray reflectometry) operate using the same general principles, neutron measurements are advantageous in a few significant ways. Most notably, since the technique probes nuclear contrast, rather than electron density, it is more sensitive for measuring some elements, especially lighter elements (hydrogen, carbon, nitrogen, oxygen, etc.). Sensitivity to isotopes also allows contrast to be greatly (and selectively) enhanced for some systems of interest using isotopic substitution, and multiple experiments that differ only by isotopic substitution can be used to resolve the phase problem that is general to scattering techniques. Finally, neutrons are highly penetrating and typically non-perturbing: which allows for great flexibility in sample environments, and the use of delicate sample materials (e.g., biological specimens). By contrast x-ray exposure may damage some materials, and laser light can modify some materials (e.g. photoresists). Also, optical techniques may include ambiguity due to optical anisotropy (birefringence), which complementary neutron measurements can resolve. Dual polarisation interferometry is one optical method which provides analogous results to neutron reflectometry at comparable resolution although the underpinning mathematical model is somewhat simpler, i.e. it can only derive a thickness (or birefringence) for a uniform layer density.
Disadvantages of neutron reflectometry include the higher cost of the required infrastructure, the fact that some materials may become radioactive upon exposure to the beam, and insensitivity to the chemical state of constituent atoms. Moreover, the relatively lower flux and higher background of the technique (when compared to x-ray reflectivity) limit the maximum value of 
References[edit]
- ^ Dalliant, Jean; Gibaud, Alain, eds. (2009). X-ray and Neutron Reflectivity. Lecture Notes in Physics. 770. Berlin Heidelberg: Springer. p. 183. ISBN 9783540885870.
A plain bearing, or more commonly sliding contact bearing and slide bearing (in railroading sometimes called a solid bearing, journal bearing, or friction bearing[1]), is the simplest type of bearing, comprising just a bearing surface and no rolling elements. Therefore, the journal (i.e., the part of the shaft in contact with the bearing) slides over the bearing surface. The simplest example of a plain bearing is a shaft rotating in a hole. A simple linear bearing can be a pair of flat surfaces designed to allow motion; e.g., a drawer and the slides it rests on[2] or the ways on the bed of a lathe.
Plain bearings, in general, are the least expensive type of bearing. They are also compact and lightweight, and they have a high load-carrying capacity.[3]
https://en.wikipedia.org/wiki/Plain_bearing
Giant magnetoresistance (GMR) is a quantum mechanical magnetoresistance effect observed in multilayers composed of alternating ferromagnetic and non-magnetic conductive layers. The 2007 Nobel Prize in Physics was awarded to Albert Fert and Peter Grünberg for the discovery of GMR.
The effect is observed as a significant change in the electrical resistance depending on whether the magnetization of adjacent ferromagnetic layers are in a parallel or an antiparallel alignment. The overall resistance is relatively low for parallel alignment and relatively high for antiparallel alignment. The magnetization direction can be controlled, for example, by applying an external magnetic field. The effect is based on the dependence of electron scattering on spin orientation.
The main application of GMR is in magnetic field sensors, which are used to read data in hard disk drives, biosensors, microelectromechanical systems (MEMS) and other devices.[1] GMR multilayer structures are also used in magnetoresistive random-access memory (MRAM) as cells that store one bit of information.
In literature, the term giant magnetoresistance is sometimes confused with colossal magnetoresistance of ferromagnetic and antiferromagnetic semiconductors, which is not related to a multilayer structure.[2][3]
https://en.wikipedia.org/wiki/Giant_magnetoresistance
The neutron detection temperature, also called the neutron energy, indicates a free neutron's kinetic energy, usually given in electron volts. The term temperature is used, since hot, thermal and cold neutrons are moderated in a medium with a certain temperature. The neutron energy distribution is then adapted to the Maxwellian distribution known for thermal motion. Qualitatively, the higher the temperature, the higher the kinetic energy of the free neutrons. The momentum and wavelength of the neutron are related through the de Broglie relation. The large wavelength of slow neutrons allows for the large cross section.[1]
Neutron energy distribution ranges[edit]
| Neutron energy | Energy range |
|---|---|
| 0.0–0.025 eV | Cold neutrons |
| 0.025 eV | Thermal neutrons |
| 0.025–0.4 eV | Epithermal neutrons |
| 0.4–0.5 eV | Cadmium neutrons |
| 0.5–1 eV | EpiCadmium neutrons |
| 1–10 eV | Slow neutrons |
| 10–300 eV | Resonance neutrons |
| 300 eV–1 MeV | Intermediate neutrons |
| 1–20 MeV | Fast neutrons |
| > 20 MeV | Ultrafast neutrons |
But different ranges with different names are observed in other sources.[4]
The following is a detailed classification:
Thermal[edit]
A thermal neutron is a free neutron with a kinetic energy of about 0.025 eV (about 4.0×10−21 J or 2.4 MJ/kg, hence a speed of 2.19 km/s), which is the energy corresponding to the most probable speed at a temperature of 290 K (17 °C or 62 °F), the mode of the Maxwell–Boltzmann distribution for this temperature.
After a number of collisions with nuclei (scattering) in a medium (neutron moderator) at this temperature, those neutrons which are not absorbed reach about this energy level.
Thermal neutrons have a different and sometimes much larger effective neutron absorption cross-section for a given nuclide than fast neutrons, and can therefore often be absorbed more easily by an atomic nucleus, creating a heavier, often unstable isotope of the chemical element as a result. This event is called neutron activation.
Epithermal[edit]
- Neutrons of energy greater than thermal
- Greater than 0.025 eV
Cadmium[edit]
- Neutrons which are strongly absorbed by cadmium
- Less than 0.5 eV.
Epicadmium[edit]
- Neutrons which are not strongly absorbed by cadmium
- Greater than 0.5 eV.
Slow[edit]
- Neutrons of energy slightly greater than epicadmium neutrons.
- Less than 1 to 10 eV.
Resonance[edit]
- Refers to neutrons which are strongly susceptible to non-fission capture by U-238.
- 1 eV to 300 eV
Intermediate[edit]
- Neutrons that are between slow and fast
- Few hundred eV to 0.5 MeV.
Fast[edit]
- A fast neutron is a free neutron with a kinetic energy level close to 1 MeV (100 TJ/kg), hence a speed of 14,000 km/s, or higher. They are named fast neutrons to distinguish them from lower-energy thermal neutrons, and high-energy neutrons produced in cosmic showers or accelerators.
