Community ecology – original research
Published: 07 August 2018
High resources and infectious disease facilitate invasion by a freshwater crustacean
Catherine L. Searle, Baylie R. Hochstedler, Abigail M. Merrick, Juliana K. Ilmain & Maggie A. Wigren
Oecologia volume 188, pages 571–581 (2018)Cite this article
It is well-established that both resources and infectious disease can influence species invasions, but little is known regarding interactive effects of these two factors. We performed a series of experiments to understand how resources and parasites can jointly affect the ability of a freshwater invasive zooplankton to establish in a population of a native zooplankton. In a life history trial, we found that both species increased offspring production to the same degree as algal resources increased, suggesting that changes in resources would have similar effects on both species. In a microcosm experiment simulating an invasion, we found that the invasive species reached its highest densities when there was a combination of both high resources and the presence of a shared parasite, but not for each of these conditions alone (i.e., a significant resource x parasite interaction). This result can be explained by changes in native host population density; high resource levels initially led to an increase in the density of the native host, which caused larger epidemics when the parasite was present. This high infection prevalence caused a subsequent reduction in native host density, increasing available resources and allowing the invasive species to establish relatively dense populations. Thus, in this system, native communities with a combination of high resource levels and parasitism may be the most vulnerable to invasions. More generally, our results suggest that parasitism and resource availability can have interactive, non-additive effects on the outcome of invasions.
https://link.springer.com/article/10.1007/s00442-018-4237-9
The predation paradox: Synergistic and antagonistic interactions between grazing by crustacean predator and infection by cyanophages promotes bloom formation in filamentous cyanobacteria
Sigitas Šulčius, Kristina Slavuckytė, Ričardas Paškauskas
First published: 20 April 2017 https://doi.org/10.1002/lno.10559
In this study, we assessed the impact of synergistic/antagonistic interactions between grazing by crustacean predator (Daphnia magna) and infection by cyanophage (Vb-AphaS-CL131) on the population dynamics of the harmful bloom-forming filamentous cyanobacterium Aphanizomenon flos-aquae. We observed synergistic effect of cyanophage infection on D. magna survival and grazing trough the lysis-induced shift in cyanobacteria population structure toward the shorter filaments. However, lysis-mediated- and grazing-enhanced removal of short A. flos-aquae filaments resulted in the dominance of grazing- and infection-resistant A. flos-aquae population. In addition, the presence of D. magna generated a trait-mediated response in the A. flos-aquae population by promoting the aggregation of filaments into colony-like structures. Experiments using temperate A. flos-aquae colony isolates from natural environment demonstrated that colony-embedded A. flos-aquae filaments are insensitive to the addition of both D. magna and cyanophages. Therefore, we propose that interactions between crustacean grazers and cyanophages may promote the emergence of defensive A. flos-aquae genotypes and population traits that eventually lead to bloom formation in aquatic environments.
https://aslopubs.onlinelibrary.wiley.com/doi/full/10.1002/lno.10559
NADIS
Ectoparasites of Cattle
Cattle in the UK are affected by a wide range of ectoparasites. Some of these are simply nuisance pests affecting grazing patterns and causing mild irritation. Others however can transmit diseases and cause significant production losses, resulting in severe welfare concerns if the infestation is not addressed.
Lice and mites live on the skin surface, either feeding on skin cells or blood. Infestation is most commonly seen in the winter and presents with itching, hair loss and occasionally anaemia if blood loss is significant.
Ticks are seen in specific geographical areas of the UK, and are important vectors of bacterial, viral and protozoal infections in cattle in those areas.
Flies feed on the blood, sweat, tears, saliva, urine and faeces of cattle, acting as nuisance pests causing disrupted grazing and reducing productivity, and as vectors of disease. On rare occasions immature fly larvae (maggots) may cause disease as a result of infestation of pre-existing wounds – known as fly strike.
The impact these ectoparasites have on the animal is dependent on genetic predisposition, age, health and nutritional status of the individual; the parasites typically affect young animals, older animals and those with concurrent health problems.
https://nadis.org.uk/disease-a-z/cattle/ectoparasites-of-cattle/
UF IFAS
EXTERNAL PARASITES ON BEEF CATTLE1
P. E. Kaufman, P. G. Koehler, and J. F. Butler 2
Arthropod pests limit production in the beef cattle industry by affecting animals in many ways. External parasites are the most serious threat since they feed on body tissues such as blood, skin, and hair. The wounds and skin irritation produced by these parasites often result in discomfort and irritation for the animal. More significant, however, is that any blood-sucking arthropod may transmit diseases from infected animals to healthy ones. In addition, arthropod pests also may reduce weight gains, cause losses in milk and meat production, produce general weakness, cause mange and severe dermatitis, and create sites for secondary invasion of disease organisms. In general, infected livestock cannot be healthy or efficiently managed to realize optimum production levels.
