Anticholinergics (anticholinergic agent) are a group of substances that blocks the action of the neurotransmitter called acetylcholine (ACh) at synapsesin the central and peripheral nervous system.[1][2]
These agents inhibit the parasympathetic nervous system by selectively blocking the binding of ACh to its receptor in nerve cells. The nerve fibers of the parasympathetic system are responsible for the involuntary movement of smooth muscles present in the gastrointestinal tract, urinary tract, lungs, sweat glands, and many other parts of the body.[3]
In broad terms, anticholinergics are divided into two categories in accordance with their specific targets in the central and peripheral nervous system and at the neuromuscular junction:[3] antimuscarinic agents, and antinicotinic agents (ganglionic blockers, neuromuscular blockers).[4]
The term "anticholinergic" is typically used to refer to antimuscarinics which competitively inhibit the binding of ACh to muscarinic acetylcholine receptors; such agents do not antagonize the binding at nicotinic acetylcholine receptors at the neuromuscular junction, although the term is sometimes used to refer to agents which do so.[3][5]
https://en.wikipedia.org/wiki/Anticholinergic
The U.S. Centers for Disease Control and Prevention (CDC) breaks biological agents into three categories: Category A, Category B, and Category C. Category A agents pose the greatest threat to the U.S. Criteria for being a Category "A" agent include high rates of morbidity and mortality; ease of dissemination and communicability; ability to cause a public panic; and special action required by public health officials to respond. Category A agents include anthrax, botulism, plague, smallpox, and viral hemorrhagic fevers.
https://en.wikipedia.org/wiki/Biological_agent
Aphanizomenon is a genus of cyanobacteria that inhabits freshwater lakes and can cause dense blooms. They are unicellular organisms that consolidate into linear (non-branching) chains called trichomes. Parallel trichomes can then further unite into aggregates called rafts.[1] Since Aphanizomenon is a genus in the cyanobacteria phylum. Bacteria in the Cyanobacteria phylum are known for using photosynthesis to create energy and therefore use sunlight as their energy source.[2] Aphanizomenon bacteria also play a big role in the Nitrogen cycle since they can perform nitrogen fixation. Studies on the species Aphanizomenon flos-aquae have shown that it can regulate buoyancy through light-induced changes in turgor pressure.[3] It is also able to move by means of gliding, though the specific mechanism by which this is possible is not yet known.
AZIV BLO (pheno; phenomenon; zinomenon; zomenon )
Overcoming phosphate limitation[edit]
Aphanizomenon may become dominant in a water body partially due to their ability to induce phosphate-limitation in other phytoplankton while also increasing phosphate availability to itself through release of cylindrospermopsin.[4] The cylindrospermopsin causes other phytoplankton to increase their alkaline phosphataseactivity, increasing inorganic phosphate availability in the water to Aphanizomenon during times when phosphate becomes limiting.
https://en.wikipedia.org/wiki/Aphanizomenon
Serratia marcescens (/səˈreɪʃiə mɑːrˈsɛsɪnz/)[3][failed verification] is a species of rod-shaped, Gram-negative bacteriain the family Yersiniaceae. It is a facultative anaerobe and an opportunistic pathogen. It was discovered in 1819 by Bartolomeo Bizio in Padua, Italy.[4] S. marcescens is commonly involved in hospital-acquired infections (HAIs), particularly catheter-associated bacteremia, urinary tract infections, and wound infections,[5][6] and is responsible for 1.4% of HAI cases in the United States.[7] It is commonly found in the respiratory and urinary tracts of hospitalized adults and in the gastrointestinal systems of children.
Due to its abundant presence in the environment, and its preference for damp conditions, S. marcescens is commonly found growing in bathrooms (especially on tile grout, shower corners, toilet water lines, and basins), where it manifests as a pink, pink-orange, or orange discoloration and slimy film feeding off phosphorus-containing materials or fatty substances such as soap and shampoo residue.
Once established, complete eradication of the organism is often difficult, but can be accomplished by application of a bleach-based disinfectant. Rinsing and drying surfaces after use can also prevent the establishment of the bacterium by removing its food source and making the environment less hospitable.
S. marcescens may also be found in environments such as dirt and the subgingival biofilm of teeth. Due to this, and because S. marcescens produces a reddish-orange tripyrrole dye called prodigiosin, it may cause staining of the teeth. The biochemical pathway for the production of prodigiosin by S. marcescens has been characterized by analyzing what intermediates become accumulated in specific mutants.[8]
S. marcescens is a motile organism and can grow in temperatures ranging from 5–40 °C and in pH levels ranging from 5 to 9. It is differentiated from other Gram-negative bacteria by its ability to perform casein hydrolysis, which allows it to produce extracellular metalloproteinases which are believed to function in cell-to-extracellular matrix interactions. Since this bacterium is a facultative anaerobe, meaning that it can grow in either the presence of oxygen (aerobic) or in the absence of oxygen (anaerobic), it is capable of nitrate reduction under anaerobic conditions. Therefore, nitrate tests are positive since nitrate is generally used as the final electron acceptor rather than oxygen. S. marcescens also exhibits tyrosine hydrolysis and citrate degradation.[9][4]Citrate is used by S. marcescens to produce pyruvic acid, thus it can rely on citrate as a carbon source and test positive for citrate utilization.[4] In identifying the organism, one may also perform a methyl red test, which determines if a microorganism performs mixed-acid fermentation. S. marcescensresults in a negative test. Another determination of S. marcescens is its capability to produce lactic acid by oxidative and fermentative metabolism. Therefore, S. marcescens is lactic acid O/F+.[10]
| Serratia marcescens | |
|---|---|
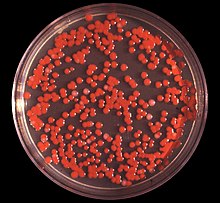 | |
| S. marcescens on an agar plate | |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Proteobacteria |
| Class: | Gammaproteobacteria |
| Order: | Enterobacterales |
| Family: | Yersiniaceae |
| Genus: | Serratia |
| Species: | S. marcescens |
| Binomial name | |
| Serratia marcescens | |
In humans, S. marcescens can cause an opportunistic infection in several sites,[12] including the urinary tract, respiratory tract, wounds,[7] and the eye, where it may cause conjunctivitis, keratitis, endophthalmitis, and tear duct infections.[13] It is also a rare cause of endocarditis and osteomyelitis (particularly in people who use intravenous drugs recreationally), pneumonia, and meningitis.[6][7] Most S. marcescens strains are resistant to several antibiotics because of the presence of R-factors, which are a type of plasmid that carry one or more genes that encode resistance; all are considered intrinsically resistant to ampicillin, macrolides, and first-generation cephalosporins (such as cephalexin).[6]
In elkhorn coral, S. marcescens is the cause of the disease known as white pox disease.[14] In silkworms, it can also cause a lethal disease, especially in association with other pathogens.[15]
In research laboratories employing Drosophila fruit flies, infection of them with S. marcescens is common. It manifests as a pink discoloration or plaque in or on larvae, pupae, or the usually starch and sugar-based food (especially when improperly prepared).
