Pseudomonas syringae is a rod-shaped, Gram-negative bacterium with polar flagella. As a plant pathogen, it can infect a wide range of species, and exists as over 50 different pathovars,[1] all of which are available to researchers from international culture collections such as the NCPPB, ICMP, and others.
Pseudomonas syringae is a member of the genus Pseudomonas, and based on 16S rRNA analysis, it has been placed in the P. syringae group.[2] It is named after the lilac tree (Syringa vulgaris), from which it was first isolated.[3]
A phylogenomic analysis of 494 complete genomes from the entire Pseudomonas genus showed that P. syringae does not form a monophyletic species in the strict sense, but a wider evolutionary group that also included other species as well, such as Pseudomonas avellanae, Pseudomonas savastanoi, Pseudomonas amygdali, and Pseudomonas cerasi.[4]
Pseudomonas syringae tests negative for arginine dihydrolase and oxidase activity, and forms the polymer levan on sucrosenutrient agar. Many, but not all, strains secrete the lipodepsinonapeptide plant toxin syringomycin,[5] and it owes its yellow fluorescent appearance when cultured in vitro on King's B medium to production of the siderophore pyoverdin.[6]
Pseudomonas syringae also produces ice nucleation active (INA) proteins which cause water (in plants) to freeze at fairly high temperatures (−1.8 to −3.8 °C (28.8 to 25.2 °F)), resulting in injury.[7] Since the 1970s, P. syringae has been implicated as an atmospheric "biological ice nucleator", with airborne bacteria serving as cloud condensation nuclei. Recent evidence has suggested the species plays a larger role than previously thought in producing rain and snow. They have also been found in the cores of hailstones, aiding in bioprecipitation.[8] These INA proteins are also used in making artificial snow.[9]
Pseudomonas syringae pathogenesis is dependent on effector proteins secreted into the plant cell by the bacterial type III secretion system. Nearly 60 different type III effector families encoded by hop genes have been identified in P. syringae.[10] Type III effectors contribute to pathogenesis chiefly through their role in suppressing plant defense. Owing to early availability of the genome sequence for three P. syringae strains and the ability of selected strains to cause disease on well-characterized host plants, including Arabidopsis thaliana, Nicotiana benthamiana, and the tomato, P. syringae has come to represent an important model system for experimental characterization of the molecular dynamics of plant-pathogen interactions.[11]
| Pseudomonas syringae | |
|---|---|
 | |
| Cultures of Pseudomonas syringae | |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Proteobacteria |
| Class: | Gammaproteobacteria |
| Order: | Pseudomonadales |
| Family: | Pseudomonadaceae |
| Genus: | Pseudomonas |
| Species group: | Pseudomonas syringae group |
| Species: | P. syringae |
| Binomial name | |
| Pseudomonas syringae Van Hall, 1904 | |
| Type strain | |
| ATCC 19310 CCUG 14279 | |
| Pathovars | |
P. s. pv. aceris | |
https://en.wikipedia.org/wiki/Pseudomonas_syringae
Alkaliphiles are a class of extremophilic microbes capable of survival in alkaline (pH roughly 8.5–11) environments, growing optimally around a pH of 10. These bacteria can be further categorized as obligate alkaliphiles (those that require high pH to survive), facultative alkaliphiles (those able to survive in high pH, but also grow under normal conditions) and haloalkaliphiles (those that require high salt content to survive).[1]
https://en.wikipedia.org/wiki/Alkaliphile
Lipophilic bacteria (fat-loving bacteria) are bacteria that may proliferate in lipids.
They include lipophilic corynebacteria.[1]
Cutibacterium acnes is a type of lipophilic bacteria,[2] releasing fatty acids and worsening comedones in acne.