Fast neutrons are produced by nuclear processes:
- Nuclear fission produces neutrons with a mean energy of 2 MeV (200 TJ/kg, i.e. 20,000 km/s), which qualifies as "fast". However the range of neutrons from fission follows a Maxwell–Boltzmann distribution from 0 to about 14 MeV in the center of momentum frame of the disintegration, and the mode of the energy is only 0.75 MeV, meaning that fewer than half of fission neutrons qualify as "fast" even by the 1 MeV criterion.[5]
- Spontaneous fission is a type of radioactive decay that some heavy elements undergo. Examples include plutonium-240 and californium-252.
- Nuclear fusion: deuterium–tritium fusion produces neutrons of 14.1 MeV (1400 TJ/kg, i.e. 52,000 km/s, 17.3% of the speed of light) that can easily fission uranium-238 and other non-fissile actinides.
- Neutron emission occurs in situations in which a nucleus contains enough excess neutrons that the separation energy of one or more neutrons becomes negative (i.e. excess neutrons "drip" out of the nucleus). Unstable nuclei of this sort will often decay in less than one second.
Fast neutrons are usually undesirable in a steady-state nuclear reactor because most fissile fuel has a higher reaction rate with thermal neutrons. Fast neutrons can be rapidly changed into thermal neutrons via a process called moderation. This is done through numerous collisions with (in general) slower-moving and thus lower-temperature particles like atomic nuclei and other neutrons. These collisions will generally speed up the other particle and slow down the neutron and scatter it. Ideally, a room temperature neutron moderator is used for this process. In reactors, heavy water, light water, or graphite are typically used to moderate neutrons.

Ultrafast[edit]
- Relativistic
- Greater than 20 MeV
Other classifications[edit]
- Pile
- Neutrons of all energies present in nuclear reactors
- 0.001 eV to 15 MeV.
- Ultracold
- Neutrons with sufficiently low energy to be reflected and trapped
- Upper bound of 335 neV
Fast-neutron reactor and thermal-neutron reactor compared[edit]
Most fission reactors are thermal-neutron reactors that use a neutron moderator to slow down ("thermalize") the neutrons produced by nuclear fission. Moderation substantially increases the fission cross section for fissile nuclei such as uranium-235 or plutonium-239. In addition, uranium-238 has a much lower capture cross section for thermal neutrons, allowing more neutrons to cause fission of fissile nuclei and propagate the chain reaction, rather than being captured by 238U. The combination of these effects allows light water reactors to use low-enriched uranium. Heavy water reactors and graphite-moderated reactors can even use natural uranium as these moderators have much lower neutron capture cross sections than light water.[6]
An increase in fuel temperature also raises U-238's thermal neutron absorption by Doppler broadening, providing negative feedback to help control the reactor. When the coolant is a liquid that also contributes to moderation and absorption (light water or heavy water), boiling of the coolant will reduce the moderator density, which can provide positive or negative feedback (a positive or negative void coefficient), depending on whether the reactor is under- or over-moderated.
Intermediate-energy neutrons have poorer fission/capture ratios than either fast or thermal neutrons for most fuels. An exception is the uranium-233 of the thorium cycle, which has a good fission/capture ratio at all neutron energies.
Fast-neutron reactors use unmoderated fast neutrons to sustain the reaction and require the fuel to contain a higher concentration of fissile material relative to fertile materialU-238. However, fast neutrons have a better fission/capture ratio for many nuclides, and each fast fission releases a larger number of neutrons, so a fast breeder reactor can potentially "breed" more fissile fuel than it consumes.
Fast reactor control cannot depend solely on Doppler broadening or on negative void coefficient from a moderator. However, thermal expansion of the fuel itself can provide quick negative feedback. Perennially expected to be the wave of the future, fast reactor development has been nearly dormant with only a handful of reactors built in the decades since the Chernobyl accident due to low prices in the uranium market, although there is now a revival with several Asian countries planning to complete larger prototype fast reactors in the next few years.
See also[edit]
https://en.wikipedia.org/wiki/Neutron_temperature
In materials that exhibit antiferromagnetism, the magnetic moments of atoms or molecules, usually related to the spins of electrons, align in a regular pattern with neighboring spins (on different sublattices) pointing in opposite directions. This is, like ferromagnetismand ferrimagnetism, a manifestation of ordered magnetism.
Generally, antiferromagnetic order may exist at sufficiently low temperatures, but vanishes at and above the Néel temperature – named after Louis Néel, who had first identified this type of magnetic ordering.[1] Above the Néel temperature, the material is typically paramagnetic.
https://en.wikipedia.org/wiki/Antiferromagnetism
A metallomesogen is a metal complex that exhibit liquid crystalline behavior. Thus, they adopt ordered structures in the molten phase, e.g. smectic and nematic phases. The dominant interactions responsible for their phase behavior are the nonbonding contacts between organic substituents. Two early classes of such materials are based on substituted ferrocenes and dithiolene complexes.[1]
https://en.wikipedia.org/wiki/Metallomesogen
Chalcogenide glass (pronounced hard ch as in chemistry) is a glass containing one or more chalcogens (sulfur, selenium and tellurium, but excluding oxygen). Such glasses are covalently bonded materials and may be classified as covalent network solids. Polonium is also a chalcogen but is not used because of its strong radioactivity. Chalcogenide materials behave rather differently from oxides, in particular their lower band gaps contribute to very dissimilar optical and electrical properties.
The classical chalcogenide glasses (mainly sulfur-based ones such as As-S or Ge-S) are strong glass-formers and possess glasses within large concentration regions. Glass-forming abilities decrease with increasing molar weight of constituent elements; i.e., S > Se > Te.
Chalcogenide compounds such as AgInSbTe and GeSbTe are used in rewritable optical disks and phase-change memory devices. They are fragile glass-formers: by controlling heating and annealing (cooling), they can be switched between an amorphous (glassy) and a crystalline state, thereby changing their optical and electrical properties and allowing the storage of information.
https://en.wikipedia.org/wiki/Chalcogenide_glass
A phosphor is a substance that exhibits the phenomenon of luminescence; it emits light when exposed to some type of radiant energy. The term is used both for fluorescent or phosphorescent substances which glow on exposure to ultraviolet or visible light, and cathodoluminescent substances which glow when struck by an electron beam (cathode rays) in a cathode ray tube.