External parasites such as lice, flies, ticks, cattle grubs, and mites are a serious problem to livestock breeders. These pests are most prevalent during the spring and summer months; however, Florida's warm climate permits many pests to live year-round.
https://edis.ifas.ufl.edu/publication/IG130
USDA
Animal and Plant Health Inspection Service
U.S. DEPARTMENT OF AGRICULTURE
Animal Health / Animal Disease Information / Aquaculture /
Aquatic Animal Diseases
Last Modified: May 5, 2021 Print
Information on diseases that pose a significant threat to commercial aquaculture industry sectors in the U.S.
Infectious diseases affect cultured aquatic animal populations similar to the impacts seen in other livestock sectors; loss of production, increased costs associated with treatment, and loss of trade or markets. APHIS encourages aquaculture producers to work with aquatic animal veterinarians and other aquatic animal health experts to address health issues on the production site.
APHIS encourages the reporting of detections of all OIE-listed aquatic animal diseases to state animal health officials (SAHO).
https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/animal-disease-information/aquaculture/aquatic-animal-diseases/index
Texas A&M
Anaplasmosis in Beef Cattle
https://agrilifeextension.tamu.edu/library/ranching/anaplasmosis-in-beef-cattle/
Philos Trans R Soc Lond B Biol Sci. 2009 Sep 27; 364(1530): 2763–2776.
doi: 10.1098/rstb.2009.0089
PMCID: PMC2865092
PMID: 19687044
Zoonotic helminth infections with particular emphasis on fasciolosis and other trematodiases
Mark W. Robinson* and John P. Dalton
Zoonotic infections are among the most common on earth and are responsible for >60 per cent of all human infectious diseases. Some of the most important and well-known human zoonoses are caused by worm or helminth parasites, including species of nematodes (trichinellosis), cestodes (cysticercosis, echinococcosis) and trematodes (schistosomiasis). However, along with social, epidemiological and environmental changes, together with improvements in our ability to diagnose helminth infections, several neglected parasite species are now fast-becoming recognized as important zoonotic diseases of humans, e.g. anasakiasis, several fish-borne trematodiasis and fasciolosis. In the present review, we discuss the current disease status of these primary helminth zoonotic infections with particular emphasis on their diagnosis and control. Advances in molecular biology, proteomics and the release of helminth genome-sequencing project data are revolutionizing parasitology research. The use of these powerful experimental approaches, and their potential benefits to helminth biology are also discussed in relation to the future control of helminth infections of animals and humans.
Keywords: zoonosis, helminth, nematode, trematode, cestode, Fasciola
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2865092/
Crustacean parasites
Of all the metazoan groups discussed in this book, the crustaceans are the most diverse and ubiquitous. Among them, the copepods are dominant. They, jointly with the monogeneans, are the most speciose group of metazoan ectoparasites of marine fishes; in addition, they infect a wide range of marine invertebrates. Thousands of species are already known, but many potential host groups have not been examined, and for this reason even approximate estimates of species numbers are impossible. Reflecting the diversity of hosts, copepods show an amazing variety of adaptations which secure infection of and survival on the hosts. Many copepods have great eco- nomic importance as agents of disease in wild and aquacultured fish populations. Isopods are primarily found in warm waters, they infect fish but also other crustaceans. Larval isopod para- sites of the family Gnathiidae are abundant on the gills of tropical marine fish and represent a primary source of food for cleaner fish. Most branchiurans occur in fresh water, but a few spe- cies of the genus Argulus are ectoparasites on the skin of marine fish. The tiny tantulocarids are ectoparasites of other crustaceans. To date only 28 species have been described, and little is known about their biology. Thoracica and Rhizocephala are included in the Cirripedia. Few spe- cies of the Thoracica are parasitic (on dogfish and polychaetes), whereas the rhizocephalans parasitise other crustaceans. The latter are particularly fascinating because of their extreme sexual dimorphism, the extreme reduction of morphological complexity in the parasitic female, and their ability to change the behaviour of host crabs which benefits the parasite. The Ascotho- racida infect various echinoderms and cnidarians. Amphipoda use many groups of marine ani- mals as hosts, including medusae, siphonophores, ctenophores, and thaliaceans. Others (including the whale-lice) infect various marine mammals. When occurring in large numbers, whale-lice may even damage very large humpback whales.
https://isopods.nhm.org/pdfs/27570/27570.pdf
WHO
Foodborne trematode infections
https://www.who.int/news-room/fact-sheets/detail/foodborne-trematode-infections
Science Direct (ScienceDirect)
Intermediate Host
Intermediate hosts are required for the life cycle to be completed, and, generally, morphological change in the parasite will also occur in intermediate hosts.