However, the group of lipophilic bacteria are not pathogenic, i.e. they don't cause food poisoning or food infection[3]
In terms of evolution, lipophilism can be regarded as fine-tuning the metabolism to lipophilic habitats. Some bacteria do not only accelerate their metabolism using lipids prevailing in their environment, some of them cannot proliferate without external lipid supply. For example, some Corynebacteria, such as Corynebacterium uropygiale,[4]lost their ability to produce certain fatty acids by themselves. On the one hand, this renders the bacteria vulnerable to environmental changes. On the other hand, energy can be saved as there is no need to put effort into lipid synthesis.[4]
Many lipophilic bacteria are a good source of biosurfactants, hence are used commercially, e.g. Bacillus licheniformis. These kinds of bacteria produce biosurfactants which replace chemically produced surfactants. Biosurfactans are degradable unlike the chemical ones.[citation needed]
https://en.wikipedia.org/wiki/Lipophilic_bacteria
Radioresistance is the level of ionizing radiation that organisms are able to withstand.
Ionizing-radiation-resistant organisms (IRRO) were defined as organisms for which the dose of acute ionizing radiation (IR) required to achieve 90% reduction (D10) is greater than 1000 gray (Gy) [1]
Radioresistance is surprisingly high in many organisms, in contrast to previously held views. For example, the study of environment, animals and plants around the Chernobyl disaster area has revealed an unexpected survival of many species, despite the high radiation levels. A Brazilian study in a hill in the state of Minas Geraiswhich has high natural radiation levels from uranium deposits, has also shown many radioresistant insects, worms and plants.[2][3] Certain extremophiles, such as the bacteria Deinococcus radiodurans and the tardigrades, can withstand large doses of ionizing radiation on the order of 5,000 Gy.[4][5][6]
It has been found in radiation biology experiments that if a group of cells are irradiated then as the dose increases the number of cells which survive decrease. It has also been found that if a population of cells are given a dose before being set aside (without being irradiated) for a length of time before being irradiated again then the radiation has less of an ability to cause cell death. The human body contains many types of cells and a human can be killed by the loss of a single tissue in a vital organ[citation needed]. For many short term radiation deaths (3 days to 30 days) the loss of cells forming blood cells (bone marrow) and the cells in the digestive system (wall of the intestines) cause death.
One possible explanation for the existence of radioresistance is that it is an example of co-opted adaptation or exaptation, where radioresistance could be an indirect consequence of the evolution of a different, linked adaptation that has been positively selected for by evolution. For example, the desiccation-adaptation hypothesis proposes that the extreme temperatures present in the habitats of hyperthermophiles like Deinococcus radiodurans cause cellular damage that is virtually identical to damage typically caused by ionizing radiation, and that the cellular repair mechanisms that have evolved to repair this heat or desiccation damage are generalizable to radiation damage as well, allowing D. radiodurans to survive extreme doses of ionizing radiation.[12] Exposure to gamma radiation leads to cellular DNA damage including alterations in nitrogenous base-pairing, sugar-phosphate backbone damage, and double-stranded DNA lesions.[13] The extraordinarily efficient cellular repair mechanisms that Deinoccocus species like D. radiodurans have evolved to repair heat-damage are likely also capable of reversing the effects of DNA damage wrought by ionizing radiation, such as by piecing back together any components of their genome that have been fragmented by the radiation.[14][15][16]
Bacillus sp. producing unusually radiation (and peroxide) resistant spores, have been isolated from spacecraft assembly facilities, and are thought of as candidates that could ride piggyback on spacecraft through interplanetary transfer.[17][18][19][20][21] Genome analysis of some of these radiation resistant spore producers have thrown some light on the genetic traits that could be responsible for the resistances observed.[22] [23][24][25]
https://en.wikipedia.org/wiki/Radioresistance
Ex-Rad (or Ex-RAD; recilisib sodium (INN, USAN); development code ON 01210.Na) is an experimental drug being developed by Onconova Therapeutics and the U.S. Department of Defense.[1] It is being studied as a radiation protection agent.[2] Chemically, it is the sodium salt of 4-carboxystyryl-4-chlorobenzylsulfone.[3]
https://en.wikipedia.org/wiki/Ex-Rad
Xanthoria elegans, commonly known as the elegant sunburst lichen,[1] is a lichenized species of fungus in the genus Xanthoria, family Teloschistaceae. Recognized by its bright orange or red pigmentation, this species grows on rocks, often near bird or rodent perches. It has a circumpolar and alpine distribution. It was one of the first lichens to be used for the rock-face dating method known as lichenometry.
| Xanthoria elegans | |
|---|---|
 | |
| Scientific classification | |
| Kingdom: | |
| Division: | |
| Class: | |
| Order: | |
| Family: | |
| Genus: | |
| Species: | X. elegans |
| Binomial name | |
| Xanthoria elegans | |
https://en.wikipedia.org/wiki/Xanthoria_elegans
Entolimod (CBLB502) is being developed by Cleveland Biolabs, Inc. for dual indications under the U.S. Food & Drug Administration’s (FDA) animal efficacy rule as a pivotal-stage radiation countermeasure, and under the FDA’s traditional drug approval pathway as a cancer treatment.
