Phosphorous acid is the compound described by the formula H3PO3. This acidis diprotic (readily ionizes two protons), not triprotic as might be suggested by this formula. Phosphorous acid is an intermediate in the preparation of other phosphorus compounds. Organic derivatives of phosphorous acid, compounds with the formula RPO3H2, are called phosphonic acids.

| IUPAC name phosphonic acid | |
| Other names Dihydroxyphosphine oxide Dihydroxy(oxo)-λ5-phosphane |
https://en.wikipedia.org/wiki/Phosphorous_acid
Phosphorus oxoacid is a generic name for any acid whose molecule consists of atoms of phosphorus, oxygen, and hydrogen.[1]There is a potentially infinite number of such compounds. Some of them are unstable and have not been isolated, but the derived anions and organic groups are present in stable salts and esters. The most important ones — in biology, geology, industry, and chemical research — are the phosphoric acids, whose esters and salts are the phosphates.
In general, any hydrogen atom bonded to an oxygen atom is acidic, meaning that the –OH group can lose a proton H+
leaving a negatively charged –O−
group and thus turning the acid into a phosphorus oxoanion. Each additional proton lost has an associated acid dissociation constant Ka1, Ka2 Ka3, ..., often expressed by its cologarithm (pKa1, pKa2, pKa3, ...). Hydrogen atoms bonded directly to phosphorus are generally not acidic.
https://en.wikipedia.org/wiki/Phosphorus_acid
Phosphoric acid, also known as orthophosphoric acid or phosphoric(V) acid, is a weak acid with the chemical formula H
3PO
4. The pure compound is a colorless solid.
All three hydrogens are acidic to varying degrees and can be lost from the moleculeas H+ ions (protons). When all three H+ ions are removed, the result is an orthophosphate ion PO43−, commonly called "phosphate". Removal of one or two protons gives dihydrogen phosphate ion H
2PO−
4, and the hydrogen phosphate ion HPO2−
4, respectively. Orthophosphoric acid also forms esters, called organophosphates.[15]
Phosphoric acid is commonly encountered in chemical laboratories as an 85% aqueous solution, which is a colourless, odourless, and non-volatile syrupy liquid. Although phosphoric acid does not meet the strict definition of a strong acid, the 85% solution can still severely irritate the skin and damage the eyes.
The name "orthophosphoric acid" can be used to distinguish this specific acid from other "phosphoric acids", such as pyrophosphoric acid. Nevertheless, the term "phosphoric acid" often means this specific compound; and that is the current IUPAC nomenclature.
https://en.wikipedia.org/wiki/Phosphoric_acid
Phosphonates and phosphonic acids are organophosphorus compounds containing C−PO(OH)2 or C−PO(OR)2 groups (where R = alkyl, aryl). Phosphonic acids, typically handled as salts, are generally nonvolatile solids that are poorly soluble in organic solvents, but soluble in water and common alcohols. Many commercially important compounds are phosphonates, including glyphosate (the active molecule of the herbicide "Roundup"), and ethephon, a widely used plant growth regulator. Bisphosphonates are popular drugs for treatment of osteoporosis.[1]
In biology and medicinal chemistry, phosphonate groups are used as stable bioisoteres for phosphate, such as in the antiviral nucleotide analog, Tenofovir, one of the cornerstones of anti-HIV therapy. And there is an indication that phosphonate derivatives are "promising ligands for nuclear medicine."[2]
Not to be confused with phosphate.
https://en.wikipedia.org/wiki/Phosphonate
A phosphene is the phenomenon of seeing light without light entering the eye. The word phosphene comes from the Greek words phos (light) and phainein (to show). Phosphenes that are induced by movement or sound may be associated with optic neuritis.[1][2]
Phosphenes can be induced by mechanical, electrical, or magnetic stimulation of the retina or visual cortex, or by random firing of cells in the visual system. Phosphenes have also been reported by meditators[3] (called nimitta), people who endure long periods without visual stimulation (the prisoner's cinema), or those who ingest psychedelic drugs.[4]
https://en.wikipedia.org/wiki/Phosphene
 | |||
| Names | |||
|---|---|---|---|
| IUPAC name Phosphane | |||
| Other names Phosphamine Phosphorus trihydride Phosphorated hydrogen | |||
| Identifiers | |||
| |||
3D model (JSmol) | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.029.328 | ||
| EC Number |
| ||
| 287 | |||
PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| UN number | 2199 | ||
CompTox Dashboard (EPA) | |||
| Properties | |||
| PH3 | |||
| Molar mass | 33.99758 g/mol | ||
| Appearance | Colourless gas | ||
| Odor | fish-like or garlic-like[1] | ||
| Density | 1.379 g/l, gas (25 °C) | ||
| Melting point | −132.8 °C (−207.0 °F; 140.3 K) | ||
| Boiling point | −87.7 °C (−125.9 °F; 185.5 K) | ||
| 31.2 mg/100 ml (17 °C) | |||
| Solubility | Soluble in alcohol, ether, CS2 slightly soluble in benzene, chloroform, ethanol | ||
| Vapor pressure | 41.3 atm (20 °C)[1] | ||
| Conjugate acid | Phosphonium (chemical formula PH+ 4) | ||
Refractive index(nD) | 2.144 | ||
| Viscosity | 1.1×10−5 Pa⋅s | ||
| Structure | |||
| Trigonal pyramidal | |||
| 0.58 D | |||
| Thermochemistry | |||
Heat capacity (C) | 37 J/mol⋅K | ||
Std molar entropy (S | 210 J/mol⋅K[2] | ||
Std enthalpy of formation(ΔfH⦵298) | 5 kJ/mol[2] | ||
Gibbs free energy(ΔfG˚) | 13 kJ/mol | ||
| Hazards | |||
| Safety data sheet | ICSC 0694 | ||
| GHS pictograms |     | ||
| NFPA 704(fire diamond) | |||
| Flash point | Flammable gas | ||
| 38 °C (100 °F; 311 K) (see text) | |||
| Explosive limits | 1.79–98%[1] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) | 3.03 mg/kg (rat, oral) | ||
LC50 (median concentration) | 11 ppm (rat, 4 hr)[3] | ||
LCLo (lowest published) | 1000 ppm (mammal, 5 min) 270 ppm (mouse, 2 hr) 100 ppm (guinea pig, 4 hr) 50 ppm (cat, 2 hr) 2500 ppm (rabbit, 20 min) 1000 ppm (human, 5 min)[3] | ||
| NIOSH (US health exposure limits): | |||
PEL(Permissible) | TWA 0.3 ppm (0.4 mg/m3)[1] | ||
REL(Recommended) | TWA 0.3 ppm (0.4 mg/m3), ST 1 ppm (1 mg/m3)[1] | ||
IDLH (Immediate danger) | 50 ppm[1] | ||
| Related compounds | |||
Other cations | |||
Related compounds | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Phosphine (IUPAC name: phosphane) is a colourless, flammable, very toxic gas compound with the chemical formula PH3, classed as a pnictogen hydride. Pure phosphine is odourless, but technical grade samples have a highly unpleasant odourlike rotting fish, due to the presence of substituted phosphine and diphosphane(P2H4). With traces of P2H4 present, PH3 is spontaneously flammable in air (pyrophoric), burning with a luminous flame. Phosphine is a highly toxic respiratory poison, and is immediately dangerous to life or health at 50 ppm. Phosphine has a trigonal pyramidal structure.
Phosphine is also the general name given to the class of organophosphorus compounds of substituted phosphanes—a class of phosphanes in which the hydrogen atoms have been replaced with organic derivative, having a general formula PR3. Organophosphines are important in catalysts where they complex (adhere) to various metal ions; complexes derived from a chiral phosphine can catalyse reactions to give chiral, enantioenriched products.
https://en.wikipedia.org/wiki/Phosphine
 | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name Carbonyl dichloride[2] | |||
| Other names Carbonyl chloride CG Carbon dichloride oxide Carbon oxychloride Chloroformyl chloride Dichloroformaldehyde Dichloromethanone Dichloromethanal | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.792 | ||
| EC Number |
| ||
PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| UN number | 1076 | ||
CompTox Dashboard (EPA) | |||
| Properties | |||
| COCl2, also CCl2O | |||
| Molar mass | 98.92 g/mol | ||
| Appearance | Colorless gas | ||
| Odor | Suffocating, like musty hay[3] | ||
| Density | 4.248 g/L (15 °C, gas) 1.432 g/cm3 (0 °C, liquid) | ||
| Melting point | −118 °C (−180 °F; 155 K) | ||
| Boiling point | 8.3 °C (46.9 °F; 281.4 K) | ||
| Insoluble, reacts[4] | |||
| Solubility | Soluble in benzene, toluene, acetic acid Decomposes in alcohol and acid | ||
| Vapor pressure | 1.6 atm (20°C)[3] | ||
| −48·10−6 cm3/mol | |||
| Structure | |||
| Planar, trigonal | |||
| 1.17 D | |||
| Hazards | |||
| Safety data sheet | [1] | ||
| GHS pictograms |    [5] [5] | ||
| GHS Signal word | Danger | ||
| H280, H330, H314[5] | |||
| P260, P280, P303+361+353+315, P304+340+315, P305+351+338+315, P403, P405[5] | |||
| NFPA 704(fire diamond) | |||
| Flash point | Non-flammable | ||
Threshold limit value (TLV) | 0.1 ppm | ||
| Lethal dose or concentration (LD, LC): | |||
LC50 (median concentration) | 500 ppm (human, 1 min) 340 ppm (rat, 30 min) 438 ppm (mouse, 30 min) 243 ppm (rabbit, 30 min) 316 ppm (guinea pig, 30 min) 1022 ppm (dog, 20 min) 145 ppm (monkey, 1 min)[6] | ||
LCLo (lowest published) | 3 ppm (human, 2.83 h) 30 ppm (human, 17 min) 50 ppm (mammal, 5 min) 88 ppm (human, 30 min) 46 ppm (cat, 15 min) 50 ppm (human, 5 min) 2.7 ppm (mammal, 30 min)[6] | ||
| NIOSH (US health exposure limits): | |||
PEL(Permissible) | TWA 0.1 ppm (0.4 mg/m3)[3] | ||
REL(Recommended) | TWA 0.1 ppm (0.4 mg/m3) C 0.2 ppm (0.8 mg/m3) [15-minute][3] | ||
IDLH (Immediate danger) | 2 ppm[3] | ||
| Related compounds | |||
Related compounds | Thiophosgene Formaldehyde Carbonic acid Urea Carbon monoxide Chloroformic acid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Phosgene is the organic chemical compound with the formula COCl2. It is a colorless gas; in low concentrations, its odor resembles that of freshly cut hay or grass.[7] Phosgene is a valued industrial building block, especially for the production of precursors of polyurethanes and polycarbonate plastics.
Phosgene is very poisonous and was used as a chemical weapon during World War I, where it was responsible for 85,000 deaths.
In addition to its industrial production, small amounts occur from the breakdown and the combustion of organochlorine compounds.[8]
https://en.wikipedia.org/wiki/Phosgene
Magnetophosphenes are flashes of light (phosphenes) that are seen when one is subjected to a changing magnetic field such as when in an MRI. This changing field causes current within the retina or visual cortex resulting in the illusion of light.[1] In one series, 8 out of 1023 people having an MRI experienced flashing lights.[2]
Magnetophosphenes have been proposed as an explanation for ball lightning.[3]
https://en.wikipedia.org/wiki/Magnetophosphene
Ivabradine, sold under the brand name Procoralan among others, is a medicationused for the symptomatic management of stable heart-related chest pain and heart failure not fully managed by beta blockers.[1]
Ivabradine acts by allowing negative chronotropy in the sinoatrial structure thus reducing the heart rate via specific inhibition of the pacemaker current, a mechanism different from that of beta blockers and calcium channel blockers, two commonly prescribed antianginal classes of cardiac pharmaceuticals. Ivabradine has no apparent inotropic properties and may be a cardiotonic agent.
https://en.wikipedia.org/wiki/Ivabradine
Tuesday, September 7, 2021
09-07-2021-1403 - Pseudohalogens analoge analogue psuedo
Pseudohalogens are polyatomic analogues of halogens, whose chemistry, resembling that of the true halogens, allows them to substitute for halogens in several classes of chemical compounds.[1] Pseudohalogens occur in pseudohalogen molecules, inorganic molecules of the general forms Ps–Ps or Ps–X (where Ps is a pseudohalogen group), such as cyanogen; pseudohalide anions, such as cyanide ion; inorganic acids, such as hydrogen cyanide; as ligands in coordination complexes, such as ferricyanide; and as functional groups in organic molecules, such as the nitrile group. Well-known pseudohalogen functional groups include cyanide, cyanate, thiocyanate, and azide.
Common pseudohalogens and their nomenclature[edit]
Many pseudohalogens are known by specialized common names according to where they occur in a compound. Well-known ones include (the true halogen chlorine is listed for comparison):
| Group | Dimer | Hydrogen compound | Pseudohalide | Ligand name | In organic compounds | Formula | Structural formula |
|---|---|---|---|---|---|---|---|
| chloro | chlorine | hydrochloride | chloride | chlorido- chloro- | -yl chloride | ~ Cl | −Cl |
| cyano | cyanogen | hydrogen cyanide, prussic acid, formonitrile | cyanide | cyanido- cyano- | -nitrile -yl cyanide | ~ CN | −C≡N |
| cyapho | cyaphogen | phosphaethyne | cyaphide | cyaphido- cyapho- | -yl cyaphide | ~ CP | −C≡P |
| isocyano | hydrogen isocyanide, isohydrocyanic acid | isocyanide | isocyanido- isocyano- | -isonitrile -yl isocyanide | ~ NC | −N+ ≡C− | |
| hydroxyl | hydrogen peroxide | water | hydroxide | hydroxido- hydroxy- | -ol | ~ OH | −O−H |
| sulfanyl | hydrogen disulfide | hydrogen sulfide | hydrosulfide | sulfanido- thiolato- | -thiol -yl mercaptane | ~ SH | −S−H |
| cyanate | cyanic acid | cyanate | cyanato- | -yl cyanate | ~ OCN | −O−C≡N | |
| isocyanate | diisocyanogen | isocyanic acid | isocyanate | isocyanato- | -yl isocyanate | ~ NCO | −N=C=O |
| fulminate | fulminic acid | fulminate | fulminato- | -nitrile oxide -yl fulminate | ~ CNO | −C≡N+ –O− | |
| thiocyanate, rhodanide | thiocyanogen | thiocyanic acid | thiocyanate | thiocyanato- | -yl thiocyanate | ~ SCN | −S−C≡N |
| isothiocyanate | isothiocyanic acid | isothiocyanate | isothiocyanato- | -yl isothiocyanate | ~ NCS | −N=C=S | |
| selenocyanate, selenorhodanide | selenocyanogen | selenocyanic acid | selenocyanate | ~ SeCN | −Se−C≡N | ||
| tellurocyanate,[2] tellurorhodanide | tellurocyanogen | tellurocyanic acid | tellurocyanate | ~ TeCN | −Te−C≡N | ||
| azide | hexazine | hydrazoic acid | azide | azido- | -yl azide | ~ N3 | −N− −N+ ≡N ↕ −N=N+ =N− |
| nitrogen monoxide | dinitrogen dioxide | nitroxyl | nitrosyl | nitroso- | ~ NO | •N=O | |
| nitrogen dioxide | nitrogen tetroxide | nitryl | nitro- | ~ NO2 | −NO2 | ||
| cobalt carbonyl | dicobalt octacarbonyl | cobalt tetracarbonyl hydride | tetracarbonylcobaltate | ~ Co(CO)4 | −Co(C≡O)4 | ||
| trinitromethanide | hexanitroethane | nitroform, trinitromethane | trinitromethanide | trinitromethanido- | -yl trinitromethanide | ~ C(NO2)3 | −C(NO2)3 |
| tricyanomethanide | hexacyanoethane | cyanoform, tricyanomethane | tricyanomethanide | tricyanomethanido- | -yl tricyanomethanide | ~ C(CN)3 | −C(CN)3 |
https://en.wikipedia.org/wiki/Pseudohalogen
Phosphonates and phosphonic acids are organophosphorus compounds containing C−PO(OH)2 or C−PO(OR)2 groups (where R = alkyl, aryl). Phosphonic acids, typically handled as salts, are generally nonvolatile solids that are poorly soluble in organic solvents, but soluble in water and common alcohols. Many commercially important compounds are phosphonates, including glyphosate (the active molecule of the herbicide "Roundup"), and ethephon, a widely used plant growth regulator. Bisphosphonates are popular drugs for treatment of osteoporosis.[1]
In biology and medicinal chemistry, phosphonate groups are used as stable bioisoteres for phosphate, such as in the antiviral nucleotide analog, Tenofovir, one of the cornerstones of anti-HIV therapy. And there is an indication that phosphonate derivatives are "promising ligands for nuclear medicine."[2]
Not to be confused with phosphate.
https://en.wikipedia.org/wiki/Phosphonate
https://en.wikipedia.org/wiki/Functional_analog
https://en.wikipedia.org/wiki/Derivative_(chemistry)
https://en.wikipedia.org/wiki/Structural_analog
Thursday, September 2, 2021
BSE is an infectious disease believed to be due to a misfolded protein, known as a prion.[3][6]Cattle are believed to have been infected from being fed meat and bone meal (MBM) that contained the remains of other cattle who spontaneously developed the disease or scrapie-infected sheep products.[3] The outbreak increased throughout the United Kingdom due to the practice of feeding meat-and-bone meal to young calves of dairy cows.[3][8] In the brain, the agent causes native cellular prion protein to deform into the misfolded state, which then goes on to deform further prion protein in an exponential cascade. This results in protein aggregates, which then form dense plaque fibers.
