09-10-2021-2152 - 2799/00-01,2
https://en.wikipedia.org/wiki/Feline_infectious_peritonitis
https://en.wikipedia.org/wiki/Immune_disorder
https://en.wikipedia.org/wiki/Vasculitis
https://en.wikipedia.org/wiki/Sodium_thiosulfate
https://en.wikipedia.org/wiki/Copper(II)_sulfate
https://en.wikipedia.org/wiki/Dimercaprol
https://en.wikipedia.org/wiki/Dimercaptosuccinic_acid
https://en.wikipedia.org/wiki/Arsenic_poisoning
https://en.wikipedia.org/wiki/Physostigmine
https://en.wikipedia.org/wiki/Cyprodenate
https://en.wikipedia.org/wiki/Bemegride
https://en.wikipedia.org/wiki/Oxime
https://en.wikipedia.org/wiki/Obidoxime
https://en.wikipedia.org/wiki/Pralidoxime
https://en.wikipedia.org/wiki/Doxapram
https://en.wikipedia.org/wiki/Direct_factor_Xa_inhibitors
https://en.wikipedia.org/wiki/Heparin
https://en.wikipedia.org/wiki/Amyl_nitrite
https://en.wikipedia.org/wiki/Nitrite
https://en.wikipedia.org/wiki/Hydroxocobalamin
https://en.wikipedia.org/wiki/4-Dimethylaminophenol
https://en.wikipedia.org/wiki/Sodium_nitrite
https://en.wikipedia.org/wiki/Prussian_blue
https://en.wikipedia.org/wiki/Ethylenediaminetetraacetic_acid
https://en.wikipedia.org/wiki/Dimercaprol
https://en.wikipedia.org/wiki/Potassium_iodide
https://en.wikipedia.org/wiki/Iodine-131
https://en.wikipedia.org/wiki/Methylene_blue
https://en.wikipedia.org/wiki/Prednisolone/promethazine
- Berlin blue
- Ferric ferrocyanide
- Ferric hexacyanoferrate
- Iron(III) ferrocyanide
- Iron(III) hexacyanoferrate(II)
- Parisian blue
- https://en.wikipedia.org/wiki/Prussian_blue
Tuesday, September 7, 2021
09-07-2021-1333 - cyanogen (CN)2 1815 prussia
Cyanogen is the chemical compound with the formula (CN)2. It is a colorless, toxic gas with a pungent odor. The molecule is a pseudohalogen. Cyanogen molecules consist of two CN groups – analogous to diatomic halogen molecules, such as Cl2, but far less oxidizing. The two cyano groups are bonded together at their carbon atoms: N≡C−C≡N, although other isomers have been detected.[6] The name is also used for the CN radical,[7] and hence is used for compounds such as cyanogen bromide (NCBr).[8]
Cyanogen is the anhydride of oxamide:
- H2NC(O)C(O)NH2 → NCCN + 2 H2O
although oxamide is manufactured from cyanogen by hydrolysis:[9]
- NCCN + 2 H2O → H2NC(O)C(O)NH2
NamesPreferred IUPAC name Oxalonitrile[4]https://en.wikipedia.org/wiki/Cyanogen
Oxalonitrile[4]https://en.wikipedia.org/wiki/Cyanogen
Prussian blue (also known as Berlin blue or, in painting, Parisian or Paris blue) is a dark blue pigment produced by oxidation of ferrous ferrocyanide salts. It has the chemical formula FeIII
4[FeII
(CN)
6]
3. Turnbull's blue is chemically identical, but is made from different reagents, and its slightly different color stems from different impurities.
Prussian blue was the first modern synthetic pigment. It is prepared as a very fine colloidal dispersion, because the compound is not soluble in water. It contains variable amounts[1] of other ions and its appearance depends sensitively on the size of the colloidal particles. The pigment is used in paints, and it is the traditional "blue" in blueprints and aizuri-e (藍摺り絵) Japanese woodblock prints.
In medicine, orally administered Prussian blue is used as an antidote for certain kinds of heavy metal poisoning, e.g., by thallium(I) and radioactive isotopes of caesium. The therapy exploits the compound's ion-exchange properties and high affinity for certain "soft" metal cations.
It is on the World Health Organization's List of Essential Medicines, the most important medications needed in a basic health system.[2] Prussian blue lent its name to prussic acid (hydrogen cyanide) derived from it. In German, hydrogen cyanide is called Blausäure ("blue acid"). French chemist Joseph Louis Gay-Lussacgave cyanide its name, from the Ancient Greek word κύανος (kyanos, "blue"), because of its Prussian blue color.