Entolimod is a recombinant protein that acts as an agonist of toll-like receptor 5 (TLR5), an innate immunity receptor. Entolimod activation of TLR5 triggers NF-κB signaling, mobilizing an innate immune response that drives expression of numerous genes, including inhibitors of apoptosis, scavengers of reactive oxygen species, and a spectrum of protective or regenerative cytokines.
https://en.wikipedia.org/wiki/Entolimod
Radiotrophic fungi are fungi that can use radiation as an energy source to stimulate growth. Radiotrophic fungi have been found in extreme environments such as in the Chernobyl Nuclear Power Plant.
Most known radiotrophic fungi utilize melanin in some capacity to survive.[1] The process of using radiation and melanin for energy has been termed radiosynthesis, and is thought to be analogous to anaerobic respiration.[2] However, it is not known if multi-step processes such as photosynthesis or chemosynthesis are used in radiosynthesis.
https://en.wikipedia.org/wiki/Radiotrophic_fungus
A piezophile (from Greek "piezo-" for pressure and "-phile" for loving) is an organism with optimal growth under high hydrostatic pressure or, more operationally, an organism that have its maximum rate of growth at a hydrostatic pressure equal or above 10 MPa (= 99 atm = 1,450 psi), when tested over all permissible temperatures.[1]Originally, the term barophile was used for these organisms, but since the prefix "baro-" stands for weight, the term piezophile should be given preference.[2] Like all definitions of extremophiles, the definition of piezophiles is anthropocentric, and humans consider that moderate values for hydrostatic pressure are those around 1 atm (= 0.1 MPa = 14.7 psi). Hyperpiezophile is defined as an organism that have their maximum rate of growth above 50 MPa (= 493 atm = 7,252 psi).[3]
The current record for highest hydrostatic pressure where growth was observed is 130 MPa (= 1,283 atm = 18,855 psi), by the archaea Thermococcus piezophilus.[4]Obligate piezophiles refers to organisms that are unable to grow under lower hydrostatic pressures, such as 0.1 MPa. In contrast, piezotolerant organisms are those that have their maximum rate of growth at a hydrostatic pressure under 10 MPa, but that nevertheless are able to grow at lower rates under higher hydrostatic pressures.
Most of the Earth's biosphere (in terms of volume) is subject to high hydrostatic pressure, and the piezosphere comprises the deep sea (at the depth of 1,000 m and greater) plus the deep subsurface (which can extend up to 5,000 m beneath the seafloor or the continental surface).[3][5] The deep sea has a mean temperature around 1 to 3 °C, and it is dominated by psychropiezophiles, in contrast to the deep subsurface and hydrothermal vents in the seafloor, which are dominated by thermopiezophiles that prosper on temperatures above 45 °C (113 °F).