https://en.wikipedia.org/wiki/Bovine_spongiform_encephalopathy
Tuesday, September 7, 2021
09-07-2021-1333 - cyanogen (CN)2 1815 prussia
Cyanogen is the chemical compound with the formula (CN)2. It is a colorless, toxic gas with a pungent odor. The molecule is a pseudohalogen. Cyanogen molecules consist of two CN groups – analogous to diatomic halogen molecules, such as Cl2, but far less oxidizing. The two cyano groups are bonded together at their carbon atoms: N≡C−C≡N, although other isomers have been detected.[6] The name is also used for the CN radical,[7] and hence is used for compounds such as cyanogen bromide (NCBr).[8]
Cyanogen is the anhydride of oxamide:
- H2NC(O)C(O)NH2 → NCCN + 2 H2O
although oxamide is manufactured from cyanogen by hydrolysis:[9]
- NCCN + 2 H2O → H2NC(O)C(O)NH2
NamesPreferred IUPAC name Systematic IUPAC nameOxalonitrile[4]Other namesEthanedinitrile[4]CyanogenIdentifiers
Systematic IUPAC nameOxalonitrile[4]Other namesEthanedinitrile[4]CyanogenIdentifiers
Bis(nitridocarbon)(C—C)[1]
Dicyan[2][3]
Carbon nitride[2]
Oxalic acid dinitrile[3]
Dicyanogen
Nitriloacetonitrile- 460-19-5
 3D model (JSmol)1732464ChEBI
3D model (JSmol)1732464ChEBI- CHEBI:29308
 ChemSpider
ChemSpider- 9605
 ECHA InfoCard100.006.643
ECHA InfoCard100.006.643  EC Number1090MeSHcyanogen
EC Number1090MeSHcyanogen- 207-306-5
PubChem CIDRTECS numberUNII- GT1925000
- 534Q0F66RK
 UN number1026CompTox Dashboard (EPA)Properties(CN)2Molar mass52.034 g/mol AppearanceColourless gasOdorpungent, almond-likeDensity950 mg mL−1 (at −21 °C)Melting point−28 °C (−18 °F; 245 K) Boiling point−21.1 °C; −6.1 °F; 252.0 K 45 g/100 mL (at 20 °C)Solubilitysoluble in ethanol, ethyl etherVapor pressure5.1 atm (21 °C)[5]Henry's law1.9 μmol Pa−1 kg−1-21.6·10−6 cm3/mol
UN number1026CompTox Dashboard (EPA)Properties(CN)2Molar mass52.034 g/mol AppearanceColourless gasOdorpungent, almond-likeDensity950 mg mL−1 (at −21 °C)Melting point−28 °C (−18 °F; 245 K) Boiling point−21.1 °C; −6.1 °F; 252.0 K 45 g/100 mL (at 20 °C)Solubilitysoluble in ethanol, ethyl etherVapor pressure5.1 atm (21 °C)[5]Henry's law1.9 μmol Pa−1 kg−1-21.6·10−6 cm3/mol
constant (kH)Refractive index(nD)1.327 (18 °C)ThermochemistryStd molar241.57 J K−1 mol−1
entropy (So298)Std enthalpy of309.07 kJ mol−1
formation(ΔfH⦵298)Std enthalpy of−1.0978–−1.0942 MJ mol−1HazardsMain hazardsforms cyanide in the body; flammable[5]Safety data sheetinchem.orgGHS pictograms
combustion(ΔcH⦵298)

 GHS Signal wordDangerH220, H331, H400, H410P210, P261, P271, P273, P304+340, P311, P321, P377, P381, P391, P403, P403+233, P405, P501NFPA 704(fire diamond)Explosive limits6.6–32%[5]NIOSH (US health exposure limits):PEL(Permissible)none[5]REL(Recommended)TWA 10 ppm (20 mg/m3)[5]IDLH (Immediate danger)N.D.[5]Related compoundsRelated alkanenitrilesRelated compoundsDBNPA
GHS Signal wordDangerH220, H331, H400, H410P210, P261, P271, P273, P304+340, P311, P321, P377, P381, P391, P403, P403+233, P405, P501NFPA 704(fire diamond)Explosive limits6.6–32%[5]NIOSH (US health exposure limits):PEL(Permissible)none[5]REL(Recommended)TWA 10 ppm (20 mg/m3)[5]IDLH (Immediate danger)N.D.[5]Related compoundsRelated alkanenitrilesRelated compoundsDBNPA
- 9605
- CHEBI:29308
- 460-19-5
- https://en.wikipedia.org/wiki/Cyanogen
- Acetic acid, acetone, oxalic acid.
Sialic acids are a class of alpha-keto acid sugars with a nine-carbon backbone.[1] The term "sialic acid" (from the Greek for saliva, σίαλον - síalon) was first introduced by Swedish biochemist Gunnar Blix in 1952. The most common member of this group is N-acetylneuraminic acid (Neu5Ac or NANA) found in animals and some prokaryotes.
Sialic acids are found widely distributed in animal tissues and related forms are found to a lesser extent in other organisms like in some micro-algae,[2] bacteria and archaea.[3][4][5][6] Sialic acids are commonly part of glycoproteins, glycolipids or gangliosides, where they decorate the end of sugar chains at the surface of cells or soluble proteins.[7] However, sialic acids have been also observed in Drosophila embryos and other insects.[8] Generally, plants seem not contain or display sialic acids.[9]
In humans the brain has the highest sialic acid content, where these acids play an important role in neural transmission and ganglioside structure in synaptogenesis.[7] More than 50 kinds of sialic acid are known, all of which can be obtained from a molecule of neuraminic acid by substituting its amino group of one of its hydroxyl groups.[1] In general, the amino group bears either an acetyl or a glycolyl group, but other modifications have been described. These modifications along with linkages have shown to be tissue specific and developmentally regulated expressions, so some of them are only found on certain types of glycoconjugates in specific cells.[8] The hydroxyl substituents may vary considerably; acetyl, lactyl, methyl, sulfate, and phosphate groups have been found.[10]
https://en.wikipedia.org/wiki/Sialic_acid
Elemental phosphorus can exist in several allotropes, the most common of which are white and red solids. Solid violet and black allotropes are also known. Gaseous phosphorus exists as diphosphorus and atomic phosphorus.
https://en.wikipedia.org/wiki/Allotropes_of_phosphorus
Phenylphosphine is an organophosphorus compound with the chemical formula C6H5PH2. It is the phosphorus analog of aniline. Like other primary phosphines, phenylphosphine has an intense penetrating odor and is highly oxidizable. It is mainly used as a precursor to other organophosphorus compounds. It can function as a ligand in coordination chemistry.[2]
https://en.wikipedia.org/wiki/Phenylphosphine
Phosphole is the organic compound with the chemical formula C
4H
4PH; it is the phosphorus analog of pyrrole. The term phosphole also refers to substituted derivatives of the parent heterocycle. These compounds are of theoretical interest but also serve as ligands for transition metals and as precursors to more complex organophosphorus compounds.Triphosphole, C
2H
3P
3, is a heterocycle with 3 phosphorus atoms.Pentaphosphole, P
5H, is a cyclic compound with 5 phosphorus atoms.oxyanion hole
https://en.wikipedia.org/wiki/Phosphole
Phosphinidenes (IUPAC: phosphanylidenes, formerly phosphinediyls) are low-valent phosphorus compounds analogous to carbenes and nitrenes, having the general structure RP.[1][2] The "free" form of these compounds is conventionally described as having a singly-coordinated phosphorus atom containing only 6 electrons in its valence level.[2] Most phosphinidenes are highly reactive and short-lived, thereby complicating empirical studies on their chemical properties.[3][4] In the last few decades, several strategies have been employed to stabilize phosphinidenes (e.g. π-donation, steric protection, transition metal complexation),[2][3]and researchers have developed a number of reagents and systems that can generate and transfer phosphinidenes as reactive intermediates in the synthesis of various organophosphorus compounds.[5][6][7][8]
https://en.wikipedia.org/wiki/Phosphinidene
Thiotepa (INN[3]), sold under the brand name Tepadina, is a medication used to treat cancer.[1][2][4]
Thiotepa is an organophosphorus compound with the formula SP(NC2H4)3.[5] It is an analog of N,N′,N′′-triethylenephosphoramide (TEPA), which contains tetrahedral phosphorus and is structurally akin to phosphate. It is manufactured by heating aziridine with thiophosphoryl chloride.[citation needed]
https://en.wikipedia.org/wiki/Thiotepa
Phosphorine (IUPAC name: phosphinine) is a heavier element analog of pyridine, containing a phosphorus atom instead of an aza- moiety. It is also called phosphabenzene and belongs to the phosphaalkene class. It is a colorless liquid that is mainly of interest in research.
Phosphorine is an air-sensitive oil[2] but is otherwise stable when handled using air-free techniques (however, substituted derivatives can often be handled under air without risk of decomposition).[3][4] In contrast, silabenzene, a related heavy-element analogue of benzene, is not only air- and moisture-sensitive but also thermally unstable without extensive steric protection.
https://en.wikipedia.org/wiki/Phosphorine
Phosphirenium ions (R
1R
2C
2PY
1Y+
2) are a series of organophosphorus compounds containing unsaturated three-membered ring phosphorus (V) heterocycles and σ*-aromaticity is believed to be present in such molecules. Many of the salts containing phosphirenium ions have been isolated and characterized by NMR spectroscopy and X-ray crystallography.https://en.wikipedia.org/wiki/Phosphirenium_ion
Phenylpiracetam (INN: fonturacetam,[1] brand names Phenotropil Фенотропил, Carphedon), is a phenylated analog of the drug piracetam. It was developed in 1983 as a medication for Soviet Cosmonauts to treat the prolonged stresses of working in space. Phenylpiracetam was created at the Russian Academy of Sciences Institute of Biomedical Problems in an effort led by psychopharmacologist Valentina Ivanovna Akhapkina (Валентина Ивановна Ахапкина).[2][unreliable source?] In Russia it is now available as a prescription drug. Research on animals has indicated that phenylpiracetam may have anti-amnesic, antidepressant, anticonvulsant, anxiolytic, and memory enhancement effects.[3][4]
https://en.wikipedia.org/wiki/Phenylpiracetam
Hypothetical types of biochemistry are forms of biochemistry agreed to be scientifically viable but not proven to exist at this time.[2] The kinds of living organisms currently known on Earth all use carbon compounds for basic structural and metabolic functions, water as a solvent, and DNAor RNA to define and control their form. If life exists on other planets or moons it may be chemically similar, though it is also possible that there are organisms with quite different chemistries[3] – for instance, involving other classes of carbon compounds, compounds of another element, or another solvent in place of water.
The possibility of life-forms being based on "alternative" biochemistries is the topic of an ongoing scientific discussion, informed by what is known about extraterrestrial environments and about the chemical behaviour of various elements and compounds. It is of interest in synthetic biologyand is also a common subject in science fiction.
The element silicon has been much discussed as a hypothetical alternative to carbon. Silicon is in the same group as carbon on the periodic table and, like carbon, it is tetravalent. Hypothetical alternatives to water include ammonia, which, like water, is a polar molecule, and cosmically abundant; and non-polar hydrocarbon solvents such as methane and ethane, which are known to exist in liquid form on the surface of Titan.
False-color Cassini radar mosaic of Titan's north polar region; the blue areas are lakes of liquid hydrocarbons.
"The existence of lakes of liquid hydrocarbons on Titan opens up the possibility for solvents and energy sources that are alternatives to those in our biosphere and that might support novel life forms altogether different from those on Earth."—NASA Astrobiology Roadmap 2008[1]https://en.wikipedia.org/wiki/Hypothetical_types_of_biochemistry
A raster scan, or raster scanning, is the rectangular pattern of image capture and reconstruction in television. By analogy, the term is used for raster graphics, the pattern of image storage and transmission used in most computer bitmap image systems. The word raster comes from the Latin word rastrum (a rake), which is derived from radere (to scrape); see also rastrum, an instrument for drawing musical staff lines. The pattern left by the lines of a rake, when drawn straight, resembles the parallel lines of a raster: this line-by-line scanning is what creates a raster. It is a systematic process of covering the area progressively, one line at a time. Although often a great deal faster, it is similar in the most general sense to how one's gaze travels when one reads lines of text. The data to be drawn is stored in an area of memory called the refresh buffer or frame buffer. This memory area holds the values for each pixel on the screen. These values are retrieved from the refresh buffer and painted onto the screen one row at a time.
https://en.wikipedia.org/wiki/Raster_scan
Patient R.B.[edit]
Patient R.B. was a normally functioning man until the age of 52. At age 50, he had been diagnosed with angina and had surgery for heart problems on two occasions. After an ischemic episode (reduction of blood to the brain) that was caused from a heart bypass surgery, R.B. demonstrated a loss of anterograde memory, but almost no loss of retrograde memory, with the exception of a couple of years before his surgery, and presented no sign of any other cognitive impairment. It wasn't until after his death that researchers had the chance to examine his brain, when they found his lesions were restricted to the CA1 portion of the hippocampus. This case study led to important research involving the role of the hippocampus and the function of memory.[60]
https://en.wikipedia.org/wiki/Amnesia
A silabenzene is a heteroaromatic compound containing one or more silicon atoms instead of carbon atoms in benzene. A single substitution gives silabenzene proper; additional substitutions give a disilabenzene (3 theoretical isomers), trisilabenzene(3 isomers), etc.
Silabenzenes have been the targets of many theoretical and synthetic studies by organic chemists interested in the question of whether analogs of benzene with Group IV elements heavier than carbon, e.g., silabenzene, stannabenzene and germabenzene—so-called "heavy benzenes"—exhibit aromaticity.
Although several heteroaromatic compounds bearing nitrogen, oxygen, and sulfuratoms have been known since the early stages of organic chemistry, silabenzene had been considered to be a transient, un-isolable compound and was detected only in low-temperature matrices or as its Diels-Alder adduct for a long time. In recent years, however, a kinetically stabilized silabenzene and other heavy aromaticcompounds with silicon or germanium atoms have been reported.
https://en.wikipedia.org/wiki/Silabenzene
Phosphorus(III)
All four symmetrical trihalides are well known: gaseous PF3, the yellowish liquids PCl3 and PBr3, and the solid PI3. These materials are moisture sensitive, hydrolysing to give phosphorous acid. The trichloride, a common reagent, is produced by chlorination of white phosphorus:
- P4 + 6 Cl2 → 4 PCl3
The trifluoride is produced from the trichloride by halide exchange. PF3 is toxic because it binds to haemoglobin.
https://en.wikipedia.org/wiki/Phosphorus
Phosphorus pentoxide is a chemical compound with molecular formula P4O10 (with its common name derived from its empirical formula, P2O5). This white crystalline solid is the anhydride of phosphoric acid. It is a powerful desiccant and dehydrating agent.
https://en.wikipedia.org/wiki/Phosphorus_pentoxide
Although phosphorus (15P) has 23 isotopes from 25P to 47P, only 31P is stable; as such, phosphorus is considered a monoisotopic element. The longest-lived radioactive isotopes are 33P with a half-life of 25.34 days and 32P with a half-life of 14.268 days. All others have half-lives of under 2.5 minutes, most under a second. The least stable is 25P with a half-life shorter than 30 nanoseconds.
https://en.wikipedia.org/wiki/Isotopes_of_phosphorus
A phosphor is a substance that exhibits the phenomenon of luminescence; it emits light when exposed to some type of radiant energy. The term is used both for fluorescent or phosphorescent substances which glow on exposure to ultraviolet or visible light, and cathodoluminescent substances which glow when struck by an electron beam (cathode rays) in a cathode ray tube.
When a phosphor is exposed to radiation, the orbital electrons in its molecules are excited to a higher energy level; when they return to their former level they emit the energy as light of a certain color. Phosphors can be classified into two categories: fluorescent substances which emit the energy immediately and stop glowing when the exciting radiation is turned off, and phosphorescent substances which emit the energy after a delay, so they keep glowing after the radiation is turned off, decaying in brightness over a period of milliseconds to days.
Fluorescent materials are used in applications in which the phosphor is excited continuously: cathode ray tubes (CRT) and plasma video display screens, fluoroscope screens, fluorescent lights, scintillation sensors, and white LEDs, and luminous paints for black light art. Phosphorescent materials are used where a persistent light is needed, such as glow-in-the-dark watch faces and aircraft instruments, and in radar screens to allow the target 'blips' to remain visible as the radar beam rotates. CRT phosphors were standardized beginning around World War II and designated by the letter "P" followed by a number.
Phosphorus, the light-emitting chemical element for which phosphors are named, emits light due to chemiluminescence, not phosphorescence.[1]
https://en.wikipedia.org/wiki/Phosphor
Although phosphorus (15P) has 23 isotopes from 25P to 47P, only 31P is stable; as such, phosphorus is considered a monoisotopic element. The longest-lived radioactive isotopes are 33P with a half-life of 25.34 days and 32P with a half-life of 14.268 days. All others have half-lives of under 2.5 minutes, most under a second. The least stable is 25P with a half-life shorter than 30 nanoseconds.