| Prussian blue | |
|---|---|
| Hex triplet | #003153 |
| HSV (h, s, v) | (205°, 100%, 32%) |
| sRGBB (r, g, b) | (0, 49, 83) |
| Source | [1] |
| ISCC–NBS descriptor | Dark blue |
| B: Normalized to [0–255] (byte) | |
Prussian blue pigment is significant since it was the first stable and relatively lightfastblue pigment to be widely used following the loss of knowledge regarding the synthesis of Egyptian blue. European painters had previously used a number of pigments such as indigo dye, smalt, and Tyrian purple, and the extremely expensive ultramarine made from lapis lazuli. Japanese painters and woodblock print artists, likewise, did not have access to a long-lasting blue pigment until they began to import Prussian blue from Europe.[3]
Prussian blue Fe
7(CN)
18 (also (Fe
4[Fe(CN)
6]
3) · xH
2O) was probably synthesized for the first time by the paint maker Diesbach in Berlin around 1706.[4] Most historical sources do not mention a first name of Diesbach. Only Berger refers to him as Johann Jacob Diesbach.[5] The pigment is believed to have been accidentally created when Diesbach used potash tainted with blood to create some red cochinealdye. The original dye required potash, ferric sulfate, and dried cochineal. Instead, the blood, potash, and iron sulfate reacted to create a compound known as iron ferrocyanide, which, unlike the desired red pigment, has a very distinct blue hue.[6] It was named Preußisch blau and Berlinisch Blau in 1709 by its first trader.[7]

Prussian blue is a microcrystalline blue powder. It is insoluble, but the crystallites tend to form a colloid. Such colloids can pass through fine filters.[1] Despite being one of the oldest known synthetic compounds, the composition of Prussian blue remained uncertain for many years. Its precise identification was complicated by three factors:
- Prussian blue is extremely insoluble, but also tends to form colloids
- Traditional syntheses tend to afford impure compositions
- Even pure Prussian blue is structurally complex, defying routine crystallographic analysis
Medicine[edit]
Prussian blue's ability to incorporate monovalent metallic cations (Me+) makes it useful as a sequestering agent for certain toxic heavy metals. Pharmaceutical-grade Prussian blue in particular is used for people who have ingested thallium (Tl+) or radioactivecaesium (134Cs+, 137Cs+) . According to the International Atomic Energy Agency, an adult male can eat at least 10 g of Prussian blue per day without serious harm. The U.S. Food and Drug Administration has determined the "500-mg Prussian blue capsules, when manufactured under the conditions of an approved New Drug Application, can be found safe and effective therapy" in certain poisoning cases.[28][29] Radiogardase (Prussian blue in soluble capsules [30]) is a commercial product for the removal of caesium-137 from the intestine, so indirectly from the bloodstream by intervening in the enterohepatic circulation of caesium-137,[31]reducing the internal residency time (and exposure) by about two-thirds. In particular, it was used to absorb 137
Cs+
from those poisoned in the Goiânia accident.[1]
Pigment[edit]
Because it is easily made, cheap, nontoxic, and intensely colored, Prussian blue has attracted many applications. It was adopted as a pigment very soon after its invention and was almost immediately widely used in oil paints, watercolor, and dyeing.[27] The dominant uses are for pigments: about 12,000 tonnes of Prussian blue are produced annually for use in black and bluish inks. A variety of other pigments also contain the material.[20] Engineer's blue and the pigment formed on cyanotypes—giving them their common name blueprints. Certain crayons were once colored with Prussian blue (later relabeled midnight blue). It is also a popular pigment in paints. Similarly, Prussian blue is the basis for laundry bluing.
Nanoparticles of Prussian blue are used as pigments in some cosmetics ingredients, according to the European Union Observatory for Nanomaterials.
Tuesday, September 7, 2021
09-07-2021-1334 - oxamide oxanamide
Oxamide is the organic compound with the formula (CONH2)2. This white crystalline solid is soluble in ethanol, slightly soluble in water and insoluble in diethyl ether. Oxamide is the diamide derived from oxalic acid.

https://en.wikipedia.org/wiki/Oxamide
Oxanamide (Quiactin) is an anxiolytic and muscle relaxant which can produce sedative and hypnotic effects in sufficiently high doses.[1] An uncontrolled trial on patients treated in a clinical gynecology practice published in 1959 found that oxanamide was efficacious in the treatment of a
nxiety resulting from premenstrual syndrome, menopause, and various other causes, with minimal sedation or other side effects.[2]
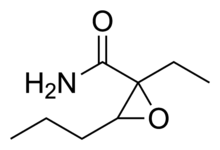
https://en.wikipedia.org/wiki/Oxanamide
By machinists and toolmakers[edit]
Engineer's blue, Prussian blue in an oily base, is the traditional material used for spotting metal surfaces such as surface plates and bearings for hand scraping. A thin layer of nondrying paste is applied to a reference surface and transfers to the high spots of the workpiece. The toolmaker then scrapes, stones, or otherwise removes the marked high spots. Prussian blue is preferable because it will not abrade the extremely precise reference surfaces as many ground pigments may.