The high pressures experienced by these organisms can cause the normal fluid cell membrane to become waxy and relatively impermeable to nutrients. The high pressure decreases the ability of the subunits of multimeric proteins to interact. Thus, large protein complexes must interact to decrease pressure-related effects and regulate processes such as protein and DNA synthesis, which are sensitive to high pressure. Piezophilic bacteria have a high proportion of fatty acids in their cytoplasmic membrane, which allows membranes to remain functional and keep from gelling at high pressures.[6]
https://en.wikipedia.org/wiki/Piezophile
A thermoacidophile is an extremophilic microorganism that is both thermophilic and acidophilic; i.e., it can grow under conditions of high temperature and low pH.[1] The large majority of thermoacidophiles are archaea (particularly the crenarchaeota and euryarchaeota) or bacteria, though occasional eukaryotic examples have been reported.[2][3] Thermoacidophiles can be found in hot springs and solfataric environments, within deep sea vents, or in other environments of geothermal activity.[1]:602They also occur in polluted environments, such as in acid mine drainage.[4]
An apparent tradeoff has been described between adaptation to high temperature and low pH; relatively few examples are known that are tolerant of the extremes of both environments (pH < 2, growth temperature > 80°C).[1]:602 Many thermoacidophilic archaea have aerobic or microaerophilic metabolism,[1]:602 although obligately anaerobic examples (e.g. the Acidilobales) have also been identified.[5]
Sequencing the genome of a thermoacidophilic eukaryote, the red algae Galdieria sulphuraria, revealed that its environmental adaptations likely originated from horizontal gene transfer from thermoacidophilic archaea and bacteria.[2]
https://en.wikipedia.org/wiki/Thermoacidophile
A xerophile (from Greek xēros 'dry', and philos 'loving')[1] is an extremophilic organism that can grow and reproduce in conditions with a low availability of water, also known as water activity. Water activity (aw) is measured as the humidity above a substance relative to the humidity above pure water (Aw = 1.0). Xerophiles are "xerotolerant", meaning tolerant of dry conditions. They can often survive in environments with water activity below 0.8; above which is typical for most life on Earth. Typically xerotolerance is used with respect to matric drying, where a substance has a low water concentration. These environments include arid desert soils. The term osmotolerance is typically applied to organisms that can grow in solutions with high solute concentrations (salts, sugars), such as halophiles.
The common food preservation method of reducing water activities may not prevent the growth of xerophilic organisms, often resulting in food spoilage. Some mold and yeast species are xerophilic. Mold growth on bread is an example of food spoilage by xerophilic organisms.
Examples of xerophiles include Trichosporonoides nigrescens[2] and cacti.
https://en.wikipedia.org/wiki/Xerophile
Lithophiles are micro-organisms that can live within the pore interstices of sedimentary and even fractured igneous rocks to depths of several kilometers.
Some are known to live on surface rocks, and make use of photosynthesis for energy.
Those that live in deeper rocks cannot use photosynthesis to gather energy, but instead extract energy from minerals around them. They live in cracks in the rock where water seeps down. The water contains dissolved carbon dioxide (CO2) which the organisms use for their carbon needs.[1] They have been detected in rocks down to depths of nearly three km, where the temperature is approximately 75 °C.
Terrestrial Lithophiles can be found in canyons primarily composed of granite, an igneous rock, and soils saturated with fractured rock.[2] Organisms from the genus Elliptochloris, a subaerial photosynthetic green algae,[3] demonstrate lithophilic preferences through colonization in granite cracks and in proximity to terrestrial lichens.[2]Lithophilic lichens from the genus Collema form tight symbiotic relationships between fungi and photosynthetic algae such as Elliptochloris in order to produce necessary saturated fatty acid secondary metabolites.[4] Lithophilic algal species colonizing fractured rock outcroppings individually exhibit coccal morphological shape while aggregating into an elliptical or globular arrangement during adulthood.[5]
Lithobiontic Ecological Niches further classify lithophiles into sub-categories determined by their spatial niche specificity. The term, Lithic, refers to an association with rock and can be further explained by the term, lithobiontic, regarded as organisms living both on, and within rock surfaces.[6] Sub-surface rock organisms, endoliths, primarily exhibit niche preference within fissures, cavities, or tunnels of various rocks. While many endoliths degrade and effectively excavate the available carbonate rock surface, many are preyed upon by select gastropod, and echinoderm species. This habitat preference can be further threatened by suspension feeding organisms searching for acquired shelter.[6]
https://en.wikipedia.org/wiki/Lithophile
An oligotroph is an organism that can live in an environment that offers very low levels of nutrients. They may be contrasted with copiotrophs, which prefer nutritionally rich environments. Oligotrophs are characterized by slow growth, low rates of metabolism, and generally low population density. Oligotrophic environments are those that offer little to sustain life. These environments include deep oceanic sediments, caves, glacial and polar ice, deep subsurface soil, aquifers, ocean waters, and leached soils.
Examples of oligotrophic organisms are the cave-dwelling olm; the bacterium, Pelagibacter ubique, which is the most abundant organism in the oceans with an estimated 2 × 1028 individuals in total; and the lichens with their extremely low metabolic rate.