A rare clinical form of gastroenteritis occurring in early infancy caused by infection with S. marcescens. The red color of the diaper can be mistaken for hematuria (blood in the urine), which may cause unnecessary investigations by the physicians.[16]
S. marcescens causes cucurbit yellow vine disease, leading to sometimes serious losses in melon fields.[17]
Professor Jim Burritt and his students at the University of Wisconsin-Stout have discovered a new strain of S. marcescens in bee blood (haemolymph) from hives decimated by winterkill. His research findings have been published and the new strain was named sicaria, which means assassin in Latin. The professor states that S. marcescens sicaria "may contribute to the wintertime failure of honey bee colonies".[18][19]
https://en.wikipedia.org/wiki/Serratia_marcescens
Casein (/ˈkeɪsiːn/ KAY-see-n, from Latin caseus "cheese") is a family of related phosphoproteins (αS1, αS2, β, κ). These proteins are commonly found in mammalian milk, comprising about 80% of the proteins in cow's milk and between 20% and 60% of the proteins in human milk.[1] Sheep and buffalo milkhave a higher casein content than other types of milk with human milk having a particularly low casein content.[2]
Casein has a wide variety of uses, from being a major component of cheese, to use as a food additive.[3] The most common form of casein is sodium caseinate.[4] In milk, casein undergoes phase separation to form colloidal casein micelles, a type of secreted biomolecular condensate.[5]
As a food source, casein supplies amino acids, carbohydrates, and two essential elements, calcium and phosphorus.[6]
https://en.wikipedia.org/wiki/Casein
White pox disease (also "acroporid serratiosis" and "patchy necrosis"), first noted in 1996 on coral reefs near the Florida keys, is a coral disease affecting Elkhorn coral (Acropora palmata) throughout the Caribbean. It causes irregular white patches or blotches on the coral that result from the loss of coral tissue. These patches distinguish white pox disease from white band disease which produces a distinctive white band where the coral skeleton has been denuded. The blotches caused by this disease are also clearly differentiated from coral bleaching and scars caused by coral-eating snails.[1] It is very contagious, spreading to nearby coral.[2]
At the locations where white pox disease has been observed, it is estimated to have reduced the living tissue in elkhorn corals by 50–80%.[3] In the Florida Keys National Marine Sanctuary (FKNMS), the losses of living coral are estimated to average around 88%.[1] Elkhorn coral was formerly the dominant shallow water reef-building coral throughout the Caribbean but now is listed as a threatened species, due in part to the disease.[4] Elkhorn coral is the first species of coral to be listed as threatened in the United States.[5]
https://en.wikipedia.org/wiki/White_pox_disease
In elkhorn coral, S. marcescens is the cause of the disease known as white pox disease.[14] In silkworms, it can also cause a lethal disease, especially in association with other pathogens.[15]
https://en.wikipedia.org/wiki/Serratia_marcescens
S. marcescens was discovered in 1819 by Venetian pharmacist Bartolomeo Bizio, as the cause of an episode of blood-red discoloration of polenta in the city of Padua.[22] Bizio named the organism four years later in honor of Serafino Serrati, a physicist who developed an early steamboat; the epithet marcescens (Latin for 'decaying') was chosen because of the dyestuff's rapid deterioration (Bizio's observations led him to believe that the organism decayed into a mucilage-like substance upon reaching maturity).[23] Serratia was later renamed Monas prodigiosus and Bacillus prodigiosus before Bizio's original name was restored in the 1920s.[22]
Until the 1950s, S. marcescens was erroneously believed to be a nonpathogenic "saprophyte",[7] and its reddish coloration was used in school experiments to track infections. During the Cold War, it was used as a simulant in biological warfare testing by the U.S. military,[24] which studied it in field tests as a substitute for the tularemia bacterium, which was being weaponized at the time.
On 26 and 27 September 1950, the U.S. Navy conducted a secret experiment named "Operation Sea-Spray" in which balloons filled with S. marcescenswere released and burst over urban areas of the San Francisco Bay Area in California. Although the Navy later claimed the bacteria were harmless, beginning on September 29, 11 patients at a local hospital developed very rare, serious urinary tract infections. One of the afflicted patients, Edward J. Nevin, died.[25] Cases of pneumonia in San Francisco also increased after S. marcescens was released.[26][27] (That the simulant bacteria caused these infections and death has never been conclusively established.) Nevin's son and grandson lost a lawsuit they brought against the government between 1981 and 1983, on the grounds that the government is immune,[28] and that the chance that the sprayed bacteria caused Nevin's death was minute.[29]The bacterium was also combined with phenol and an anthrax simulant and sprayed across south Dorset by US and UK military scientists as part of the DICE trials which ran from 1971 to 1975.[30]
https://en.wikipedia.org/wiki/Serratia_marcescens
Studies on the species Aphanizomenon flos-aquae have shown that it can regulate buoyancy through light-induced changes in turgor pressure.[3]
Overcoming phosphate limitation[edit]
Aphanizomenon may become dominant in a water body partially due to their ability to induce phosphate-limitation in other phytoplankton while also increasing phosphate availability to itself through release of cylindrospermopsin.[4] The cylindrospermopsin causes other phytoplankton to increase their alkaline phosphataseactivity, increasing inorganic phosphate availability in the water to Aphanizomenon during times when phosphate becomes limiting.
All species in the cyanobacteria phylum can perform photosynthesis. They use a similar photosynthesis to plants, using two photosystems which is called the Z-scheme. This is different from other photosynthetic bacteria that only use one photosystem and do not have thylakoids. Cyanobacteria species such as Aphanizomenon also use Oxygen as their final electron acceptor in the Electron Transport Chain, which is also different from other photosynthetic bacteria, which perform a type of photosynthesis called anoxygenic photosynthesis.[5]
Aphanizomenon are a special type of cyanobacteria called heterocysts, which are capable of producing biologically-useful nitrogen (ammonium) by the process of nitrogen fixation from atmospheric nitrogen.
A large proportion (between 35-50%) of fixed nitrogen may be released into the surrounding water, providing an important source of biologically-available nitrogen to the ecosystem.[6][7] Since Aphanizomenon are one of the few species of bacteria that can perform nitrogen fixation, other bacterial species that use nitrogen ions as a reactant will start to rely on the species as a source of usable nitrogen. This will cause a bacterial bloom to form, which is a condition under which the number of bacterial colonies in a area will suddenly increase.[8]
Aphanizomenon can produce algal blooms from producing usable nitrogen causing other bacterial species to form colonies around the Aphanizomenon. Algal Blooms formed from Aphanizomenon species tend to be very toxic and create a variety of toxins. These blooms may also create dead zones in the water. This ends up being bad for the ecosystem, since it can hurt many of the plants and animals living around it.[9]
Aphanizomenon species may produce cyanotoxins including cylindrospermospin (CYN), lipopolysaccharides (LPS), anatoxin-a, saxitoxin and BMAA.[10][11]Though not all Aphanizomenon produce cyanotoxins, many do. CYNs are a toxin that is especially toxic for the liver and kidney, thought to inhibit protein synthesis. LPSs are found in the cellular membrane of gram-negative bacterial cells and is released when the cellular membrane is degraded. The releasing of LPSs in animals can cause a severe immune response causing it to be very toxic for animals. Anatoxin-a is a type of anatoxin, it is normally released during algal blooms in lakes, causing exposure to animals around it. Anatoxin-a is toxic to the nerves in animals and is very lethal to humans with a lethal dose thought to be less than 5mg.[12] Similarly to anatoxin-a, BMAAs are another type of neurotoxin that lingers inside animals for longer than anatoxin-a. It will keep affecting animals even after a algal bloom dies down. Last, saxitoxins is yet another type of neurotoxin known to be released by a species of Aphanizomenon. It interrupts nerve transmissions to and from the brain, causing it to be very toxic.[13]
Aphanizomenon may form large colonies as a defense against herbivore grazing, especially Daphnia in freshwater. [14]
https://en.wikipedia.org/wiki/Aphanizomenon
Daphnia is a genus of small planktonic crustaceans, 0.2–6.0 mm (0.01–0.24 in) in length. Daphnia are members of the order Cladocera, and are one of the several small aquatic crustaceans commonly called water fleasbecause their saltatory (Wiktionary) swimming style resembles the movements of fleas. Daphnia spp. live in various aquatic environments ranging from acidic swamps to freshwater lakes and ponds.
The two most commonly found species of Daphnia are D. pulex (small and most common) and D. magna (large). They are often associated with a related genus in the order Cladocera: Moina, which is in the Moinidae family instead of the Daphniidae, and is much smaller than D. pulex (roughly half the maximum length).
| Daphnia | |
|---|---|
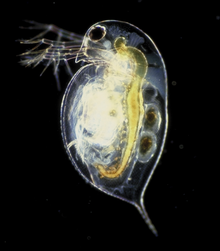 | |
| Daphnia pulex | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Subphylum: | Crustacea |
| Class: | Branchiopoda |
| Order: | Cladocera |
| Family: | Daphniidae |
| Genus: | Daphnia Müller, 1785 |
| Subgenera | |
| |
| Diversity | |
| > 200 spp. | |
| Synonyms [1] | |
| |
This experiment can also be performed using caffeine, nicotine, or adrenaline, each producing an increase in the heart rate.[citation needed]
The blood/circulation system is an open circulatory system, with only the heart and blood without blood vessels, and when the heart spouts blood, since the shell of the water flea is attached to the back and head, and blood is transmitted all over the body along the water that enters the gap. Because the crust is transparent, you can see the heart beating if you observe it well under a microscope. Its heart beats fast because it's small.