In analytical chemistry[edit]
Prussian blue is formed in the Prussian blue assay for total phenols. Samples and phenolic standards are given acidic ferric chloride and ferricyanide, which is reduced to ferrocyanide by the phenols. The ferric chloride and ferrocyanide react to form Prussian blue. Comparing the absorbance at 700 nm of the samples to the standards allows for the determination of total phenols or polyphenols.[33][34]
https://en.wikipedia.org/wiki/Prussian_blue
Astrochemistry is the study of the abundance and reactions of molecules in the Universe, and their interaction with radiation.[1]The discipline is an overlap of astronomy and chemistry. The word "astrochemistry" may be applied to both the Solar System and the interstellar medium. The study of the abundance of elements and isotope ratios in Solar System objects, such as meteorites, is also called cosmochemistry, while the study of interstellar atoms and molecules and their interaction with radiation is sometimes called molecular astrophysics. The formation, atomic and chemical composition, evolution and fate of molecular gas clouds is of special interest, because it is from these clouds that solar systems form.
Observations of solar spectra as performed by Athanasius Kircher (1646), Jan Marek Marci (1648), Robert Boyle (1664), and Francesco Maria Grimaldi (1665) all predated Newton's 1666 work which established the spectral nature of light and resulted in the first spectroscope.[2] Spectroscopy was first used as an astronomical technique in 1802 with the experiments of William Hyde Wollaston, who built a spectrometer to observe the spectral lines present within solar radiation.[3] These spectral lines were later quantified through the work of Joseph Von Fraunhofer.
While radio astronomy was developed in the 1930s, it was not until 1937 that any substantial evidence arose for the conclusive identification of an interstellar molecule[6] – up until this point, the only chemical species known to exist in interstellar space were atomic. These findings were confirmed in 1940, when McKellar et al. identified and attributed spectroscopic lines in an as-of-then unidentified radio observation to CH and CN molecules in interstellar space.[7] In the thirty years afterwards, a small selection of other molecules were discovered in interstellar space: the most important being OH, discovered in 1963 and significant as a source of interstellar oxygen,[8] and H2CO (Formaldehyde), discovered in 1969 and significant for being the first observed organic, polyatomic molecule in interstellar space[9]
The discovery of interstellar formaldehyde – and later, other molecules with potential biological significance such as water or carbon monoxide – is seen by some as strong supporting evidence for abiogenetic theories of life: specifically, theories which hold that the basic molecular components of life came from extraterrestrial sources. This has prompted a still ongoing search for interstellar molecules which are either of direct biological importance – such as interstellar glycine, discovered in 2009[10] – or which exhibit biologically relevant properties like Chirality – an example of which (propylene oxide) was discovered in 2016[11] – alongside more basic astrochemical research.
Moreover, such methods are completely blind to molecules that have no dipole. For example, by far the most common molecule in the universe is H2 (hydrogen gas), but it does not have a dipole moment, so it is invisible to radio telescopes.
Moreover, such methods are completely blind to molecules that have no dipole. For example, by far the most common molecule in the universe is H2 (hydrogen gas), but it does not have a dipole moment, so it is invisible to radio telescopes. Moreover, such methods cannot detect species that are not in the gas-phase. Since dense molecular clouds are very cold (10 to 50 K [−263.1 to −223.2 °C; −441.7 to −369.7 °F]), most molecules in them (other than hydrogen) are frozen, i.e. solid. Instead, hydrogen and these other molecules are detected using other wavelengths of light. Hydrogen is easily detected in the ultraviolet (UV) and visible ranges from its absorption and emission of light (the hydrogen line). Moreover, most organic compounds absorb and emit light in the infrared (IR) so, for example, the detection of methane in the atmosphere of Mars[15] was achieved using an IR ground-based telescope, NASA's 3-meter Infrared Telescope Facility atop Mauna Kea, Hawaii. NASA's researchers use airborne IR telescope SOFIA and space telescope Spitzer for their observations, researches and scientific operations.[16][17] Somewhat related to the recent detection of methane in the atmosphere of Mars. Christopher Oze, of the University of Canterbury in New Zealand and his colleagues reported, in June 2012, that measuring the ratio of hydrogen and methane levels on Mars may help determine the likelihood of life on Mars.[18][19] According to the scientists, "...low H2/CH4 ratios (less than approximately 40) indicate that life is likely present and active."[18] Other scientists have recently reported methods of detecting hydrogen and methane in extraterrestrial atmospheres.[20][21]
Infrared astronomy has also revealed that the interstellar medium contains a suite of complex gas-phase carbon compounds called polyaromatic hydrocarbons, often abbreviated PAHs or PACs. These molecules, composed primarily of fused rings of carbon (either neutral or in an ionized state), are said to be the most common class of carbon compound in the galaxy. They are also the most common class of carbon molecule in meteorites and in cometary and asteroidal dust (cosmic dust). These compounds, as well as the amino acids, nucleobases, and many other compounds in meteorites, carry deuterium and isotopes of carbon, nitrogen, and oxygen that are very rare on earth, attesting to their extraterrestrial origin. The PAHs are thought to form in hot circumstellar environments (around dying, carbon-rich red giant stars).