When a phosphor is exposed to radiation, the orbital electrons in its molecules are excited to a higher energy level; when they return to their former level they emit the energy as light of a certain color. Phosphors can be classified into two categories: fluorescentsubstances which emit the energy immediately and stop glowing when the exciting radiation is turned off, and phosphorescentsubstances which emit the energy after a delay, so they keep glowing after the radiation is turned off, decaying in brightness over a period of milliseconds to days.
Fluorescent materials are used in applications in which the phosphor is excited continuously: cathode ray tubes (CRT) and plasma video display screens, fluoroscope screens, fluorescent lights, scintillation sensors, and white LEDs, and luminous paints for black lightart. Phosphorescent materials are used where a persistent light is needed, such as glow-in-the-dark watch faces and aircraft instruments, and in radar screens to allow the target 'blips' to remain visible as the radar beam rotates. CRT phosphors were standardized beginning around World War II and designated by the letter "P" followed by a number.
Phosphorus, the light-emitting chemical element for which phosphors are named, emits light due to chemiluminescence, not phosphorescence.[1]
https://en.wikipedia.org/wiki/Phosphor
Potassium bromide (K Br) is a salt, widely used as an anticonvulsant and a sedative in the late 19th and early 20th centuries, with over-the-counter use extending to 1975 in the US. Its action is due to the bromide ion (sodium bromide is equally effective). Potassium bromide is used as a veterinary drug, as an antiepileptic medication for dogs.
Under standard conditions, potassium bromide is a white crystalline powder. It is freely soluble in water; it is not soluble in acetonitrile. In a dilute aqueous solution, potassium bromide tastes sweet, at higher concentrations it tastes bitter, and tastes salty when the concentration is even higher.[by how much?] These effects are mainly due to the properties of the potassium ion—sodium bromide tastes salty at any concentration. In high concentration, potassium bromide strongly irritates the gastric mucous membrane, causing nausea and sometimes vomiting (a typical effect of all soluble potassium salts).[citation needed]
https://en.wikipedia.org/wiki/Potassium_bromide
Barium fluoride (BaF2) is an inorganic compound with the formula BaF2. It is a colorless solid that occurs in nature as the rare mineral frankdicksonite.[9] Under standard conditions it adopts the fluorite structure and at high pressure the PbCl2 structure.[10]Like CaF2, it is resilient to and insoluble in water.
Above ca. 500 °C, BaF2 is corroded by moisture, but in dry environments it can be used up to 800 °C. Prolonged exposure to moisture degrades transmission in the vacuum UV range. It is less resistant to water than calcium fluoride, but it is the most resistant of all the optical fluorides to high-energy radiation, though its far ultraviolet transmittance is lower than that of the other fluorides. It is quite hard, very sensitive to thermal shock and fractures quite easily.
https://en.wikipedia.org/wiki/Barium_fluoride
Arsenic triselenide (As2Se3) is an inorganic chemical compound, a selenide of arsenic.
Amorphous arsenic triselenide is used as a chalcogenide glass for infrared optics. When purified, it transmits light with wavelengths between ca. 0.7 and 19 µm.[3]
Arsenic triselenide is covalently bonded. Even so, the arsenic has a formal oxidation state of +3.
Solution processed thin film As2Se3[edit]
Thin film selenide glasses have emerged as an important material for integrated photonics due to its high refractive index, mid-IR transparency and high non-linear optical indices. High-quality As2Se3 glass films can be deposited from spin coating method from ethylenediamine solutions.[4]

https://en.wikipedia.org/wiki/Arsenic_triselenide
Liquid crystals (LCs) are a state of matter which has properties between those of conventional liquids and those of solid crystals. For instance, a liquid crystal may flow like a liquid, but its molecules may be oriented in a crystal-like way. There are many different types of liquid-crystal phases, which can be distinguished by their different optical properties (such as textures). The contrasting areas in the textures correspond to domains where the liquid-crystal molecules are oriented in different directions. Within a domain, however, the molecules are well ordered. LC materials may not always be in a liquid-crystal state of matter (just as water may turn into ice or water vapor).
Liquid crystals can be divided into thermotropic, lyotropic and metallotropic phases. Thermotropic and lyotropic liquid crystals consist mostly of organic molecules, although a few minerals are also known. Thermotropic LCs exhibit a phase transition into the liquid-crystal phase as temperature is changed. Lyotropic LCs exhibit phase transitions as a function of both temperature and concentration of the liquid-crystal molecules in a solvent (typically water). Metallotropic LCs are composed of both organic and inorganic molecules; their liquid-crystal transition depends not only on temperature and concentration, but also on the inorganic-organic composition ratio.
Examples of liquid crystals can be found both in the natural world and in technological applications. Widespread liquid-crystal displays use liquid crystals. Lyotropic liquid-crystalline phases are abundant in living systems but can also be found in the mineral world. For example, many proteins and cell membranes are liquid crystals. Other well-known examples of liquid crystals are solutions of soap and various related detergents, as well as the tobacco mosaic virus, and some clays.
https://en.wikipedia.org/wiki/Liquid_crystal
Hydrogen silsesquioxane (HSQ) is class of inorganic compounds with the chemical formula [HSiO3/2]n.[1] Such clusters are specific representatives of the family of silsesquioxanes with the formula [RSiO3/2]n (R = alkyl, halide, alkoxide, etc.). The most widely studied member of the hydrogen silsesquioxanes is the cubic cluster H8Si8O12.
HSQ has been used in photolithography and Electron-beam lithography due to the fine resolution achievable (~10 nm).[2] Thickness of the coated resist has been reported to play a major role in the achievable resolution.[3]
Cross-linking of the HSQ can is achieved through exposure to e-beam or EUV radiation with wavelengths shorter than 157 nm.
A collection of practical knowledge for using HSQ is provided by Georgia Tech[1].
https://en.wikipedia.org/wiki/Hydrogen_silsesquioxane
Calcite is a carbonate mineral and the most stable polymorph of calcium carbonate (CaCO3). The Mohs scale of mineral hardness, based on scratch hardness comparison, defines value 3 as "calcite".