Due to its intermediate size, Daphnia spp. use both diffusion and circulatory methods, producing hemoglobin in low-oxygen environments.[4]
aphnia spp. are typically filter feeders, ingesting mainly unicellular algae and various sorts of organic detritus including protists and bacteria[2][11] Beating of the legs produces a constant current through the carapace, which brings such material into the digestive tract. The trapped food particles are formed into a food bolus which then moves down the digestive tract until voided through the anus located on the ventral surface of the terminal appendage.[11] The second and third pairs of legs are used in the organisms' filter-feeding, ensuring large, unabsorbable particles are kept out, while the other sets of legs create the stream of water rushing into the organism.[11]
Daphnia spp. are known to show behavioral changes or modifications to their morphology in the presence of predator kairomones (chemical signals), including larger size at hatching, increased bulkiness, and the development of “neck-teeth". For example, juveniles of D. pulex have a larger size after hatching, along with developing neck-teeth at the back of the head, when in the presence of Chaoborus kairomones. These morphological defenses have shown to reduce mortality due to Chaoborus predation, which is a gape-limited predator. Chitin-related genes (deacetylases) are thought to play an important part in the expression/development of these morphological defenses in Daphnia. Chitin-modifying enzymes (chitin deacetylases) have been shown to catalyse the N-deacetylation of chitin to influence the protein-binding affinity of these chitin filaments.[13]
Towards the end of the growing season, however, the mode of reproduction changes, and the females produce tough "resting eggs" or "winter eggs".[2]When environmental conditions deteriorate (e.g. crowding), some of the asexually produced offspring develop into males.[2] The females start producing haploid sexual eggs, which the males fertilise. In species without males, resting eggs are also produced asexually and are diploid. In either case, the resting eggs are protected by a hardened coat (consisting of two chitinous plates) called the ephippium, and are cast off at the female's next molt. The ephippia can withstand periods of extreme cold, drought, or poor food availability, and hatch – when conditions improve – into females (They are close to being classed as extremophiles) .[2]
The diagram on the left shows the lifecycle of Pasteuria ramosa, a bacterial parasite of Daphnia. Susceptible hosts acquire the infection from spores in the sediment or in suspension. The parasite develops mainly in the host's body cavity and muscle tissue, increasing in density and eventually expanding to occupy the entire host. Typical effects on the host are sterility and gigantism. Spores are released mainly after the host dies and sinks to the substrate, and sometimes directly to the water via clumsy predation.
https://en.wikipedia.org/wiki/Daphnia
A protist (/ˈproʊtɪst/) is any eukaryotic organism (that is, an organism whose cells contain a cell nucleus) that is not an animal, plant, or fungus. While it is likely that protists share a common ancestor (the last eukaryotic common ancestor),[2] the exclusion of other eukaryotes means that protists do not form a natural group, or clade.[a] Therefore, some protists may be more closely related to animals, plants, or fungi than they are to other protists; however, like algae, invertebrates, or protozoans, the grouping is used for convenience. The study of protists is termed protistology.[3]
| Protist Temporal range: Paleoproterozoic[a] – Present | |
|---|---|
 | |
| Scientific classification | |
| Domain: | Eukaryota |
| Groups included | |
Supergroups[1] and typical phyla
Many others; | |
| Cladistically included but traditionally excluded taxa | |
The term Protista was first used by Ernst Haeckel in 1866. Protists were traditionally subdivided into several groups based on similarities to the "higher" kingdoms such as:[4]
- Protozoa
- These unicellular "animal-like" (heterotrophic, and sometimes parasitic) organisms are further sub-divided based on characteristics such as motility, such as the (flagellated) Flagellata, the (ciliated) Ciliophora, the (phagocytic) amoeba, and the (spore-forming) Sporozoa.
- Protophyta
- These "plant-like" (autotrophic) organisms are composed mostly of unicellular algae. The dinoflagelates, diatoms and Euglena-like flagellates are photosynthetic protists.
- Molds
- "Mold" generally refer to fungi; but slime molds and water molds are "fungus-like" (saprophytic) protists, although some are pathogens. Two separate types of slime molds exist, the cellular and acellular forms.
Some protists, sometimes called ambiregnal protists, have been considered to be both protozoa and algae or fungi (e.g., slime molds and flagellatedalgae), and names for these have been published under either or both of the ICN and the ICZN.[16][17] Conflicts, such as these – for example the dual-classification of Euglenids and Dinobryons, which are mixotrophic – is an example of why the kingdom Protista was adopted.
These traditional subdivisions, largely based on superficial commonalities, have been replaced by classifications based on phylogenetics (evolutionaryrelatedness among organisms). Molecular analyses in modern taxonomy have been used to redistribute former members of this group into diverse and sometimes distantly related phyla. For instance, the water molds are now considered to be closely related to photosynthetic organisms such as Brown algae and Diatoms, the slime molds are grouped mainly under Amoebozoa, and the Amoebozoa itself includes only a subset of the "Amoeba" group, and significant number of erstwhile "Amoeboid" genera are distributed among Rhizarians and other Phyla.
However, the older terms are still used as informal names to describe the morphology and ecology of various protists. For example, the term protozoa is used to refer to heterotrophic species of protists that do not form filaments.
https://en.wikipedia.org/wiki/Protist
A glassworm is a type of larva of a midge genus called Chaoborus. They are also known as phantom midge larvae, because they are transparent. They can be found commonly in lakes all over the world and can be up to 2 cm (0.8 in). The adults are sometimes called phantom midges or lake flies.[1]
| Glassworm | |
|---|---|
 | |
 | |
| Aquatic larvae (above) and winged adult (below) | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Diptera |
| Family: | Chaoboridae |
| Genus: | Chaoborus Lichtenstein, 1800 |
| Synonyms | |
Sayomyia Coquillett, 1903 | |
https://en.wikipedia.org/wiki/Glassworm
The rotifers (/ˈroʊtɪfərz/, from Latin rota "wheel" and -fer "bearing"), commonly called wheel animals or wheel animalcules,[1] make up a phylum (Rotifera /roʊˈtɪfərə/) of microscopic and near-microscopic pseudocoelomateanimals.
They were first described by Rev. John Harris in 1696, and other forms were described by Antonie van Leeuwenhoek in 1703.[2] Most rotifers are around 0.1–0.5 mm long (although their size can range from 50 μm to over 2 mm),[1] and are common in freshwater environments throughout the world with a few saltwater species.
Some rotifers are free swimming and truly planktonic, others move by inchworming along a substrate, and some are sessile, living inside tubes or gelatinous holdfasts that are attached to a substrate. About 25 species are colonial (e.g., Sinantherina semibullata), either sessile or planktonic. Rotifers are an important part of the freshwater zooplankton, being a major foodsource and with many species also contributing to the decomposition of soil organic matter.[3] Most species of the rotifers are cosmopolitan, but there are also some endemic species, like Cephalodella vittata to Lake Baikal.[4] Recent barcoding evidence, however, suggests that some 'cosmopolitan' species, such as Brachionus plicatilis, B. calyciflorus, Lecane bulla, among others, are actually species complexes.[5][6] In some recent treatments, rotifers are placed with acanthocephalans in a larger cladecalled Syndermata.