Infrared astronomy has also been used to assess the composition of solid materials in the interstellar medium, including silicates, kerogen-like carbon-rich solids, and ices. This is because unlike visible light, which is scattered or absorbed by solid particles, the IR radiation can pass through the microscopic interstellar particles, but in the process there are absorptions at certain wavelengths that are characteristic of the composition of the grains.[22] As above with radio astronomy, there are certain limitations, e.g. N2 is difficult to detect by either IR or radio astronomy.
Such IR observations have determined that in dense clouds (where there are enough particles to attenuate the destructive UV radiation) thin ice layers coat the microscopic particles, permitting some low-temperature chemistry to occur. Since hydrogen is by far the most abundant molecule in the universe, the initial chemistry of these ices is determined by the chemistry of the hydrogen. If the hydrogen is atomic, then the H atoms react with available O, C and N atoms, producing "reduced" species like H2O, CH4, and NH3. However, if the hydrogen is molecular and thus not reactive, this permits the heavier atoms to react or remain bonded together, producing CO, CO2, CN, etc. These mixed-molecular ices are exposed to ultraviolet radiation and cosmic rays, which results in complex radiation-driven chemistry.[22] Lab experiments on the photochemistry of simple interstellar ices have produced amino acids.[23] The similarity between interstellar and cometary ices (as well as comparisons of gas phase compounds) have been invoked as indicators of a connection between interstellar and cometary chemistry. This is somewhat supported by the results of the analysis of the organics from the comet samples returned by the Stardust mission but the minerals also indicated a surprising contribution from high-temperature chemistry in the solar nebula.
Research is progressing on the way in which interstellar and circumstellar molecules form and interact, e.g. by including non-trivial quantum mechanical phenomena for synthesis pathways on interstellar particles.[25] This research could have a profound impact on our understanding of the suite of molecules that were present in the molecular cloud when our solar system formed, which contributed to the rich carbon chemistry of comets and asteroids and hence the meteorites and interstellar dust particles which fall to the Earth by the ton every day.
The sparseness of interstellar and interplanetary space results in some unusual chemistry, since symmetry-forbidden reactions cannot occur except on the longest of timescales. For this reason, molecules and molecular ions which are unstable on Earth can be highly abundant in space, for example the H3+ ion.
Astrochemistry overlaps with astrophysics and nuclear physics in characterizing the nuclear reactions which occur in stars, as well as the structure of stellar interiors. If a star develops a largely convective envelope, dredge-up events can occur, bringing the products of nuclear burning to the surface. If the star is experiencing significant mass loss, the expelled material may contain molecules whose rotational and vibrational spectral transitions can be observed with radio and infrared telescopes. An interesting example of this is the set of carbon stars with silicate and water-ice outer envelopes. Molecular spectroscopy allows us to see these stars transitioning from an original composition in which oxygen was more abundant than carbon, to a carbon star phase where the carbon produced by helium burning is brought to the surface by deep convection, and dramatically changes the molecular content of the stellar wind.[26][27]
In October 2011, scientists reported that cosmic dust contains organic matter ("amorphous organic solids with a mixed aromatic-aliphatic structure") that could be created naturally, and rapidly, by stars.[28][29][30]
On August 29, 2012, and in a world first, astronomers at Copenhagen University reported the detection of a specific sugar molecule, glycolaldehyde, in a distant star system. The molecule was found around the protostellar binary IRAS 16293-2422, which is located 400 light years from Earth.[31][32] Glycolaldehyde is needed to form ribonucleic acid, or RNA, which is similar in function to DNA. This finding suggests that complex organic molecules may form in stellar systems prior to the formation of planets, eventually arriving on young planets early in their formation.[33]
In September, 2012, NASA scientists reported that polycyclic aromatic hydrocarbons (PAHs), subjected to interstellar medium (ISM) conditions, are transformed, through hydrogenation, oxygenation and hydroxylation, to more complex organics – "a step along the path toward amino acids and nucleotides, the raw materials of proteins and DNA, respectively".[34][35] Further, as a result of these transformations, the PAHs lose their spectroscopic signature which could be one of the reasons "for the lack of PAH detection in interstellar ice grains, particularly the outer regions of cold, dense clouds or the upper molecular layers of protoplanetary disks."[34][35]
In February 2014, NASA announced the creation of an improved spectral database [36] for tracking polycyclic aromatic hydrocarbons (PAHs) in the universe. According to scientists, more than 20% of the carbon in the universe may be associated with PAHs, possible starting materials for the formation of life. PAHs seem to have been formed shortly after the Big Bang, are widespread throughout the universe, and are associated with new stars and exoplanets.[37]
On August 11, 2014, astronomers released studies, using the Atacama Large Millimeter/Submillimeter Array (ALMA) for the first time, that detailed the distribution of HCN, HNC, H2CO, and dust inside the comae of comets C/2012 F6 (Lemmon) and C/2012 S1 (ISON).[38][39]
For the study of the recourses of chemical elements and molecules in the universe is developed the mathematical model of the molecules composition distribution in the interstellar environment on thermodynamic potentials by professor M.Yu. Dolomatov using methods of the probability theory, the mathematical and physical statistics and the equilibrium thermodynamics.[40][41][42]Based on this model are estimated the resources of life-related molecules, amino acids and the nitrogenous bases in the interstellar medium. The possibility of the oil hydrocarbons molecules formation is shown. The given calculations confirm Sokolov's and Hoyl's hypotheses about the possibility of the oil hydrocarbons formation in Space. Results are confirmed by data of astrophysical supervision and space researches.