Other polymorphs of calcium carbonate are the minerals aragonite and vaterite. Aragonite will change to calcite over timescales of days or less at temperatures exceeding 300 °C,[5][6] and vaterite is even less stable.
https://en.wikipedia.org/wiki/Calcite
Magnesium oxide (MgO), or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium (see also oxide). It has an empirical formula of MgO and consists of a lattice of Mg2+ ions and O2− ions held together by ionic bonding. Magnesium hydroxide forms in the presence of water (MgO + H2O → Mg(OH)2), but it can be reversed by heating it to remove moisture.
Magnesium oxide was historically known as magnesia alba (literally, the white mineral from Magnesia – other sources give magnesia alba as MgCO3), to differentiate it from magnesia negra, a black mineral containing what is now known as manganese.
While "magnesium oxide" normally refers to MgO, magnesium peroxide MgO2 is also known as a compound. According to evolutionary crystal structure prediction,[8] MgO2 is thermodynamically stable at pressures above 116 GPa (gigapascals), and a semiconducting suboxide Mg3O2 is thermodynamically stable above 500 GPa. Because of its stability, MgO is used as a model system for investigating vibrational properties of crystals.[9]
https://en.wikipedia.org/wiki/Magnesium_oxide
Racemic acid is an old name for an optically inactive or racemic form of tartaric acid. It is an equal mixture of two mirror-image isomers (enantiomers), optically active in opposing directions. It occurs naturally in grape juice.
Tartaric acid's sodium-ammonium salt is unusual among racemic mixtures in that during crystallization it can separate out into two kinds of crystals, each composed of one isomer, and whose macroscopic shapes are mirror images of each other. Thus, Louis Pasteur was able before 1850 to separate the two enantiomers by picking apart the crystals.[1] Pasteur announced his intention to resolve racemic acid in:
- Pasteur, Louis (1848) "Sur les relations qui peuvent exister entre la forme cristalline, la composition chimique et le sens de la polarisation rotatoire"[2]
while he presented his resolution of racemic acid into separate optical isomers in:
- Pasteur, Louis (1850) "Recherches sur les propriétés spécifiques des deux acides qui composent l'acide racémique"[3]
In the latter paper, Pasteur sketches from natural concrete reality chiral polytopes quite possibly for the first time. The optical property of tartaric acid was first observed in 1832 by Jean Baptiste Biot, who observed its ability to rotate polarized light.[4][5] It remains unknown whether Arthur Cayley or Ludwig Schläfli, or other contemporary mathematicians who studied polytopes, knew of the French work.
In two modern-day re-enactments performed in Japan of the Pasteur experiment,[6][7] it was established that the preparation of crystals was not very reproducible. The crystals deformed, but they were large enough to inspect with the naked eye (microscope not required).
https://en.wikipedia.org/wiki/Racemic_acid
Reflectance paper is a surface that contains a lattice of mirrored dimples. The paper is printed with color and the angle-dependent reflectance function for each pixel of an image captured with a light field camera such as Lytro. The image then displays differently depending on the angle of incident light in the viewing environment. The technique can be used for example to display the image of a sculpture with its direction-dependent shadow depending on the incidence angle of the light.[1]
History[edit]
As of 2012, researchers at the University of California, Santa Cruz, Hewlett-Packard Laboratories and 3M had together created the first such paper, using a hexagonal lattice of millimeter-sized dimples. Dimple depth was 50 µm, representing 70% of a hemisphere. Mirroring used silver or sputtered aluminum. A 32×32 matrix of light-field information was printed on a transparent mask over the dimples.[1][2]
https://en.wikipedia.org/wiki/Reflectance_paper
Sapphire is a precious gemstone, a variety of the mineral corundum, consisting of aluminium oxide (α-Al2O3) with trace amounts of elements such as iron, titanium, chromium, vanadium, or magnesium. It is typically blue, but natural "fancy" sapphires also occur in yellow, purple, orange, and green colors; "parti sapphires" show two or more colors. Red corundum stones also occur, but are called rubies not sapphires.[2] Pink-colored corundum may be classified either as ruby or sapphire depending on locale. Commonly, natural sapphires are cut and polished into gemstones and worn in jewelry. They also may be created synthetically in laboratories for industrial or decorative purposes in large crystal boules. Because of the remarkable hardness of sapphires – 9 on the Mohs scale (the third hardest mineral, after diamond at 10 and moissanite at 9.5) – sapphires are also used in some non-ornamental applications, such as infrared optical components, high-durability windows, wristwatch crystals and movement bearings, and very thin electronic wafers, which are used as the insulating substrates of special-purpose solid-state electronicssuch as integrated circuits and GaN-based blue LEDs. Sapphire is the birthstone for September and the gem of the 45th anniversary. A sapphire jubilee occurs after 65 years.[3]
https://en.wikipedia.org/wiki/Sapphire
Speculum metal is a mixture of around two-thirds copper and one-third tin, making a white brittle alloy that can be polished to make a highly reflective surface. It was used historically to make different kinds of mirrors from personal grooming aids to optical devices until it was replaced by more modern materials such as metal-coated glass mirrors.
Large speculum metal mirrors are hard to manufacture, and the alloy is prone to tarnish, requiring frequent re-polishing. However, it was the only practical choice for large mirrors in high-precision optical equipment between mid-17th and mid-19th century, before the invention of glass silvering.
Speculum metal was noted for its use in the metal mirrors of reflecting telescopes, and famous examples of its use were Newton's telescope, the Leviathan of Parsonstown, and William Herschel's telescope used to discover the planet Uranus. A major difficulty with its use in telescopes is that the mirrors could not reflect as much light as modern mirrors and would tarnish rapidly.