In June 2021, biologists reported the restoration of bdelloid rotifers after being frozen for 24,000 years in the Siberian permafrost.[7]
| Rotifera | |
|---|---|
 | |
| Rotifera | |
 | |
| Pulchritia dorsicornuta | |
| Scientific classification | |
| Kingdom: | Animalia |
| Subkingdom: | Eumetazoa |
| Clade: | ParaHoxozoa |
| Clade: | Bilateria |
| Clade: | Nephrozoa |
| (unranked): | Protostomia |
| (unranked): | Spiralia |
| Clade: | Gnathifera |
| Phylum: | Rotifera Cuvier, 1798 |
| Classes and other subgroups | |
https://en.wikipedia.org/wiki/Rotifer
Bdelloidea /ˈdɛlɔɪdiə/ (Greek βδέλλα, bdella, "leech") is a class of rotifers found in freshwater habitats all over the world. There are over 450 described species of bdelloid rotifers (or 'bdelloids'),[1] distinguished from each other mainly on the basis of morphology.[2] The main characteristics that distinguish bdelloids from related groups of rotifers are exclusively parthenogenetic reproduction and the ability to survive in dry, harsh environments by entering a state of desiccation-induced dormancy (anhydrobiosis) at any life stage.[3] They are often referred to as "ancient asexuals" due to their unique asexual history that spans back to over 25 million years ago through fossil evidence.[4] Bdelloid rotifers are microscopic organisms, typically between 150 and 700 µm in length.[3] Most are slightly too small to be seen with the naked eye, but appear as tiny white dots through even a weak hand lens, especially in bright light. In June 2021, biologists reported the restoration of bdelloid rotifers after being frozen for 24,000 years in the Siberian permafrost.[5]
| Bdelloid rotifers | |
|---|---|
 | |
| SEM showing morphological variation of bdelloid rotifers and their jaws | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Rotifera |
| Superclass: | Eurotatoria |
| Class: | Bdelloidea Hudson, 1884 |
https://en.wikipedia.org/wiki/Bdelloidea
| Animals | |
|---|---|
| Scientific classification | |
| Domain: | Eukaryota |
| (unranked): | Unikonta |
| (unranked): | Obazoa |
| (unranked): | Opisthokonta |
| (unranked): | Holozoa |
| (unranked): | Filozoa |
| Kingdom: | Animalia Linnaeus, 1758 |
| Major divisions | |
| Synonyms | |
| |
The rotifers (/ˈroʊtɪfərz/, from Latin rota "wheel" and -fer "bearing"), commonly called wheel animals or wheel animalcules,[1] make up a phylum (Rotifera /roʊˈtɪfərə/) of microscopic and near-microscopic pseudocoelomateanimals.
They were first described by Rev. John Harris in 1696, and other forms were described by Antonie van Leeuwenhoek in 1703.[2] Most rotifers are around 0.1–0.5 mm long (although their size can range from 50 μm to over 2 mm),[1] and are common in freshwater environments throughout the world with a few saltwater species.
Some rotifers are free swimming and truly planktonic, others move by inchworming along a substrate, and some are sessile, living inside tubes or gelatinous holdfasts that are attached to a substrate. About 25 species are colonial (e.g., Sinantherina semibullata), either sessile or planktonic. Rotifers are an important part of the freshwater zooplankton, being a major foodsource and with many species also contributing to the decomposition of soil organic matter.[3] Most species of the rotifers are cosmopolitan, but there are also some endemic species, like Cephalodella vittata to Lake Baikal.[4] Recent barcoding evidence, however, suggests that some 'cosmopolitan' species, such as Brachionus plicatilis, B. calyciflorus, Lecane bulla, among others, are actually species complexes.[5][6] In some recent treatments, rotifers are placed with acanthocephalans in a larger cladecalled Syndermata.
In June 2021, biologists reported the restoration of bdelloid rotifers after being frozen for 24,000 years in the Siberian permafrost.[7]
https://en.wikipedia.org/wiki/Rotifer
Acanthocephala /əˌkænθoʊˈsɛfələ/[3] (Greek ἄκανθος, akanthos, thorn + κεφαλή, kephale, head) is a phylum of parasitic worms known as acanthocephalans, thorny-headed worms, or spiny-headed worms, characterized by the presence of an eversible proboscis, armed with spines, which it uses to pierce and hold the gut wall of its host. Acanthocephalans have complex life cycles, involving at least two hosts, which may include invertebrates, fish, amphibians, birds, and mammals.[4][5][6] About 1420 species have been described.[7][8]
The Acanthocephala were thought to be a discrete phylum. Recent genome analysis has shown that they are descended from, and should be considered as, highly modified rotifers.[9] This unified taxon is known as Syndermata.
| Acanthocephala | |
|---|---|
 | |
| Corynosoma wegeneri | |
| Scientific classification | |
| Kingdom: | Animalia |
| Subkingdom: | Eumetazoa |
| Clade: | ParaHoxozoa |
| Clade: | Bilateria |
| Clade: | Nephrozoa |
| (unranked): | Protostomia |
| (unranked): | Spiralia |
| Clade: | Gnathifera |
| Phylum: | Acanthocephala Koelreuter, 1771[1][2] |
| Classes | |
Oligacanthorhynchida is an order containing a single parasitic worm family, Oligacanthorhynchidae,[1] that attach themselves to the intestinal wall of terrestrial vertebrates.
| Oligacanthorhynchidae | |
|---|---|
 | |
| Adult Macracanthorhynchus hirudinaceus | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Acanthocephala |
| Class: | Archiacanthocephala |
| Order: | Oligacanthorhynchida |
| Family: | Oligacanthorhynchidae Petrochenko, 1956 |
Contents
- 1Taxonomy and description
- 2Species
- 3Hosts
- 4Notes
- 5References
Protostomia /proʊtoʊˈstoʊmiə/ is the clade of animals once thought to be characterized by the formation of the organism's mouth before its anus during embryonic development. This nature has since been discovered to be extremely variable among Protostomia's members, although the reverse is typically true of its sister clade, Deuterostomia.[1][2] Some examples of protostomes are arthropods, molluscs, and tardigrades.
Together with the Deuterostomia and Xenacoelomorpha, these form the clade Bilateria, animals with bilateral symmetry and three germ layers.[3]
https://en.wikipedia.org/wiki/Protostome
Xenacoelomorpha[2] /ˌzɛnəˌsɛloʊˈmɔːrfə/ is a small phylum of bilaterian invertebrate animals, consisting of two sister groups: xenoturbellids and acoelomorphs. This new phylum was named in February 2011 and suggested based on morphological synapomorphies (physical appearances shared by the animals in the clade),[3] which was then confirmed by phylogenomic analyses of molecular data (similarities in the DNA of the animals within the clade).[2][4]
The sensory organs include a statocyst (for balance) and some groups have two unicellular ocelli (simple eyes).[9][10]Xenacoelomorpha 
Xenoturbella japonica, a xenacoelomorph member (xenoturbellids) 
Proporus sp., another xenacoelomorph member (acoelomorphs) Scientific classification 
Kingdom: Animalia Subkingdom: Eumetazoa Clade: ParaHoxozoa Clade: Bilateria Phylum: Xenacoelomorpha
Philippe et al. 2011[1]Subphyla https://en.wikipedia.org/wiki/Xenacoelomorpha
Schizocoely (adjective forms: schizocoelous or schizocoelic) is a process by which some animal embryos develop. The schizocoely mechanism occurs when secondary body cavities (coeloms) are formed by splitting a solid mass of mesodermal embryonic tissue.[1][2]
Animals called protostomes develop through schizocoely for which they are also known as schizocoelomates.