Etymologically, the word "oligotroph" is a combination of the Greek adjective oligos (ὀλίγος)[1] meaning "few" and the adjective trophikos (τροφικός)[2]) meaning "feeding".
https://en.wikipedia.org/wiki/Oligotroph
Metallotolerants are extremophiles that are able to survive in environments with a high concentration of dissolved heavy metals in solution. Metallotolerants may be found in environments containing arsenic, cadmium, copper, and zinc. Known metallotolerants include Ferroplasma sp. and Cupriavidus metallidurans.
Metallotolerants adapt to their environment by reducing energy loss by excreting less.
Sinorhizobium sp. M14 is a metallotolerant bacterium. [1]
https://en.wikipedia.org/wiki/Metallotolerant
Osmophilic organisms are microorganisms adapted to environments with high osmotic pressures, such as high sugar concentrations. Osmophiles are similar to halophiles (salt-loving organisms) in that a critical aspect of both types of environment is their low water activity, aW. High sugar concentrations represent a growth-limiting factor for many microorganisms, yet osmophiles protect themselves against this high osmotic pressure by the synthesis of osmoprotectants such as alcohols and amino acids. Many osmophilic microorganisms are yeasts; a variety of bacteria are also osmophilic.
Osmophilic yeasts are important because they cause spoilage in the sugar and sweet goods industry, with products such as fruit juices, fruit juice concentrates, liquid sugars (such as golden syrup), honey and in some cases marzipan. Among the most osmophilic are:
| Organism | Minimum aW |
|---|---|
| Saccharomyces rouxii | 0.62 |
| Saccharomyces bailii | 0.80 |
| Debaryomyces | 0.83 |
| Wallemia sebi | 0.87 |
| Saccharomyces cerevisiae | 0.90 |
https://en.wikipedia.org/wiki/Osmophile
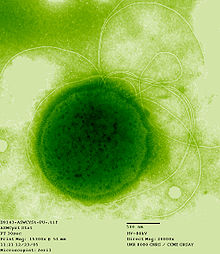
Thermococcus gammatolerans is an archaea extremophile and the most radiation-resistant organism known to exist.
Discovered in 2003 in a submarine hydrothermal vent in the Guaymas Basin about 2,000 m deep off the coast of California, Thermococcus gammatolerans thrives in temperatures between 55 and 95 °C with an optimum development around 88 °C. Its optimal growth pH is 6, favoring the presence of sulfur (S), which is reduced to hydrogen sulfide (H
2S). It is the organism with the strongest known resistance to radiation, supporting a radiation of gamma rays from 30,000 gray (Gy).[1]
Along with the genera Palaeococcus and Pyrococcus, Thermococcus belongs to the Thermococcaceae family, sole family of the Thermococci (called "Protoarchaea" by Cavalier-Smith), a class in the phylum Euryarchaeota of Archaea.[2] Thermococcusspecies live in extremely hot environments such as hydrothermal vents with a growth optimum temperature above 80 °C. Thermococcus and Pyrococcus (literally "ball of fire") are both chemoorganotrophic anaerobic required. Thermococcus spp. prefer 70–95 °C, whereas Pyrococcus species prefer 70–100 °C.
The resistance to ionizing radiation of T. gammatolerans is enormous. While a dose of 5 Gy is sufficient to kill a human, and a dose of 60 Gy is able to kill all cells in a colony of E. coli, Thermococcus gammatolerans can withstand doses up to 30,000 Gy, and an instantaneous dose up to 5,000 Gy with no loss of viability.
