Warm antibody autoimmune hemolytic anemia (WAIHA) is the most common form of autoimmune haemolytic anemia.[1] About half of the cases are of unknown cause, with the other half attributable to a predisposing condition or medications being taken. Contrary to cold autoimmune hemolytic anemia (e.g., cold agglutinin disease and paroxysmal cold hemoglobinuria) which happens in cold temperature (28–31 °C), WAIHA happens at body temperature.[citation needed]
https://en.wikipedia.org/wiki/Warm_antibody_autoimmune_hemolytic_anemia
Cold autoimmune hemolytic anemia caused by cold-reacting antibodies. Autoantibodies that bind to the erythrocyte membrane leading to premature erythrocyte destruction (hemolysis) characterize autoimmune hemolytic anemia.
https://en.wikipedia.org/wiki/Cold_autoimmune_hemolytic_anemia
Cold agglutinin disease (CAD) is a rare autoimmune disease characterized by the presence of high concentrations of circulating cold sensitive antibodies, usually IgM and autoantibodies that are also active at temperatures below 30 °C (86 °F),[1] directed against red blood cells, causing them to agglutinate and undergo lysis.[2] It is a form of autoimmune hemolytic anemia, specifically one in which antibodies bind red blood cells only at low body temperatures, typically 28–31 °C.
When affected people's blood is exposed to cold temperatures (32 °F (0 °C; 273 K) to 50 °F (10 °C; 283 K)), certain proteins that normally attack bacteria (IgM antibodies) attach themselves to red blood cells and bind them together into clumps (agglutination). This eventually causes red blood cells to be prematurely destroyed (hemolysis) leading to anemia and other associated signs and symptoms.[3][4]
Cold agglutinin disease can be primary (unknown cause) or secondary, due to an underlying condition such as an infection, another autoimmune disease, or certain cancers. Treatment depends on many factors including the severity of the condition, the signs and symptoms present in each person, and the underlying cause.[3][4]
Cold agglutinin disease was first described in 1957.[5][6]
https://en.wikipedia.org/wiki/Cold_agglutinin_disease
IgG4-related disease (IgG4-RD), formerly known as IgG4-related systemic disease, is a chronicinflammatory condition characterized by tissue infiltration with lymphocytes and IgG4-secreting plasma cells, various degrees of fibrosis (scarring) and a usually prompt response to oral steroids. In approximately 51–70% of people with this disease, serum IgG4 concentrations are elevated during an acute phase.[1][2][3]
It is a relapsing-remitting disease associated with a tendency to mass forming, tissue-destructive lesions in multiple sites, with a characteristic histopathological appearance in whichever site is involved. Inflammation and the deposition of connective tissue in affected anatomical sites can lead to organ dysfunction, organ failure, or even death if not treated.[4]
Early detection is important to avoid organ damage and potentially serious complications.[5]Treatment is recommended in all symptomatic cases of IgG4-RD and also in asymptomatic IgG4-RD involving certain anatomical sites.[4][6]
https://en.wikipedia.org/wiki/IgG4-related_disease
Thrombotic thrombocytopenic purpura (TTP) is a blood disorder that results in blood clots forming in small blood vessels throughout the body.[2] This results in a low platelet count, low red blood cells due to their breakdown, and often kidney, heart, and brain dysfunction.[1] Symptoms may include large bruises, fever, weakness, shortness of breath, confusion, and headache.[2][3] Repeated episodes may occur.[3]
In about half of cases a trigger is identified, while in the remainder the cause remains unknown.[3]Known triggers include bacterial infections, certain medications, autoimmune diseases such as lupus, and pregnancy.[3] The underlying mechanism typically involves antibodies inhibiting the enzymeADAMTS13.[1] This results in decreased break down of large multimers of von Willebrand factor(vWF) into smaller units.[1] Less commonly TTP is inherited from a person's parents, known as Upshaw–Schulman syndrome, such that ADAMTS13 dysfunction is present from birth.[5] Diagnosis is typically based on symptoms and blood tests.[2] It may be supported by measuring activity of or antibodies against ADAMTS13.[2]
With plasma exchange the risk of death has decreased from more than 90% to less than 20%.[1]Immunosuppressants, such as glucocorticoids, and rituximab may also be used.[3] Platelet transfusions are generally not recommended.[6]
About 1 per 100,000 people are affected.[3] Onset is typically in adulthood and women are more often affected.[3] About 10% of cases begin in childhood.[3] The condition was first described by Eli Moschcowitz in 1924.[3] The underlying mechanism was determined in the 1980s and 1990s.[3]
| Thrombotic thrombocytopenic purpura | |
|---|---|
| Other names | Moschcowitz syndrome,[1] idiopathic thrombotic thrombocytopenic purpura[2] |
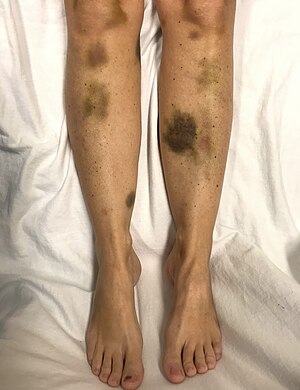 | |
| Spontaneous bruising in a woman with critically low platelets | |
https://en.wikipedia.org/wiki/Thrombotic_thrombocytopenic_purpura
Sarcoidosis (also known as Besnier-Boeck-Schaumann disease) is a disease involving abnormal collections of inflammatory cells that form lumps known as granulomata.[2] The disease usually begins in the lungs, skin, or lymph nodes.[2] Less commonly affected are the eyes, liver, heart, and brain.[2] Any organ, however, can be affected.[2] The signs and symptoms depend on the organ involved.[2] Often, no, or only mild, symptoms are seen.[2] When it affects the lungs, wheezing, coughing, shortness of breath, or chest pain may occur.[3] Some may have Löfgren syndrome with fever, large lymph nodes, arthritis, and a rash known as erythema nodosum.[2]
The cause of sarcoidosis is unknown.[2] Some believe it may be due to an immune reaction to a trigger such as an infection or chemicals in those who are genetically predisposed.[12][13] Those with affected family members are at greater risk.[4] Diagnosis is partly based on signs and symptoms, which may be supported by biopsy.[6] Findings that make it likely include large lymph nodes at the root of the lung on both sides, high blood calcium with a normal parathyroid hormone level, or elevated levels of angiotensin-converting enzyme in the blood.[6] The diagnosis should only be made after excluding other possible causes of similar symptoms such as tuberculosis.[6]
Sarcoidosis may resolve without any treatment within a few years.[2][5] However, some people may have long-term or severe disease.[5] Some symptoms may be improved with the use of anti-inflammatory drugs such as ibuprofen.[8] In cases where the condition causes significant health problems, steroids such as prednisone are indicated.[9] Medications such as methotrexate, chloroquine, or azathioprine may occasionally be used in an effort to decrease the side effects of steroids.[9] The risk of death is 1–7%.[5] The chance of the disease returning in someone who has had it previously is less than 5%.[2]
In 2015, pulmonary sarcoidosis and interstitial lung disease affected 1.9 million people globally and they resulted in 122,000 deaths.[10][11] It is most common in Scandinavians, but occurs in all parts of the world.[14] In the United States, risk is greater among black people as opposed to white people.[14]It usually begins between the ages of 20 and 50.[4] It occurs more often in women than men.[4]Sarcoidosis was first described in 1877 by the English doctor Jonathan Hutchinson as a non-painful skin disease.[15]
https://en.wikipedia.org/wiki/Sarcoidosis
No comments:
Post a Comment