In July 2015, scientists reported that upon the first touchdown of the Philae lander on comet 67/P's surface, measurements by the COSAC and Ptolemy instruments revealed sixteen organic compounds, four of which were seen for the first time on a comet, including acetamide, acetone, methyl isocyanate and propionaldehyde.[43][44][45]
See also[edit]
- Astrobotany – Study of plants grown in spacecraft
- Astrobiology – Science concerned with life in the universe
- Astrophysics – Branch of astronomy
- Astrosciences
- Hemolithin – Protein claimed to be of extraterrestrial origin
- Interstellar medium – Matter and radiation in the space between the star systems in a galaxy
- List of interstellar and circumstellar molecules – Molecules detected in space
- Molecular astrophysics
- Nucleocosmochronology – Technique to determine timescales for astrophysical objects and events
- Recombination – Epoch at which charged electrons and protons first became bound to form electrically neutral hydrogen atoms
- Reionization – Process that caused matter to reionize early in the history of the Universe
https://en.wikipedia.org/wiki/Astrochemistry
- 09-07-2021-1555 - ion exchange resin polymer ion t...
- 09-07-2021-1553 - Hexazine (also known as hexaazab...
- 09-07-2021-1510 - Dog Litter Dog Breeding Similari...
- Elevator (feat. Timbaland) - Flo Rida
- 09-07-2021-1403 - 2592/3-4,5
- 09-07-2021-1403 - Pseudohalogens analoge analogue ...
- 09-07-2021-1401 - Methylidynephosphane (phosphaethyne
- 09-07-2021-1400 - phosphaalkyne (IUPAC name: alkyl...
- 09-07-2021-1358 - Cyaphide
- 09-07-2021-1352 - Cyanogen iodide or iodine cyanid...
- 09-07-2021-1353 - 2586/7-8,9
- 09-07-2021-1349 - cyanide was first isolated by th...
- 09-07-2021-1346 - Diacetylene (also known as butad...
- 09-07-2021-1345 - Dicyanoacetylene, also called ca...
- 09-07-2021-1342 - 2581/2-3,4
- 09-07-2021-1341 - Free Radicals Reactive Intermedi...
- 09-07-2021-1339 - Octatetraynyl radical (C8H)
- 09-07-2021-1338 - Ketenimines R1R2C=C=NR3 ketene ...
- 09-07-2021-1337 - Molecules detected in outer space
- 09-07-2021-1336 - Ethyl formate ester formed when ...
- 09-07-2021-1335 - Formamide methanamide formic aci...
- 09-07-2021-1334 - 2574/5-6,7
- 09-07-2021-1334 - oxamide oxanamide
- 09-07-2021-1333 - cyanogen (CN)2 1815 prussia
- 09-07-2021-1329 - Explosion in a nitrogenous ferti...
- 09-07-2021-1325 - Dust 1998
- 09-07-2021-1315 - Coronavirus Cases
- 09-07-2021-0103 - Crustacean Methroxetrate (shrink...
- 09-06-2021-0138 - Mycosubtilin
- 09-06-2021-0137 - Sulfamide (IUPAC name: sulfuric ...
- 09-06-2021-0136 - sulfonamide
- 09-06-2021-0134 - Pyrazole
- 09-06-2021-0132 - 1,9-Pyrazoloanthrone anthrone
- 09-06-2021-0124 - 2453/4-5,6
- 09-06-2021-0123 - proteolipid
- 09-06-2021-0120 - pyrazole sulfonamide HIV 1 HIV A...
- 09-06-2021-0122 - acylation
- 09-06-2021-0118 - Ion Channel Modulators
- 09-06-2021-0115 - Arylcyclohexylamines, also known...
- 09-06-2021-0114 - Methoxphenidine (methoxydiphenid...
- 09-06-2021-0109 - Remacemide
- 09-06-2021-0108 - 2445/6-7,8
- 09-06-2021-0107 - hexapradol INN
- 09-06-2021-0106 - Diphenidine (1,2-DEP, DPD, DND)
- Saltwater - Chicane
- Missy Elliott - One Minute Man (feat. Ludacris) [O...
- 09-06-2021-0054 - Thirteen Service Members Dead Gr...
- 09-06-2021-0033 - Post-translational modification
- 09-06-2021-0029 - PALMING METHOD SYNAPTIC DETER - ...
- 09-06-2021-0024 - 2437/8-9,10
- 09-06-2021-0023 - palmitoylation fatty acids palmi...
- 09-06-2021-0022 - myristoylation lipidation modifi...
- 09-05-2021-0035 - Apicomplexa genera fusiona
- 09-05-2021-0033 - Buparvaquone is a hydroxynaphtho...