List of isotopes[edit]
| Nuclide[2] [n 1] | Z | N | Isotopic mass (Da)[3] [n 2][n 3] | Half-life [n 4] | Decay mode [n 5] | Daughter isotope [n 6] | Spin and parity [n 7][n 4] | Natural abundance (mole fraction) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Excitation energy | Normal proportion | Range of variation | |||||||||||||||||
| 25P | 15 | 10 | 25.02119(43)# | <30 ns | p | 24Si | (1/2+)# | ||||||||||||
| 26P[n 8] | 15 | 11 | 26.01178(21)# | 43.7(6) ms | β+ (63.2%) | 26Si | (3+) | ||||||||||||
| β+, p (36.8%) | 25Al | ||||||||||||||||||
| 26mP | 164.4(1) keV | 120(9) ns | IT | 26P | |||||||||||||||
| 27P | 15 | 12 | 26.999224(28) | 260(80) ms | β+ (99.93%) | 27Si | 1/2+ | ||||||||||||
| β+, p (.07%) | 26Al | ||||||||||||||||||
| 28P | 15 | 13 | 27.9923266(12) | 270.3(5) ms | β+ (99.99%) | 28Si | 3+ | ||||||||||||
| β+, p (.0013%) | 27Al | ||||||||||||||||||
| β+, α (8.6×10−4%) | 24Mg | ||||||||||||||||||
| 29P | 15 | 14 | 28.9818004(4) | 4.142(15) s | β+ | 29Si | 1/2+ | ||||||||||||
| 30P | 15 | 15 | 29.97831349(7) | 2.498(4) min | β+ | 30Si | 1+ | ||||||||||||
| 31P | 15 | 16 | 30.9737619986(7) | Stable | 1/2+ | 1.0000 | |||||||||||||
| 32P | 15 | 17 | 31.97390764(4) | 14.268(5) d | β− | 32S | 1+ | Trace | |||||||||||
| 33P | 15 | 18 | 32.9717257(12) | 25.35(11) d | β− | 33S | 1/2+ | ||||||||||||
| 34P | 15 | 19 | 33.9736459(9) | 12.43(10) s | β− | 34S | 1+ | ||||||||||||
| 35P | 15 | 20 | 34.9733141(20) | 47.3(8) s | β− | 35S | 1/2+ | ||||||||||||
| 36P | 15 | 21 | 35.978260(14) | 5.6(3) s | β− | 36S | 4− | ||||||||||||
| 37P | 15 | 22 | 36.97961(4) | 2.31(13) s | β− | 37S | (1/2+) | ||||||||||||
| 38P | 15 | 23 | 37.98430(8) | 0.64(14) s | β− (87.5%) | 38S | |||||||||||||
| β−, n (12.5%) | 37S | ||||||||||||||||||
| 39P | 15 | 24 | 38.98629(12) | 282(24) ms | β− (73.2%) | 39S | 1/2+# | ||||||||||||
| β−, n (26.8%) | 38S | ||||||||||||||||||
| 40P | 15 | 25 | 39.99129(16) | 150(8) ms | β− (84.2%) | 40S | (2−,3−) | ||||||||||||
| β−, n (15.8%) | 39S | ||||||||||||||||||
| 41P | 15 | 26 | 40.99465(13) | 101(5) ms | β− (70%) | 41S | 1/2+# | ||||||||||||
| β−, n (30%) | 40S | ||||||||||||||||||
| 42P | 15 | 27 | 42.00108(34) | 48.5(15) ms | β− (50%) | 42S | |||||||||||||
| β−, n (50%) | 41S | ||||||||||||||||||
| 43P | 15 | 28 | 43.00502(60) | 35.8(13) ms | β−, n | 42S | 1/2+# | ||||||||||||
| β− | 43S | ||||||||||||||||||
| 44P | 15 | 29 | 44.01122(54)# | 18.5(25) ms | β− | 44S | |||||||||||||
| 45P | 15 | 30 | 45.01675(54)# | 8# ms [>200 ns] | β− | 45S | 1/2+# | ||||||||||||
| 46P | 15 | 31 | 46.02466(75)# | 4# ms [>200 ns] | β− | 46S | |||||||||||||
| 47P[4] | 15 | 32 | 47.03190(86)# | 2# ms | β− | 47S | |||||||||||||
| This table header & footer: | |||||||||||||||||||
- ^ mP – Excited nuclear isomer.
- ^ ( ) – Uncertainty (1σ) is given in concise form in parentheses after the corresponding last digits.
- ^ # – Atomic mass marked #: value and uncertainty derived not from purely experimental data, but at least partly from trends from the Mass Surface (TMS).
- ^ a b # – Values marked # are not purely derived from experimental data, but at least partly from trends of neighboring nuclides (TNN).
- ^ Modes of decay:
IT: Isomeric transition n: Neutron emission p: Proton emission - ^ Bold symbol as daughter – Daughter product is stable.
- ^ ( ) spin value – Indicates spin with weak assignment arguments.
- ^ Has 1 halo proton
Phosphorus-32[edit]
32P, a beta-emitter (1.71 MeV) with a half-life of 14.3 days, is used routinely in life-science laboratories, primarily to produce radiolabeled DNA and RNA probes, e.g. for use in Northern blots or Southern blots. Because the high-energy beta particles produced penetrate skin and corneas, and because any 32P ingested, inhaled, or absorbed is readily incorporated into bone and nucleic acids, OSHA requires that a lab coat, disposable gloves, and safety glasses or goggles be worn when working with 32P, and that working directly over an open container be avoided in order to protect the eyes.[citation needed] Monitoring personal, clothing, and surface contamination is also required. In addition, due to the high energy of the beta particles, shielding this radiation with the normally used dense materials (e.g. lead), gives rise to secondary emission of X-rays via a process known as bremsstrahlung, meaning braking radiation. Therefore, shielding must be accomplished with low-density materials, e.g. Plexiglas, Lucite, plastic, wood, or water.
Phosphorus-33[edit]
33P, a beta-emitter (0.25 MeV) with a half-life of 25.4 days. It is used in life-science laboratories in applications in which lower energy beta emissions are advantageous such as DNA sequencing. 33P can be used to label nucleotides. It is less energetic than 32P, giving a better resolution. A disadvantage is its higher cost compared to 32P, as most of the bombarded 31P will have acquired only one neutron, while only some will have acquired two or more. Its maximum specific activity is 5118 Ci/mol.
https://en.wikipedia.org/wiki/Isotopes_of_phosphorus
Phosphorus(I) and phosphorus(II)
These compounds generally feature P–P bonds.[14] Examples include catenated derivatives of phosphine and organophosphines. Compounds containing P=P double bonds have also been observed, although they are rare.
Phosphides and phosphines
Phosphides arise by reaction of metals with red phosphorus. The alkali metals (group 1) and alkaline earth metals can form ionic compounds containing the phosphide ion, P3−. These compounds react with water to form phosphine. Other phosphides, for example Na3P7, are known for these reactive metals. With the transition metals as well as the monophosphides there are metal-rich phosphides, which are generally hard refractory compounds with a metallic lustre, and phosphorus-rich phosphides which are less stable and include semiconductors.[14] Schreibersite is a naturally occurring metal-rich phosphide found in meteorites. The structures of the metal-rich and phosphorus-rich phosphides can be complex.
Phosphine (PH3) and its organic derivatives (PR3) are structural analogues of ammonia (NH3), but the bond angles at phosphorus are closer to 90° for phosphine and its organic derivatives. It is an ill-smelling, toxic compound. Phosphorus has an oxidation number of −3 in phosphine. Phosphine is produced by hydrolysis of calcium phosphide, Ca3P2. Unlike ammonia, phosphine is oxidised by air. Phosphine is also far less basic than ammonia. Other phosphines are known which contain chains of up to nine phosphorus atoms and have the formula PnHn+2.[14] The highly flammable gas diphosphine (P2H4) is an analogue of hydrazine.
Oxoacids
Phosphorous oxoacids are extensive, often commercially important, and sometimes structurally complicated. They all have acidic protons bound to oxygen atoms, some have nonacidic protons that are bonded directly to phosphorus and some contain phosphorus - phosphorus bonds.[14] Although many oxoacids of phosphorus are formed, only nine are commercially important, and three of them, hypophosphorous acid, phosphorous acid, and phosphoric acid, are particularly important.
| Oxidation state | Formula | Name | Acidic protons | Compounds |
|---|---|---|---|---|
| +1 | HH2PO2 | hypophosphorous acid | 1 | acid, salts |
| +3 | H2HPO3 | phosphorous acid | 2 | acid, salts |
| +3 | HPO2 | metaphosphorous acid | 1 | salts |
| +3 | H3PO3 | (ortho)phosphorous acid | 3 | acid, salts |
| +4 | H4P2O6 | hypophosphoric acid | 4 | acid, salts |
| +5 | (HPO3)n | metaphosphoric acids | n | salts (n = 3,4,6) |
| +5 | H(HPO3)nOH | polyphosphoric acids | n+2 | acids, salts (n = 1-6) |
| +5 | H5P3O10 | tripolyphosphoric acid | 3 | salts |
| +5 | H4P2O7 | pyrophosphoric acid | 4 | acid, salts |
| +5 | H3PO4 | (ortho)phosphoric acid | 3 | acid, salts |
Nitrides
The PN molecule is considered unstable, but is a product of crystalline phosphorus nitride decomposition at 1100 K. Similarly, H2PN is considered unstable, and phosphorus nitride halogens like F2PN, Cl2PN, Br2PN, and I2PN oligomerise into cyclic Polyphosphazenes. For example, compounds of the formula (PNCl2)n exist mainly as rings such as the trimerhexachlorophosphazene. The phosphazenes arise by treatment of phosphorus pentachloride with ammonium chloride:
When the chloride groups are replaced by alkoxide (RO−), a family of polymers is produced with potentially useful properties.[43]
Sulfides
Phosphorus forms a wide range of sulfides, where the phosphorus can be in P(V), P(III) or other oxidation states. The three-fold symmetric P4S3 is used in strike-anywhere matches. P4S10 and P4O10 have analogous structures.[44] Mixed oxyhalides and oxyhydrides of phosphorus(III) are almost unknown.
Organophosphorus compounds
Compounds with P-C and P-O-C bonds are often classified as organophosphorus compounds. They are widely used commercially. The PCl3 serves as a source of P3+ in routes to organophosphorus(III) compounds. For example, it is the precursor to triphenylphosphine:
- PCl3 + 6 Na + 3 C6H5Cl → P(C6H5)3 + 6 NaCl
Treatment of phosphorus trihalides with alcohols and phenols gives phosphites, e.g. triphenylphosphite:
- PCl3 + 3 C6H5OH → P(OC6H5)3 + 3 HCl
Similar reactions occur for phosphorus oxychloride, affording triphenylphosphate:
- OPCl3 + 3 C6H5OH → OP(OC6H5)3 + 3 HCl
History
Etymology
The name Phosphorus in Ancient Greece was the name for the planet Venus and is derived from the Greek words (φῶς = light, φέρω = carry), which roughly translates as light-bringer or light carrier.[16] (In Greek mythology and tradition, Augerinus (Αυγερινός = morning star, still in use today), Hesperus or Hesperinus (΄Εσπερος or Εσπερινός or Αποσπερίτης = evening star, still in use today) and Eosphorus (Εωσφόρος = dawnbearer, not in use for the planet after Christianity) are close homologues, and also associated with Phosphorus-the-morning-star).
According to the Oxford English Dictionary, the correct spelling of the element is phosphorus. The word phosphorous is the adjectival form of the P3+ valence: so, just as sulfur forms sulfurous and sulfuric compounds, phosphorus forms phosphorous compounds (e.g., phosphorous acid) and P5+ valence phosphoric compounds (e.g., phosphoric acids and phosphates).
Discovery
The discovery of phosphorus, the first element to be discovered that was not known since ancient times,[45] is credited to the German alchemist Hennig Brand in 1669, although other chemists might have discovered phosphorus around the same time.[46] Brand experimented with urine, which contains considerable quantities of dissolved phosphates from normal metabolism.[16]Working in Hamburg, Brand attempted to create the fabled philosopher's stone through the distillation of some salts by evaporating urine, and in the process produced a white material that glowed in the dark and burned brilliantly. It was named phosphorus mirabilis ("miraculous bearer of light").[47]
Brand's process originally involved letting urine stand for days until it gave off a terrible smell. Then he boiled it down to a paste, heated this paste to a high temperature, and led the vapours through water, where he hoped they would condense to gold. Instead, he obtained a white, waxy substance that glowed in the dark. Brand had discovered phosphorus. We now know that Brand produced ammonium sodium hydrogen phosphate, (NH
4)NaHPO
4. While the quantities were essentially correct (it took about 1,100 litres [290 US gal] of urine to make about 60 g of phosphorus), it was unnecessary to allow the urine to rot first. Later scientists discovered that fresh urine yielded the same amount of phosphorus.[29]
Brand at first tried to keep the method secret,[48] but later sold the recipe for 200 thalers to D. Krafft from Dresden,[16] who could now make it as well, and toured much of Europe with it, including England, where he met with Robert Boyle. The secret that it was made from urine leaked out and first Johann Kunckel (1630–1703) in Sweden (1678) and later Boyle in London (1680) also managed to make phosphorus, possibly with the aid of his assistant, Ambrose Godfrey-Hanckwitz, who later made a business of the manufacture of phosphorus.
Boyle states that Krafft gave him no information as to the preparation of phosphorus other than that it was derived from "somewhat that belonged to the body of man". This gave Boyle a valuable clue, so that he, too, managed to make phosphorus, and published the method of its manufacture.[16] Later he improved Brand's process by using sand in the reaction (still using urine as base material),
- 4 NaPO
3 + 2 SiO
2 + 10 C → 2 Na
2SiO
3 + 10 CO + P
4
Robert Boyle was the first to use phosphorus to ignite sulfur-tipped wooden splints, forerunners of our modern matches, in 1680.[49]
Phosphorus was the 13th element to be discovered. Because of its tendency to spontaneously combust when left alone in air, it is sometimes referred to as "the Devil's element".[50]
Bone ash and guano
In 1769, Johan Gottlieb Gahn and Carl Wilhelm Scheele showed that calcium phosphate (Ca
3(PO
4)
2) is found in bones, and they obtained elemental phosphorus from bone ash. Antoine Lavoisierrecognised phosphorus as an element in 1777.[51] Bone ash was the major source of phosphorus until the 1840s. The method started by roasting bones, then employed the use of clay retorts encased in a very hot brick furnace to distill out the highly toxic elemental phosphorus product.[52] Alternately, precipitated phosphates could be made from ground-up bones that had been de-greased and treated with strong acids. White phosphorus could then be made by heating the precipitated phosphates, mixed with ground coal or charcoal in an iron pot, and distilling off phosphorus vapour in a retort.[53]Carbon monoxide and other flammable gases produced during the reduction process were burnt off in a flare stack.
In the 1840s, world phosphate production turned to the mining of tropical island deposits formed from bird and bat guano (see also Guano Islands Act). These became an important source of phosphates for fertiliser in the latter half of the 19th century.[54]
Phosphate rock
Phosphate rock, which usually contains calcium phosphate, was first used in 1850 to make phosphorus, and following the introduction of the electric arc furnace by James Burgess Readman in 1888[55] (patented 1889),[56] elemental phosphorus production switched from the bone-ash heating, to electric arc production from phosphate rock. After the depletion of world guano sources about the same time, mineral phosphates became the major source of phosphate fertiliser production. Phosphate rock production greatly increased after World War II, and remains the primary global source of phosphorus and phosphorus chemicals today. See the article on peak phosphorus for more information on the history and present state of phosphate mining. Phosphate rock remains a feedstock in the fertiliser industry, where it is treated with sulfuric acid to produce various "superphosphate" fertiliser products.
Incendiaries
White phosphorus was first made commercially in the 19th century for the match industry. This used bone ash for a phosphate source, as described above. The bone-ash process became obsolete when the submerged-arc furnace for phosphorus productionwas introduced to reduce phosphate rock.[57][58] The electric furnace method allowed production to increase to the point where phosphorus could be used in weapons of war.[27][59] In World War I, it was used in incendiaries, smoke screens and tracer bullets.[59] A special incendiary bullet was developed to shoot at hydrogen-filled Zeppelins over Britain (hydrogen being highly flammable).[59] During World War II, Molotov cocktails made of phosphorus dissolved in petrol were distributed in Britain to specially selected civilians within the British resistance operation, for defence; and phosphorus incendiary bombs were used in war on a large scale. Burning phosphorus is difficult to extinguish and if it splashes onto human skin it has horrific effects.[14]
Early matches used white phosphorus in their composition, which was dangerous due to its toxicity. Murders, suicides and accidental poisonings resulted from its use. (An apocryphal tale tells of a woman attempting to murder her husband with white phosphorus in his food, which was detected by the stew's giving off luminous steam).[27] In addition, exposure to the vapours gave match workers a severe necrosis of the bones of the jaw, known as "phossy jaw". When a safe process for manufacturing red phosphorus was discovered, with its far lower flammability and toxicity, laws were enacted, under the Berne Convention (1906), requiring its adoption as a safer alternative for match manufacture.[60] The toxicity of white phosphorus led to discontinuation of its use in matches.[61] The Allies used phosphorus incendiary bombs in World War II to destroy Hamburg, the place where the "miraculous bearer of light" was first discovered.[47]
Production
Most production of phosphorus-bearing material is for agriculture fertilisers. For this purpose, phosphate minerals are converted to phosphoric acid. It follows two distinct chemical routes, the main one being treatment of phosphate minerals with sulfuric acid. The other process utilises white phosphorus, which may be produced by reaction and distillation from very low grade phosphate sources. The white phosphorus is then oxidised to phosphoric acid and subsequently neutralised with base to give phosphate salts. Phosphoric acid produced from white phosphorus is relatively pure and is the main route for the production of phosphates for all purposes, including detergent production.