| Thermococcus gammatolerans | |
|---|---|
 | |
| Thermococcus gammatolerans | |
| Scientific classification | |
| Domain: | |
| Phylum: | |
| Class: | |
| Order: | |
| Family: | |
| Genus: | |
| Species: | T. gammatolerans |
| Binomial name | |
| Thermococcus gammatolerans Jolivet , 2003 | |
https://en.wikipedia.org/wiki/Thermococcus_gammatolerans
Pyrolobus fumarii, (literally the "firelobe of the chimney"), is a species of archaea known for its ability to live at extremely high temperatures that kill most organisms.[1][2]
It was first discovered in 1997 in a black smoker hydrothermal vent at the Mid-Atlantic Ridge, setting the upper temperature threshold for known life to exist at 113°C (235.4°F), but more recently Methanopyrus kandleri has been discovered which can survive temperatures up to 122°C. (251.6°F) [3][4] The species "freezes" or solidifies and ceases growth at temperatures of 90°C (194°F) and below.[5]
Strain 121, a microbe from the same family found at a vent in the Pacific Ocean, survived and multiplied during a 10-hour interval spent at 121°C (249.8°F) in an autoclave.[3]
| Pyrolobus fumarii | |
|---|---|
 | |
| Scientific classification | |
| Domain: | |
| Phylum: | |
| Class: | |
| Order: | |
| Family: | |
| Genus: | Pyrolobus |
| Binomial name | |
| Pyrolobus fumarii Blöch, Rachel, Burggraf, Hafenbradl, Jannasch & Stetter, 1997 | |
https://en.wikipedia.org/wiki/Pyrolobus_fumarii
Pyrococcus furiosus is an extremophilic species of Archaea. It can be classified as a hyperthermophile because it thrives best under extremely high temperatures—higher than those preferred of a thermophile. It is notable for having an optimum growth temperature of 100 °C (a temperature that would destroy most living organisms), and for being one of the few organisms identified as possessing aldehyde ferredoxin oxidoreductase enzymes containing tungsten, an element rarely found in biological molecules.
| Pyrococcus furiosus | |
|---|---|
 | |
| Pyrococcus furiosus | |
| Scientific classification | |
| Domain: | |
| Kingdom: | |
| Phylum: | |
| Class: | |
| Order: | |
| Family: | |
| Genus: | |
| Species: | P. furiosus |
| Binomial name | |
| Pyrococcus furiosus Erauso et al. 1993 | |
https://en.wikipedia.org/wiki/Pyrococcus_furiosus
Strain 121 (Geogemma barossii) is a single-celled microbe of the domain Archaea. First discovered 320 km (200 mi) off Puget Sound near a hydrothermal vent, it is a hyperthermophile, able to reproduce at 121 °C (250 °F), hence its name. It was (at the time of its discovery) the only known form of life that could tolerate such high temperatures. A temperature of 130 °C (266 °F) is biostatic for Strain 121, meaning that although growth is halted, the archaeon remains viable, and can resume reproducing once it has been transferred to a cooler medium.
The ability to grow at 121 °C (250 °F) is significant because medical equipment is exposed to this temperature for sterilization in an autoclave. Prior to the 2003 discovery of Strain 121, a fifteen-minute exposure to autoclave temperatures was believed to kill all living organisms. However, Strain 121 is not infectious in humans, because it cannot grow at temperatures near 37 °C (99 °F).
Strain 121 metabolizes by reducing iron oxide.
| Strain 121 | |
|---|---|
| Scientific classification | |
| Domain: | |
| Kingdom: | |
| Phylum: | |
| Class: | |
| Order: | |
| Family: | |
| Genus: | |
| Species: | Geogemma barossii |
https://en.wikipedia.org/wiki/Strain_121
Deinococcus radiodurans is an extremophilic bacterium and one of the most radiation-resistant organisms known. It can survive cold, dehydration, vacuum, and acid, and therefore is known as a polyextremophile. It has been listed as the world's toughest known bacterium in The Guinness Book Of World Records.[1]
| Deinococcus radiodurans | |
|---|---|
 | |
| A tetrad of D. radiodurans | |
| Scientific classification | |
| Domain: | |
| Kingdom: | |
| Phylum: | |
| Class: | |
| Order: | |
| Family: | |
| Genus: | |
| Species: | D. radiodurans |
| Binomial name | |
| Deinococcus radiodurans Brooks & Murray, 1981 | |
Snottite, also snoticle, is a microbial mat of single-celled extremophilic bacteria which hang from the walls and ceilings of caves and are similar to small stalactites, but have the consistency of nasal mucus. In the Frasassi Caves in Italy, over 70% of cells in Snottite have been identified as Acidithiobacillus thiooxidans, with smaller populations including an archaeon in the uncultivated 'G-plasma' clade of Thermoplasmatales (>15%) and a bacterium in the Acidimicrobiaceae family (>5%).[1]
The bacteria derive their energy from chemosynthesis of volcanic sulfur compounds including H2S and warm-water solution dripping down from above, producing sulfuric acid. Because of this, their waste products are highly acidic (approaching pH=0), with similar properties to battery acid.[2] Researchers at the University of Texas have suggested that this sulfuric acid may be a more significant cause of cave formation than the usual explanation offered of the carbonic acid formed from carbon dioxide dissolved in water.[3]
Snottites were brought to attention by researchers Diana Northup and Penny Boston studying them (and other organisms) in a toxic sulfur cave called Cueva de Villa Luz(Cave of the Lighted House), in Tabasco, Mexico. The term "snottite" was given to these cave features by Jim Pisarowicz in 1986.