- 09-05-2021-0029 - 2422/3-4,5
- 09-05-2021-0027 - Amido black 10B azo western blot
- 09-05-2021-0022 - Semicarbazide
- 09-05-2021-0021 - Naphthalenesulfonic acids
- 09-05-2021-0020 - 1-Naphthols
- 09-05-2021-0016 - Trypan blue is an azo dye
- 09-05-2021-0015 - Stramenopile
- 09-05-2021-0014 - Rhizaria
- 09-05-2021-0013 - alveolates protist
- 09-05-2021-0012 - 2413/4-5,6
- 09-05-2021-0011 - SAR or Harosa (informally the SA...
- 09-05-2021-0009 - Imidocarb
- 09-05-2021-0008 - Cytauxzoon felis
- 09-05-2021-0007 - Ivermectin
- 09-05-2021-0006 - Praziquantel
- 09-05-2021-0004 - Niclosamide
- 09-05-2021-0001 - Hymenolepiasis (rat tapeworm, dw...
- 09-04-2021-2355 - Flea Borne Disease Murine Typhus...
- 09-04-2021-2349 - Babesiosis or piroplasmosis Bab...
- 09-04-2021-2346 - Vasculitis
- 09-04-2021-2316 - EMA
- 09-04-2021-2250 - piranha or piraña
- 09-04-2021-2237 - Robert Swan Mueller III (directo...
- 09-04-2021-2230 - R. Mueller, Grigory, Nikiya, etc...
- 09-04-2021-2217 - Agent Muller, Mueller, etc.
- 09-04-2021-2215 - phosphorylation
- 09-04-2021-2215 - 2396/7-8,9
- 09-04-2021-2214 - dephosphorylation
- 09-04-2021-2213 - 1,3-Bisphosphoglyceric acid (1,3...
- Sing Sing Sing - Benny Goodman
- Sisqo - Thong Song (Official Music Video)
- 09-04-2021-1924 - 2390/1-2,3
- 09-04-2021-1923 - Stimulus Check Four Update
- 09-04-2021-1905 - Please do not accept donation/et...
- 09-04-2021-1825 - Great Depression 1930 USA NAC
- 09-04-2021-1822 - House - Collections 1. Advertis...
- 09-04-2021-1613 - 3-Phosphoglyceric acid (3PG, 3-P...
- 09-04-2021-1609 - Fumaric Acid
- 09-04-2021-1607 - 2381/2-3,4
- 09-04-2021-1523 - Infrastructure continued... draf...
- 09-04-2021-1515 - Semi-Automatic
- Remy Ma - Conceited (There's Something About Remy)
- 09-04-2021-1448 - CIA Observatory Nine
- 09-04-2021-1326 - Story Rubes & Lee (Draft 1; Over...
- 09-03-2021-2248 - Rubys & Lee Wallace Bettey (Simi...
- 09-3-2021-2043 - Admissions To Home Care and Hospice
- 09-03-2021-1819 - Maine State Hospice Options. htt...
- 09-03-2021-1800 - COVID-19 4.55
- The Pretty Reckless - 25 (Official Music Video)
- 08-30-2021-1455 - Stimulus checks: Will payments t...
- 08-29-2021 - 0246 - Mass Vaccination Program - C...
- 08-30-2021-0070 - Vaccinia @ HIV or AIDS 1999 Equu...
- 08-29-2021-2149 - Cellulose 2046, 2047, 2048-2050
- 08-29-2021-2150 - 2047/8-9,00
- 08-29-2021-2147 - Pyrophosphoric acid diphosphoric...
- 08-29-2021-2145 - Phosphoric acid weak acid acetic...
- 08-29-2021-2139 - phosphodiester bond hydroxyl gro...
- 08-29-2021-2137 - Phosphoesters form the backbone ...
- 08-29-2021-214 - Vacuolar-type ATPase (V-ATPase) A...
- 08-29-2021-2107 - Biochemistry Phosphorous draft
- 08-29-2021-2105 - 2039/40-1,2
- 08-29-2021-2102 - Phosphorus - Phosphorous - pho...
- 08-29-2021-2100 - phosphate phosphorous
- 08-29-2021-2059 - phosphorylase phosphorylases
- 08-29-2021-2057 - Phosphorolysis
- 08-29-2021-2054 - Inorganic pyrophosphatase (or in...
- 08-29-2021-2052 - Pyrophosphatases tobacco acid py...
- 08-29-2021-2050 - 2032/3-4,5
- 08-29-2021-2049 - proton ATPase ADP ATP phosphate ...
- 08-29-2021-2048 - P-type ATPases E1-E2 ATPases P-t...
- 08-29-2021-2043 - Amidophosphoribosyltransferase p...
- 08-29-2021-2040 - Hydrolase Lyase Oxidoreductase
- 08-29-2021-2037 - catalytic triad (trihydrogen cat...
- 08-29-2021-2036 - oxyanion hole
- 08-29-2021-2032 - HIV-1 protease PR aspartyl retro...
- 08-29-2021-2031 - Viral infectivity factor Vif BC ...