Schizocoelous development often occurs in protostomes,[1][5][6] as in phyla Mollusca, Annelida, and Arthropoda. However, some deuterostomes like enteropneusts can exhibit schizocoely as well.[7]
https://en.wikipedia.org/wiki/Schizocoely
Nematomorpha (sometimes called Gordiacea, and commonly known as horsehair worms, hairsnakes,[1][2][3] or Gordian worms) are a phylum of parasitoid animals superficially similar to nematode worms in morphology, hence the name. Most species range in size from 50 to 100 millimetres (2.0 to 3.9 in) long, reaching 2 metres in extreme cases, and 1 to 3 millimetres (0.039 to 0.118 in) in diameter. Horsehair worms can be discovered in damp areas, such as watering troughs, swimming pools, streams, puddles, and cisterns. The adult worms are free-living, but the larvae are parasitic on arthropods, such as beetles, cockroaches, mantids, orthopterans, and crustaceans.[4] About 351 freshwater species are known[5] and a conservative estimate suggests that there may be about 2000 freshwater species worldwide.[6] The name "Gordian" stems from the legendary Gordian knot. This relates to the fact that nematomorphs often tie themselves in knots.[7]
Nematomorpha 
Paragordius tricuspidatus Scientific classification 
Kingdom: Animalia Subkingdom: Eumetazoa Clade: ParaHoxozoa Clade: Bilateria Clade: Nephrozoa (unranked): Protostomia Superphylum: Ecdysozoa Clade: Nematoida Phylum: Nematomorpha
Vejdovsky, 1886Orders and families - Gordioidea Rauther, 1930
- Chordodidae May, 1919
- Gordiidae May, 1919
- Nectonematoidea Rauther, 1930
- Nectonemidae Ward, 1892

https://en.wikipedia.org/wiki/Nematomorpha
Loricifera 
Pliciloricus enigmaticus Scientific classification 
Kingdom: Animalia Subkingdom: Eumetazoa Clade: ParaHoxozoa Clade: Bilateria Clade: Nephrozoa (unranked): Protostomia Superphylum: Ecdysozoa Phylum: Loricifera
Kristensen, 1983[2]Order: Nanaloricida
Kristensen, 1983[2]Families Loricifera (from Latin, lorica, corselet (armour) + ferre, to bear) is a phylum of very small to microscopic marine cycloneuralian sediment-dwelling animals that had been determined to be 37 described species, in nine genera,[3][4][5] but in 2021 has increased to 43 species.[6] Aside from these described species, there are approximately 100 more that have been collected and not yet described.[4] Their sizes range from 100 μm to ca. 1 mm.[7] They are characterised by a protective outer case called a lorica and their habitat is in the spaces between marine gravel to which they attach themselves. The phylum was discovered in 1983 by Reinhardt Kristensen, near Roscoff, France.[8] They are among the most recently discovered groups of Metazoans.[9] They attach themselves quite firmly to the substrate, and hence remained undiscovered for so long.[5] The first specimen was collected in the 1970s, and later described in 1983.[9] They are found at all depths, in different sediment types, and in all latitudes.[5]
https://en.wikipedia.org/wiki/Loricifera
A protist (/ˈproʊtɪst/) is any eukaryotic organism (that is, an organism whose cells contain a cell nucleus) that is not an animal, plant, or fungus. While it is likely that protists share a common ancestor (the last eukaryotic common ancestor),[2] the exclusion of other eukaryotes means that protists do not form a natural group, or clade.[a] Therefore, some protists may be more closely related to animals, plants, or fungi than they are to other protists; however, like algae, invertebrates, or protozoans, the grouping is used for convenience. The study of protists is termed protistology.[3]
https://en.wikipedia.org/wiki/Protist
Daphnia is a genus of small planktonic crustaceans, 0.2–6.0 mm (0.01–0.24 in) in length. Daphnia are members of the order Cladocera, and are one of the several small aquatic crustaceans commonly called water fleasbecause their saltatory (Wiktionary) swimming style resembles the movements of fleas. Daphnia spp. live in various aquatic environments ranging from acidic swamps to freshwater lakes and ponds.
The two most commonly found species of Daphnia are D. pulex (small and most common) and D. magna (large). They are often associated with a related genus in the order Cladocera: Moina, which is in the Moinidae family instead of the Daphniidae, and is much smaller than D. pulex (roughly half the maximum length).
https://en.wikipedia.org/wiki/Daphnia
Aphanizomenon is a genus of cyanobacteria that inhabits freshwater lakes and can cause dense blooms. They are unicellular organisms that consolidate into linear (non-branching) chains called trichomes. Parallel trichomes can then further unite into aggregates called rafts.[1] Since Aphanizomenon is a genus in the cyanobacteria phylum. Bacteria in the Cyanobacteria phylum are known for using photosynthesis to create energy and therefore use sunlight as their energy source.[2] Aphanizomenon bacteria also play a big role in the Nitrogen cycle since they can perform nitrogen fixation. Studies on the species Aphanizomenon flos-aquae have shown that it can regulate buoyancy through light-induced changes in turgor pressure.[3] It is also able to move by means of gliding, though the specific mechanism by which this is possible is not yet known.
https://en.wikipedia.org/wiki/Aphanizomenon#Overcoming_phosphate_limitation
Protostomia /proʊtoʊˈstoʊmiə/ is the clade of animals once thought to be characterized by the formation of the organism's mouth before its anus during embryonic development. This nature has since been discovered to be extremely variable among Protostomia's members, although the reverse is typically true of its sister clade, Deuterostomia.[1][2] Some examples of protostomes are arthropods, molluscs, and tardigrades.
Together with the Deuterostomia and Xenacoelomorpha, these form the clade Bilateria, animals with bilateral symmetry and three germ layers.[3]
https://en.wikipedia.org/wiki/Protostome
The rotifers (/ˈroʊtɪfərz/, from Latin rota "wheel" and -fer "bearing"), commonly called wheel animals or wheel animalcules,[1] make up a phylum (Rotifera /roʊˈtɪfərə/) of microscopic and near-microscopic pseudocoelomateanimals.
They were first described by Rev. John Harris in 1696, and other forms were described by Antonie van Leeuwenhoek in 1703.[2] Most rotifers are around 0.1–0.5 mm long (although their size can range from 50 μm to over 2 mm),[1] and are common in freshwater environments throughout the world with a few saltwater species.
Some rotifers are free swimming and truly planktonic, others move by inchworming along a substrate, and some are sessile, living inside tubes or gelatinous holdfasts that are attached to a substrate. About 25 species are colonial (e.g., Sinantherina semibullata), either sessile or planktonic. Rotifers are an important part of the freshwater zooplankton, being a major foodsource and with many species also contributing to the decomposition of soil organic matter.[3] Most species of the rotifers are cosmopolitan, but there are also some endemic species, like Cephalodella vittata to Lake Baikal.[4] Recent barcoding evidence, however, suggests that some 'cosmopolitan' species, such as Brachionus plicatilis, B. calyciflorus, Lecane bulla, among others, are actually species complexes.[5][6] In some recent treatments, rotifers are placed with acanthocephalans in a larger cladecalled Syndermata.
In June 2021, biologists reported the restoration of bdelloid rotifers after being frozen for 24,000 years in the Siberian permafrost.[7]
https://en.wikipedia.org/wiki/Rotifer
Onychophora /ɒnɪˈkɒfərə/ (from Ancient Greek ονυχής, onyches, "claws"; and φέρειν, pherein, "to carry"), commonly known as velvet worms (due to their velvety texture and somewhat wormlike appearance) or more ambiguously as peripatus /pəˈrɪpətəs/ (after the first described genus, Peripatus), is a phylum of elongate, soft-bodied, many-legged panarthropods.[1][2] In appearance they have variously been compared to worms with legs, caterpillars, and slugs.[3] They prey upon smaller animals such as insects, which they catch by squirting an adhesive slime.
Approximately 200 species of velvet worms have been described, although the true number of species is likely greater. The two extant families of velvet worms are Peripatidae and Peripatopsidae. They show a peculiar distribution, with the peripatids being predominantly equatorial and tropical, while the peripatopsids are all found south of the equator. It is the only phylum within Animalia that is wholly endemic to terrestrial environments.[4][5]Velvet worms are generally considered close relatives of the Arthropoda and Tardigrada, with which they form the proposed taxon Panarthropoda.[6] This makes them of palaeontological interest, as they can help reconstruct the ancestral arthropod. In modern zoology, they are particularly renowned for their curious mating behaviour and for bearing live young.
https://en.wikipedia.org/wiki/Onychophora
The flatworms, flat worms, Platyhelminthes, or platyhelminths (from the Greek πλατύ, platy, meaning "flat" and ἕλμινς (root: ἑλμινθ-), helminth-, meaning "worm")[4] are a phylum of relatively simple bilaterian, unsegmented, soft-bodied invertebrates. Unlike other bilaterians, they are acoelomates (having no body cavity), and have no specialized circulatory and respiratory organs, which restricts them to having flattened shapes that allow oxygen and nutrients to pass through their bodies by diffusion. The digestive cavity has only one opening for both ingestion (intake of nutrients) and egestion (removal of undigested wastes); as a result, the food cannot be processed continuously.
https://en.wikipedia.org/wiki/Flatworm
The annelids /ˈænəlɪdz/ (Annelida /əˈnɛlɪdə/, from Latin anellus, "little ring"[2][a]), also known as the ringed worms or segmented worms, are a large phylum, with over 22,000 extant species including ragworms, earthworms, and leeches. The species exist in and have adapted to various ecologies – some in marine environments as distinct as tidal zones and hydrothermal vents, others in fresh water, and yet others in moist terrestrial environments.
https://en.wikipedia.org/wiki/Annelid
The Chaetognatha /kiːˈtɒɡnəθə/ or chaetognaths /ˈkiːtɒɡnæθs/ (meaning bristle-jaws) are a phylum of predatory marine worms that are a major component of plankton worldwide. Commonly known as arrow worms, about 20% of the known Chaetognatha species are benthic, and can attach to algae and rocks. They are found in all marine waters, from surface tropical waters and shallow tide pools to the deep sea and polar regions. Most chaetognaths are transparent and are torpedo shaped, but some deep-sea species are orange. They range in size from 2 to 120 millimetres (0.1 to 4.7 in).