https://en.wikipedia.org/wiki/Speculum_metal
A split-ring resonator (SRR) is an artificially produced structure common to metamaterials. Their purpose is to produce the desired magnetic susceptibility (magnetic response) in various types of metamaterials up to 200 terahertz. These media create the necessary strong magnetic coupling to an applied electromagnetic field, not otherwise available in conventional materials. For example, an effect such as negative permeability is produced with a periodic array of split ring resonators.[4]
A single cell SRR has a pair of enclosed loops with splits in them at opposite ends. The loops are made of nonmagneticmetal like copper and have a small gap between them. The loops can be concentric, or square, and gapped as needed. A magnetic flux penetrating the metal rings will induce rotating currents in the rings, which produce their own flux to enhance or oppose the incident field (depending on the SRRs resonant properties). This field pattern is dipolar. The small gaps between the rings produces large capacitance values which lower the resonating frequency. Hence the dimensions of the structure are small compared to the resonant wavelength. This results in low radiative losses, and very high quality factors.[4][5][6]
https://en.wikipedia.org/wiki/Split-ring_resonator
In chemistry, a sulfoselenide is a compound containing both metal sulfides and metal selenides. Because metal sulfides and metal selenides have similar crystal structures, they exhibit some mutual solubility, forming solid solutions. Since the ionic radius sulfide of (S2−) is however much smaller than that for selenide (Se2−), the solubility ranges can be only limited. For example, pyrite (FeS2) will accept only a few percent of selenium in place of sulfur. A broader range is seen for the solid solution of cadmium sulfide and cadmium selenide. CdS is yellow and CdSe is red. The sulfoselenides of cadmium are orange. They are used as an artist's pigment.[1]
https://en.wikipedia.org/wiki/Sulfoselenide
Super black is a surface treatment developed at the National Physical Laboratory (NPL) in the United Kingdom. It absorbs approximately 99.6% of visible light at normal incidence, while conventional black paint absorbs about 97.5%. At other angles of incidence, super black is even more effective: at an angle of 45°, it absorbs 99.9% of light.
Technology[edit]
The technology to create super black involves chemically etching a nickel–phosphorus alloy.[1][2]
Applications of super black are in specialist optical instruments for reducing unwanted reflections. The disadvantage of this material is its low optical thickness, as it is a surface treatment. As a result, infrared light of a wavelength longer than a few micrometers penetrates through the dark layer and has much higher reflectivity. The reported spectral dependence increases from about 1% at 3 µm to 50% at 20 µm.[3]
In 2009, a competitor to the super black material, Vantablack, was developed based on carbon nanotubes. It has a relatively flat reflectance in a wide spectral range.[4]
In 2011, NASA and the US Army began funding research in the use of nanotube-based super black coatings in sensitive optics.[5] Nanotube-based superblack arrays and coatings have recently become commercially available.[6]
See also[edit]
https://en.wikipedia.org/wiki/Super_black
Yogo sapphires are blue sapphires, a colored variety of corundum, found in Montana, primarily in Yogo Gulch (part of the Little Belt Mountains) in Judith Basin County, Montana. Yogo sapphires are typically cornflower blue, a result of trace amounts of ironand titanium. They have high uniform clarity and maintain their brilliance under artificial light. Because Yogo sapphires occur within a vertically dipping resistive igneous dike, mining efforts have been sporadic and rarely profitable. It is estimated that at least 28 million carats (5.6 t or 5.5 long tons or 6.2 short tons) of Yogo sapphires are still in the ground. Jewelry containing Yogo sapphires was given to First Ladies Florence Harding and Bess Truman; in addition, many gems were sold in Europe, though promoters' claims that Yogo sapphires are in the crown jewels of England or the engagement ring of Princess Diana are dubious. Today, several Yogo sapphires are part of the Smithsonian Institution's gem collection.
Yogo sapphires were not initially recognized or valued. Gold was discovered at Yogo Creek in 1866, and though "blue pebbles" were noticed alongside gold in the stream alluvium by 1878, it was not until 1894 that the "blue pebbles" were recognized as sapphires. Sapphire mining began in 1895 after a local rancher named Jake Hoover sent a cigar box of gems he had collected to an assay office, which in turn sent them to Tiffany's in New York, where an appraiser pronounced them "the finest precious gemstones ever found in the United States".[2] Hoover then purchased the original mother lode from a sheepherder, later selling it to other investors. This became the highly profitable "English Mine", which flourished from 1899 until the 1920s. A second operation, the "American Mine", was owned by a series of investors in the western section of the Yogo dike, but was less profitable and bought out by the syndicate that owned the English Mine. In 1984, a third set of claims, known as the Vortex mine, opened.
The term "Yogo sapphire" is the preferred wording for gems found in the Yogo Gulch, whereas "Montana sapphire" generally refers to gems found in other Montana locations. More gem-quality sapphires are produced in Montana than anywhere else in North America. Sapphires were first discovered in Montana in 1865, in alluvium along the Missouri River. Finds in other locations in the western half of the state occurred in 1889, 1892, and 1894. The Rock Creek location, near Phillipsburg, is the most productive site in Montana, and its gems inspired the name of the nearby Sapphire Mountains. In 1969, the sapphire was co-designated along with the agate as Montana's state gemstones.
In the early 1980s, Intergem Limited, which controlled most of the Yogo sapphire mining at the time, rocked the gem world by marketing Yogo sapphires as the world's only guaranteed "untreated" sapphire, exposing a practice of the time wherein 95 percent of all the world's sapphires were heat-treated to enhance their natural color. Although Intergem went out of business, the gems it mined appeared on the market through the 1990s because the company had paid its salesmen in sapphires during its financial demise. Citibank had obtained a large stock of Yogo sapphires as a result of Intergem's collapse, and after keeping them in a vault for nearly a decade, sold its collection in 1994 to a Montana jeweler. Mining activity today is largely confined to hobby miners in the area; the major mines are currently inactive.
https://en.wikipedia.org/wiki/Yogo_sapphire
Yttrium aluminium garnet (YAG, Y3Al5O12) is a synthetic crystalline material of the garnet group. It is a cubic yttrium aluminiumoxide phase, with other examples being YAlO3 (YAP[2]) in a hexagonal or an orthorhombic, perovskite-like form, and the monoclinic Y4Al2O9 (YAM[3]).[4]
YAG, like garnet and sapphire, has no uses as a laser medium when pure. However, after being doped with an appropriate ion, YAG is commonly used as a host material in various solid-state lasers.[5] Rare earth elements such as neodymium and erbiumcan be doped into YAG as active laser ions, yielding Nd:YAG and Er:YAG lasers, respectively. Cerium-doped YAG (Ce:YAG) is used as a phosphor in cathode ray tubes and white light-emitting diodes, and as a scintillator.