- 08-29-2021-2018 - Examination of SARS-CoV-2 sequen...
- 08-29-2021-2013 - Variant gamma beta alpha proteob...
- 08-29-2021-2009 - five clusters of mink variants o...
- 08-29-2021-2005 - COVID-19 Death 4.5
- 08-29-2021-1939 - 2019/20-1,2
- 101 Dalmatians - Family scene (HD)
- The Aristocats - Duchess meets Amelia and Abigail ...
- Sleeping Beauty | Once Upon A Dream | Lyric Video ...
- The Jungle Book | The Bare Necessities Song | Disn...
- Scooby Doo Theme Song
- It's a Binky!
- Winnie The Pooh Monster FrankenPooh
- winnie the pooh heffalumps and woozles song
- I Wanna Scare Myself--Boo to You, Winnie the Pooh
- Aladdin - Prince Ali
- Aladdin - Prince Ali [High Quality]
- Will Smith - Prince Ali (From "Aladdin")
- Aladdin - SNL
- Poor Unfortunate Souls Lyrics
- Britney Spears - Everytime (Official HD Video)


Popova died on 8 May 1896 (Gregorian calendar; 26 April in the Julian Calendar),[1][2] (the date is sometimes given as 1897 in English sources) as a result of an explosion which occurred while she was attempting to synthesize H-C≡P (methylidynephosphane), a chemical similar to hydrogen cyanide.[5] She was 28.
Aftermath[edit]
H-C≡P, the chemical that she was trying to synthesize at the time of her death, was not successfully created until 1961 from phosphine and carbon.[6] It is extremely pyrophoric and polymerizes easily at temperatures above −120 °C. Its triple point is −124 °C and it burns spontaneously even at low temperatures when exposed to air.[6]
Popova is credited with classifying dibenzyl ketone. This laid the foundation for synthetic acrylic resins created from acetone cyanohydrin.[1]
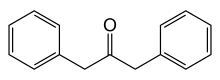

Acetone cyanohydrin is an intermediate en route to methyl methacrylate.[6] Treatment with sulfuric acid gives the sulfate ester of the methacrylamide,[clarification needed]methanolysis of which gives ammonium bisulfate and methyl methacrylate.[7]
It is used as a surrogate in place of HCN, as illustrated by its use as a precursor to lithium cyanide:[8]
- (CH3)2C(OH)CN + LiH → (CH3)2CO + LiCN + H2
In transhydrocyanation, an equivalent of HCN is transferred from acetone cyanohydrin to another acceptor, with acetone as byproduct. The transfer is an equilibrium process, initiated by base. The reaction can be driven by trapping reactions or by the use of a superior HCN acceptor, such as an aldehyde.[9] In the hydrocyanation reaction of butadiene, the transfer is irreversible.[10]
| Preferred IUPAC name 2-Hydroxy-2-methylpropanenitrile[2] | |
| Other names |
| Related compounds | |
|---|---|
Related alkanenitriles | |
https://en.wikipedia.org/wiki/Acetone_cyanohydrin
Category:Cyanohydrins
| Wikimedia Commons has media related to Cyanohydrins.Subcategories |
This category has only the following subcategory.
C
Pages in category "Cyanohydrins"
The following 6 pages are in this category, out of 6 total. This list may not reflect recent changes (learn more).
https://en.wikipedia.org/wiki/Category:Cyanohydrins
Glycolonitrile is produced by reacting formaldehyde with hydrogen cyanide under acidic conditions. This reaction is catalysed by base.[clarification needed].[5] Glycolonitrile polymerizes under alkaline conditions above pH 7.0. As the product of polymerization is an amine with a basic character, the reaction is self-catalysed, gaining in speed with ongoing conversion.