There are more than 120 modern species assigned to over 20 genera.[3] Despite the limited diversity of species, the number of individuals is large.[4]
Arrow worms are usually considered a type of protostome that do not belong to either Ecdysozoa or Lophotrochozoa.
https://en.wikipedia.org/wiki/Chaetognatha
Kimberella is an extinct genus of bilaterian known only from rocks of the Ediacaran period. The slug-like organism fed by scratching the microbial surface on which it dwelt in a manner similar to the gastropods, although its affinity with this group is contentious.
Specimens were first found in Australia's Ediacara Hills, but recent research has concentrated on the numerous finds near the White Sea in Russia, which cover an interval of time from 555 to 558 million years ago.[2] As with many fossils from this time, its evolutionary relationships to other organisms are hotly debated. Paleontologists initially classified Kimberella as a type of jellyfish, but since 1997 features of its anatomy and its association with scratch marks resembling those made by a radula have been interpreted as signs that it may have been a mollusc. Although some paleontologists dispute its classification as a mollusc, it is generally accepted as being at least a bilaterian.
The classification of Kimberella is important for scientific understanding of the Cambrian explosion: if it was a mollusc or at least a protostome, the protostome and deuterostome lineages must have diverged significantly before 555 million years ago. Even if it was a bilaterian but not a mollusc, its age would indicate that animals were diversifying well before the start of the Cambrian.
https://en.wikipedia.org/wiki/Kimberella
Bromelia is the type genus of the plant family Bromeliaceae, subfamily Bromelioideae. Bromelia species are widespread across much of Latin America and the West Indies,[1] and are characterized by flowers with a deeply cleft calyx. The genus is named after the Swedish medical doctor and botanist Olof Bromelius (1639-1705).
The type species is B. karatas.
https://en.wikipedia.org/wiki/Bromelia
Deinacanthon is a genus of the botanical family Bromeliaceae, subfamily Bromelioideae. The genus name is from the Greek “deinos” - terrible and “anthos” - flower.[1]
https://en.wikipedia.org/wiki/Deinacanthon
Deinacanthon urbanianum Scientific classification Kingdom: (unranked): (unranked): (unranked): Order: Family: Subfamily: Genus: Species: D. urbanianumBinomial name Deinacanthon urbanianum (Mez) MezSynonyms Bromelia urbaniana (Mez) L.B.Sm.
Deinacanthon urbanianum is a plant species in the genus Deinacanthon. This species is native to Bolivia.
https://en.wikipedia.org/wiki/Deinacanthon_urbanianum
In taxonomy, the Thermoplasmatales are an order of the Thermoplasmata.[1] All are acidophiles, growing optimally at pH below 2. Picrophilus is currently the most acidophilic of all known organisms, being capable of growing at a pH of -0.06.[2] Many of these organisms do not contain a cell wall, although this is not true in the case of Picrophilus. Most members of the Thermotoplasmata are thermophilic.
Thermoplasmatales Scientific classification Domain: Kingdom: Phylum: Class: ThermoplasmataReysenbach 2002Order: ThermoplasmatalesReysenbach 2002Families - Thermogymnomonas Itoh et al. 2007
- Ferroplasmaceae Golyshina et al. 2000
- Acidiplasma Golyshina et al. 2009
- Ferroplasma Golyshina et al. 2000 emend. Hawkes et al. 2008
- Picrophilaceae Schleper et al. 1996
- Picrophilus Schleper et al. 1996
- ThermoplasmataceaeReysenbach 2002
- Thermoplasma Darland et al. 1970
https://en.wikipedia.org/wiki/Thermoplasmatales
Ferroplasma is a genus of Archaea that belong to the family Ferroplasmaceae. Members of the Ferroplasma are typically acidophillic, pleomorphic, irregularly shaped cocci.[1][2]
The archaean family Ferroplasmaceae was first described in the early 2000s.[3] To date very few species of Ferroplasma have been isolated and characterized. Isolated species include Ferroplasma acidiphilum, Ferroplasma acidarmanus, and Ferroplasma thermophilum.[1][4] A fourth isolate Ferroplasma cupricumulans was later determined to belong to a separate genus.[5][6] All known Ferroplasma sp. are iron-oxidizers.
Iron is the fourth most abundant mineral in earth's crust. As iron-oxidizers Ferroplasma sp. participate in the biogeochemical of iron. Ferroplasma sp. are often identified at acid mine drainage (AMD) sites.[3] When ferrous iron (Fe2+) is oxidized to ferric iron (Fe3+) at mine sites, Fe3+ spontaneously reacts with water and iron-sulfur compounds like pyrite to produce sulfate and hydrogen ions.[8] During this reaction ferrous iron, which can be utilized by Ferroplasma, is also regenerated leading to a "propagation cycle" where pH is lowered. The reaction can be described by the following equation:
Ferroplasma species are often present at AMD sites where they participate in this cycle through the biotic oxidation of ferrous iron.[8]
Ferroplasma sp. may have important applications for bioleaching metals. Microbial bioleaching occurs naturally in the highly acidic environments that are home to Ferroplasma sp. Harnessing the power of bioleaching to recover metal from low quality ores and waste material is energetically advantageous compared to smelting and purifying.[9][10] It also produces fewer toxic byproducts. Studies have shown that the inclusion of Ferroplasma thermophilumalong with the bacteria Acidithiobacillus caldus and Leptospirillum ferriphilum can bioaugment the leaching process of chalcopyrite and increase the rate at which copper is recovered.[11]
Ferroplasma acidiphilum has been shown to grow as a chemomixotroph and to grow synergistically with the acidophilic bacteria Leptospirillum ferriphilum.[12] The strain Ferroplasma acidiphilum YT is a facultative anaerobe with all the required genes for arginine fermentation.[13] Although it is unclear whether Ferroplasma acidiphilum YT uses its arginine fermentation pathway, the pathway itself is an ancient metabolism that traces back to the last universal common ancestor (LUCA) of the three domains of life.[13][14]
Ferroplasma acidiphilum[edit]
Ferroplasma acidiphilum has been shown to grow as a chemomixotroph and to grow synergistically with the acidophilic bacteria Leptospirillum ferriphilum.[12] The strain Ferroplasma acidiphilum YT is a facultative anaerobe with all the required genes for arginine fermentation.[13] Although it is unclear whether Ferroplasma acidiphilum YT uses its arginine fermentation pathway, the pathway itself is an ancient metabolism that traces back to the last universal common ancestor (LUCA) of the three domains of life.[13][14]
Ferroplasma acidarmanus[edit]
Ferroplasma acidarmanus Fer1 was isolated from mine samples collected at Iron Mountain, California.[15] Iron Mountain (CA) is a former mine that is known for its acid mine drainage (AMD) and heavy metal contamination. In addition to being acidophilic, F. acidarmanus Fer1 is highly resistant to both copper and arsenic.[15][16]
Ferroplasma cupricumulans (formerly Ferroplasma cyprexacervatum)[edit]
In 2006 Ferroplasma cupricumulans was isolated from leachate solution collected from the Myanmar Ivanhoe Copper company (MICCL) mining site in Myanmar.[5] It was noted to be the first slightly thermophilic member of the genus Ferroplasma. However, in 2009 a new genus of acidophilic, thermophilic archaea, Acidiplasma, was identified. It was proposed that, based on 16S rRNA similarity and DNA-DNA hybridization, be transferred to the genus Acidiplasma and renamed Acidiplasma cupricumulans.[6]
Ferroplasma thermophilum[edit]
In 2008, Zhou, et al. described the isolation of the organism Ferroplasma thermophilum L1T from a chalcopyrite column reactor that was inoculated with acid mine drainage (AMD) from the Daye copper mine in China’s Hubei province.[4] In aerobic conditions with low concentrations of yeast extract F. thermophilum grows by oxidizing ferrous iron.[4] However, in anaerobic conditions F. thermophilum reduces ferric iron and sulfate.[4] This makes F. thermophilum ecologically important for iron and sulfur cycling at pyrite-rich mine sites.
https://en.wikipedia.org/wiki/Ferroplasma
Pages in category "Metallotolerants"
The following 3 pages are in this category, out of 3 total. This list may not reflect recent changes (learn more).
https://en.wikipedia.org/wiki/Category:Metallotolerants
Cupriavidus metallidurans strain CH34 (renamed from Ralstonia metallidurans[1] and previously known as Ralstonia eutropha and Alcaligenes eutrophus[2]) is a non-spore-forming, Gram-negative bacterium which is adapted to survive several forms of heavy metal stress.[3][4] [5]Therefore, it is an ideal subject to study heavy metal disturbance of cellular processes. This bacterium shows a unique combination of advantages not present in this form in other bacteria.
