Flavivirus is a genus of positive-strand RNA viruses in the family Flaviviridae. The genus includes the West Nile virus, dengue virus, tick-borne encephalitis virus, yellow fever virus, Zika virus and several other viruses which may cause encephalitis,[3] as well as insect-specific flaviviruses (ISFs) such as cell fusing agent virus (CFAV), Palm Creek virus (PCV), and Parramatta River virus (PaRV).[4] While dual-host flaviviruses can infect vertebrates as well as arthropods, insect-specific flaviviruses are restricted to their competent arthropods.[5]
Flaviviruses are named for the yellow fever virus; the word flavus means 'yellow' in Latin, and yellow fever in turn is named from its propensity to cause yellow jaundice in victims.[6]
Flaviviruses share several common aspects: common size (40–65 nm), symmetry (enveloped, icosahedral nucleocapsid), nucleic acid (positive-sense, single-stranded RNAaround 10,000–11,000 bases), and appearance under the electron microscope.
Most of these viruses are primarily transmitted by the bite from an infected arthropod(mosquito or tick), and hence are classified as arboviruses. Human infections with most of these arboviruses are incidental, as humans are unable to replicate the virus to high enough titers to reinfect the arthropods needed to continue the virus lifecycle – humans are then a dead end host. The exceptions to this are the yellow fever virus, dengue virusand zika virus. These three viruses still require mosquito vectors but are well-enough adapted to humans as to not necessarily depend upon animal hosts (although they continue to have important animal transmission routes, as well).
Other virus transmission routes for arboviruses include handling infected animal carcasses, blood transfusion, sex, child birth and consumption of unpasteurised milk products. Transmission from nonhuman vertebrates to humans without an intermediate vector arthropod however mostly occurs with low probability. For example, early tests with yellow fever showed that the disease is not contagious.
The known non-arboviruses of the flavivirus family reproduce in either arthropods or vertebrates, but not both, with one odd member of the genus affecting a nematode.[7]
| Flavivirus | |
|---|---|
 | |
| A TEM micrograph of Yellow fever virus | |
 | |
| Zika virus capsid model, colored by chains, PDB entry 5ire[2] |
Species[edit]
In the genus Flavivirus there are 53 defined species:[40]
- Apoi virus
- Aroa virus
- Bagaza virus
- Banzi virus
- Bouboui virus
- Bukalasa bat virus
- Cacipacore virus
- Carey Island virus
- Cowbone Ridge virus
- Dakar bat virus
- Dengue virus
- Edge Hill virus
- Entebbe bat virus
- Gadgets Gully virus
- Ilheus virus
- Israel turkey meningoencephalomyelitis virus
- Japanese encephalitis virus
- Jugra virus
- Jutiapa virus
- Kadam virus
- Kedougou virus
- Kokobera virus
- Koutango virus
- Kyasanur Forest disease virus
- Langat virus
- Louping ill virus
- Meaban virus
- Modoc virus
- Montana myotis leukoencephalitis virus
- Murray Valley encephalitis virus
- Ntaya virus
- Omsk hemorrhagic fever virus
- Phnom Penh bat virus
- Powassan virus
- Rio Bravo virus
- Royal Farm virus
- Saboya virus
- Saint Louis encephalitis virus
- Sal Vieja virus
- San Perlita virus
- Saumarez Reef virus
- Sepik virus
- Tembusu virus
- Tick-borne encephalitis virus
- Tyuleniy virus
- Uganda S virus
- Usutu virus
- Wesselsbron virus
- West Nile virus
- Yaounde virus
- Yellow fever virus
- Yokose virus
- Zika virus
Sorted by vector
https://en.wikipedia.org/wiki/Flavivirus
Louping-ill (/ˈlaʊpɪŋɪl/) is an acute viral disease primarily of sheep that is characterized by a biphasic fever, depression, ataxia, muscularincoordination, tremors, posterior paralysis, coma, and death. Louping-ill is a tick-transmitted disease whose occurrence is closely related to the distribution of the primary vector, the sheep tick Ixodes ricinus. It also causes disease in red grouse, and can affect humans. The name 'louping-ill' is derived from an old Scottish word describing the effect of the disease in sheep whereby they 'loup' or spring into the air.[1]
| Louping ill | |
|---|---|
| Other names | Ovine encephalomyelitis, infectious encephalomyelitis of sheep, trembling-ill |
 | |
| Nonsuppurative encephalitis in goat affected by louping ill. A) Cerebellum with necrosis of Purkinje cells. Hematoxylin and eosin (H&E) stain; scale bar = 100 µm. Inset: necrosis of Purkinje cells. H&E stain; scale bar = 20 µm. B) Midbrain. Area of neurophagia (arrow) surrounded by microglial cells. Necrosis of neurons can be also seen. H&E stain; scale bar = 50 µm. C) Lymphoid perivascular cuff in midbrain. H&E stain; scale bar = 50 µm. D) Spinal cord, gray matter. Focal microgliosis (crosses) and neurons undergoing necrosis (arrows). H&E stain; scale bar = 50 µm. | |
| Specialty | Veterinary medicine |
https://en.wikipedia.org/wiki/Louping_ill
The Flavivirus capsid hairpin cHP is a conserved RNA hairpin structure identified within the capsid coding region of several flavivirus genomes. These positive strand RNA genomes are translated as a single polypeptide and subsequently cleaved into constituent proteins, the first of which is the capsid protein. The cHP hairpin is located within the capsid coding region between two AUG start codons. The cHP cis element has been shown to direct translation start from the suboptimal first start codon.[1] The ability of cHP to direct initiation from the first start codon is proportional to its thermodynamic stability, is position dependent, and is sequence independent. It has been demonstrated that both AUGs and the conserved cHP are necessary for efficient viral replication in human and mosquito cells.[1]
mosquito t cell.
| flavivirus capsid hairpin cHP | |
|---|---|
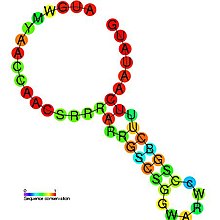 Predicted secondary structure and sequence conservation of cHP |
https://en.wikipedia.org/wiki/Flavivirus_capsid_hairpin_cHP
Defective interfering particles (DIPs), also known as defective interfering viruses, are spontaneously generated virus mutants in which a critical portion of the particle's genome has been lost due to defective replication or non-homologous recombination.[2][3] The mechanism of their formation is presumed to be as a result of template-switching during replication of the viral genome, although non-replicative mechanisms involving direct ligation of genomic RNA fragments have also been proposed.[4][5] DIPs are derived from and associated with their parent virus, and particles are classed as DIPs if they are rendered non-infectious due to at least one essential gene of the virus being lost or severely damaged as a result of the defection.[6] A DIP can usually still penetrate host cells, but requires another fully functional virus particle (the 'helper' virus) to co-infect a cell with it, in order to provide the lost factors.[7][8]
DIPs were first observed as early as the 1950s by Von Magnus and Schlesinger, both working with influenza viruses.[9] However, the formalization of DIPs terminology was in 1970 by Huang and Baltimore when they noticed the presence of ‘stumpy’ particles of vesicular stomatitis virus in electron micrographs.[10] DIPs can occur within nearly every class of both DNA and RNA viruses both in clinical and laboratory settings including poliovirus, SARS coronavirus, measles, alphaviruses, respiratory syncytial virus and influenza virus.[11][12][13][14][15][16][17][18]
https://en.wikipedia.org/wiki/Defective_interfering_particle

No comments:
Post a Comment