The BBC series Wonders of the Solar System saw Professor Brian Cox examining snottites and positing that if there is life on Mars, it may be similarly primitive and hidden beneath the surface of the Red Planet.
https://en.wikipedia.org/wiki/Snottite
Spirochaeta americana is a relatively newly discovered[1] single-celled extremophile. This haloalkaliphilic and obligately anaerobic bacterium can be found in the bleach-like highly alkaline, salty, deep waters of California's Mono Lake.[1][2]
| Spirochaeta americana | |
|---|---|
 | |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Spirochaetes |
| Order: | Spirochaetales |
| Family: | Spirochaetaceae |
| Genus: | Spirochaeta |
| Species: | S. americana |
| Binomial name | |
| Spirochaeta americana | |
https://en.wikipedia.org/wiki/Spirochaeta_americana
GFAJ-1 is a strain of rod-shaped bacteria in the family Halomonadaceae. It is an extremophile that was isolated from the hypersaline and alkaline Mono Lake in eastern California by geobiologist Felisa Wolfe-Simon, a NASA research fellow in residence at the US Geological Survey. In a 2010 Science journal publication,[1] the authors claimed that the microbe, when starved of phosphorus, is capable of substituting arsenic for a small percentage of its phosphorus to sustain its growth.[2][3]Immediately after publication, other microbiologists and biochemists expressed doubt about this claim which was robustly criticized in the scientific community. Subsequent independent studies published in 2012 found no detectable arsenate in the DNA of GFAJ-1, refuted the claim, and demonstrated that GFAJ-1 is simply an arsenate-resistant, phosphate-dependent organism.[4][5][6][7]
| GFAJ-1 | |
|---|---|
 | |
| Magnified cells of bacterium GFAJ-1 grown in medium containing arsenate | |
| Scientific classification | |
| Domain: | |
| Phylum: | |
| Class: | |
| Order: | |
| Family: | |
https://en.wikipedia.org/wiki/GFAJ-1
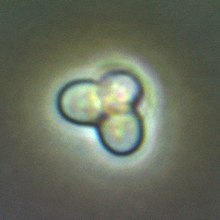
Cyanidioschyzon merolae is a small (2μm), club-shaped, unicellular haploid red alga adapted to high sulfur acidic hot spring environments (pH 1.5, 45 °C).[2][3] The cellular architecture of C. merolae is extremely simple, containing only a single chloroplastand a single mitochondrion and lacking a vacuole and cell wall.[4] In addition, the cellular and organelle divisions can be synchronized. For these reasons, C. merolae is considered an excellent model system for study of cellular and organelle division processes, as well as biochemistry and structural biology.[5][6][7] The organism's genome was the first full algal genome to be sequenced in 2004;[8] its plastid was sequenced in 2000 and 2003, and its mitochondrion in 1998.[9] The organism has been considered the simplest of eukaryotic cells for its minimalist cellular organization.[10]
| Cyanidioschyzon | |
|---|---|
 | |
| Scientific classification | |
| (unranked): | Archaeplastida |
| Division: | Rhodophyta |
| Class: | Cyanidiophyceae |
| Order: | Cyanidiales |
| Family: | Cyanidiaceae |
| Genus: | Cyanidioschyzon |
| Species: | C. merolae |
| Binomial name | |
| Cyanidioschyzon merolae P.De Luca, R.Taddei & L.Varano, 1978[ | |
Paralvinella sulfincola, also known as the sulfide worm, is a species of polychaete worm of the Alvinellidae family that thrives on undersea hot-water vents. It dwells within tubes in waters surrounding hydrothermal vents, in close proximity to super-heated fluids reaching over 300 °C. The upper thermal limit for this polychaete is unknown; however, it is unlikely they can survive in constant temperatures over 50 °C. It may tentatively be named a metazoan extremophile or, more specifically, a thermophile.