In the early 1990s, Albright and Wilson's purified wet phosphoric acid business was being adversely affected by phosphate rock sales by China and the entry of their long-standing Moroccan phosphate suppliers into the purified wet phosphoric acid business.[62]
Peak phosphorus
In 2017, the USGS estimated 68 billion tons of world reserves, where reserve figures refer to the amount assumed recoverable at current market prices; 0.261 billion tons were mined in 2016.[63] Critical to contemporary agriculture, its annual demand is rising nearly twice as fast as the growth of the human population.[37]
The production of phosphorus may have peaked already (as per 2011), leading to the possibility of global shortages by 2040.[64] In 2007, at the rate of consumption, the supply of phosphorus was estimated to run out in 345 years.[65] However, some scientists now believe that a "peak phosphorus" will occur in 30 years and that "At current rates, reserves will be depleted in the next 50 to 100 years."[66] Cofounder of Boston-based investment firm and environmental foundation Jeremy Grantham wrote in Nature in November 2012 that consumption of the element "must be drastically reduced in the next 20-40 years or we will begin to starve."[37][67] According to N.N. Greenwood and A. Earnshaw, authors of the textbook, Chemistry of the Elements, however, phosphorus comprises about 0.1% by mass of the average rock, and consequently the Earth's supply is vast, although dilute.[14]
Elemental phosphorus
Presently, about 1,000,000 short tons (910,000 t) of elemental phosphorus is produced annually. Calcium phosphate (phosphate rock), mostly mined in Florida and North Africa, can be heated to 1,200–1,500 °C with sand, which is mostly SiO
2, and coke(refined coal) to produce vaporised P
4. The product is subsequently condensed into a white powder under water to prevent oxidation by air. Even under water, white phosphorus is slowly converted to the more stable red phosphorus allotrope. The chemical equation for this process when starting with fluoroapatite, a common phosphate mineral, is:
- 4 Ca5(PO4)3F + 18 SiO2 + 30 C → 3 P4 + 30 CO + 18 CaSiO3 + 2 CaF2
Side products from this process include ferrophosphorus, a crude form of Fe2P, resulting from iron impurities in the mineral precursors. The silicate slag is a useful construction material. The fluoride is sometimes recovered for use in water fluoridation. More problematic is a "mud" containing significant amounts of white phosphorus. Production of white phosphorus is conducted in large facilities in part because it is energy intensive. The white phosphorus is transported in molten form. Some major accidents have occurred during transportation; train derailments at Brownston, Nebraska and Miamisburg, Ohio led to large fires. The worst incident in recent times was an environmental contamination in 1968 when the sea was polluted from spillage and/or inadequately treated sewage from a white phosphorus plant at Placentia Bay, Newfoundland.[68]
Another process by which elemental phosphorus is extracted includes calcining tricalcium phosphate at high temperatures (1500 °C):[69]
- 2 Ca3(PO4)2 + 6 SiO2 + 10 C → 6 CaSiO3 + 10 CO + P4
Historically, before the development of mineral-based extractions, white phosphorus was isolated on an industrial scale from bone ash.[70] In this process, the tricalcium phosphate in bone ash is converted to monocalcium phosphate with sulfuric acid:
- Ca3(PO4)2 + 2 H2SO4 → Ca(H2PO4)2 + 2 CaSO4
Monocalcium phosphate is then dehydrated to the corresponding metaphosphate:
- Ca(H2PO4)2 → Ca(PO3)2 + 2 H2O
When ignited to a white heat (~1300C) with charcoal, calcium metaphosphate yields two-thirds of its weight of white phosphorus while one-third of the phosphorus remains in the residue as calcium orthophosphate:
- 3 Ca(PO3)2 + 10 C → Ca3(PO4)2 + 10 CO + P4
Applications
Fertiliser
Phosphorus is an essential plant nutrient (the most often limiting nutrient, after nitrogen),[71] and the bulk of all phosphorus production is in concentrated phosphoric acids for agriculture fertilisers, containing as much as 70% to 75% P2O5. That led to large increase in phosphate (PO43−) production in the second half of the 20th century.[37] Artificial phosphate fertilisation is necessary because phosphorus is essential to all living organisms; it is involved in energy transfers, strength of root and stems, photosynthesis, the expansion of plant roots, formation of seeds and flowers, and other important factors effecting overall plant health and genetics.[71]
Natural phosphorus-bearing compounds are mostly inaccessible to plants because of the low solubility and mobility in soil.[72] Most phosphorus is very stable in the soil minerals or organic matter of the soil. Even when phosphorus is added in manure or fertilizer it can become fixed in the soil. Therefore, the natural cycle of phosphorus is very slow. Some of the fixed phosphorus is released again over time, sustaining wild plant growth, however, more is needed to sustain intensive cultivation of crops.[73] Fertiliser is often in the form of superphosphate of lime, a mixture of calcium dihydrogen phosphate (Ca(H2PO4)2), and calcium sulfate dihydrate (CaSO4·2H2O) produced reacting sulfuric acid and water with calcium phosphate.
Processing phosphate minerals with sulfuric acid for obtaining fertiliser is so important to the global economy that this is the primary industrial market for sulfuric acid and the greatest industrial use of elemental sulfur.[74]
| Widely used compounds | Use |
|---|---|
| Ca(H2PO4)2·H2O | Baking powder and fertilisers |
| CaHPO4·2H2O | Animal food additive, toothpowder |
| H3PO4 | Manufacture of phosphate fertilisers |
| PCl3 | Manufacture of POCl3 and pesticides |
| POCl3 | Manufacture of plasticiser |
| P4S10 | Manufacturing of additives and pesticides |
| Na5P3O10 | Detergents |
Organophosphorus
White phosphorus is widely used to make organophosphorus compounds through intermediate phosphorus chlorides and two phosphorus sulfides, phosphorus pentasulfide and phosphorus sesquisulfide.[75] Organophosphorus compounds have many applications, including in plasticisers, flame retardants, pesticides, extraction agents, nerve agents and water treatment.[14][76]
Metallurgical aspects
Phosphorus is also an important component in steel production, in the making of phosphor bronze, and in many other related products.[77][78] Phosphorus is added to metallic copper during its smelting process to react with oxygen present as an impurity in copper and to produce phosphorus-containing copper (CuOFP) alloys with a higher hydrogen embrittlement resistance than normal copper.[79]
Matches
The first striking match with a phosphorus head was invented by Charles Sauria in 1830. These matches (and subsequent modifications) were made with heads of white phosphorus, an oxygen-releasing compound (potassium chlorate, lead dioxide, or sometimes nitrate), and a binder. They were poisonous to the workers in manufacture,[80]sensitive to storage conditions, toxic if ingested, and hazardous when accidentally ignited on a rough surface.[81][82] Production in several countries was banned between 1872 and 1925.[83] The international Berne Convention, ratified in 1906, prohibited the use of white phosphorus in matches.
In consequence, phosphorous matches were gradually replaced by safer alternatives. Around 1900 french chemists Henri Sévène and Emile David Cahen invented the modern strike-anywhere match, wherein the white phosphorus was replaced by phosphorus sesquisulfide (P4S3), a non-toxic and non-pyrophoric compound that ignites under friction. For a time these safer strike-anywhere matches were quite popular but in the long run they were superseded by the modern safety match.
Safety matches are very difficult to ignite on any surface other than a special striker strip. The strip contains non-toxic red phosphorus and the match head potassium chlorate, an oxygen-releasing compound. When struck, small amounts of abrasionfrom match head and striker strip are mixed intimately to make a small quantity of Armstrong's mixture, a very touch sensitive composition. The fine powder ignites immediately and provides the initial spark to set off the match head. Safety matches separate the two components of the ignition mixture until the match is struck. This is the key safety advantage as it prevents accidental ignition. Nonetheless, safety matches, invented in 1844 by Gustaf Erik Pasch and market ready by the 1860s, didn't gain consumer acceptance until the prohibition of white phosphorus. Using a dedicated striker strip was considered clumsy.[17][75][84]
Water softening
Sodium tripolyphosphate made from phosphoric acid is used in laundry detergents in some countries, but banned for this use in others.[19] This compound softens the water to enhance the performance of the detergents and to prevent pipe/boiler tube corrosion.[85]
Miscellaneous
- Phosphates are used to make special glasses for sodium lamps.[19]
- Bone-ash, calcium phosphate, is used in the production of fine china.[19]
- Phosphoric acid made from elemental phosphorus is used in food applications such as soft drinks, and as a starting point for food grade phosphates.[75] These include mono-calcium phosphate for baking powder and sodium tripolyphosphate.[75]Phosphates are used to improve the characteristics of processed meat and cheese, and in toothpaste.[75]
- White phosphorus, called "WP" (slang term "Willie Peter") is used in military applications as incendiary bombs, for smoke-screening as smoke pots and smoke bombs, and in tracer ammunition. It is also a part of an obsolete M34 White Phosphorus US hand grenade. This multipurpose grenade was mostly used for signaling, smoke screens, and inflammation; it could also cause severe burns and had a psychological impact on the enemy.[86][87] Military uses of white phosphorus are constrained by international law.
- 32P and 33P are used as radioactive tracers in biochemical laboratories.[88]
Inorganic phosphorus in the form of the phosphate PO3−
4 is required for all known forms of life.[89] Phosphorus plays a major role in the structural framework of DNA and RNA. Living cells use phosphate to transport cellular energy with adenosine triphosphate(ATP), necessary for every cellular process that uses energy. ATP is also important for phosphorylation, a key regulatory event in cells. Phospholipids are the main structural components of all cellular membranes. Calcium phosphate salts assist in stiffening bones.[14] Biochemists commonly use the abbreviation "Pi" to refer to inorganic phosphate.[90]
Every living cell is encased in a membrane that separates it from its surroundings. Cellular membranes are composed of a phospholipid matrix and proteins, typically in the form of a bilayer. Phospholipids are derived from glycerol with two of the glycerol hydroxyl (OH) protons replaced by fatty acids as an ester, and the third hydroxyl proton has been replaced with phosphate bonded to another alcohol.[91]
An average adult human contains about 0.7 kg of phosphorus, about 85–90% in bones and teeth in the form of apatite, and the remainder in soft tissues and extracellular fluids (~1%). The phosphorus content increases from about 0.5 weight% in infancy to 0.65-1.1 weight% in adults. Average phosphorus concentration in the blood is about 0.4 g/L, about 70% of that is organic and 30% inorganic phosphates.[92] An adult with healthy diet consumes and excretes about 1-3 grams of phosphorus per day, with consumption in the form of inorganic phosphate and phosphorus-containing biomolecules such as nucleic acids and phospholipids; and excretion almost exclusively in the form of phosphate ions such as H
2PO−
4 and HPO2−
4. Only about 0.1% of body phosphate circulates in the blood, paralleling the amount of phosphate available to soft tissue cells.
Bone and teeth enamel
The main component of bone is hydroxyapatite as well as amorphous forms of calcium phosphate, possibly including carbonate. Hydroxyapatite is the main component of tooth enamel. Water fluoridation enhances the resistance of teeth to decay by the partial conversion of this mineral to the still harder material called fluoroapatite:[14]
- Ca
5(PO
4)
3OH + F−
→ Ca
5(PO
4)
3F + OH−
Phosphorus deficiency
In medicine, phosphate deficiency syndrome may be caused by malnutrition, by failure to absorb phosphate, and by metabolic syndromes that draw phosphate from the blood (such as in refeeding syndrome after malnutrition[93]) or passing too much of it into the urine. All are characterised by hypophosphatemia, which is a condition of low levels of soluble phosphate levels in the blood serum and inside the cells. Symptoms of hypophosphatemia include neurological dysfunction and disruption of muscle and blood cells due to lack of ATP. Too much phosphate can lead to diarrhoea and calcification (hardening) of organs and soft tissue, and can interfere with the body's ability to use iron, calcium, magnesium, and zinc.[94]
Phosphorus is an essential macromineral for plants, which is studied extensively in edaphology to understand plant uptake from soil systems. Phosphorus is a limiting factor in many ecosystems; that is, the scarcity of phosphorus limits the rate of organism growth. An excess of phosphorus can also be problematic, especially in aquatic systems where eutrophication sometimes leads to algal blooms.[37]
Organic compounds of phosphorus form a wide class of materials; many are required for life, but some are extremely toxic. Fluorophosphate esters are among the most potent neurotoxins known. A wide range of organophosphorus compounds are used for their toxicity as pesticides (herbicides, insecticides, fungicides, etc.) and weaponised as nerve agents against enemy humans. Most inorganic phosphates are relatively nontoxic and essential nutrients.[14]
The white phosphorus allotrope presents a significant hazard because it ignites in air and produces phosphoric acid residue. Chronic white phosphorus poisoning leads to necrosis of the jaw called "phossy jaw". White phosphorus is toxic, causing severe liver damage on ingestion and may cause a condition known as "Smoking Stool Syndrome".[103]
In the past, external exposure to elemental phosphorus was treated by washing the affected area with 2% copper sulfate solution to form harmless compounds that are then washed away. According to the recent US Navy's Treatment of Chemical Agent Casualties and Conventional Military Chemical Injuries: FM8-285: Part 2 Conventional Military Chemical Injuries, "Cupric (copper(II)) sulfate has been used by U.S. personnel in the past and is still being used by some nations. However, copper sulfate is toxic and its use will be discontinued. Copper sulfate may produce kidney and cerebral toxicity as well as intravascular hemolysis."[104]
The manual suggests instead "a bicarbonate solution to neutralise phosphoric acid, which will then allow removal of visible white phosphorus. Particles often can be located by their emission of smoke when air strikes them, or by their phosphorescence in the dark. In dark surroundings, fragments are seen as luminescent spots. Promptly debride the burn if the patient's condition will permit removal of bits of WP (white phosphorus) that might be absorbed later and possibly produce systemic poisoning. DO NOT apply oily-based ointments until it is certain that all WP has been removed. Following complete removal of the particles, treat the lesions as thermal burns."[note 1][citation needed] As white phosphorus readily mixes with oils, any oily substances or ointments are not recommended until the area is thoroughly cleaned and all white phosphorus removed.
People can be exposed to phosphorus in the workplace by inhalation, ingestion, skin contact, and eye contact. The Occupational Safety and Health Administration (OSHA) has set the phosphorus exposure limit (Permissible exposure limit) in the workplace at 0.1 mg/m3 over an 8-hour workday. The National Institute for Occupational Safety and Health (NIOSH) has set a Recommended exposure limit (REL) of 0.1 mg/m3 over an 8-hour workday. At levels of 5 mg/m3, phosphorus is immediately dangerous to life and health.[105]
US DEA List I status
Phosphorus can reduce elemental iodine to hydroiodic acid, which is a reagent effective for reducing ephedrine or pseudoephedrine to methamphetamine.[106] For this reason, red and white phosphorus were designated by the United States Drug Enforcement Administration as List I precursor chemicals under 21 CFR 1310.02 effective on November 17, 2001.[107] In the United States, handlers of red or white phosphorus are subject to stringent regulatory controls.[107][108][109]
See also
The phosphorus cycle is the biogeochemical cycle that describes the movement of phosphorus through the lithosphere, hydrosphere, and biosphere. Unlike many other biogeochemical cycles, the atmosphere does not play a significant role in the movement of phosphorus, because phosphorus and phosphorus-based compounds are usually solids at the typical ranges of temperature and pressure found on Earth. The production of phosphine gas occurs in only specialized, local conditions. Therefore, the phosphorus cycle should be viewed from whole Earth system and then specifically focused on the cycle in terrestrial and aquatic systems.
On the land, phosphorus gradually becomes less available to plants over thousands of years, since it is slowly lost in runoff. Low concentration of phosphorus in soils reduces plant growth and slows soil microbial growth, as shown in studies of soil microbial biomass. Soil microorganisms act as both sinks and sources of available phosphorus in the biogeochemical cycle.[1] Short-term transformation of phosphorus is chemical, biological, or microbiological. In the long-term global cycle, however, the major transfer is driven by tectonic movement over geologic time.[2]
Humans have caused major changes to the global phosphorus cycle through shipping of phosphorus minerals, and use of phosphorus fertilizer, and also the shipping of food from farms to cities, where it is lost as effluent.
Phosphorus in the environment[edit]
Ecological function[edit]
Phosphorus is an essential nutrient for plants and animals. Phosphorus is a limiting nutrient for aquatic organisms. Phosphorus forms parts of important life-sustaining molecules that are very common in the biosphere. Phosphorus does enter the atmosphere in very small amounts when the dust is dissolved in rainwater and seaspray but remains mostly on land and in rock and soil minerals. Eighty percent of the mined phosphorus is used to make fertilizers. Phosphates from fertilizers, sewage and detergents can cause pollution in lakes and streams. Over-enrichment of phosphate in both fresh and inshore marine waters can lead to massive algae blooms. In fresh water, the death and decay of these blooms leads to eutrophication. An example of this is the Canadian Experimental Lakes Area.