- Its genome has been fully sequenced (preliminary, annotated sequence data were obtained from the DOE Joint Genome Institute)
- It is not pathogenic, therefore, models of the cell can also be tested in artificial environments similar to its natural habitats.
- It is related to the plant pathogen Ralstonia solanacearum.[6]
- It is of ecological importance since related bacteria are predominant in mesophilic heavy metal-contaminated environments.[2][7]
- It is of industrial importance and used for heavy metal remediation and sensing.[4]
- It is an aerobic chemolithoautotroph, facultatively able to grow in a mineral salts medium in the presence of H2, O2, and CO2 without an organic carbon source.[8] The energy-providing subsystem of the cell under these conditions is composed only of the hydrogenase, the respiratory chain, and the F1F0-ATPase. This keeps this subsystem simple and clearly separated from the anabolic subsystems that starts with the Calvin cycle for CO2-fixation.
- It is able to degrade xenobiotics even in the presence of high heavy metal concentrations.[9]
- Finally, strain CH34 is adapted to the outlined harsh conditions by a multitude of heavy-metal resistance systems that are encoded by the two indigenous megaplasmids pMOL28 and pMOL30 on the bacterial chromosome(s).[3][4][10]
Also it plays a vital role, together with the species Delftia acidovorans, in the formation of gold nuggets, by precipitating metallic gold from a solution of gold(III) chloride, a compound highly toxic to most other microorganisms.[11][12][13]
https://en.wikipedia.org/wiki/Cupriavidus_metallidurans
Cupriavidus necator is a Gram-negative soil bacterium of the class Betaproteobacteria.[1]
Cupriavidus necator has gone through a series of name changes. In the first half of the 20th century, many micro-organisms were isolated for their ability to use hydrogen. Hydrogen-metabolizing chemolithotrophic organisms were clustered into the group Hydrogenomonas.[2] C. necator was originally named Hydrogenomonas eutrophusbecause it fell under the Hydrogenomonas classification and was “well nourished and robust”.[3] Some of the original H. eutrophus cultures isolated were by Bovell and Wilde.[4][5] After characterizing cell morphology, metabolism and GC content, the Hydrogenomonas nomenclature was disbanded because it comprised many species of microorganisms.[2] H. eutrophus was then renamed Alcaligenes eutropha because it was a micro-organism with degenerated peritrichous flagellation.[3][6] Investigating phenotype, lipid composition, fatty acidcomposition and 16S rRNA analysis, A. eutropha was found to belong to the genus Ralstonia and named Ralstonia eutropha.[1] Upon further study of the genus, Ralstonia was found to comprise two phenotypically distinct clusters. The new genus Wautersia was created from one of these clusters which included R. eutropha. In turn R. eutropha was renamed Wautersia eutropha.[7] Looking at DNA-DNA hybridization and phenotype comparison with Cupriavidus necator, W. eutropha was found to be the same species as previously described C. necator. Because C. necator was named in 1987 far before the name change to R. eutropha and W. eutropha, the name C. necator was assigned to R. eutropha according to Rule 23a of the International Code of Nomenclature of Bacteria.[8]
Cupriavidus necator is a hydrogen-oxidizing bacterium (“knallgas” bacterium) capable of growing at the interface of anaerobic and aerobic environments. It can easily adapt between heterotrophic and autotrophic lifestyles. Both organic compounds and hydrogen can be used as a source of energy[9] C. necatorcan perform aerobic or anaerobic respiration by denitrification of nitrate and/or nitrite to nitrogen gas.[10] When growing under autotrophic conditions, C. necator fixes carbon through the reductive pentose phosphate pathway.[11] It is known to produce and sequester polyhydroxyalkanoate (PHA) plastics when exposed to excess amounts of sugar substrate. PHA can accumulate to levels around 90% of the cell's dry weight.[12] To better characterize the lifestyle of C. necator, the genomes of two strains have been sequenced.[9][13]
Cupriavidus necator can use hydrogen gas as a source of energy when growing under autotrophic conditions. It contains four different hydrogenases that have [Ni-Fe] active sites and all perform this reaction:[14][15]
- H2 2H+ + 2e−
The hydrogenases of C. necator are like other typical [Ni-Fe] hydrogenases because they are made up of a large and a small subunit. The large subunit is where the [Ni-Fe] active site resides and the small subunit is composed of [Fe-S] clusters.[16] However, the hydrogenases of C. necator are different from typical [Ni-Fe] hydrogenases because they are tolerant to oxygen and are not inhibited by CO.[14] While the four hydrogenases perform the same reaction in the cell, each hydrogenase is linked to a different cellular process. The differences between the regulatory hydrogenase, membrane-bound hydrogenase, soluble hydrogenase and actinobacterial hydrogenase in C. necator are described below.
The first hydrogenase is a regulatory hydrogenase (RH) that signals to the cell hydrogen is present. The RH is a protein containing large and small [Ni-Fe] hydrogenase subunits attached to a histidine protein kinase subunit.[17] The hydrogen gas is oxidized at the [Ni-Fe] center in the large subunit and in turn reduces the [Fe-S] clusters in the small subunit. It is unknown whether the electrons are transferred from the [Fe-S] clusters to the protein kinase domain.[14] The histidine protein kinase activates a response regulator. The response regulator is active in the dephosphorylated form. The dephosphorylated response regulator promotes the transcription of the membrane bound hydrogenase and soluble hydrogenase.[18]
The membrane-bound hydrogenase (MBH) is linked to the respiratory chain through a specific cytochrome b-related protein in C. necator.[19] Hydrogen gas is oxidized at the [Ni-Fe] active site in the large subunit and the electrons are shuttled through the [Fe-S] clusters in the small subunit to the cytochrome b-like protein.[14] The MBH is located on the outer cytoplasmic membrane. It recovers energy for the cell by funneling electrons into the respiratory chain and by increasing the proton gradient.[19] The MBH in C. necator is not inhibited by CO and is tolerant to oxygen.[20]
NAD+-reducing hydrogenase[edit]
The NAD+-reducing hydrogenase (soluble hydrogenase, SH) creates a NADH-reducing equivalence by oxidizing hydrogen gas. The SH is a heterohexameric protein[21] with two subunits making up the large and small subunits of the [Ni-Fe] hydrogenase and the other two subunits comprising a reductase module similar to the one of Complex I.[22] The [Ni-Fe] active site oxidized hydrogen gas which transfers electrons to a FMN-a cofactor, then to a [Fe-S] cluster relay of the small hydrogenase subunit and the reductase module, then to another FMN-b cofactor and finally to NAD+.[14] The reducing equivalences are then used for fixing carbon dioxide when C. necator is growing autotrophically.