From: Encyclopedia of Food Safety, 2014
https://www.sciencedirect.com/topics/earth-and-planetary-sciences/intermediate-host
CDC
Fascioliasis is an infectious disease caused by Fasciola parasites, which are flat worms referred to as liver flukes.
The adult (mature) flukes are found in the bile ducts and liver of infected people and animals, such as sheep and cattle. In general, fascioliasis is more common in livestock and other animals than in people.
Two Fasciola species (types) infect people. The main species is Fasciola hepatica, which is also known as “the common liver fluke” and “the sheep liver fluke.” A related species, Fasciola gigantica, also can infect people.
https://www.cdc.gov/parasites/fasciola/gen_info/faqs.html
Biology
Causal Agent:
More than 30 species of trematodes (flukes) of the genus Paragonimus have been reported which infect animals and humans. Among the more than 10 species reported to infect humans, the most common is P. westermani, the oriental lung fluke.
Life Cycle:
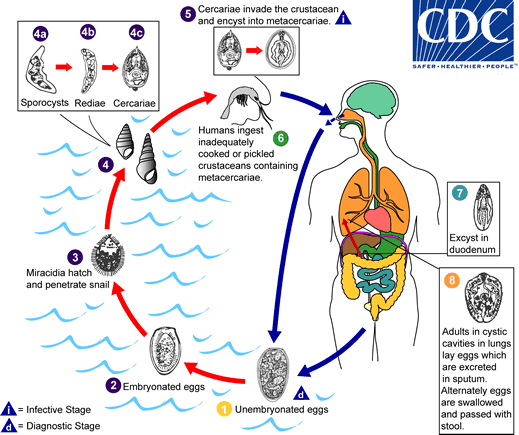
Paragonimus kifecycle
The eggs are excreted unembryonated in the sputum, or alternately they are swallowed and passed with stool The number 1. In the external environment, the eggs become embryonated The number 2, and miracidia hatch and seek the first intermediate host, a snail, and penetrate its soft tissues The number 3. Miracidia go through several developmental stages inside the snail The number 4: sporocysts The number 4a, rediae The number 4b, with the latter giving rise to many cercariae The number 4c, which emerge from the snail. The cercariae invade the second intermediate host, a crustacean such as a crab or crayfish, where they encyst and become metacercariae. This is the infective stage for the mammalian host The number 5. Human infection with P. westermani occurs by eating inadequately cooked or pickled crab or crayfish that harbor metacercariae of the parasite The number 6. The metacercariae excyst in the duodenum The number 7, penetrate through the intestinal wall into the peritoneal cavity, then through the abdominal wall and diaphragm into the lungs, where they become encapsulated and develop into adults The number 8 (7.5 to 12 mm by 4 to 6 mm). The worms can also reach other organs and tissues, such as the brain and striated muscles, respectively. However, when this takes place completion of the life cycles is not achieved, because the eggs laid cannot exit these sites. Time from infection to oviposition is 65 to 90 days.
https://www.cdc.gov/parasites/paragonimus/biology.html
https://www.oie.int/doc/ged/D8933.PDF
https://www.ajtmh.org/view/journals/tpmd/14/4/article-p581.xml
https://scholarworks.gsu.edu/iph_theses/436/
https://zookeys.pensoft.net/article/4268/
https://onlinelibrary.wiley.com/doi/epdf/10.1111/raq.12482
https://www.otago.ac.nz/parasitegroup/PDF%20papers/Friesenetal2019-Hydrobiol.pdf
https://scholarworks.wm.edu/cgi/viewcontent.cgi?article=2792&context=vimsarticles
https://www.biorxiv.org/content/10.1101/2020.12.17.423222v1
https://pubmed.ncbi.nlm.nih.gov/21669853/
https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/crustacean-disease
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7172356/
https://www.sciencedirect.com/science/article/pii/S2213224417300196
https://spo.nmfs.noaa.gov/sites/default/files/pdf-content/MFR/mfr375-6/mfr375-61.pdf
https://isopods.nhm.org/pdfs/27570/27570.pdf
https://www.oie.int/fileadmin/Home/eng/Health_standards/aahm/current/chapitre_general_information_2_2.pdf
https://www.frontiersin.org/articles/10.3389/fimmu.2020.574721/full
https://www.adfg.alaska.gov/static/species/disease/pdfs/crustaceandiseases/prokaryotic_intracytoplasmic_inclusions.pdf
Google Search: crustacean infection
above. tlc - no scrubs
No comments:
Post a Comment