These freshwater algal blooms should not be confused with those in saltwater environments. Recent research suggests that the predominant pollutant responsible for algal blooms in saltwater estuaries and coastal marine habitats is nitrogen.[3]
Phosphorus occurs most abundantly in nature as part of the orthophosphate ion (PO4)3−, consisting of a P atom and 4 oxygen atoms. On land most phosphorus is found in rocks and minerals. Phosphorus-rich deposits have generally formed in the ocean or from guano, and over time, geologic processes bring ocean sediments to land. Weathering of rocks and minerals release phosphorus in a soluble form where it is taken up by plants, and it is transformed into organic compounds. The plants may then be consumed by herbivores and the phosphorus is either incorporated into their tissues or excreted. After death, the animal or plant decays, and phosphorus is returned to the soil where a large part of the phosphorus is transformed into insoluble compounds. Runoff may carry a small part of the phosphorus back to the ocean. Generally with time (thousands of years) soils become deficient in phosphorus leading to ecosystem retrogression.[4]
Major pools in aquatic systems[edit]
There are four major pools of phosphorus in freshwater ecosystems: dissolved inorganic phosphorus (DIP), dissolved organic phosphorus (DOP), particulate organic phosphorus (POP), and particulate inorganic phosphorus (PIP). Dissolved material is defined as substances that pass through a 0.45 μm filter.[5] DIP consists mainly of orthophosphate (PO43-) and polyphosphate, while DOP consists of DNA and phosphoproteins. Particulate matter are the substances that get caught on a 0.45 μm filter and do not pass through. POP consists of both living and dead organisms, while PIP mainly consists of hydroxyapatite, Ca5(PO4)3OH .[5]
Biological function[edit]
The primary biological importance of phosphates is as a component of nucleotides, which serve as energy storage within cells (ATP) or when linked together, form the nucleic acids DNA and RNA. The double helix of our DNA is only possible because of the phosphate ester bridge that binds the helix. Besides making biomolecules, phosphorus is also found in bone and the enamel of mammalian teeth, whose strength is derived from calcium phosphate in the form of hydroxyapatite. It is also found in the exoskeleton of insects, and phospholipids (found in all biological membranes).[6] It also functions as a buffering agent in maintaining acid base homeostasis in the human body.[7]
Phosphorus cycling[edit]
| Part of a series on |
| Biogeochemical cycles |
|---|
 |
Phosphates move quickly through plants and animals; however, the processes that move them through the soil or ocean are very slow, making the phosphorus cycle overall one of the slowest biogeochemical cycles.[2][8]
The global phosphorus cycle includes four major processes:
- (i) tectonic uplift and exposure of phosphorus-bearing rocks such as apatite to surface weathering;[9]
- (ii) physical erosion, and chemical and biological weathering of phosphorus-bearing rocks to provide dissolved and particulate phosphorus to soils,[10] lakes and rivers;
- (iii) riverine and subsurface transportation of phosphorus to various lakes and run-off to the ocean;
- (iv) sedimentation of particulate phosphorus (e.g., phosphorus associated with organic matter and oxide/carbonate minerals) and eventually burial in marine sediments (this process can also occur in lakes and rivers).[11]
In terrestrial systems, bioavailable P (‘reactive P’) mainly comes from weathering of phosphorus-containing rocks. The most abundant primary phosphorus-mineral in the crust is apatite, which can be dissolved by natural acids generated by soil microbes and fungi, or by other chemical weathering reactions and physical erosion.[12] The dissolved phosphorus is bioavailable to terrestrial organisms and plants and is returned to the soil after their decay. Phosphorus retention by soil minerals (e.g., adsorption onto iron and aluminum oxyhydroxides in acidic soils and precipitation onto calcite in neutral-to-calcareous soils) is usually viewed as the most important processes in controlling terrestrial P-bioavailability in the mineral soil.[13] This process can lead to the low level of dissolved phosphorus concentrations in soil solution. Various physiological strategies are used by plants and microorganisms for obtaining phosphorus from this low level of phosphorus concentration.[14]
Soil phosphorus is usually transported to rivers and lakes and can then either be buried in lake sediments or transported to the ocean via river runoff. Atmospheric phosphorus deposition is another important marine phosphorus source to the ocean.[15] In surface seawater, dissolved inorganic phosphorus, mainly orthophosphate (PO43-), is assimilated by phytoplankton and transformed into organic phosphorus compounds.[11][15] Phytoplankton cell lysis releases cellular dissolved inorganic and organic phosphorus to the surrounding environment. Some of the organic phosphorus compounds can be hydrolyzed by enzymes synthesized by bacteria and phytoplankton and subsequently assimilated.[15] The vast majority of phosphorus is remineralized within the water column, and approximately 1% of associated phosphorus carried to the deep sea by the falling particles is removed from the ocean reservoir by burial in sediments.[15] A series of diagenetic processes act to enrich sediment pore water phosphorus concentrations, resulting in an appreciable benthic return flux of phosphorus to overlying bottom waters. These processes include
- (i) microbial respiration of organic matter in sediments,
- (ii) microbial reduction and dissolution of iron and manganese (oxyhydr)oxides with subsequent release of associated phosphorus, which connects the phosphorus cycle to the iron cycle,[16] and
- (iii) abiotic reduction of iron (oxyhydr)oxides by hydrogen sulfide and liberation of iron-associated phosphorus.[11]
Additionally,
- (iv) phosphate associated with calcium carbonate and
- (v) transformation of iron oxide-bound phosphorus to vivianite play critical roles in phosphorus burial in marine sediments.[17][18]
These processes are similar to phosphorus cycling in lakes and rivers.
Although orthophosphate (PO43-), the dominant inorganic P species in nature, is oxidation state (P5+), certain microorganisms can use phosphonate and phosphite (P3+ oxidation state) as a P source by oxidizing it to orthophosphate.[19] Recently, rapid production and release of reduced phosphorus compounds has provided new clues about the role of reduced P as a missing link in oceanic phosphorus.[20]
Phosphatic minerals[edit]
The availability of phosphorus in an ecosystem is restricted by the rate of release of this element during weathering. The release of phosphorus from apatite dissolution is a key control on ecosystem productivity. The primary mineral with significant phosphorus content, apatite [Ca5(PO4)3OH] undergoes carbonation.[2][21]
Little of this released phosphorus is taken up by biota (organic form), whereas a larger proportion reacts with other soil minerals. This leads to precipitation into unavailable forms in the later stage of weathering and soil development. Available phosphorus is found in a biogeochemical cycle in the upper soil profile, while phosphorus found at lower depths is primarily involved in geochemical reactions with secondary minerals. Plant growth depends on the rapid root uptake of phosphorus released from dead organic matter in the biochemical cycle. Phosphorus is limited in supply for plant growth. Phosphates move quickly through plants and animals; however, the processes that move them through the soil or ocean are very slow, making the phosphorus cycle overall one of the slowest biogeochemical cycles.[2][8]
Low-molecular-weight (LMW) organic acids are found in soils. They originate from the activities of various microorganisms in soils or may be exuded from the roots of living plants. Several of those organic acids are capable of forming stable organo-metal complexes with various metal ions found in soil solutions. As a result, these processes may lead to the release of inorganic phosphorus associated with aluminum, iron, and calcium in soil minerals. The production and release of oxalic acid by mycorrhizalfungi explain their importance in maintaining and supplying phosphorus to plants.[2][22]
The availability of organic phosphorus to support microbial, plant and animal growth depends on the rate of their degradation to generate free phosphate. There are various enzymes such as phosphatases, nucleases and phytase involved for the degradation. Some of the abiotic pathways in the environment studied are hydrolytic reactions and photolytic reactions. Enzymatic hydrolysis of organic phosphorus is an essential step in the biogeochemical phosphorus cycle, including the phosphorus nutrition of plants and microorganisms and the transfer of organic phosphorus from soil to bodies of water.[1] Many organisms rely on the soil derived phosphorus for their phosphorus nutrition.[citation needed]
Eutrophication[edit]
Eutrophication is an enrichment of water by nutrient that lead to structural changes to the aquatic ecosystem such as algae bloom, deoxygenation, reduction of fish species. The primary source that contributes to the eutrophication is considered as nitrogen and phosphorus. When these two elements exceed the capacity of the water body, eutrophication occurs. Phosphorus that enters lakes will accumulate in the sediments and the biosphere, it also can be recycled from the sediments and the water system.[23] Drainage water from agricultural land also carries phosphorus and nitrogen.[24] Since a large amount of phosphorus is in the soil contents, so the overuse of fertilizers and over-enrichment with nutrients will lead to increasing the amount of phosphorus concentration in agricultural runoff. When eroded soil enters the lake, both phosphorus and the nitrogen in the soil contribute to eutrophication, and erosion caused by deforestation which also results from uncontrolled planning and urbanization.[25]
Wetland[edit]
Wetlands are frequently applied to solve the issue of eutrophication. Nitrate is transformed in wetlands to free nitrogen and discharged to the air. Phosphorus is adsorbed by wetland soils which are taken up by the plants. Therefore, wetlands could help to reduce the concentration of nitrogen and phosphorus to remit and solve the eutrophication. However, wetland soils can only hold a limited amount of phosphorus. To remove phosphorus continually, it is necessary to add more new soils within the wetland from remnant plant stems, leaves, root debris, and undecomposable parts of dead algae, bacteria, fungi, and invertebrates.[24]
Human influences[edit]
Nutrients are important to the growth and survival of living organisms, and hence, are essential for development and maintenance of healthy ecosystems. Humans have greatly influenced the phosphorus cycle by mining phosphorus, converting it to fertilizer, and by shipping fertilizer and products around the globe. Transporting phosphorus in food from farms to cities has made a major change in the global Phosphorus cycle. However, excessive amounts of nutrients, particularly phosphorus and nitrogen, are detrimental to aquatic ecosystems. Waters are enriched in phosphorus from farms' run-off, and from effluent that is inadequately treated before it is discharged to waters. The input of P in agricultural runoff can accelerate the eutrophication of P-sensitive surface waters.[26]Natural eutrophication is a process by which lakes gradually age and become more productive and may take thousands of years to progress. Cultural or anthropogenic eutrophication, however, is water pollution caused by excessive plant nutrients; this results in excessive growth in the algal population; when this algae dies its putrefaction depletes the water of oxygen. Such eutrophication may also give rise to toxic algal bloom. Both these effects cause animal and plant death rates to increase as the plants take in poisonous water while the animals drink the poisoned water. Surface and subsurface runoff and erosion from high-phosphorus soils may be major contributing factors to this fresh water eutrophication. The processes controlling soil Phosphorus release to surface runoff and to subsurface flow are a complex interaction between the type of phosphorus input, soil type and management, and transport processes depending on hydrological conditions.[27][28]
Repeated application of liquid hog manure in excess to crop needs can have detrimental effects on soil phosphorus status. Also, application of biosolids may increase available phosphorus in soil.[29] In poorly drained soils or in areas where snowmelt can cause periodic waterlogging, reducing conditions can be attained in 7–10 days. This causes a sharp increase in phosphorus concentration in solution and phosphorus can be leached. In addition, reduction of the soil causes a shift in phosphorus from resilient to more labile forms. This could eventually increase the potential for phosphorus loss. This is of particular concern for the environmentally sound management of such areas, where disposal of agricultural wastes has already become a problem. It is suggested that the water regime of soils that are to be used for organic wastes disposal is taken into account in the preparation of waste management regulations.[30]
Human interference in the phosphorus cycle occurs by overuse or careless use of phosphorus fertilizers. This results in increased amounts of phosphorus as pollutants in bodies of water resulting in eutrophication. Eutrophication devastates water ecosystems by inducing anoxic conditions.[25]
See also[edit]
https://en.wikipedia.org/wiki/Phosphorus_cycle
https://www.ducksters.com/science/chemistry/phosphorus.php
https://pubchem.ncbi.nlm.nih.gov/element/Phosphorus
| Toxic metals (cadmium |
|---|
https://en.wikipedia.org/wiki/Sodium_thiosulfate
Category:Polyatomic nonmetals
Subcategories
This category has the following 4 subcategories, out of 4 total.
Pages in category "Polyatomic nonmetals"
The following 5 pages are in this category, out of 5 total. This list may not reflect recent changes (learn more).
C
P
S
Category:Reactive nonmetals
From Wikipedia, the free encyclopedia
Jump to navigationJump to search
Main section: Nonmetal#Reactive nonmetal
Pages in category "Reactive nonmetals"
The following 12 pages are in this category, out of 12 total. This list may not reflect recent changes (learn more).
B
Bromine
C
Carbon
Chlorine
F
Fluorine
H
Hydrogen
I
Iodine
N
Nitrogen
O
Oxygen
P
Phosphorus
R
Reactive nonmetal
S
Selenium
Sulfur
https://en.wikipedia.org/wiki/Category:Reactive_nonmetals
Category:Pyrotechnic fuels
| Wikimedia Commons has media related to Pyrotechnic fuels. |
Pages in category "Pyrotechnic fuels"
The following 17 pages are in this category, out of 17 total. This list may not reflect recent changes (learn more).
I
Z
09-07-2021-1553 - Hexazine (also known as hexaazabenzene)
Hexazine (also known as hexaazabenzene) is a hypothetical allotrope of nitrogen composed of 6 nitrogen atoms arranged in a ring-like structure analogous to that of benzene. It would be the final member of the azabenzene (azine) series, in which all of the methine groups of the benzene molecule have been replaced with nitrogen atoms. The two last members of this series, hexazine and pentazine, have not been observed, although all other members of the azine series have (such as pyridine, pyrimidine, pyridazine, pyrazine, triazines, and tetrazines).

https://en.wikipedia.org/wiki/Hexazine
09-07-2021-1401 - Methylidynephosphane (phosphaethyne
Methylidynephosphane (phosphaethyne) is a chemical compound which was the first phosphaalkyne compound discovered, containing the unusual C≡P carbon-phosphorus triple bond.

https://en.wikipedia.org/wiki/Methylidynephosphane
Tuesday, September 7, 2021
09-07-2021-1358 - Cyaphide
Cyaphide, P≡C−, is the phosphorus analogue of cyanide. It is not known as a discrete salt, however In silico measurements reveal that the −1 charge in this ion is located mainly on carbon (0.65), as opposed to phosphorus.

https://en.wikipedia.org/wiki/Cyaphide
Biperiden, sold under the brand name Akineton among others, is a medication used to treat Parkinson disease and certain drug-induced movement disorders.[1] It is not recommended for tardive dyskinesias.[2] It is taken by mouth, injection into a vein, or muscle.[1][2]
Common side effects include blurred vision, dry mouth, sleepiness, constipation, and confusion.[1] It should not be used in people with a bowel obstruction or glaucoma.[1]It is unclear if use in pregnancy or breastfeeding is safe.[3] Biperiden is in the anticholinergic family of medication.[1]
Biperiden was approved for medical use in the United States in 1959.[1] It is on the World Health Organization's List of Essential Medicines, the safest and most effective medicines needed in a health system.[4] Biperiden is no longer marketed in the United States.[5][6][7]
https://en.wikipedia.org/wiki/Biperiden
Pralidoxime (2-pyridine aldoxime methyl chloride) or 2-PAM, usually as the chloride or iodide salts, belongs to a family of compounds called oximes that bind to organophosphate-inactivated acetylcholinesterase.[1] It is used to treat organophosphate poisoning[2] in conjunction with atropine and either diazepam or midazolam. It is a white solid.
https://en.wikipedia.org/wiki/Pralidoxime
Cyprodenate (Actebral) is a stimulant drug.[1] It was used for counteracting the effects of benzodiazepine tranquillizer drugs before the development of newer antidotes such as flumazenil.[2] It produces dimethylethanolamine as a metabolite.[citation needed]
https://en.wikipedia.org/wiki/Cyprodenate
Physostigmine (also known as eserine from éséré, the West African name for the Calabar bean) is a highly toxic parasympathomimetic alkaloid, specifically, a reversible cholinesterase inhibitor. It occurs naturally in the Calabar bean and the Manchineel tree.
The chemical was synthesized for the first time in 1935 by Percy Lavon Julian and Josef Pikl. It is available in the U.S. under the trade names Antilirium and Isopto Eserine, and as eserine salicylate and eserine sulfate. Today, physostigmine is most commonly used for its medicinal value. However, before its discovery by Sir Robert Christison in 1846, it was much more prevalent as an ordeal poison. The positive medical applications of the drug were first suggested in the gold medal-winning final thesis of Thomas Richard Fraser at the University of Edinburgh in 1862.[1]
https://en.wikipedia.org/wiki/Physostigmine
Bemegride (trademarked as Megimide) is a central nervous system stimulant.[1]The drug was first made in 1911.[2] It has been used in hypnotic overdose.[1]
As with other chemoreceptor agonists, it is a potent emetic at doses above those normally used in management of barbiturate overdose although emesis and aspiration are a concern during treatment.
It is a controlled substance.[1]
https://en.wikipedia.org/wiki/Bemegride
Heparin, also known as unfractionated heparin (UFH), is a medication and naturally occurring glycosaminoglycan.[2][3] As a medication it is used as an anticoagulant.[2] Specifically it is also used in the treatment of heart attacks and unstable angina.[2] It is given by injection into a vein or under the skin.[2] Other uses include inside test tubes and kidney dialysis machines.[3][4]
Common side effects include bleeding, pain at the injection site, and low blood platelets.[2] Serious side effects include heparin-induced thrombocytopenia.[2]Greater care is needed in those with poor kidney function.[2]
Heparin is contraindicated for suspected cases of vaccine-induced pro-thrombotic immune thrombocytopenia (VIPIT) secondary to SARS-CoV-2 vaccination, as heparin may further increase the risk of bleeding in an anti-PF4/heparin complex autoimmune manner, in favor of alternative anticoagulant medications (such as argatroban or danaparoid).[5][6][7]
Heparin appears to be relatively safe for use during pregnancy and breastfeeding.[8]Heparin is produced by basophils and mast cells in all mammals.[9]
The discovery of heparin was announced in 1916.[10] It is on the World Health Organization's List of Essential Medicines.[11] A fractionated version of heparin, known as low molecular weight heparin, is also available.[12]
https://en.wikipedia.org/wiki/Heparin
Hydroxocobalamin, also known as vitamin B12a and hydroxycobalamin, is a vitamin found in food and used as a dietary supplement.[2] As a supplement it is used to treat vitamin B12 deficiency including pernicious anemia.[2][3] Other uses include treatment for cyanide poisoning, Leber's optic atrophy, and toxic amblyopia.[4][5] It is given by injection into a muscle or vein.[3]
Side effects are generally few.[3] They may include diarrhea, low blood potassium, allergic reactions, and high blood pressure.[3] Normal doses are considered safe in pregnancy.[1] Hydroxocobalamin is the natural form of vitamin B12 and a member of the cobalamin family of compounds.[6][7] Hydroxocobalamin, or another form of vitamin B12, are required for the body to make DNA.[7]
Hydroxocobalamin was first isolated in 1949.[8] It is on the World Health Organization's List of Essential Medicines.[9] Hydroxocobalamin is available as a generic medication.[3] Commercially it is made using one of a number of types of bacteria.[10]
https://en.wikipedia.org/wiki/Hydroxocobalamin
Amyl nitrite is a chemical compound with the formula C5H11ONO. A variety of isomers are known, but they all feature an amyl group attached to the nitritefunctional group. The alkyl group is unreactive and the chemical and biological properties are mainly due to the nitrite group. Like other alkyl nitrites, amyl nitrite is bioactive in mammals, being a vasodilator, which is the basis of its use as a prescription medicine. As an inhalant, it also has a psychoactive effect, which has led to its recreational use with its smell being described as that of old socks or dirty feet.[1] It is also referred to as banapple gas.[2]
It was first documented in 1844 and came into medical use in 1867.[3]
https://en.wikipedia.org/wiki/Amyl_nitrite
Polyclonal antibodies (pAbs) are antibodies that are secreted by different B cell lineages within the body (whereas monoclonal antibodies come from a single cell lineage). They are a collection of immunoglobulin molecules that react against a specific antigen, each identifying a different epitope.
https://en.wikipedia.org/wiki/Polyclonal_antibodies
Category:Antitoxins
This is a list of articles about antitoxins — including antisera and antivenins — used (or formerly used) to treat disease in humans or animals.