The active site of the SH of C. necator H16 has been extensively studied because C. necator H16 can be produced in large amounts, can be genetically manipulated, and can be analyzed with spectrographic techniques. However, no crystal structure is currently available for the C. necator H16 soluble hydrogenase in the presence of oxygen to determine the interactions of the active site with the rest of the protein.[14]
Typical anaerobic [Ni-Fe] hydrogenases[edit]
The [Ni-Fe] hydrogenase from Desulfovibrio vulgaris and D. gigas have similar protein structures to each other and represent typical [Ni-Fe] hydrogenases.[14][23][24][25] The large subunit contains the [Ni-Fe] active site buried deep in the core of the protein and the small subunit contains [Fe-S] clusters. The Ni atom is coordinated to the Desulfovibrio hydrogenase by 4 cysteine ligands. Two of these same cysteine ligands also bridge the Fe of the [Ni-Fe] active site.[23][24] The Fe atom also contains three ligands, one CO and two CN that complete the active site.[26] These additional ligands might contribute to the reactivity or help stabilize the Fe atom in the low spin +2 oxidation state.[23] Typical [NiFe] hydrogenases like those of D. vulgaris and D. gigas are poisoned by oxygen because an oxygen atom binds strongly to the NiFe active site.[20]
C. necator oxygen-tolerant SH[edit]
The SH in C. necator are unique for other organisms because it is oxygen tolerant.[27] The active site of the SH has been studied to learn why this protein is tolerant to oxygen. A recent study showed that oxygen tolerance as implemented in the SH is based on a continuous catalytically driven detoxification of O2 [Ref missing]. The genes encoding this SH can be up-regulated under heterotrophic growth condition using glycerol in the growth media [28] and this enables aerobic production and purification of the same enzyme.[29]
The oxygen-tolerant hydrogenases of C. necator have been studied for diverse purposes. C. necator was studied as an attractive organism to help support life in space. It can fix carbon dioxide as a carbon source, use the urea in urine as a nitrogen source, and use hydrogen as an energy source to create dense cultures that could be used as a source of protein.[30][31]
Electrolysis of water is one way of creating oxygenic atmosphere in space and C. necator was investigated to recycle the hydrogen produced during this process.[32]
Oxygen-tolerant hydrogenases are being used to investigate biofuels. Hydrogenases from C. necator have been used to coat electrode surfaces to create hydrogen fuel cells tolerant to oxygen and carbon monoxide[20] and to design hydrogen-producing light complexes.[33] In addition, the hydrogenases from C. necator have been used to create hydrogen sensors.[34] Genetically modified C. necator can produce isobutanol from CO
2 that can directly substitute or blend with gasoline. The organism emits the isobutanol without having to be destroyed to obtain it.[35]Researchers at UCLA have genetically modified a strain of the species C. necator (formerly known as R. eutropha H16) to produce isobutanol from CO2feedstock using electricity produced by a solar cell. The project, funded by the U.S. Dept. of Energy, is a potential high energy-density electrofuel that could use existing infrastructure to replace oil as a transportation fuel.[36]
https://en.wikipedia.org/wiki/Cupriavidus_necator
Pandoraea oxalativorans Scientific classification Kingdom: Phylum: Class: Order: Family: Genus: Species: P. oxalativoransBinomial name Pandoraea oxalativorans Sahin et al. 2011[1]Type strain CCM 7677, DSM 23570, NBRC 106091, TA25[2] Pandoraea oxalativorans is a Gram-negative, aerobic, non-spore-forming bacterium of the genus Pandoraea.[3][4]
https://en.wikipedia.org/wiki/Pandoraea_oxalativorans
Polynucleobacter is a genus of Proteobacteria, originally established by Heckmann and Schmidt (1987)[1] to exclusively harbor obligate endosymbionts of ciliates belonging to the genus Euplotes.
Recently, several new Polynucleobacter species were described,[2][3][4][5][6][7][8][9] which all represent free-living (i.e. not host-associated) planktonic freshwater bacteria. Thus, the genus Polynucleobacter currently includes one species containing obligate endosymbionts of ciliates and nine species representing free-living planktonic strains. The type strains of the planktonic species were isolated from freshwater systems located in Armenia, Austria, China, France, Germany, Norway, Uganda, and the United States. Currently, the genus harbors 17 species.
Free-living Poynucleobacter bacteria represent important members of bacterioplankton in freshwater systems such as lakes, ponds, and streams.[10]
Two genome projects are finished on P. necessarius strains: one project on an obligately freeliving strain isolated from an acidic freshwater pond,[11] and one project on an obligate endosymbiont of the ciliate Euplotes aediculatus.[12]
Analyses of the genome sequences resulted in the discovery of a conserved RNA motif.https://en.wikipedia.org/wiki/Polynucleobacter
Cupriavidus alkaliphilus is a bacterium of the genus Cupriavidus and the family Burkholderiaceae which was isolated from the rhizosphere of agricultural plants which grow on alkaline soils in northeast Mexico.[3][4]
https://en.wikipedia.org/wiki/Cupriavidus_alkaliphilus
The ciliates are a group of protozoans characterized by the presence of hair-like organelles called cilia, which are identical in structure to eukaryotic flagella, but are in general shorter and present in much larger numbers, with a different undulating pattern than flagella. Cilia occur in all members of the group (although the peculiar Suctoriaonly have them for part of their life-cycle) and are variously used in swimming, crawling, attachment, feeding, and sensation.
Ciliates are an important group of protists, common almost anywhere there is water — in lakes, ponds, oceans, rivers, and soils. About 4,500 unique free-living species have been described, and the potential number of extant species is estimated at 27,000–40,000.[2] Included in this number are many ectosymbiotic and endosymbioticspecies, as well as some obligate and opportunistic parasites. Ciliate species range in size from as little as 10 µmin some colpodeans to as much as 4 mm in length in some geleiids, and include some of the most morphologicallycomplex protozoans.[3][4]
In most systems of taxonomy, "Ciliophora" is ranked as a phylum[5] under any of several kingdoms, including Chromista,[6] Protista[7] or Protozoa.[8] In some older systems of classification, such as the influential taxonomic works of Alfred Kahl, ciliated protozoa are placed within the class "Ciliata"[9][10] (a term which can also refer to a genus of fish). In the taxonomic scheme endorsed by the International Society of Protistologists, which eliminates formal rank designations such as "phylum" and "class", "Ciliophora" is an unranked taxon within Alveolata.[11][12]
https://en.wikipedia.org/wiki/Ciliate
Suctoria are ciliates that become sessile in their developed stage and then lose their redundant cilia. They feed by extracellular digestion.[1] They were originally thought to feed by suction – hence their name.[2] In fact, they use specialized microtubules to ensnare and manipulate their prey.[2] They live in both freshwater and marine environments, including some that live on the surface of aquatic animals, and typically feed on other ciliates. Instead of a single cytostome, each cell feeds by means of several specialized tentacles. These are supported by microtubules and phyllae, and have toxic extrusomes called haptocysts at the tip, which they attach to prey. They then suck the prey's cytoplasm directly into a food vacuole inside the cell, where they digest and absorb its contents. Most suctoria are around 15-30 μm in size, with a non-contractile stalk and often a lorica or shell.
Suctoria reproduce primarily by budding, producing swarmers that lack both tentacles and stalks but have cilia. They may also reproduce through conjugation, which is peculiar in involving cells of different size and often involves total fusion. The way buds form is the primary distinction between different orders of suctoria. Among the Exogenida, including common genera like Podophrya and Sphaerophrya, they appear directly on the cell surface. Among the Endogenida, for instance Tokophrya and Acineta, they form in an internal pouch and escape through an opening—and among the Evaginogenida, they form in a pouch that inverts before they are released.
Once the swarmers have found a place to attach themselves, they quickly develop stalks and tentacles. The cilia are lost, but the underlying infraciliature persists throughout the entire life-cycle. This has a structure that, together with other ultrastructural similarities, places the suctoria within the class Phyllopharyngea.
Suctoria 
Scientific classification 
(unranked): Diaphoretickes Clade: TSAR Clade: SAR Infrakingdom: Alveolata Phylum: Ciliophora Class: Phyllopharyngea Subclass: Suctoria
Claparède & Lachmann1858(unranked): Diaphoretickes https://en.wikipedia.org/wiki/Suctoria
Category:SAR supergroup subclasses
Pages in category "SAR supergroup subclasses"
The following 23 pages are in this category, out of 23 total. This list may not reflect recent changes (learn more).
https://en.wikipedia.org/wiki/Category:SAR_supergroup_subclasses
- Gordioidea Rauther, 1930








No comments:
Post a Comment