Glycolonitrile can react with ammonia to give aminoacetonitrile, which can be hydrolysed to give glycine:
- HOCH2CN + NH3 → H2NCH2CN + H2O
- H2NCH2CN + 2 H2O → H2NCH2CO2H + NH3
The industrially important chelating agent EDTA is prepared from glycolonitrile and ethylenediamine followed by hydrolysis of the resulting tetranitrile. Nitrilotriacetic acidis prepared similarly.[5]
![]()
- Hydrogen cyanide
- Thiocyanic acid
- Cyanogen iodide
- Cyanogen bromide
- Cyanogen chloride
- Cyanogen fluoride
- Acetonitrile
- Aminoacetonitrile
- Cyanogen
- Propanenitrile
- Aminopropionitrile
- Malononitrile
- Pivalonitrile
- Acetone cyanohydrin
https://en.wikipedia.org/wiki/Glycolonitrile
Hydrogen cyanide, sometimes called prussic acid, is a chemical compound with the chemical formula HCN. It is a colorless, extremely poisonous, and flammableliquid that boils slightly above room temperature, at 25.6 °C (78.1 °F). HCN is produced on an industrial scale and is a highly valued precursor to many chemical compounds ranging from polymers to pharmaceuticals.[8]
| Related compounds | |
|---|---|
Related alkanenitriles | |
https://en.wikipedia.org/wiki/Hydrogen_cyanide
Glycolonitrile, also called hydroxyacetonitrile or formaldehyde cyanohydrin, is the organic compound with the formula HOCH2CN. It is the simplest cyanohydrin and it is derived from formaldehyde.[3] It is a colourless liquid that dissolves in water and ether. Because glycolonitrile decomposes readily into formaldehyde and hydrogen cyanide, it is listed as an extremely hazardous substance. In January 2019, astronomers reported the detection of glycolonitrile, another possible building block of life among other such molecules, in outer space.[4]
![]()
| Names | |
|---|---|
| Preferred IUPAC name Hydroxyacetonitrile | |
Other names
|
| Related compounds | |
|---|---|
Related alkanenitriles | |
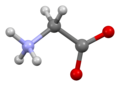
Glycine (symbol Gly or G;[6] /ˈɡlaɪsiːn/)[7] is an amino acid that has a single hydrogenatom as its side chain. It is the simplest stable amino acid (carbamic acid is unstable), with the chemical formula NH2‐CH2‐COOH. Glycine is one of the proteinogenic amino acids. It is encoded by all the codons starting with GG (GGU, GGC, GGA, GGG). Glycine is integral to the formation of alpha-helices in secondary protein structure due to its compact form. For the same reason, it is the most abundant amino acid in collagen triple-helices. Glycine is also an inhibitory neurotransmitter – interference with its release within the spinal cord (such as during a Clostridium tetani infection) can cause spastic paralysis due to uninhibited muscle contraction.
Glycine is a colorless, sweet-tasting crystalline solid. It is the only achiralproteinogenic amino acid. It can fit into hydrophilic or hydrophobic environments, due to its minimal side chain of only one hydrogen atom. The acyl radical is glycyl.
| |||
| |||
https://en.wikipedia.org/wiki/Glycine
Adiponitrile is the organic compound with the formula (CH2)4(CN)2. This dinitrile, a viscous, colourless liquid, is an important precursor to the polymer nylon 66. In 2005, about one million tonnes were produced.[4]
| Names | |
|---|---|
| Preferred IUPAC name Hexanedinitrile[1] | |
Other names
|
| Related compounds | |
|---|---|
Related alkanenitriles | Glutaronitrile |
Related compounds | hexanedioic acid hexanedihydrazide hexanedioyl dichloride hexanediamide |
Production[edit]
In 2018 there was about 1.5 million tons of capacity. The main producers of adiponitrile are: [8] [9]
- Ascend: Decatur,AL 400kt, being expanded to 580kt by 2022
- Invista: Victoria,TX and Orange,TX.
- Invista and BASFjv 'Butachimie': Chalampé (France). Production to be increased from 100kt in 2020 to 600kt.
- Asahi Kasei
BASF closed the 128kt ADN plant at Seal Sands in 2009 [10]
In 2015, Shandong Runxing New Material 100kt plant suffered an explosion and was not reopened.[8] In 2022, Invista plans to open a 300-400kt plant in Shanghai[11]
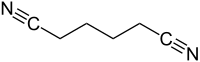
Early routes[edit]
Because of the industrial value of adiponitrile, many methods have been developed for its synthesis. Early industrial routes started from furfural and later by the chlorination of butadiene to give 1,4-dichloro-2-butene, which with sodium cyanide, converts to 3-hexenedinitrile, which in turn can be hydrogenated to adiponitrile:[4]
- ClCH2CH=CHCH2Cl + 2 NaCN → NCCH2CH=CHCH2CN + 2 NaCl
- NCCH2CH=CHCH2CN + H2 → NC(CH2)4CN
Adiponitrile has also been produced from adipic acid, by dehydration of the diamide, but this route is rarely employed.
Modern routes[edit]
The majority of adiponitrile is prepared by the nickel-catalysed hydrocyanation of butadiene, as discovered at DuPont, pioneered by Drinkard. The net reaction is:
- CH2=CHCH=CH2 + 2 HCN → NC(CH2)4CN

The process involves several stages, the first of which involves monohydrocyanation (addition of one molecule of HCN), affording isomers of pentenenitriles as well as 2- and 3-methylbutenenitriles. These unsaturated nitriles are subsequently isomerized to the 3-and 4-pentenenitriles. In the final stage, these pentenenitriles are subjected to a second hydrocyanation, in an anti-Markovnikov sense, to produce adiponitrile.[4]
3-pentenenitrile, formed in the first hydrocyanation, can undergo alkene metathesis to give dicyanobutenes, which are readily hydrogenated as described above. A useful byproduct of the production of adiponitrile is 2-methylglutaronitrile.
The other major industrial route involves hydrodimerization, starting from acrylonitrile:[5][6]
- 2 CH2=CHCN + 2 e− + 2 H+ → NCCH2CH2CH2CH2CN
The electrolytic coupling of acrylonitrile was discovered at Monsanto Company.
https://en.wikipedia.org/wiki/Adiponitrile


No comments:
Post a Comment