Pages in category "Antitoxins"
The following 7 pages are in this category, out of 7 total. This list may not reflect recent changes (learn more).
https://en.wikipedia.org/wiki/Category:Antitoxins
https://en.wikipedia.org/wiki/Antivenom

Names
IUPAC name
Iron(II,III) hexacyanoferrate(II,III)
Other names
Berlin blue
Ferric ferrocyanide
Ferric hexacyanoferrate
Iron(III) ferrocyanide
Iron(III) hexacyanoferrate(II)
Parisian blue
Identifiers
CAS Number
14038-43-8

3D model (JSmol)
Interactive image
ChEBI
CHEBI:30069

ChEMBL
ChEMBL2096629

ChemSpider
20074656

ECHA InfoCard 100.034.418
EC Number
237-875-5
Gmelin Reference 1093743
PubChem CID
2724251
UNII
TLE294X33A

CompTox Dashboard (EPA)
DTXSID9047756
show
InChI
show
SMILES
Properties
Chemical formula C18Fe7N18
Molar mass 859.239 g·mol−1
Appearance Blue opaque crystals
Solubility in water Insoluble
Pharmacology
ATC code V03AB31 (WHO)
Routes of
administration Oral
Hazards
Safety data sheet MSDS Prussian blue
Related compounds
Other cations Potassium ferrocyanide
Sodium ferrocyanide
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
 verify (what is
verify (what is 
 ?)
?)Infobox references
Prussian blue
 Color coordinates
Color coordinatesHex triplet #003153
HSV (h, s, v) (205°, 100%, 32%)
sRGBB (r, g, b) (0, 49, 83)
Source [1]
ISCC–NBS descriptor Dark blue
B: Normalized to [0–255] (byte)
Prussian blue (also known as Berlin blue or, in painting, Parisian or Paris blue) is a dark blue pigment produced by oxidation of ferrous ferrocyanide salts. It has the chemical formula FeIII
4[FeII
(CN)
6]
3. Turnbull's blue is chemically identical, but is made from different reagents, and its slightly different color stems from different impurities.
Prussian blue was the first modern synthetic pigment. It is prepared as a very fine colloidal dispersion, because the compound is not soluble in water. It contains variable amounts[1] of other ions and its appearance depends sensitively on the size of the colloidal particles. The pigment is used in paints, and it is the traditional "blue" in blueprints and aizuri-e (藍摺り絵) Japanese woodblock prints.
In medicine, orally administered Prussian blue is used as an antidote for certain kinds of heavy metal poisoning, e.g., by thallium(I) and radioactive isotopes of caesium. The therapy exploits the compound's ion-exchange properties and high affinity for certain "soft" metal cations.
It is on the World Health Organization's List of Essential Medicines, the most important medications needed in a basic health system.[2] Prussian blue lent its name to prussic acid (hydrogen cyanide) derived from it. In German, hydrogen cyanide is called Blausäure ("blue acid"). French chemist Joseph Louis Gay-Lussacgave cyanide its name, from the Ancient Greek word κύανος (kyanos, "blue"), because of its Prussian blue color.
https://en.wikipedia.org/wiki/Prussian_blue
Griseofulvin is an antifungal medication used to treat a number of types of dermatophytoses (ringworm).[1] This includes fungal infections of the nails and scalp, as well as the skin when antifungal creams have not worked.[2] It is taken by mouth.[1]
Common side effects include allergic reactions, nausea, diarrhea, headache, trouble sleeping, and feeling tired.[1] It is not recommended in people with liver failure or porphyria.[1] Use during or in the months before pregnancy may result in harm to the baby.[1][2] Griseofulvin works by interfering with fungal mitosis.[1]
Griseofulvin was discovered in 1939 from the soil fungus Penicillium griseofulvum.[3][4][5] It is on the World Health Organization's List of Essential Medicines.[6]
The drug binds to tubulin, interfering with microtubule function, thus inhibiting mitosis. It binds to keratin in keratin precursor cells and makes them resistant to fungal infections. The drug reaches its site of action only when hair or skin is replaced by the keratin-griseofulvin complex. Griseofulvin then enters the dermatophyte through energy-dependent transport processes and binds to fungal microtubules. This alters the processing for mitosis and also underlying information for deposition of fungal cell walls.
Metabolism Liver (demethylation and glucuronidation)
It is produced industrially by fermenting the fungus Penicillium griseofulvum.[10][11][12]
The first step in the biosynthesis of griseofulvin by P. griseofulvin is the synthesis of the 14-carbon poly-β-keto chain by a type I iterative polyketide synthase (PKS) via iterative addition of 6 malonyl-CoA to an acyl-CoA starter unit. The 14-carbon poly-β-keto chain undergoes cyclization/aromatization, using cyclase/aromatase, respectively, through a Claisen and aldol condensation to form the benzophenoneintermediate. The benzophenone intermediate is then methylated via S-adenosyl methionine (SAM) twice to yield griseophenone C. The griseophenone C is then halogenated at the activated site ortho to the phenol group on the left aromatic ring to form griseophenone B. The halogenated species then undergoes a single phenolic oxidation in both rings forming the two oxygen diradical species. The right oxygen radical shifts alpha to the carbonyl via resonance allowing for a stereospecific radical coupling by the oxygen radical on the left ring forming a tetrahydrofuranone species.[13] The newly formed grisan skeleton with a spiro center is then O-methylated by SAM to generate dehydrogriseofulvin. Ultimately, a stereoselective reduction of the olefin on dehydrogriseofulvin by NADPH affords griseofulvin.[14][15]
https://en.wikipedia.org/wiki/Griseofulvin
Self-assembled monolayers (SAM) of organic molecules are molecular assemblies formed spontaneously on surfaces by adsorption and are organized into more or less large ordered domains.[1][2] In some cases molecules that form the monolayer do not interact strongly with the substrate. This is the case for instance of the two-dimensional supramolecular networks[3] of e.g. perylenetetracarboxylic dianhydride(PTCDA) on gold[4] or of e.g. porphyrins on highly oriented pyrolitic graphite(HOPG).[5] In other cases the molecules possess a head group that has a strong affinity to the substrate and anchors the molecule to it.[1] Such a SAM consisting of a head group, tail and functional end group is depicted in Figure 1. Common head groups include thiols, silanes, phosphonates, etc.
SAMs are created by the chemisorption of "head groups" onto a substrate from either the vapor or liquid phase[6][7] followed by a slow organization of "tail groups".[8]Initially, at small molecular density on the surface, adsorbate molecules form either a disordered mass of molecules or form an ordered two-dimensional "lying down phase",[6] and at higher molecular coverage, over a period of minutes to hours, begin to form three-dimensional crystalline or semicrystalline structures on the substrate surface.[9] The "head groups" assemble together on the substrate, while the tail groups assemble far from the substrate. Areas of close-packed molecules nucleate and grow until the surface of the substrate is covered in a single monolayer.
Adsorbate molecules adsorb readily because they lower the surface free-energy of the substrate[1] and are stable due to the strong chemisorption of the "head groups." These bonds create monolayers that are more stable than the physisorbed bonds of Langmuir–Blodgett films.[10][11] A Trichlorosilane based "head group", for example in a FDTSmolecule, reacts with a hydroxyl group on a substrate, and forms very stable, covalent bond [R-Si-O-substrate] with an energy of 452 kJ/mol. Thiol-metal bonds are on the order of 100 kJ/mol, making them fairly stable in a variety of temperatures, solvents, and potentials.[9] The monolayer packs tightly due to van der Waals interactions,[1][11]thereby reducing its own free energy.[1] The adsorption can be described by the Langmuir adsorption isotherm if lateral interactions are neglected. If they cannot be neglected, the adsorption is better described by the Frumkin isotherm.[9]
https://en.wikipedia.org/wiki/Self-assembled_monolayer
Antifungals (D01 and J02)
hidevte
Xenobiotic-sensing receptor modulators
CAR
Agonists: 6,7-Dimethylesculetin Amiodarone Artemisinin Benfuracarb Carbamazepine Carvedilol Chlorpromazine Chrysin CITCO Clotrimazole Cyclophosphamide Cypermethrin DHEA (prasterone) Efavirenz Ellagic acid Griseofulvin Methoxychlor Mifepristone Nefazodone Nevirapine Nicardipine Octicizer Permethrin Phenobarbital Phenytoin Pregnanedione (5β-dihydroprogesterone) Reserpine TCPOBOP Telmisartan Tolnaftate Troglitazone Valproic acid
Antagonists: 3,17β-Estradiol 3α-Androstanol 3α-Androstenol 3β-Androstanol 17-Androstanol AITC Ethinylestradiol Meclizine Nigramide J Okadaic acid PK-11195 S-07662 T-0901317
PXR
Agonists: 17α-Hydroxypregnenolone 17α-Hydroxyprogesterone Δ4-Androstenedione Δ5-Androstenediol Δ5-Androstenedione AA-861 Allopregnanediol Allopregnanedione (5α-dihydroprogesterone) Allopregnanolone (brexanolone) Alpha-Lipoic acid Ambrisentan AMI-193 Amlodipine besylate Antimycotics Artemisinin Aurothioglucose Bile acids Bithionol Bosentan Bumecaine Cafestol Cephaloridine Cephradine Chlorpromazine Ciglitazone Clindamycin Clofenvinfos Chloroxine Clotrimazole Colforsin Corticosterone Cyclophosphamide Cyproterone acetate Demecolcine Dexamethasone DHEA (prasterone) DHEA-S (prasterone sulfate) Dibunate sodium Diclazuril Dicloxacillin Dimercaprol Dinaline Docetaxel Docusate calcium Dodecylbenzenesulfonic acid Dronabinol Droxidopa Eburnamonine Ecopipam Enzacamene Epothilone B Erythromycin Famprofazone Febantel Felodipine Fenbendazole Fentanyl Flucloxacillin Fluorometholone Griseofulvin Guggulsterone Haloprogin Hetacillin potassium Hyperforin Hypericum perforatum (St John's wort) Indinavir sulfate Lasalocid sodium Levothyroxine Linolenic acid LOE-908 Loratadine Lovastatin Meclizine Metacycline Methylprednisolone Metyrapone Mevastatin Mifepristone Nafcillin Nicardipine Nicotine Nifedipine Nilvadipine Nisoldipine Norelgestromin Omeprazole Orlistat Oxatomide Paclitaxel Phenobarbital Piperine Plicamycin Prednisolone Pregnanediol Pregnanedione (5β-dihydroprogesterone) Pregnanolone Pregnenolone Pregnenolone 16α-carbonitrile Proadifen Progesterone Quingestrone Reserpine Reverse triiodothyronine Rifampicin Rifaximin Rimexolone Riodipine Ritonavir Simvastatin Sirolimus Spironolactone Spiroxatrine SR-12813 Suberoylanilide Sulfisoxazole Suramin Tacrolimus Tenylidone Terconazole Testosterone isocaproate Tetracycline Thiamylal sodium Thiothixene Thonzonium bromide Tianeptine Troglitazone Troleandomycin Tropanyl 3,5-dimethulbenzoate Zafirlukast Zeranol
Antagonists: Ketoconazole Sesamin
See also Receptor/signaling modulators
Medicine portal
Categories: AntifungalsAromatic ketonesBenzofuran ethers at the benzene ringChloroarenesCyclohexenesDisulfiram-like drugsHalogen-containing natural productsIARC Group 2B carcinogensMutagensResorcinol ethersSpiro compoundsWorld Health Organization essential medicines
Science and technology[edit]
Biology, chemistry and medicine[edit]
Phytelephas seemannii, known in Cuna as 'sam'
S-Adenosyl methionine, a common co-substrate involved in methyl group transfers
SAM, a candidate phylum of bacteria
Sustained Acoustic Medicine, a medical treatment for arthritis
SAMtools (for Sequence Alignment Map), a data storage format for DNA sequencing
Self-assembled monolayer of amphiphilic molecules
Shoot apical meristem, a plant tissue
Significance analysis of microarrays, in DNA microanalysis
Spore photoproduct lyase, an enzyme
Sorting and assembly machinery, a protein complex in the outer mitochondrial membrane
Segmental arterial mediolysis, a medical condition affecting the arteries
Physics and astronomy[edit]
Southern Annular Mode of southern hemisphere atmospheric variability
Swinging Atwood's machine
SAm, an unbarred Magellanic spiral galaxy
Benzophenone is the organic compound with the formula (C6H5)2CO, generally abbreviated Ph2CO. It is a white solid that is soluble in organic solvents. Benzophenone is a widely used building block in organic chemistry, being the parent diarylketone.
https://en.wikipedia.org/wiki/Benzophenone
Pages in category "Benzofuran ethers at the benzene ring"
The following 15 pages are in this category, out of 15 total. This list may not reflect recent changes (learn more).
0–9
5-MeO-DiBF
B
Befunolol
Brofaromine
C
Carbofuran
Carbosulfan
D
Dimemebfe
F
F-2 (drug)
F-22 (psychedelic)
FL3 (flavagline)
Fura-2
Fura-2-acetoxymethyl ester
G
Griseofulvin
K
Khellin
L
LY-320,135
S
Silvestrol
https://en.wikipedia.org/wiki/Category:Benzofuran_ethers_at_the_benzene_ring
Category:Disulfiram-like drugs
From Wikipedia, the free encyclopedia
Jump to navigationJump to search
The main article for this category is Disulfiram-like drug.
Disulfiram-like drugs that produce sensitivity to the toxic effects of alcohol (drug). Mostly acetaldehyde dehydrogenase inhibitors.
Subcategories
This category has only the following subcategory.
A
Acetaldehyde dehydrogenase inhibitors (24 P)
Pages in category "Disulfiram-like drugs"
The following 30 pages are in this category, out of 30 total. This list may not reflect recent changes (learn more).
Disulfiram
Disulfiram-like drug
B
Benznidazole
E
Etacrynic acid
G
Glibenclamide
Griseofulvin
I
Isoniazid
K
Ketoconazole
L
List of sulfonamides
M
Mepacrine
Metronidazole
N
Nilutamide
Nitrofurantoin
Nitroglycerin
Nitroimidazole
Nitrovasodilator
O
Ornidazole
P
Pargyline
Phenacetin
Phentolamine
Phenylbutazone
Pimecrolimus
Procarbazine
Propranolol
S
Sulfonamide (medicine)
Sulfonylurea
T
Tacrolimus
Tinidazole
Tolazoline
Tolbutamide
https://en.wikipedia.org/wiki/Category:Disulfiram-like_drugs
Category:Halogen-containing natural products
From Wikipedia, the free encyclopedia
Jump to navigationJump to search
Halogen-containing natural products.
Wikimedia Commons has media related to Halogen-containing natural products.
Subcategories
This category has the following 5 subcategories, out of 5 total.
A
Halogen-containing alkaloids (12 P)
B
Bromine-containing natural products (3 P)
C
Chlorine-containing natural products (3 P)
F
Fluorine-containing natural products (7 P)
I
Iodine-containing natural products (3 P)
Pages in category "Halogen-containing natural products"
The following 45 pages are in this category, out of 45 total. This list may not reflect recent changes (learn more).
A
Aplysiatoxin
Ascofuranone
B
Bromoform
Brostallicin
C
Calicheamicin
Chloramphenicol
4-Chloroindole-3-acetic acid
Chloroeremomycin
Chloroform
Chloromethane
Cyanobacterin
Cyclochlorotine
D
Dichloromethane
Dysidenin
E
Epibatidine
F
5-Fluoro-5-deoxy-D-ribose 1-phosphate
Fluoroacetic acid
G
Griseofulvin
H
Halomon
Hydrogen chloride
I
Iodomethane
K
Kaitocephalin
L
Levothyroxine
Liothyronine
M
Maitansine
MC21-A
MC21-B
O
Ochratoxin A
P
Penitrem A
Pentabromopseudilin
Phantasmidine
Prymnesin-1
Prymnesin-2
Prymnesin-B1
Pterulone
Pyoluteorin
R
Radicicol
S
Salinosporamide A
Sceptrin
SCH-202,596
T
Teicoplanin
Telavancin
Thyroid hormones
Tyrian purple
V
Vancomycin
https://en.wikipedia.org/wiki/Category:Halogen-containing_natural_products
Category:Organohalides
From Wikipedia, the free encyclopedia
Jump to navigationJump to search
Wikimedia Commons has media related to Organohalides.
The main article for this category is Halocarbon.
Subcategories
This category has the following 13 subcategories, out of 13 total.
A
Acyl halides (4 C, 1 P)
D
Dihaloacetylenes (4 P)
H
Haloalkyl groups (4 P)
Halogen-containing natural products (5 C, 45 P)
Halogenated solvents (46 P)
Halohydrins (1 C, 28 P)
Halomethanes (44 P)
Hydrochlorofluorocarbons (9 P)
O
Organobromides (4 C, 174 P)
Organochlorides (7 C, 320 P)
Organofluorides (19 C, 268 P)
Organoiodides (5 C, 38 P)
Σ
Organohalide stubs (116 P)
Pages in category "Organohalides"
The following 10 pages are in this category, out of 10 total. This list may not reflect recent changes (learn more).
Halocarbon
A
Aryl halide
B
3-Bromopyridine
H
Haloacetic acids
Haloalkane
Halogenated ether
Halonium ion
P
Polyhalogenated compound
T
Trihalide
V
Vinyl halide
https://en.wikipedia.org/wiki/Category:Organohalides
Category:Resorcinol ethers
From Wikipedia, the free encyclopedia
Jump to navigationJump to search
Subcategories
This category has the following 2 subcategories, out of 2 total.
A
Aflatoxins (7 P)
H
Hydroxyquinol ethers (10 P)
Pages in category "Resorcinol ethers"
The following 25 pages are in this category, out of 25 total. This list may not reflect recent changes (learn more).
0–9
2C-O-4
A
Ablukast
Alnespirone
Amurensin (flavonol)
Antrocamphin B
Atibeprone
B
BAY 38-7271
BAY 59-3074
Bergapten
Brodimoprim
4-Bromo-3,5-dimethoxyamphetamine
Buflomedil
C
Citropten
D
Dalbergichromene
Danielone
Daphnoretin
DIMBOA
Dimefline
Dimetofrine
E
Ensaculin
F
Flavagline
G
Griseofulvin
L
LY-320,135
S
Sinapine
T
Tubocurarine chloride
https://en.wikipedia.org/wiki/Category:Resorcinol_ethers
Category:Cyclohexenes
From Wikipedia, the free encyclopedia
Jump to navigationJump to search
Wikimedia Commons has media related to Cyclohexenes.