Nd:YAG[edit]
Neodymium-doped YAG (Nd:YAG) was developed in the early 1960s, and the first working Nd:YAG laser was invented in 1964. Neodymium-YAG is the most widely used active laser medium in solid-state lasers, being used for everything from low-power continuous-wave lasers to high-power Q-switched (pulsed) lasers with power levels measured in the kilowatts.[7] The thermal conductivity of Nd:YAG is higher and its fluorescence lifetime is about twice as long as that of Nd:YVO4 crystals, however it is not as efficient and is less stable, requiring more precisely controlled temperatures. The best absorption band of Nd:YAG for pumping the laser is centered at 807.5 nm, and is 1 nm wide.[8]
Most Nd:YAG lasers produce infrared light at a wavelength of 1064 nm. Light at this wavelength is rather dangerous to vision, since it can be focused by the eye's lens onto the retina, but the light is invisible and does not trigger the blink reflex. Nd:YAG lasers can also be used with frequency doubling or frequency tripling crystals, to produce green light with a wavelength of 532 nm or ultraviolet light at 355 nm, respectively.
The dopant concentration in commonly used Nd:YAG crystals usually varies between 0.5 and 1.4 molar percent. Higher dopant concentration is used for pulsed lasers; lower concentration is suitable for continuous-wave lasers. Nd:YAG is pinkish-purple, with lighter-doped rods being less intensely colored than heavier-doped ones. Since its absorption spectrum is narrow, the hue depends on the light under which it is observed.
https://en.wikipedia.org/wiki/Yttrium_aluminium_garnet
Neodymium is a chemical element with the symbol Nd and atomic number 60. Neodymium belongs to the lanthanide series and is a rare-earth element. It is a hard, slightly malleable silvery metal that quickly tarnishes in air and moisture. When oxidized, neodymium reacts quickly to produce pink, purple/blue and yellow compounds in the +2, +3 and +4 oxidation states.[5] Neodymium was discovered in 1885 by the Austrian chemist Carl Auer von Welsbach. It is present in significant quantities in the ore minerals monazite and bastnäsite. Neodymium is not found naturally in metallic form or unmixed with other lanthanides, and it is usually refined for general use. Although neodymium is classed as a rare-earth element, it is fairly common, no rarer than cobalt, nickel, or copper, and is widely distributed in the Earth's crust.[6] Most of the world's commercial neodymium is mined in China.
Neodymium compounds were first commercially used as glass dyes in 1927, and they remain a popular additive in glasses. The color of neodymium compounds is due to the Nd3+ ion and is often a reddish-purple, but it changes with the type of lighting, because of the interaction of the sharp light absorption bands of neodymium with ambient light enriched with the sharp visible emission bands of mercury, trivalent europium or terbium. Some neodymium-doped glasses are used in lasers that emit infrared with wavelengths between 1047 and 1062 nanometers. These have been used in extremely-high-power applications, such as experiments in inertial confinement fusion. Neodymium is also used with various other substratecrystals, such as yttrium aluminium garnet in the Nd:YAG laser.
Another important use of neodymium is as a component in the alloys used to make high-strength neodymium magnets—powerful permanent magnets.[7] These magnets are widely used in such products as microphones, professional loudspeakers, in-ear headphones, high performance hobby DC electric motors, and computer hard disks, where low magnet mass (or volume) or strong magnetic fields are required. Larger neodymium magnets are used in high-power-versus-weight electric motors (for example in hybrid cars) and generators (for example aircraft and wind turbine electric generators).[8]

https://en.wikipedia.org/wiki/Neodymium
A scintillator is a material that exhibits scintillation, the property of luminescence,[1] when excited by ionizing radiation. Luminescent materials, when struck by an incoming particle, absorb its energy and scintillate (i.e. re-emit the absorbed energy in the form of light).[a]Sometimes, the excited state is metastable, so the relaxation back down from the excited state to lower states is delayed (necessitating anywhere from a few nanoseconds to hours depending on the material). The process then corresponds to one of two phenomena: delayed fluorescence or phosphorescence. The correspondence depends on the type of transition and hence the wavelength of the emitted optical photon.
https://en.wikipedia.org/wiki/Scintillator
A Nicol prism is a type of polarizer, an optical device made from calcite crystal used to produce and analyse plane polarized light. It is made in such a way that it eliminates one of the rays by total internal reflection, i.e. the ordinary ray is eliminated and only the extraordinary ray is transmitted through the prism. It was the first type of polarizing prism, invented in 1828 by William Nicol (1770–1851) of Edinburgh. It consists of a rhombohedralcrystal of Iceland spar (a variety of calcite) that has been cut at an angle of 68° with respect to the crystal axis, cut again diagonally, and then rejoined as shown, using a layer of transparent Canada balsam as a glue.[1]
https://en.wikipedia.org/wiki/Nicol_prism
https://en.wikipedia.org/wiki/Lithium-ion_battery
https://en.wikipedia.org/wiki/Glass_casting#Graphite_casting
https://en.wikipedia.org/wiki/Glass_casting
https://en.wikipedia.org/wiki/Vacuum_fluorescent_display
https://en.wikipedia.org/wiki/Resistor#Fixed_resistor
https://en.wikipedia.org/wiki/Resistor#Series_and_parallel_resistors
https://en.wikipedia.org/wiki/Y-Δ_transform
https://en.wikipedia.org/wiki/Star-mesh_transform
https://en.wikipedia.org/wiki/Duality_(electrical_circuits)
https://en.wikipedia.org/wiki/Drum_brake#Backing_plate
https://en.wikipedia.org/wiki/Electric_arc_furnace
https://en.wikipedia.org/wiki/Die_casting
https://en.wikipedia.org/wiki/Relative_density
https://en.wikipedia.org/wiki/Cathode-ray_tube#Microchannel_plate
https://en.wikipedia.org/wiki/Brake_pad
https://en.wikipedia.org/wiki/Gunpowder