Their unique abilities to withstand high temperatures close to hydrothermal fluids enables them to prey upon sulphur-oxidising bacterial mats which grow close to the metal rich vent plume.
| Paralvinella sulfincola | |
|---|---|
 | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Annelida |
| Class: | Polychaeta |
| Order: | Terebellida |
| Family: | Alvinellidae |
| Genus: | Paralvinella |
| Species: | P. sulfincola |
| Binomial name | |
| Paralvinella sulfincola Desbruyères & Laubier, 1993 | |
Halicephalobus mephisto is a species of nematode, among a number of other roundworms, discovered by geoscientists Gaetan Borgonie and Tullis Onstott in 2011. It was detected in ore recovered from deep rock fracture water in several gold mines in South Africa 0.9 km (0.56 mi), 1.3 km (0.81 mi), and 3.6 km (2.2 mi) under the surface of the Earth.[1] Onstott said that "it scared the life out of me when I first saw them moving", and explained that "they look like black little swirly things".[2] The finding is significant[3]because no other multicellular organism had ever been detected farther than 2 km (1.2 mi) below the Earth's surface.[citation needed]
Halicephalobus mephisto is resistant to a temperature as high as 37 °C (higher than most terrestrial nematodes can tolerate),[2] it reproduces asexually, and feeds on subterranean bacteria. According to radiocarbon dating, these worms live in groundwater that is 3,000–12,000 years old.[1] The worms are also able to survive in waters with extremely low levels of oxygen, lower than one percent of the level of most oceans.[2] It is named after Mephistopheles, the Lord of the Underworld in the Faust story,[2] and alludes to the fact it is found so deep under the Earth's surface.[1]
It is the deepest-living animal ever found, able to withstand heat and crushing pressure,[4] and the first multicellular organism found at deep subsurface levels. A previously known species found at similar depths in the same study was Plectus aquatilis.[2]Borgonie said that the worm was similar to the detritus feeding species found on the surface, and probably descended from surface species. Such species are also able to survive extremes of temperature, and so, for Borgonie, the fact the first animal discovered at this depth was a worm was unsurprising.[2] The team hypothesised that the species was descended from animals on the surface that were washed down the earth's crust by rainwater.[2]
Halicephalobus mephisto worms measure from 0.5 to 0.56 mm in length. Though species in the genus Halicephalobus have few distinguishing features, H. mephisto can be differentiated from other species within its genus by its comparatively long tail, which is between 110 and 130 micrometres in length. It is somewhat closely related to the mammalian pathogen Halicephalobus gingivalis, but is more closely related to certain unnamed species of the genus.[1]
In 2019, genome sequencing of the nematode indicated that there were expansions of the 70 kilodalton heat shock protein(Hsp70) and avrRpt2 -induced gene 1 (AIG1) proteins, both of which are transcriptionally induced under heat stress.[5]
The water, in which Halicephalobus mephisto was found, contained aerobic and anaerobic bacteria. Unlike other nematodes it does not prefer Escherichia coli and will rather feed on the sulphophile endolith and depth specialist Desulforudis audaxviator. The found specimen of H. mephisto propagated through parthenogenesis.[6]
| Halicephalobus mephisto | |
|---|---|
 | |
| Head | |
| Scientific classification | |
| Kingdom: | |
| Phylum: | |
| Class: | |
| Order: | |
| Family: | |
| Genus: | |
| Species: | H. mephisto |
| Binomial name | |
| Halicephalobus mephisto | |
| Acidithiobacillales | |
|---|---|
| Scientific classification | |
| Domain: | |
| Phylum: | |
| Class: | |
| Order: | Acidithiobacillales |
| Families & Genera | |
Acidithiobacillaceae | |
No comments:
Post a Comment