Subcategories
This category has only the following subcategory.
C
Cyclohexenols (14 P)
Pages in category "Cyclohexenes"
The following 133 pages are in this category, out of 133 total. This list may not reflect recent changes (learn more).
A
Abscisic acid
Abscisic aldehyde
Acarbose
Acarviosin
Alitretinoin
Alpha-isomethyl ionone
Aminoshikimic acid
Antheraxanthin
Apocarotenal
Astaxanthin
B
Batrachotoxin
Bisabolene
Bisabolol
Bisacurone
Bisnortilidine
C
Cadinenes
Cannabidiol
Cannabidivarin
Canthaxanthin
Carbestrol
3-Carene
Α-Carotene
Β-Carotene
Δ-Carotene
Ε-Carotene
Γ-Carotene
Carthamin
Carvone
Carvonic acid
CBD-DMH
Cinitapride
Citranaxanthin
Conduritol
Coronatine
Β-Cryptoxanthin
Cyclobarbital
Cyclohexenone
D
Dactylifric acid
Damascone
Dehydronorketamine
3-Dehydroshikimic acid
Diadinoxanthin
Diatoxanthin
4,7-Dihydroisoindole
2,4-Dinitrophenylmorphine
E
Echinenone
Elemene
F
Farinamycin
Fenestrel
Fenretinide
Flavoxanthin
4'-Fluorocannabidiol
(6S)-6-Fluoroshikimic acid
Food orange 7
G
Grapefruit mercaptan
Griseofulvin
H
Hagemann's ester
Hawkinsin
Hernandulcin
Hexobarbital
HU-320
HU-331
3'-Hydroxyechinenone
Hydroxymethylpentylcyclohexenecarboxaldehyde
I
Ionone
Isophorone
Beta-Isophorone
Isotretinoin
J
Juvabione
K
KLS-13019
Kuwanon G
L
Limonene
Lutein
M
Manoalide
Maximiscin
Megaphone (molecule)
Menitrazepam
Meso-Zeaxanthin
2-(2-(4-Methyl-3-cyclohexen-1-yl)propyl)cyclopentanone
Momordol
Mutatochrome
Myxoxanthophyll
N
Nalorphine
Navitoclax
Norsecurinine
Nortetrazepam
Nortilidine
O
O-1602
O-1918
Orestrate
Oseltamivir
P
Pelretin
Perillaldehyde
Perillartine
Perillyl alcohol
Phellandrene
Picrocrocin
Pinene
Pinnatoxin
Piperitone
R
Radalbuvir
Retinal
Retinoic acid
Retinol
Retinyl acetate
Retinyl palmitate (chole palm)
Rhodoxanthin
Rubixanthin
S
Salinosporamide A
SCH-202,596
Securinine
Sobrerol
Sporolides
SS220
T
Terpinene
Terpineol
Tetrahydrobenzaldehyde
Tetrazepam
Thialbarbital
Tilidine
Torreyanic acid
Torulene
Tretinoin
V
Valencene
Validamycin
Valienamine
Valienol
Venetoclax
4-Vinylcyclohexene
Viridicatumtoxin A
Viridicatumtoxin B
Z
Beta-Zeacarotene
Zeaxanthin
https://en.wikipedia.org/wiki/Category:Cyclohexenes
Help
Category:Chloroarenes
From Wikipedia, the free encyclopedia
Jump to navigationJump to search
Wikimedia Commons has media related to Chloroarenes.
The main article for this category is Aryl halide.
Contents
Top 0–9 A B C D E F G H I J K L M N O P Q R S T U V W X Y Z
Subcategories
This category has the following 4 subcategories, out of 4 total.
C
Chlorcyclizines (7 P)
Chloropyridines (25 P)
Chloropyrimidines (4 P)
M
Meta-Chlorophenylpiperazines (2 C, 7 P)
Pages in category "Chloroarenes"
The following 200 pages are in this category, out of approximately 917 total. This list may not reflect recent changes (learn more).
(previous page) (next page)
0–9
2C-C
3-Chloro-PCP
4,7-Dichloroquinoline
4EGI-1
5-Cl-bk-MPA
5F-JWH-398
25C-NBOH
25C-NBOMe
A
A-971432
A22 (antibiotic)
ABT-737
Aceclofenac
Acemetacin
Acifluorfen
Aclonifen
Adafenoxate
Adinazolam
Adjudin
ADX-71149
Afatinib
Afoxolaner
Aganodine
AH-7921
AL-1095
Alaproclate
Albaconazole
Alclofenac
Alda-1
Alinidine
Alisertib
Alloclamide
Almoxatone
Alpidem
Alprazolam
Alprazolam triazolobenzophenone
Altizide
Aluminium clofibrate
AM-251 (drug)
AM-0902
Ambenonium chloride
Amibegron
Aminocyclopyrachlor
Amitifadine
Amlodipine
Amodiaquine
Amoxapine
Anagrelide
Anilazine
Apraclonidine
Arbaclofen placarbil
Arfendazam
Arofylline
Arprinocid
Ascofuranone
Asenapine
Asunaprevir
Atovaquone
Atrazine
Avanafil
Azalanstat
Azapride
Azasetron
Azelastine
Azimilide
B
Baclofen
Balovaptan
Bamaluzole
Batanopride
BAY 73-6691
BD1008
BD1052
BD1063
Befiradol
Bemesetron
Benoxaprofen
Benthiocarb
Benzthiazide
Bepotastine
Besifloxacin
Bexlosteride
Bezafibrate
Biclotymol
Bifenox
Bithionol
BL-1020
BML-190
BMS-564,929
Bosutinib
Brilanestrant
Brilaroxazine
BRL-52537
5-Bromo-4-chloro-3-indolyl phosphate
Bromochlorobenzene
Bromochlorosalicylanilide
Brotizolam
BTS 74,398
Bupranolol
Bupropion
Burapitant
Butafenacil
Butanilicaine
Butoconazole
C
CA77.1
Camazepam
Canertinib
Capeserod
Capravirine
Capsazepine
Carbinoxamine
Carbonyl cyanide m-chlorophenyl hydrazone
Carboxyamidotriazole
Carburazepam
Carisbamate
Carprofen
CCG-4986
Cebaracetam
Cenobamate
Cericlamine
CGS-9896
CHIR99021
Chloracyzine
Chloramben
Chloranilic acid
Chlorbenside
Chlorbenzoxamine
Chlorbisan
Chlorcyclizine
Chlordiazepoxide
Chlorfenapyr
Chlorflurazole
Chlorhexidine
Chlorinated polycyclic aromatic hydrocarbon
Chlorisondamine
Chlormadinone
Chlormadinone acetate
Chlormadinone caproate
Chlormethenmadinone acetate
Chlormezanone
Chlormidazole
2-Chloro-m-cresol
4-Chloroindole-3-acetic acid
3-Chloro-4-fluorophenylpiperazine
1-Chloro-9,10-bis(phenylethynyl)anthracene
2-Chloro-9,10-bis(phenylethynyl)anthracene
2-Chloro-9,10-diphenylanthracene
5-Chloro-αMT
6-Chloro-MDMA
4-Chloro-o-toluidine
6-CAT
Para-Chloroamphetamine
4-Chloroaniline
2-Chlorobenzaldehyde
Chlorobenzene
Chlorobenzilate
2-Chlorobenzoic acid
3-Chlorobenzoic acid
4-Chlorobenzoic acid
Chlorohydroxyphenylglycine
7-Chlorokynurenic acid
4-Chlorokynurenine
3-Chloromethamphetamine
Para-Chloromethamphetamine
3-Chloromethcathinone
4-Chloromethcathinone
1-Chloronaphthalene
2-Chloronaphthalene
Meta-Chloroperoxybenzoic acid
Chlorophacinone
Chlorophenol
Chlorophenol red
2-Chlorophenol
3-Chlorophenol
4-Chlorophenol
4-Chlorophenoxyacetic acid
1-(3-Chlorophenyl)-4-(2-phenylethyl)piperazine
(2-Chlorophenyl)thiourea
Chlorophenylbiguanide
Para-Chlorophenylpiperazine
Chlorophenylsilatrane
Chlorophetanol
3-Chlorophthalic anhydride
4-Chlorophthalic anhydride
Chloroprednisone
Chloroprocaine
Chloropyramine
Chloroquine
Chloroquine and hydroxychloroquine during the COVID-19 pandemic
2-Chlorostyrene
Chlorothalonil
Chlorothen
8-Chlorotheophylline
Chlorotoluene
Chloroxine
Chloroxuron
Chloroxylenol
Chlorphenamine
Chlorphenesin carbamate
Chlorphenoxamine
Chlorphentermine
Chlorproethazine
Ketazocine (INN), also known as ketocyclazocine, is a benzomorphan derivative used in opioid receptor research. Ketocyclazocine is an exogenous opioid that binds to the κ opioid receptor.[1]
Activation of this receptor is known to cause sleepiness, a decrease in pain sensation and (potentially) dysphoria, paranoia, and hallucinations. It also causes an increase in urine production because it inhibits the release of vasopressin. (Vasopressin is an endogenous substance that assists in regulating fluid and electrolyte balance in the body and decreases the amount of water released into the urine.)
Unlike other opioids, substances that only bind to the κ receptor theoretically do not depress the respiratory system.
The crystal structure of ketazocine was determined in 1983.[2]
https://en.wikipedia.org/wiki/Ketazocine
From the beginning of the 18th century, Prussian blue was the predominant uniform coat color worn by the infantry and artillery regiments of the Prussian Army.[18] As Dunkelblau (dark blue), this shade achieved a symbolic importance and continued to be worn by German soldiers for ceremonial and off-duty occasions until the outbreak of World War I, when it was superseded by greenish-gray field gray (Feldgrau).[19]
6 groups shown will be missing. This illustration superimposes both possibilities at each site — water molecules or cyanide ions.
Prussian blue is strongly colored and tends towards black and dark blue when mixed into oil paints. The exact hue depends on the method of preparation, which dictates the particle size. The intense blue color of Prussian blue is associated with the energy of the transfer of electrons from Fe(II) to Fe(III). Many such mixed-valence compounds absorb certain wavelengths of visible light resulting from intervalence charge transfer. In this case, orange-red light around 680 nanometers in wavelength is absorbed, and the reflected light appears blue as a result.
Like most high-chroma pigments, Prussian blue cannot be accurately displayed on a computer display. PB is electrochromic—changing from blue to colorless upon reduction. This change is caused by reduction of the Fe(III) to Fe(II), eliminating the intervalence charge transfer that causes Prussian blue's color.
https://en.wikipedia.org/wiki/Prussian_blue
https://en.wikipedia.org/wiki/Prussian_blue
https://en.wikipedia.org/wiki/Prussian_blue
Tolnaftate (INN)[1] is a synthetic thiocarbamate used as an anti-fungal agent that may be sold without medical prescription in most jurisdictions. It is supplied as a cream, powder, spray, liquid, and liquid aerosol. Tolnaftate is used to treat fungal conditions such as jock itch, athlete's foot and ringworm.
https://en.wikipedia.org/wiki/Tolnaftate
Segmental arterial mediolysis (SAM) is a rare disorder of the arteries characterized by the development of aneurysms, blood clots, narrowing of the arteries (stenoses), and blood collections (hematomas) in the affected distribution.[1][2]
SAM most commonly affects the arteries supplying the intestines and abdominal organs.[citation needed]
In 1731, Georg Ernst Stahl published an account of the first synthesis of Prussian blue.[10] The story involves not only Diesbach, but also Johann Konrad Dippel. Diesbach was attempting to create a red lake pigment from cochineal, but obtained the blue instead as a result of the contaminated potash he was using. He borrowed the potash from Dippel, who had used it to produce his animal oil. No other known historical source mentions Dippel in this context. It is, therefore, difficult to judge the reliability of this story today. In 1724, the recipe was finally published by John Woodward.[11][12][13]
https://en.wikipedia.org/wiki/Segmental_arterial_mediolysis
Georg Ernst Stahl (22 October 1659[1] – 24 May 1734) was a German chemist, physician and philosopher. He was a supporter of vitalism, and until the late 18th century his works on phlogiston were accepted as an explanation for chemical processes.[2]
Georg Ernst Stahl | |
|---|---|
 Georg Ernst Stahl | |
| Born | 22 October 1659 |
| Died | 24 May 1734 (aged 74) Berlin, Holy Roman Empire |
| Nationality | German |
| Alma mater | University of Jena |
| Known for | Phlogiston theory Fermentation |
| Scientific career | |
| Fields | Chemistry |
| Institutions | University of Halle |
| Influences | J. J. Becher |
He was born in St. John's parish in Ansbach, Brandenburg on October 21, 1659. His father was Johann Lorentz Stahl.[4] He was raised in Pietism, which influenced his viewpoints on the world. His interests in chemistry were due to the influence a professor of medicine, Jacob Barner, and a chemist, Johann Kunckel von Löwenstjern.[5] In the late 1670s, Stahl moved to Saxe-Jena to study medicine at the University of Jena. Stahl’s success at Jena earned him a M.D. around 1683 and then he went on to teach at the same university.
Teaching at the university gained him such a good reputation that in 1687 he was hired as the personal physician to Duke Johann Ernst of Sachsen-Weimar. In 1693, he joined his old college friend Friedrich Hoffmann at the University of Halle.[5] In 1694, he held the chair of medicine at the University of Halle. From 1715 until his death, he was the physician and counselor to King Friedrich Wilhelm I of Prussia and in charge of Berlin's Medical Board.[4]
Stahl's focus was on the distinction between the living and nonliving. Although he did not support the views of iatro-mechanists, he believed that all non-living creatures are mechanical and so are living things to a certain degree.[4] His views were that nonliving things are stable throughout time and did not rapidly change. On the other hand, living things are subject to change and have a tendency to decompose, which led Stahl to work with fermentation.
Stahl professed an animistic system, in opposition to the materialism of Hermann Boerhaave and Friedrich Hoffmann.[6] His main argument on living things was that there is an agent responsible for delaying this decomposition of living things and that agent is the anima or soul of the living organism. The anima controls all of the physical processes that happen in the body. It not only just controls the mechanical aspects of it but the direction and goals of them too.[2] How the anima controls these processes is through motion. He believed that the three important motions of the body are the circulation of blood, excretion and secretion.
These beliefs were reflected in his views on medicine. He thought that medicine should deal with the body as a whole and its anima, rather than the specific parts of a body. Having knowledge on the specific mechanical parts of the body is not very useful.[2]His views had been criticized by Gottfried Leibniz, with whom he exchanged letters, later published in a book titled Negotium otiosum seu σκιαμαχία (1720).[7][8] Also, during the first part of the 18th century, Stahl's ideas on the non-physical part of the body were disregarded while his mechanistic ideas on the body were accepted in the works of Boerhaave and Hoffmann.[9]
As a physician, Stahl worked with patients and focused on the soul, or anima, as well as blood circulation and tonic motion. Animawas a vital force that when working properly would allow the subject to be healthy; however, when malfunction of the animaoccurred, so did illness. Tonic motion, to Stahl, involved the contracting and relaxing movements of the body tissue in order to serve the three main purposes. Tonic motion helped explain how animals produce heat and how fevers were caused. In Stahl's 1692 dissertation, De motu tonico vitali, Stahl explains his theory of tonic motion and how it is connected to blood flow within a subject, without citing William Harvey's blood flow and circulation theories, which lacked an explanation of irregular blood flow. Also within the dissertation, 'practitioners' are mentioned as users of his theory of tonic motion.
Stahl's theory of tonic motion was about the muscle tone of the circulatory system. During his work at Halle, Stahl oversaw patients experiencing headaches and nosebleeds. Tonic motion explained these phenomena as blood needed a natural or artificial path to flow when a part of the body is obstructed, injured, or swollen. Stahl also experimented with menstruation, finding that bloodlettingin an upper portion of the body would relieve bleeding during the period. During the next period, the wound would experience pain and swelling, which would only be relieved by an opening in the foot. He also followed this procedure as a treatment for amenorrhoea.[10]
The best of Stahl’s work in chemistry was done while he was a professor at Halle. Just like medicine, he believed that chemistry could not be reduced to mechanistic views. Although he believed in atoms, he did not believe that atomic theories were enough to describe the chemical processes that go on. He believed that atoms could not be isolated individually and that they join together to form elements. He took an empirical approach when establishing his descriptions of chemistry.[5]
Stahl used the works of Johann Joachim Becher to help him come up with explanations of chemical phenomena. The main theory that Stahl got from J. J. Becher was the theory of phlogiston. This theory did not have any experimental basis before Stahl. Becher's theories attempted in explaining chemistry as comprehensively as seemingly possible through classifying different earths according to specific reactions. Terra pinguis was a substance that escaped during combustion reactions, according to Becher.[11]Stahl, influenced by Becher's work, developed his theory of phlogiston. Phlogiston theory did not have any experimental basis before Stahl worked with metals and various other substances in order separate phlogiston from them. Stahl proposed that metals were made of calx, or ash, and phlogiston and that once a metal is heated, the phlogiston leaves only the calx within the substance. He was able to make the theory applicable to chemistry as it was one of the first unifying theories in the discipline. Phlogiston provided an explanation of various chemical phenomena and encouraged the chemists of the time to rationally work with the theory to explore more of the subject. This theory was later replaced by Antoine-Laurent Lavoisier’s theory of oxidation and caloric theory.[4] He also propounded a view of fermentation, which in some respects resembles that supported by Justus von Liebig a century and half later. Although his theory was replaced, Stahl's theory of phlogiston is seen to be the transition between alchemy and chemistry.[5]
Stahl is credited for being among the first to describe carbon monoxide as noxious carbonarii halitus (carbonic vapors) in his 1697 publication Zymotechnia fundamentalis.[12]
Works[edit]
- Zymotechnia fundamentalis (1697)
- Disquisitio de mechanismi et organismi diversitate (1706)
- Paraenesis, ad aliena a medica doctrine arcendum (1706)
- De vera diversitate corporis mixti et vivi (1706)
- Theoria medica vera (1708)
- Georgii Ernesti Stahlii opusculum chymico-physico-medicum : seu schediasmatum, a pluribus annis variis occasionibus in publicum emissorum nunc quadantenus etiam auctorum et deficientibus passim exemplaribus in unum volumen iam collectorum, fasciculus publicae luci redditus / Praemißa praefationis loco authoris epistola ad Michaelem Alberti (1715) Digital edition by the University and State Library Düsseldorf
- Specimen Beccherianum (1718)[2]
- Philosophical Principles of Universal Chemistry (1730), Peter Shaw, translator, from Open Library.