https://en.wikipedia.org/wiki/Fourier-transform_infrared_spectroscopy
https://en.wikipedia.org/wiki/Thermal_conductivity
https://en.wikipedia.org/wiki/Scanning_electron_microscope
https://en.wikipedia.org/wiki/Adsorption
https://en.wikipedia.org/wiki/Carbide-derived_carbon
https://en.wikipedia.org/wiki/Relative_permittivity
https://en.wikipedia.org/wiki/Austempering
https://en.wikipedia.org/wiki/Electron_microscope
https://en.wikipedia.org/wiki/Black-body_radiation
https://en.wikipedia.org/wiki/Slate
https://en.wikipedia.org/wiki/Diamond_tool
https://en.wikipedia.org/wiki/Oxy-fuel_welding_and_cutting
https://en.wikipedia.org/wiki/Boiling_water_reactor
https://en.wikipedia.org/wiki/Phonograph
https://en.wikipedia.org/wiki/Bell
https://en.wikipedia.org/wiki/R-value_(insulation)
https://en.wikipedia.org/wiki/Heat_flux
https://en.wikipedia.org/w/index.php?title=Special:Search&limit=20&offset=20&profile=default&search=R+value+scalar&ns0=1&searchToken=2hy50pyp32equagkj6f5drrhb
https://en.wikipedia.org/wiki/Scalar_boson
https://en.wikipedia.org/wiki/Lankford_coefficient
https://en.wikipedia.org/wiki/Singular_value_decomposition
https://en.wikipedia.org/wiki/Vacuum_expectation_value
https://en.wikipedia.org/wiki/Gradient
https://en.wikipedia.org/wiki/Ohm%27s_law
https://en.wikipedia.org/wiki/Vector_space
https://en.wikipedia.org/wiki/Quaternion#Scalar_and_vector_parts
https://en.wikipedia.org/wiki/Quantization_(signal_processing)
https://en.wikipedia.org/wiki/zero_setter
https://en.wikipedia.org/wiki/Zero_state_response
https://en.wikipedia.org/wiki/Resonance
https://en.wikipedia.org/wiki/Compression_(physics)
https://en.wikipedia.org/wiki/Oscillation
https://en.wikipedia.org/wiki/Linearity
https://en.wikipedia.org/wiki/spring
https://en.wikipedia.org/wiki/pendulum
https://en.wikipedia.org/wiki/spinor
https://en.wikipedia.org/wiki/spiral
https://en.wikipedia.org/wiki/loop
https://en.wikipedia.org/wiki/Potential_energy
https://en.wikipedia.org/wiki/Carbon_nanofiber
https://en.wikipedia.org/wiki/Terraforming
https://en.wikipedia.org/wiki/Center_of_mass
https://en.wikipedia.org/wiki/Mechanics
As the number of degrees of freedom becomes arbitrarily large, a system approaches continuity; examples include a string or the surface of a body of water. Such systems have (in the classical limit) an infinite number of normal modes and their oscillations occur in the form of waves that can characteristically propagate.
https://en.wikipedia.org/wiki/Oscillation
https://en.wikipedia.org/wiki/Anti-vibration_compound
https://en.wikipedia.org/wiki/Friction
https://en.wikipedia.org/wiki/Harmonic_oscillator#Driven_harmonic_oscillators
https://en.wikipedia.org/wiki/Aerodynamics
https://en.wikipedia.org/wiki/Angle_of_attack
https://en.wikipedia.org/wiki/phase
https://en.wikipedia.org/wiki/dihedral_angle
https://en.wikipedia.org/wiki/helicoid_catenoid
https://en.wikipedia.org/wiki/Continuum_mechanics
Asteroseismology studies the internal structure of our Sun and other stars using oscillations. These can be studied by interpreting the temporal frequency spectrum acquired through observations.[1] In the same way, the more extreme neutron stars might be studied and hopefully give us a better understanding of neutron-star interiors, and help in determining the equation of state for matter at nuclear densities. Scientists also hope to prove, or discard, the existence of so-called quark stars, or strange stars, through these studies.[2] Fundamental information can be obtained of the General Relativity Theory by observing the gravitational radiation from oscillating neutron stars.[3]
https://en.wikipedia.org/wiki/Neutron-star_oscillation
https://en.wikipedia.org/wiki/Neutron_star
https://en.wikipedia.org/wiki/Radio-quiet_neutron_star
https://en.wikipedia.org/wiki/Tuning_fork
https://en.wikipedia.org/wiki/Torsional_vibration
https://en.wikipedia.org/wiki/Lever_escapement
Helmholtz resonance or wind throb is the phenomenon of air resonance in a cavity, such as when one blows across the top of an empty bottle. The name comes from a device created in the 1850s by Hermann von Helmholtz, the Helmholtz resonator, which he used to identify the various frequencies or musical pitches present in music and other complex sounds.[1]
https://en.wikipedia.org/wiki/Helmholtz_resonance
https://en.wikipedia.org/wiki/Double_pendulum
https://en.wikipedia.org/wiki/Wilberforce_pendulum
https://en.wikipedia.org/wiki/String_vibration
https://en.wikipedia.org/wiki/Acoustic_resonance#Resonance_of_a_sphere_of_air_(vented)
https://en.wikipedia.org/wiki/Pressure
https://en.wikipedia.org/wiki/Q_factor
https://en.wikipedia.org/wiki/Oscillation
https://en.wikipedia.org/wiki/Blocking_oscillator
https://en.wikipedia.org/wiki/Butler_oscillator
https://en.wikipedia.org/wiki/Phase-shift_oscillator
https://en.wikipedia.org/wiki/Relaxation_oscillator
https://en.wikipedia.org/wiki/Crystal_oscillator
https://en.wikipedia.org/wiki/Oscillating_gene
https://en.wikipedia.org/wiki/Stall_(fluid_dynamics)
https://en.wikipedia.org/wiki/Chandler_wobble
https://en.wikipedia.org/wiki/Cyclic_model
https://en.wikipedia.org/wiki/Neutron-star_oscillation
https://en.wikipedia.org/wiki/Neutral_particle_oscillation
https://en.wikipedia.org/wiki/Quantum_harmonic_oscillator
https://en.wikipedia.org/wiki/Quantum_harmonic_oscillator#Natural_length_and_energy_scales
https://en.wikipedia.org/wiki/Nondimensionalization#Quantum_harmonic_oscillator
The mercury beating heart is an electrochemical redox reaction between the elements mercury, iron and chromium. The reaction causes a blob of mercury in water to oscillate.
The observeable reaction demonstrates an effect of a non-homogeneous electrical double layer.[1][2] It is often used as a classroom demonstration.
https://en.wikipedia.org/wiki/Mercury_beating_heart
A chemical oscillator is a complex mixture of reacting chemical compounds in which the concentration of one or more components exhibits periodic changes, They are a class of reactions that serve as an example of non-equilibrium thermodynamics with far-from-equilibrium behavior. The reactions are theoretically important in that they show that chemical reactions do not have to be dominated by equilibrium thermodynamic behavior.