- Materia medica : das ist: Zubereitung, Krafft und Würckung, derer sonderlich durch chymische Kunst erfundenen Artzneyen(1744), Vol. 1&2 Digital edition by the University and State Library Düsseldorf
- The Leibniz-Stahl Controversy (2016), transl. and edited by F. Duchesneau and J. H. Smith, Yale UP (536 pp.)
https://en.wikipedia.org/wiki/Georg_Ernst_Stahl
The phlogiston theory is a superseded scientific theory that postulated the existence of a fire-like element called phlogiston (/flɒˈdʒɪstən, floʊ-, -ɒn/)[1][2] contained within combustible bodies and released during combustion. The name comes from the Ancient Greek φλογιστόν phlogistón(burning up), from φλόξ phlóx (flame). The idea was first proposed in 1667 by Johann Joachim Becher and later put together more formally by Georg Ernst Stahl. Phlogiston theory attempted to explain chemical processes of weight increase such as combustion and rusting, now collectively known as oxidation, and was abandoned before the end of the 18th century following experiments by Antoine Lavoisier and others. Phlogiston theory led to experiments which ultimately concluded with the discovery of oxygen.
https://en.wikipedia.org/wiki/Phlogiston_theory
- 1659 births
- 1734 deaths
- People from Ansbach
- People from the Principality of Ansbach
- 18th-century German chemists
- 17th-century German physicians
- 18th-century German physicians
- 17th-century German writers
- 18th-century German writers
- University of Jena alumni
- German male writers
- Vitalists
- 17th-century German chemists
Oxford Blue is the official colour of the University of Oxford.[2] The official Oxford branding guidelines set the definition of Oxford Blue as Pantone 282, equivalent to the hex code#002147.[3]
With a hue code of 212, this colour is a very dark tone of azure.
| Oxford Blue | |
|---|---|
| Hex triplet | #002147 |
| HSV (h, s, v) | (212°, 100%, 28[1]%) |
| sRGBB (r, g, b) | (0, 33, 71) |
| Source | Oxford Branding Guidelines |
| ISCC–NBS descriptor | Dark blue |
| B: Normalized to [0–255] (byte) H: Normalized to [0–100] (hundred) | |
https://en.wikipedia.org/wiki/Oxford_Blue_(colour)
| Sapphire | |
|---|---|
 Sapphire gemstone | |
| Hex triplet | #082567 |
| HSV (h, s, v) | (222°, 86%, 22%) |
| sRGBB (r, g, b) | (8, 37, 103) |
| Source | 99Colors |
| ISCC–NBS descriptor | Deep blue |
| B: Normalized to [0–255] (byte) H: Normalized to [0–100] (hundred) | |
https://en.wikipedia.org/wiki/Shades_of_blue#Penn_blue
| White | |
|---|---|
| Hex triplet | #FFFFFF |
| HSV (h, s, v) | (0°, 0%, 100%) |
| sRGBB (r, g, b) | (255, 255, 255) |
| Source | By definition |
| ISCC–NBS descriptor | White |
| B: Normalized to [0–255] (byte) H: Normalized to [0–100] (hundred) | |
| Black | |
|---|---|
| Hex triplet | #000000 |
| HSV (h, s, v) | (?°, 0%, 0%) |
| sRGBB (r, g, b) | (0, 0, 0) |
| Source | By definition |
| B: Normalized to [0–255] (byte) H: Normalized to [0–100] (hundred) | |
| Silver | |
|---|---|
| Hex triplet | #C0C0C0 |
| HSV (h, s, v) | (?°, 0%, 75%) |
| sRGBB (r, g, b) | (192, 192, 192) |
| Source | HTML/CSS[1] |
| ISCC–NBS descriptor | Light gray |
| B: Normalized to [0–255] (byte) | |
| Medium Gray | |
|---|---|
| Hex triplet | #BEBEBE |
| HSV (h, s, v) | (?°, 0%, 75[10]%) |
| sRGBB (r, g, b) | (190, 190, 190) |
| Source | X11 |
| ISCC–NBS descriptor | Light gray |
| B: Normalized to [0–255] (byte) H: Normalized to [0–100] (hundred) | |
| Jet | |
|---|---|
| Hex triplet | #343434 |
| HSV (h, s, v) | (33°, 0%, 20[19]%) |
| sRGBB (r, g, b) | (52, 52, 52) |
| Source | ISCC-NBS |
| ISCC–NBS descriptor | Black |
| B: Normalized to [0–255] (byte) H: Normalized to [0–100] (hundred) | |
| White smoke | |
|---|---|
| Hex triplet | #F5F5F5 |
| HSV (h, s, v) | (0°, 0%, 96.1%) |
| sRGBB (r, g, b) | (245, 245, 245) |
| Source | X11 |
| ISCC–NBS descriptor | White |
| B: Normalized to [0–255] (byte) H: Normalized to [0–100] (hundred) | |
https://en.wikipedia.org/wiki/Shades_of_white
https://en.wikipedia.org/wiki/Shades_of_white#White_smoke
Sodium thiosulfate (sodium thiosulphate) is an inorganic compound with the formula Na2S2O3.xH2O. Typically it is available as the white or colorless pentahydrate, Na2S2O3·5H2O. The solid is an efflorescent (loses water readily) crystalline substance that dissolves well in water.[2]
Sodium thiosulfate is used in gold mining, water treatment, analytical chemistry, the development of silver-based photographic film and prints, and medicine. The medical uses of sodium thiosulfate include treatment of cyanide poisoning and pityriasis.[3] It is on the World Health Organization's List of Essential Medicines, the safest and most effective medicines needed in a health system.[4]
https://en.wikipedia.org/wiki/Sodium_thiosulfate
https://en.wikipedia.org/wiki/Ketazocine
https://en.wikipedia.org/wiki/Georg_Ernst_Stahl
https://en.wikipedia.org/wiki/Postpartum_infections
https://en.wikipedia.org/wiki/Prussian_blue_(medical_use)
https://en.wikipedia.org/wiki/Prussian_blue
https://en.wikipedia.org/wiki/Thallium#Thallium(I)
https://en.wikipedia.org/wiki/Caesium
https://en.wikipedia.org/wiki/Toxic_heavy_metal
Mercury, Lead, Boron, Beryllium, Bromine, Silver,
https://en.wikipedia.org/wiki/Cobalt_glass
https://en.wikipedia.org/wiki/Potash
https://en.wikipedia.org/wiki/Tetraethyllead
https://en.wikipedia.org/wiki/Lead(II)_azide
https://en.wikipedia.org/wiki/Benzophenone
Element Acute exposure
usually a day or less Chronic exposure
often months or years
Cadmium Pneumonitis (lung inflammation) Lung cancer
Osteomalacia (softening of bones)
Proteinuria (excess protein in urine; possible kidney damage)
Mercury Diarrhea
Fever
Vomiting Stomatitis (inflammation of gums and mouth)
Nausea
Nephrotic syndrome (nonspecific kidney disorder)
Neurasthenia (neurotic disorder)
Parageusia (metallic taste)
Pink Disease (pain and pink discoloration of hands and feet)
Tremor
Lead Encephalopathy (brain dysfunction)
Nausea
Vomiting Anemia
Encephalopathy
Foot drop/wrist drop (palsy)
Nephropathy (kidney disease)
Chromium Gastrointestinal hemorrhage (bleeding)
Hemolysis (red blood cell destruction)
Acute renal failure Pulmonary fibrosis (lung scarring)
Lung cancer
Arsenic Nausea
Vomiting
Diarrhea
Encephalopathy
Multi-organ effects
Arrhythmia
Painful neuropathy Diabetes
Hypopigmentation/Hyperkeratosis
Cancer
https://en.wikipedia.org/wiki/Toxic_heavy_metal
The toxic effects of arsenic, mercury and lead were known to the ancients but methodical studies of the overall toxicity of heavy metals appear to date from only 1868. In that year, Wanklyn and Chapman speculated on the adverse effects of the heavy metals "arsenic, lead, copper, zinc, iron and manganese" in drinking water. They noted an "absence of investigation" and were reduced to "the necessity of pleading for the collection of data".[30] In 1884, Blake described an apparent connection between toxicity and the atomic weight of an element.[31] The following sections provide historical thumbnails for the "classical" toxic heavy metals (arsenic, mercury and lead) and some more recent examples (chromium and cadmium).
Arsenic[edit]
Arsenic, as realgar (As
4S
4) and orpiment (As
2S
3), was known in ancient times. Strabo (64–50 BCE – c. AD 24?), a Greek geographer and historian,[32] wrote that only slaves were employed in realgar and orpiment mines since they would inevitably die from the toxic effects of the fumes given off from the ores. Arsenic-contaminated beer poisoned over 6,000 people in the Manchester area of England in 1900, and is thought to have killed at least 70 victims.[33] Clare Luce, American ambassador to Italy from 1953 to 1956, suffered from arsenic poisoning. Its source was traced to flaking arsenic-laden paint on the ceiling of her bedroom. She may also have eaten food contaminated by arsenic in flaking ceiling paint in the embassy dining room.[34] Ground water contaminated by arsenic, as of 2014, "is still poisoning millions of people in Asia".[35]
https://en.wikipedia.org/wiki/Toxic_heavy_metal
Above. arsenic level dimension filament particle proton ion EMR EMF electromagnetism electrodynamics
https://en.wikipedia.org/wiki/Light_metal
Sources[edit]
- Betz Laboratories Handbook of Industrial Water Conditioning(7th Edition) Betz Laboratories (1976)
- Kemmer, Frank N.'The NALCO Water Handbook' McGraw-Hill (1979)
- Perry, Robert H., Chilton, Cecil H. and Kirkpatrick, Sidney D. Chemical Engineers' Handbook (4th Edition) McGraw-Hill (1963)
- Woodruff, Everett B., Lammers, Herbert B. and Lammers, Thomas F. Steam-Plant Operation (5th Edition) McGraw-Hill (1984) ISBN 0-07-071732-X
https://en.wikipedia.org/wiki/Boiler_blowdown
Rural areas[edit]
Rural areas with low population density may not need formal FSM services if the local practice is to cover and rebuild latrines when they fill up. However, if this is not possible, rural areas often lack treatment facilities within a reasonable (say 30 minutes drive) distance; are difficult for tankers to access and often have limited demand for emptying making transport and treatment uneconomic, and unaffordable for most people. Therefore, options such as relocating latrines on-site, double (alternating) pit or Arborloo toilets could be considered. Also sharing decentralized FSM services and sludge treatment between nearby villages, or direct safe removal burial of waste could be considered and organized.
Alternatives to fecal sludge producing systems[edit]
Most types of dry toilets (except for pit latrines) do not generate fecal sludge but generate instead dried feces (in the case of urine-diverting dry toilets) or compost (in the case of composting toilets). For example, in the case of Arborloo toilets, nothing is ever extracted from the pit and, instead, the lightweight outhouse superstructure is moved to another shallow hole and a tree is planted on top of the filled hole.
https://en.wikipedia.org/wiki/Fecal_sludge_management
A dry toilet (or non-flush toilet, no flush toilet or toilet without a flush) is a toilet that operates without flush water, unlike a flush toilet.[1]The dry toilet may have a raised pedestal on which the user can sit, or a squat pan over which the user squats in the case of a squat toilet. In both cases, the excreta (both urine and feces) falls through a drop hole.[1]
A dry toilet can be any of the following types of toilets: a composting toilet, urine-diverting dry toilet, arborloo, container-based toilet, bucket toilet, simple pit latrine (but not those that operate on a "pour flush" basis), incinerating toilets, or freezing toilets.
The urine and feces can either become mixed at the point of dropping or stay separated, which is called urine diversion.
https://en.wikipedia.org/wiki/Dry_toilet
An incinerating toilet is a type of dry toilet that burns human feces instead of flushing them away with water, as does a flush toilet.[1]
Incinerating toilets are used only for niche applications, which include:
- Apartments with limited or difficult access to waste plumbing.
- Houses without access to drains, and where building a septic tank would be difficult or uneconomic.
- On yachts and canal barges, as an alternative to a Blackwater holding tank, which needs to be pumped out occasionally.
- On mobile homes, RVs and caravans/(trailers).
Incinerating toilets may be powered by electricity, gas, dried feces or other energy sources.[2][3] Incinerating toilets gather excrement in an integral ashpan and then incinerate it,[4] reducing it to pathogen-free ash.[5] Some will also incinerate "grey water" created from showers and sinks.
https://en.wikipedia.org/wiki/Incinerating_toilet
China's waste import ban, instated at the end of 2017, prevented foreign inflows of waste products. Starting in early 2018, the government of China, under Operation National Sword, banned the import of several types of waste, including plastics. The ban has greatly affected recycling industries worldwide,[1] as China had been the world's largest importer of waste plastics and processed hard-to-recycle plastics for other countries, especially in the West.[1]
The decision caused widespread repercussions on a global scale. In July 2018, China produced a document to the World Trade Organization regarding environmental and health issues. China requested an urgent change to be made revolving the imported waste China imports from other countries. The recommended list was pushing forward for wastes such as plastics, textile, and paper products to be banned from imports.[2]
Sorting[edit]
The first step of disposing of the waste is to divide them into different categories. Recycling standards are various from different countries. But we can divide them into two big categories, recyclable and none-recyclable waste. In general, plastic products can be fully recycled. The difficulty is the sorting process. For example, although the plastic bottle is theoretically 100% recyclable, the plastic bottle cap and the label cannot be mixed together for recycling because they are different plastic materials. The sorting machine is currently unable to unscrew the cap and tear off the label, so this step must be done manually by the sorting worker. This process obviously increases the business cost and human resources. Some illegal industries recycled mixed plastic products together to control the costs, this cause incomplete recycling of plastic which causes some unexpected environmental issues.
Burning[edit]
The general disposal method is to categorize the types of waste and dispose of them in different processes. However, a few illegal industries want to minimize the cost of disposing of the waste, so they choose the easiest way to deal with the rubbish. By inappropriate use of landfills and incinerators, earning money from the disposal of waste, rather than the secondary benefits of proper recycling waste. The burning of uncategorized waste produces toxic and contaminate air to the sky which harmful for human health. The carbon dioxide also produced by the process of burning wastes. By statistic, the global total carbon dioxide produced in 2018 was about 37.1 gigatonnes.[11] Some power plants were operated by the heat produced from the burning of waste (Waste-to-energy plant). It is a combination of disposing of waste and producing electricity which widely adopted in China's waste disposing industries.
Pyrolysis plants[edit]
Pyrolysis plants is an innovative technology that can help in the aid of waste disposal. The process of disposing of is describing as "Plastics are crushed and melted at temperatures below gasification temperature and contain less oxygen. Heat decomposes plastic polymers into smaller hydrocarbons that can be refined into diesel or even other petrochemical products, including new plastics." This technology is still in the demonstration phase and hopping to expand globally. The facilities are built in China as well. Pyrolysis plants can recycle many hard to decomposed materials that normal recyclers cannot. It will only produce a little carbon dioxide and no contamination at all. The economic profits from expensive pyrolysis plants is the determinate factor of whether to build more of these plants or not.
https://en.wikipedia.org/wiki/China%27s_waste_import_ban
Biosolids, waste, and waste management (president biden infrastructure plan?)
Major types
Agricultural wastewater Biodegradable waste Biomedical waste Brown waste Chemical waste Construction waste Demolition waste Electronic waste by country Food waste Green waste Hazardous waste Heat waste Industrial waste Industrial wastewater Litter Marine debris Mining waste Municipal solid waste Open defecation Packaging waste Post-consumer waste Radioactive waste Scrap metal Sewage Sharps waste Surface runoff Toxic waste
Bedrijfsafval.jpg
Processes
Anaerobic digestion Balefill Biodegradation Composting Garden waste dumping Illegal dumping Incineration Landfill Landfill mining Mechanical biological treatment Mechanical sorting Photodegradation Recycling Resource recovery Sewage treatment Waste collection Waste sorting Waste trade Waste treatment Waste-to-energy
Countries
Armenia Australia Bangladesh Brazil Hong Kong India Japan Kazakhstan New Zealand Russia South Korea Switzerland Taiwan Thailand Turkey United Kingdom United States
Agreements
Bamako Convention Basel Convention EU directives batteries framework incineration landfills RoHS vehicles waste water WEEE London Convention Oslo Convention OSPAR Convention
Occupations
Sanitation worker Street sweeper Waste collector Waste picker
Other topics
Blue Ribbon Commission on America's Nuclear Future China's waste import ban Cleaner production Downcycling Eco-industrial park Extended producer responsibility High-level radioactive waste management History of waste management Landfill fire Sewage regulation and administration Upcycling Waste hierarchy Waste legislation Waste minimisation Zero waste
Aegopodium podagraria1 ies.jpg Environment portal Category Category: Waste Concepts Index Journals Lists Organizations
showvte
Toilets
Categories: Toilet typesSanitationWater conservation
https://en.wikipedia.org/wiki/Dry_toilet
https://en.wikipedia.org/wiki/Dry_toilet
https://en.wikipedia.org/wiki/Waste-to-energy
https://en.wikipedia.org/wiki/Waste_treatment
https://en.wikipedia.org/wiki/Anaerobic_digestion

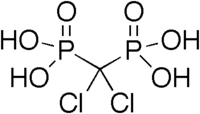






















No comments:
Post a Comment