In cases where one of the reagents has a visible color, periodic color changes can be observed. Examples of oscillating reactions are the Belousov–Zhabotinsky reaction (BZ), the Briggs–Rauscher reaction, and the Bray–Liebhafsky reaction.
https://en.wikipedia.org/wiki/Chemical_oscillator
The Briggs–Rauscher oscillating reaction is one of a small number of known oscillating chemical reactions. It is especially well suited for demonstration purposes because of its visually striking colour changes: the freshly prepared colourless solution slowly turns an amber colour, suddenly changing to a very dark blue. This slowly fades to colourless and the process repeats, about ten times in the most popular formulation, before ending as a dark blue liquid smelling strongly of iodine.
https://en.wikipedia.org/wiki/Briggs–Rauscher_reaction
https://en.wikipedia.org/wiki/Continuous_stirred-tank_reactor
https://en.wikipedia.org/wiki/shear
https://en.wikipedia.org/wiki/Rate_equation#Parallel_or_competitive_reactions
https://en.wikipedia.org/wiki/Chemical_reaction_network_theory
https://en.wikipedia.org/wiki/Flux_balance_analysis
https://en.wikipedia.org/wiki/Collision_theory
https://en.wikipedia.org/wiki/Petersen_matrix
https://en.wikipedia.org/wiki/Michaelis–Menten_kinetics
https://en.wikipedia.org/wiki/Oscillator_(cellular_automaton)
https://en.wikipedia.org/wiki/Batsuit
- US 474230 Speaking Telegraph (graphite transmitter) by Thomas Edison (Western Union) May 3, 1892
https://en.wikipedia.org/wiki/Phase_noise
https://en.wikipedia.org/wiki/Oscillator_phase_noise
https://en.wikipedia.org/wiki/Tantalum_capacitor
https://en.wikipedia.org/wiki/Structure_factor
https://en.wikipedia.org/wiki/Glossary_of_mechanical_engineering
https://en.wikipedia.org/wiki/Atmospheric_entry
https://en.wikipedia.org/wiki/Abiogenesis
https://en.wikipedia.org/wiki/Glossary_of_fuel_cell_terms
https://en.wikipedia.org/wiki/Aluminum_electrolytic_capacitor
https://en.wikipedia.org/wiki/Glossary_of_fuel_cell_terms#Bipolar_plate
https://en.wikipedia.org/wiki/Ultra-high-temperature_ceramics
https://en.wikipedia.org/wiki/Tribology
Angle-resolved photoemission spectroscopy (ARPES) is an experimental technique used in condensed matter physics to probe the allowed energies and momenta of the electrons in a material, usually a crystalline solid. It is based on the photoelectric effect, in which an incoming photon of sufficient energy ejects an electron from the surface of a material. By directly measuring the kinetic energy and emission angle distributions of the emitted photoelectrons, the technique can map the electronic band structure and Fermi surfaces. ARPES is best suited for the study of one- or two-dimensional materials. It has been used by physicists to investigate high-temperature superconductors, graphene, topological materials, quantum well states, and materials exhibiting charge density waves.
ARPES systems consist of a monochromatic light source to deliver a narrow beam of photons, a sample holder connected to a manipulator used to position the sample of a material, and an electron spectrometer. The equipment is contained within an ultra-high vacuum (UHV) environment, which protects the sample and prevents scattering of the emitted electrons. After being dispersed along two perpendicular directions with respect to kinetic energy and emission angle, the electrons are directed to a detector and counted to provide ARPES spectra—slices of the band structure along one momentum direction. Some ARPES instruments can extract a portion of the electrons alongside the detector to measure the polarization of their spin.
https://en.wikipedia.org/wiki/Angle-resolved_photoemission_spectroscopy
https://en.wikipedia.org/wiki/High-temperature_superconductivity
https://en.wikipedia.org/wiki/Angle-resolved_photoemission_spectroscopy
https://en.wikipedia.org/wiki/Quantum_well
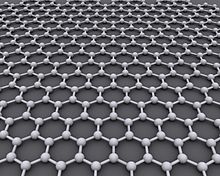
Graphene (/ˈɡræfiːn/[1]) is an allotrope of carbon consisting of a single layer of atoms arranged in a two-dimensional honeycomb lattice[2][3] nanostructure.[4] The name is derived from "graphite" and the suffix -ene, reflecting the fact that the graphite allotrope of carbon contains numerous double bonds.
https://en.wikipedia.org/wiki/Graphene
Glass-like carbon, often called glassy carbon or vitreous carbon, is a non-graphitizing, or nongraphitizable, carbon which combines glassy and ceramic properties with those of graphite. The most important properties are high temperature resistance, hardness (7 Mohs), low density, low electrical resistance, low friction, low thermal resistance, extreme resistance to chemical attack and impermeability to gases and liquids. Glassy carbon is widely used as an electrode material in electrochemistry, for high temperature crucibles, and as a component of some prosthetic devices. It can be fabricated in different shapes, sizes and sections.
The names glassy carbon and vitreous carbon have been registered as trademarks, and IUPAC does not recommend their use as technical terms.[1]
Vitreous carbon can also be produced as a foam, called reticulated vitreous carbon (RVC). This foam was first developed in the mid to late 1960s as a thermally insulating, microporous glassy carbon electrode material. RVC foam is a strong, inert, electrically and thermally conductive, and corrosion-resistant porous form of carbon with a low resistance to gas and fluid flow. Due to these characteristics, the most widespread use of RVC in scientific work is as a three-dimensional electrode in electrochemistry.[2]Additionally, RVC foams are characterized by an exceptionally high void volume, high surface area, and very high thermal resistance in non-oxidising environments, which allows for heat sterilization and facilitates manipulation in biological applications.
https://en.wikipedia.org/wiki/Glassy_carbon
https://en.wikipedia.org/wiki/Ultra-high_vacuum
https://en.wikipedia.org/wiki/Glossary_of_chemistry_terms
https://en.wikipedia.org/wiki/Timeline_of_United_States_inventions_(before_1890)
https://en.wikipedia.org/wiki/Load_following_power_plant
https://en.wikipedia.org/wiki/Victorian_Railways_box_vans#General_goods%2C_fixed-wheeled_stock
https://en.wikipedia.org/wiki/Boeing_601
https://en.wikipedia.org/wiki/Rotation_matrix
https://en.wikipedia.org/wiki/Dirac_delta_function
https://en.wikipedia.org/wiki/Quantum_state#Pure_states
https://en.wikipedia.org/wiki/Quantum_statistical_mechanics













No comments:
Post a Comment